Abstract
The Coombs test is important in hematology for detecting erythrocyte-bound IgG antibodies or in serm through agglutination methods, but its sensitivity and specificity are limited. Flow cytometry provides a more precise and sensitive alternative for quantitatively assessing RBC-bound IgG antibodies. This assessment is crucial for evaluating the risk of hemolytic reactions and ensuring safe transfusions. This study aimed to explore a new method for the detection of RBC-bound IgG antibodies in rabbits following the injection of human red blood cells. Rabbits serum treated with 2-mercaptoethanol (2-ME) were serially diluted at ratios of 1:1, 1:2, 1:4, 1:8, 1:16, 1:32, 1:64, 1:128, 1:256, 1:512, 1:1024, and 1:2048. These diluted samples were then reacted with O-type red blood cells (RBCs). Serum samples from healthy individuals were used as the control group. The tubes were kept in a water bath at 37°C for 30 min incubation. After incubation, the samples were analyzed using a flow cytometry-based assay. Additionally, the traditional Coombs tube method was used and the strength of IgG antibody and agglutination was graded. The results were analyzed using a flow cytometry-based assay, and the agglutination strength was determined using the Coombs traditional tube method for RBC-bound IgG antibodies. A significant difference was found between the rabbits serum and normal control groups (p < 0.001). IgG titers increased significantly after 1 month of immunization in rabbits compared to the titers observed after 1 week. The serum Anti-D stability test showed a coefficient of variation (CV) of 7.74%, indicating good stability of the test results. In this study, we concluded that the flow cytometry-based assay for detecting RBC-bound IgG antibodies was accurate, sensitive, and had positional value in clinical laboratories and research centers.
Keywords: agglutination, antihuman globulin, flow cytometry, immunization, serum
Introduction
In 1945, a veterinarian, Robin Coombs developed direct and indirect antiglobulin tests to identify subagglutinating and alloantibodies attached to the surface of red blood cells (RBCs). The Coombs test remains a crucial diagnostic immunohematology technique in both veterinary and human medicine, including blood banking.1,2 The Coombs test is one of the most widely used assays in medical laboratories for detecting antibodies against red blood cells (RBCs) circulating in the body, which can cause hemolysis.3,4 This test is also known as the anti-human globulin test (AHG) or antibody screening test. RBC destruction by these antibodies is diagnostically termed auto-immune hemolytic anemia (AIHA).5,6 The Coombs test is a relatively simple and non-invasive test that can provide important information about a patient’s condition. However, it should be performed by a trained medical professional in the laboratory setting. Several factors can potentially affect the accuracy of Coombs test results. These include the timing of the test, sample quality, and recent blood transfusions, which can interfere with the test’s accuracy. Additionally, technical factors such as errors in reagent preparation, incorrect interpretation of results, or instrument malfunctions can also impact the accuracy of the Coombs test. 7
There are two types of Coombs tests: the direct antiglobulin test (DAT) and the indirect antiglobulin test (IAT). The principle of the direct antiglobulin test is to detect antibodies attached directly to the red blood cells, while the indirect test looks for floating antibodies present in the liquid part of the blood called serum.8,9 Direct antiglobulin tests can be used to investigate antibodies in patients with autoimmune hemolytic anemia, alloantibody investigation in newborn babies suffering from hemolytic disease, and to document hemolytic transfusion reactions. The indirect antiglobulin test is used for detecting irregular antibodies in the patient’s serum, determining red blood cell phenotypes, and as a part of the cross-match screening process for blood transfusions.10,11
Direct antiglobulin (DAT) is the most commonly used method and is still considered the gold standard method for the diagnosis of autoimmune hemolytic anemia (AIHA). This is because DAT is not sensitive enough visually by conventional tube technique (CTT) to detect IgG less than 500 molecules per red cell.12,13 The detection of RBC agglutination is commonly performed using conventional tube methods, gel microcolumn methods, and traditional slide methods. The naked eye can observe the degree and intensity of agglutination by noting the distribution, uniformity, and overall alterations in both aggregated and non-aggregated cells within the liquid. 14 Despite the availability of blood typing equipment in diverse markets, challenges persist, including issues related to visual observation of blood samples, automation difficulties, and concerns regarding blood handling processes. 15 The outcomes are influenced by the subjective judgments of the observers. Studies indicate that the level of agglutination observed by the same individual and by different individuals can impact the reliability and accuracy of the experiment. Therefore, for the detection of RBC-bound IgG establishing of flow cytometry method has become important.16,17
The gel test (GT) is also widely used for the detection of RBC-bound IgG and is more sensitive and accurate than the conventional tube technique (CTT) for the direct antiglobulin test (DAT). However, false positive results have been found in certain studies, affecting the test’s accuracy.18,19 Flow cytometry method (FCM) is a well-known and effective analytical technology that has yielded numerous revolutionary findings in cell biology and cellular-molecular disease diagnosis. 20 Flow cytometry was a single-parameter tool for detecting the size of cells in previous studies but recently, advanced instruments have been developed that can detect up to 14 parameters simultaneously. 21 Flow cytometry can measure the fluorescence and optical characteristics of a single cell or other particles, such as microorganisms, DNA, and chromosome formation in the fluid stream passing through a beam of light. Parameters used to analyze and differentiate cells include granularity, size, and fluorescent characteristics derived from dyes or antibodies. 22
Flow cytometry in the field of immunohematology is gradually increasing due to its high sensitivity, accuracy, and reproducibility compared to other traditional laboratory technique. 23 It is being implemented as an alternative method for detecting erythrocyte agglutination. Flow cytometry allows for the precise measurement of IgG and IgM antibodies.24,25 Flow cytometry has been studied to be the most sensitive technique for detecting erythrocyte-bound antibodies and has the potential to detect as low as 30–40 molecules per red cell. Additionally, it also requires a smaller sample volume, is less time-consuming, and can be integrated into an automated system. 26 This study aimed to provide a sensitive, simple, and precise method to detect RBC-bound IgG antibodies used in clinical practice.
Materials and methods
Ethical statement
The experiments involving both animals and humans were conducted in strict accordance with the ethical guidelines outlined in the Declaration of Helsinki. The study received approval from the Ethics Committee of Dalian Medical University, with the ethical number AEE23125. Additionally, all necessary informed consent was obtained from human participants in written form. For the animal studies, the research received approval from the appropriate regulatory body, and all procedures were carried out in compliance with the relevant animal welfare guidelines and regulations.
Appropriate housing conditions for rabbits were provided, the cage size for a single rabbit was around 4–6 square feet of floor space, the temperature range was maintained as 18−24°C (64−75°F), and the humidity level was set around 30–70% to ensure the rabbit’s comfort. Environmental monitoring and control systems were implanted to regulate these factors, lighting cycle that mimics natural daylight patterns was also ensured, including both periods of light and darkness. This helps regulate the rabbit’s circadian rhythm and promotes their overall health and well-being. Proper ventilation to maintain air quality and prevent the buildup of odors, dust, or harmful gases were ensured. Furthermore, sufficient fresh air exchange is essential to maintain a healthy environment for the rabbits.
Reagents and chemicals
This study used various components, including serum obtained from albino white rabbits, red blood cells (RBCs), and serum from young, healthy volunteers with blood group-O, Antihuman globulin (AHG), 2-mercaptoethanol (2-ME), and 0.9% normal saline. AHG, monoclonal antibody reagents that specifically bind to RBC-bound IgG antibodies. The host species are humans and rabbits. It is widely employed in blood typing and compatibility testing. The AHG used in the experiment was an IgG antibody isotope manufactured by Shanghai Biological Medicine Co. Ltd. 2-ME (2-mercaptoethanol) manufactured by Changchun Boxun Biotechnology, It is commonly used in laboratory procedures to break down certain structures of IgM and facilitate specific reactions. This study also used Anti-D IgG, a commercially available biological antibody that targets red blood cells positive for the Rh (D) antigen (also referred to as the D antigen). Following the incubation of Anti-D with D antigen-positive red blood cells, anti-human globulins were introduced, resulting in a positive outcome. The Anti-D antibody is commonly used in various medical procedures, such as blood transfusions and Rh factor testing.
Collection and preparation of 50% RBCs from blood group O of young healthy volunteers
Four milliliters of blood were drawn from a healthy individual with blood group O into an EDTA tube. To prepare 50% RBCs, the whole blood was centrifuged at 3500 rpm for 5 min. After centrifugation, the supernatant plasma and buffy coat were removed, and 5 ml sterile normal saline was added to the RBCs. The tube was shaken and re-centrifuged at 3500 rpm for 5 min, the process was repeated 2–3 times until the supernatant was clear and the liquid became transparent. Finally, to collect 50% RBCs concentration, 500 µl RBC-O was added to 500 µl 0.9% saline and then used for further experimental purposes.
Administration of RBC-O for the immunization of rabbits
Albino white rabbits weighing 3–5 kg were used for the production and detection of IgG antibodies. Blood was drawn from the rabbits before the administration of any RBC injection dose and used as a control group. A 1 ml of 50% RBC-O was injected into the marginal vein of each rabbit every other day for 1 month. Blood was collected from the rabbit before each subsequent red blood cell injection.
Preparation and collection of sera from the rabbit’s blood
Blood was drawn from the rabbits every other day. To separate the serum, the blood was centrifuged at 3500 rpm for 10 min. After centrifugation, the serum was taken and placed in a water bath at 56°C for 30 min to inactivate the complement system. The serum was then stored at −20°C for future experimental studies.
Preparation of 2% RBCs from the healthy human blood group O
Blood was collected in EDTA tubes from a healthy individual with blood group O. The blood was kept in the refrigerator for 30 min and then centrifuged at 3500 rpm for 5 min. After centrifugation, the supernatant was discarded and the RBCs were washed 2–3 times until the supernatant was clear. 20 μl RBCs were added to 980 μl of 0.9% saline and then used for experimental work.
Comparison of young healthy human serum and standard anti-D antibodies using flow cytometry
Normal healthy D antigen positive human serum and anti-D antibody samples were obtained. 100 μl of anti-D serum was antibody IgG added to three Eppendorf tubes, each containing 50 μl RBC-O and 100 μl 0.9% saline. Additionally, a control sample was prepared using saline instead of serum. All the tubes were incubated in a water bath at 37°C for 30 min. In contrast, the same process was performed for the healthy serum group. The results were then analyzed using flow cytometry.
Establishment of flow cytometry for the determination of RBC-bound IgG
A flow Cytometer (Agilent NovoCytes 2040R) was used in this study. Flow cytometry is a widely used technique to analyze cells for various purposes such as phenotyping, cell counting, viability, and cell cycle assessment. In a flow cytometer, lasers produce light that is scattered by cells in the sample, which can measure and then produce signals that can be analyzed and measured using a computer system. Flow cytometry technique determines small cell populations in a very short time and is found to be a more accurate and sensitive method for detecting RBC-bound IgG antibodies. Thus, preliminary flow cytometry was used to detect RBC-bound IgG antibodies.
Use of 2-ME for inactivation of IgM antibodies
100 µl of rabbit serum was added to an equal amount of 2-ME (100 µl), mixed thoroughly, and placed in a water bath at 37°C for 1 h. After incubation, the results were analyzed using flow cytometry and the traditional tube method.
Determination of serum antibodies (IgG) of the rabbits by using flow cytometry
Rabbit sera, treated every other day with 2-ME, were serially diluted (1:1, 1:2, 1:4, 1:8, 1:16, 1:32, 1:64) in tubes containing 50 µl of 2% RBC-O and an equal amount of 100 µl saline. The tubes were incubated in a water bath at 37°C for 30 min, mixed well after incubation, and analyzed using flow cytometry. At the same time for the control group, 100 µl healthy serum of human was mixed with 50 µl of 2% RBCs and processed by flow cytometry method.
Flow cytometry parameters were set as follows: FSC-H threshold was set to more than 100,000 and the stop condition setting was 20 μl. The diameter of the sample flow was 7.2 μm and the flow rate of the sample was set at 14 μl/min. The Absolute Count Value of each sample was recorded after analyzing all samples using flow cytometry.
To compare the values of the original and control group samples, the agglutination index (IAG) was used, based on the following equation:
The IAG ranges from 0 to 1, where a higher agglutination index indicates stronger agglutination strength, agglutination strength refers to the level of clumping observed when RBCs come into contact with other RBCs through antibodies.
Quadrant gate formation for the agglutination of RBC-O based on flow cytometry
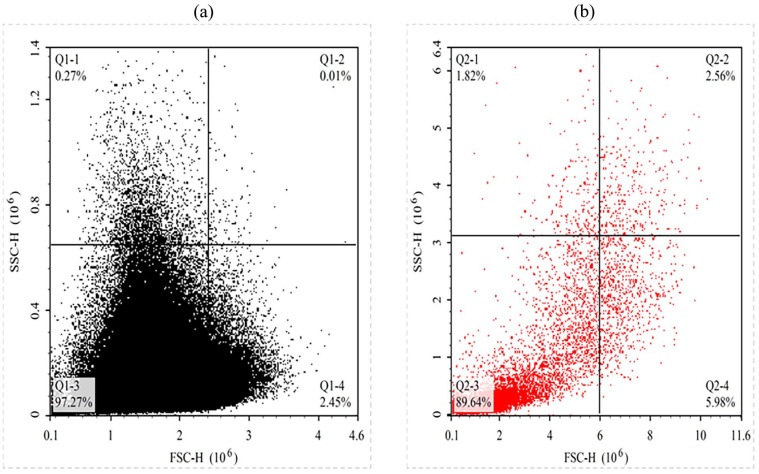
Quadrant gates were generated after obtaining the results for serum IgG and RBC-O to mark the cells of interest in the resulting map. Forward scattering (FSC-H) and Side scattering (SSC-H) were selected as the abscissa to set the quadrant gates. The purpose of this experiment was to assess the agglutination of red blood cells. The abscissa were divided and ordinated equally such as Q1-1(upper left), Q1-2(upper right), Q1-3(lower left), and Q1-4(lower right) for gating, and the results were analyzed. It was observed that most of the cells in the control and original groups were clustered in Q1-3 and Q1-4.
Determination of RBC-bound IgG antibodies using the Coombs traditional tube method
Preparation of 5% RBCs from young healthy human blood group O
Anticoagulated human blood group-O (4 ml) was centrifuged at 3500 rpm for 5 min to prepare the RBC suspension for the Coombs tube method. After centrifugation, the plasma was discarded, and the RBC pellet was then mixed with 5 ml of normal saline. The mixture was then suspended and centrifuged again until the supernatant became clear. The transparent liquid containing the RBCs were obtained after discarding the plasma. For an experimental purpose, 50 µl of the resulting 5% RBC-O suspension was added to 950 µl of 0.9% normal saline to prepare the hematocrit.
Serum antibodies (IgG) dilution and centrifugation
Rabbit serum treated with 2-ME was serially diluted (1:1, 1:2, 1:4, 1:8, 1:16, 1:32, 1:64, 1:128, 1:256, 1:512, 1:1024, and 1:2048) in tubes containing an equal amount of saline (100 µl) and 50 µl RBCs from prepared 5% RBC-O suspension for each experiment. At the same time, human healthy serum was used instead of saline in the control group. All tubes were placed in a water bath at 37°C for 30 min. After incubation, the strength of agglutination was examined. Furthermore, to remove unbound immunoglobulin all tubes were washed 3–4 times with saline. Finally, one drop of antihuman globulin (AHG) was added and all the tubes were centrifuged at 3400 rpm for 18 s, after centrifugation, the strength of agglutination was graded as 4+, 3+, 2+, 1+, ±, and −.
Experimental results of RBC-bound IgG based on flow cytometry
Rabbit serum treated with 2-ME was serially diluted (1:1, 1:2, 1:4, 1:8, 1:16, 1:32, 1:64) in tubes containing prepared 2% red blood cells of blood group O. The samples were then incubated at 37°C for 30 min, after incubation, all the tubes were washed 2-3 time with normal saline and then one drop of antihuman globulin was added to each tube and mixed vigorously. At the same time, 100 ul of healthy human serum was added to 2% RBC-O as a control sample. All tubes, including the control, were thoroughly mixed again and individually analyzed using flow cytometry-based assay. To examine antibody titers, the results were calculated, the injection duration was represented on the X-axis, and the index of agglutination (IAG) of rabbit serum on the Y-axis.
Serum (anti-D) stability test by using flow cytometry method
The serum (anti-D) was divided into ten portions, which were kept in the freezer for experimental purposes. Each day, one tube was thawed properly, and serum (100 µl) was added to the tubes containing an equal amount of 100 µl saline and (50 μl) of 2% RBC-O. In the control group, instead of anti-D only healthy serum was added. The tubes were then incubated in a water bath for 30 min. After incubation, all tubes were analyzed using flow cytometry-based assay. Finally, the coefficient of variation (CV) was calculated from data collected over 10 consecutive days.
Statistical analysis
Statistical analysis included an unpaired t-test using GraphPad Prism 8.3.0 to compare the agglutination index. A P-value < 0.05 was considered statistically significant. The coefficient of variation assessed serum stability. SPSS software version 25.0 was used for the analysis of CV.
Results
Gating results of RBC-bound IgG using flow cytometry
The flow cytometry analysis involved creating quadrant gates using FSC-H and SSC-H, coordinates, divided horizontally and vertically, respectively. Each sample was analyzed with a fixed volume parameter of 20 µl. Results indicated significance RBC agglutination in quadrants Q1-3 and Q1-4 for the original group, whereas in the control group, only RBCs were found with no agglutination in quadrants Q1-3 and Q1-4, as depicted in Figure 1a and 1b.
Figure 1.
Control (a) and original sample (b) serum agglutination results analyzed on flow cytometry.
Experimental results of gates on flow cytometry-based assay
Sample volumes of flow cytometry parameters were fixed and the quadrants of scatter plot gating were obtaining results, detailed in Table 1. The total Abs count value was higher in control group while in orignal positive group the value become decreased, indicating a significant interaction of RBCs with serum and stronger agglutination was observed. In contrast, the control group showed higher Abs count value in Q3 indicating no interaction between RBCs and the serum as shown in Table 1.
Table 1.
Quadrants gates results of serum agglutination with fixed volume of sample using flow cytometry.
| Gates | Total Abs count | Q1 | Q2 | Q3 | Q4 |
|---|---|---|---|---|---|
| Control | 52639 | 36.7 | 2.7 | 51101 | 1499 |
| Positive (1:1) | 18738 | 158 | 869 | 12741 | 4970 |
Experimental results of healthy human serum and anti-D antibodies by using flow cytometry
Following the flow cytometry analysis of the standard serum (anti-D), strong agglutination was observed, resulting in positive findings. In contrast, no agglutination was observed in the normal healthy human group, indicating negative IAG results, as shown in Table 2.
Table 2.
Comparison of healthy human serum and anti-D based on flow cytometry.
| Sample tube | Healthy human serum | Anti-D antibodies | Index of agglutination (IAG) | |
|---|---|---|---|---|
| Control | 56,910.00 | 58,720.00 | ||
| 1 | 60,414.00 | 39,643.00 | −6.15 | 32.49 |
| 2 | 61,677.00 | 38,868.00 | −8.37 | 33.81 |
| 3 | 62,290.00 | 39,291.00 | −9.45 | 33.09 |
Serum (IgG) antibody titers detected by flow cytometry-based assay
In the initial testing, no antibodies were detected against normal human RBC-O by flow cytometry, indicating that the control rabbit had not been injected with human RBCs. The Abs. Count value was higher in the control sample due to the absences of agglutination. However, in the experimental group; the Abs. count value was decreased due to serum and RBCs agglutination. After 1-month dosage period, the Abs. count value was extremely low and the IAG was observed to be the strongest. Although, RBC-O was injected every other day, the IgG antibody titers in rabbits serum were relatively low after 1 week. In contrast, with repeated doses of RBCs the IgG antibodies titers increased after 1 month, and the index of agglutination (IAG) was found to be stronger than the antibody titers at 1 week as shown in Figures 2 and 3; Table 3.
Figure 2.
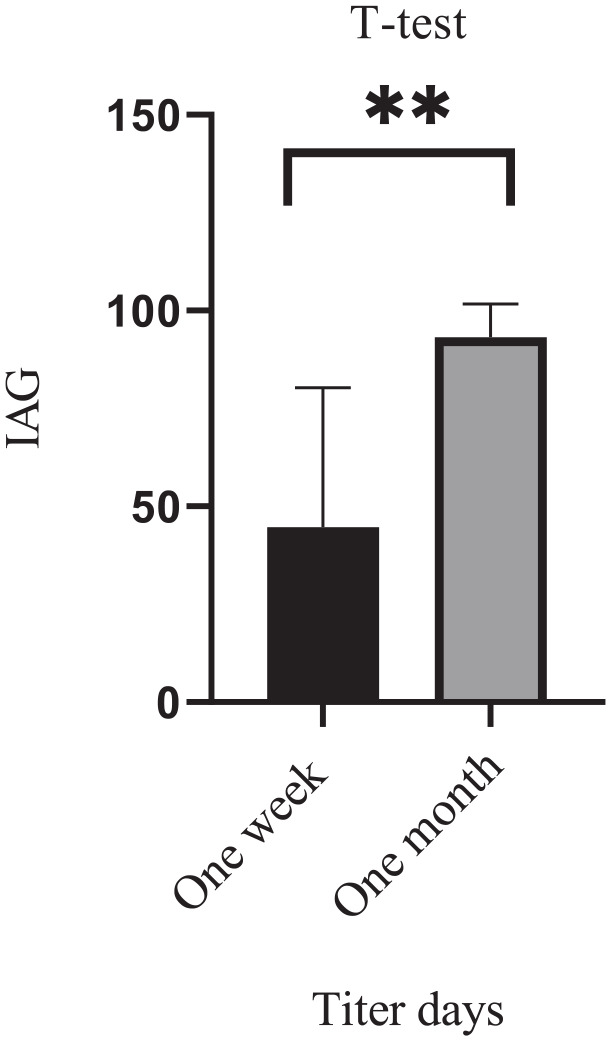
Antibody titers of rabbit sera against RBC-O after injection of 1-week and 1-month period.
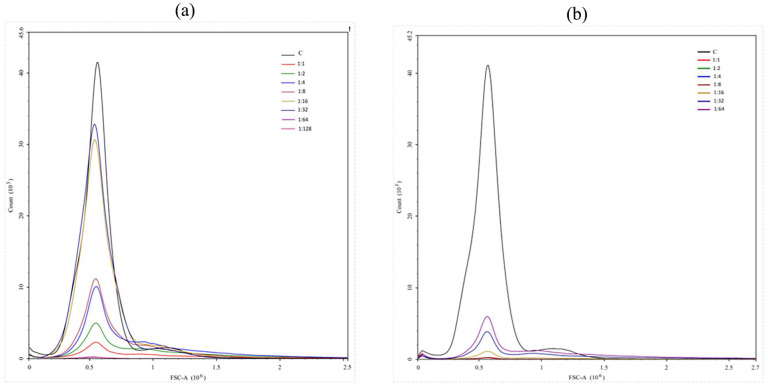
Figure 3.
RBCs agglutination titer: (a) 1 week: the peak was higher indicating lower agglutination titers, (b) end of month: the peak was lower which indicated stronger agglutination titers.
Table 3.
Agglutination titers of serum antibodies (IgG) measured by flow cytometry at 1-week and 1-month period after RBC-O injection.
| Dilution | Abs count at 1-week | IAG | Abs count at 1-month | IAG | P value |
|---|---|---|---|---|---|
| Control | 56,647.00 | 0.00 | 53,734.00 | 0.00 | 0.001 |
| 1:1 | 6678.00 | 88.21 | 609.00 | 98.87 | |
| 1:2 | 12,865.00 | 77.29 | 741.00 | 98.62 | |
| 1:4 | 22,328.00 | 60.58 | 874.00 | 98.37 | |
| 1:8 | 20,994.00 | 62.94 | 831.00 | 98.45 | |
| 1:16 | 49,602.00 | 12.44 | 2536.00 | 95.28 | |
| 1:32 | 52,397.00 | 7.50 | 7885.00 | 85.33 | |
| 1:64 | 54,383.00 | 4.00 | 12,023.00 | 77.62 |
In Table 3, the P value = 0.001 indicates that the increase in antibody titers is statistically significant, the results showed that flow cytometry has a higher reproducibility and greater sensitivity for the detection of RBC-bound IgG antibodies. This evidence proves that flow cytometry is a reliable and accurate tool for the determination of erythrocyte-bound IgG antibodies.
Serum antibodies (IgG) agglutination results based on Coombs traditional tube method
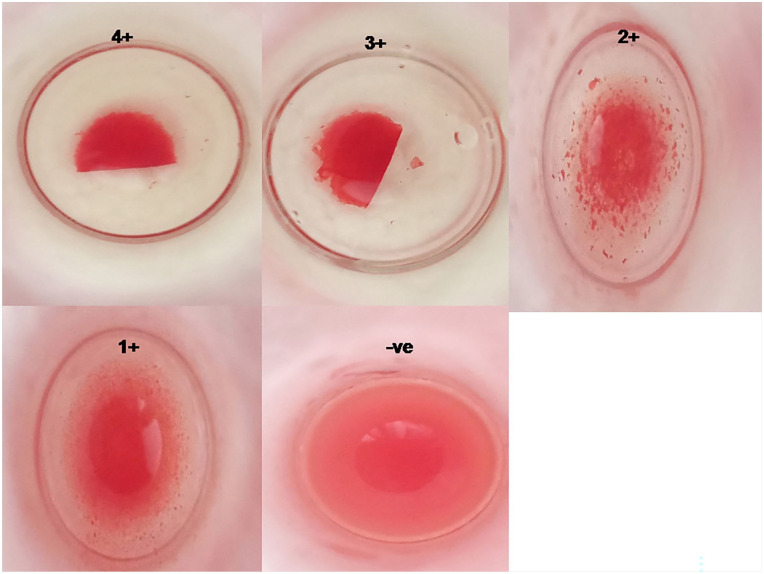
IgG titers were assessed against healthy human RBC-O, using coombs traditional method. Immunization of rabbits with RBC-O after 1 week, the IgG antibody titers were relatively low across all dosages with a titer of 1:256. In contrast, after 1 month, the antibody titers showed a significant increase compared to the initial levels. Rabbits that received higher injection doses exhibited the highest antibody titers, reaching 1:2048, indicating a positive correlation between the dose and antibody production. In both cases, the agglutination strength was stronger initially, but as the serum was diluted the strength of antibody titers decreased. Agglutination was not observed in the control samples as shown in the Table 4 and Figure 3.
Table 4.
Antibody titers of rabbit serum after immunization with RBC-O.
| Days | Control | Dilution of serum antibodies (IgG) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1:1 | 1:2 | 1:4 | 1:8 | 1:16 | 1:32 | 1:64 | 1:128 | 1:256 | 1:512 | 1:1024 | 1:2048 | ||
| 1 week | 0 | ++++ | ++++ | ++++ | ++++ | ++++ | +++ | ++ | + | ± | 0 | 0 | 0 |
| 1 month | 0 | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | +++ | +++ | ++ | + | ± |
Figure 4, shows the grading and strength of agglutination as 4+, 3+, 2+, 1+, and -ve respectively.
Figure 4.
Grading of agglutination titers based on Coombs tube test method.
Ten days serum(anti-D) stability test based on flow cytometry
Serum stability tests were performed over ten consecutively days using flow cytometry, after collecting the results, data from all 10 days were obtained and finally, the index of agglutination was analyzed using SPSS as shown in Table 5. The coefficient of variation (CV) was calculated as =7.47% indicating good stability of the serum samples.
Table 5.
Anti-D serum stability results over 10 days using flow cytometry.
| Days | All control | Anti-D Abs count | IAG | Mean ± SD | CV |
|---|---|---|---|---|---|
| 1 | 55,005.00 | 29,921.00 | 45.60 | 51.9233 ± 3.8823 | 7.47% |
| 2 | 56,033.00 | 27,911.00 | 50.19 | ||
| 3 | 56,295.00 | 29,446.00 | 47.69 | ||
| 4 | 59,802.00 | 29,175.00 | 51.21 | ||
| 5 | 58,619.00 | 26,556.00 | 54.70 | ||
| 6 | 57,402.00 | 25,121.00 | 56.24 | ||
| 7 | 55,669.00 | 28,944.00 | 48.01 | ||
| 8 | 57,023.00 | 25,317.00 | 55.60 | ||
| 9 | 56,271.00 | 25,125.00 | 55.35 | ||
| 10 | 57,160.00 | 25,927.00 | 54.64 |
Discussion
Flow cytometry is an advanced technique used for analyzing cells and particles in fluid suspensions. The principle of flow cytometry involves the use of a laser to illuminate cells or particles as they flow in a stream through a narrow channel. The laser light interacts with cells or particles, causing them to scatter light and emit fluorescence. The scattered light and fluorescence emissions are then detected using a series of photodetectors.27,28
In this study, quadrant gates were introduced to examine the agglutination of RBC-bound IgG antibodies. In control group, the total Abs.count value was higher shows no agglutination while in the original group the total Abs.count value was decreased, indicating a strong agglutination. Data in Table 1 suggest that it is not necessary for gating.
Serum from both young healthy individuals and standard ant-D serums were analyzed by flow cytometry. Agglutination was not observed in the normal serum of healthy individuals, indicating the absence of RBC-bound antibodies. On the other hand, the standard ant-D serums exhibited strong agglutination, indicating a significant aggregation of RBCs. These results highlight the high sensitivity of flow cytometry as a tool for the detection RBCs agglutination. This sensitivity allows for the accurate differentiation between healthy normal serum and serum containing RBC-bound antibodies, such as anti-D.
In this experimental work, flow cytometry was used for the detection of erythrocyte-bound IgG antibodies in rabbits. The rabbits were immunized with human RBC-O every other day over a 1-month period. Rabbits serum samples were analyzed using flow cytometry, and their results were compared after 1-week and 1-month post-immunization. The agglutination titer of RBC-bound IgG were significantly higher after 1-month immunization compared to 1-week, indicating a developed immune response and a substantial production of IgG antibodies after human RBC injections. Flow cytometry was studied to be highly effective even in the detection of small amounts of erythrocyte-bound IgG, thus, flow cytometry was declared to be a more reliable and accurate method than the traditional Coombs tube testing method for the detection of IgG antibodies.
Our findings are an agreement with those of other studies, and flow cytometry is now widely used by immunologists in various clinical laboratories for the detection of erythrocyte-bound IgG, due to its high reproducibility, sensitivity, and accuracy compared to traditional methods.29,30
In this study, the traditional Coombs tube method was used for the detection of RBC-bound IgG following the administration of red blood cells in rabbits. Initially, antibody titers remained very low after 1 week, but with increased injection doses, antibody titers were observed to be significantly raised after 1-month. However, the Coombs tube method can be influenced by subjective factors, making standardization very difficult and reducing the accuracy of the experimental outcomes compared to flow cytometry. This method depends on manual testing and the results were observed with the naked eyes, which may be subject to human errors, particularly, in high workloads. Additionally, the observation of erythrocytes-bound IgG agglutination may vary among different clinical laboratory staff due to differences in experience and techniques employed for tube shaking to resuspend the pellet, confirming other findings regarding traditional tube method. 31
The background value was determined using flow cytometry by introducing normal saline to red blood cells sourced from healthy individuals with blood group O. A cutoff value was established based on a 5% confidence interval of the background value. 32 Presence of weak agglutination above this 5% threshold indicates an abnormal or heightened level of non-specific agglutination, signifying a positive result. This positive result points towards abnormality and potential incompatibility within the standard blood grouping procedures.Therefore, we believe that flow cytometry is a highly sensitive technique that ensures and detects weaks agglutination. In contrast, the traditional tube method may not detect accurately this phenomenon, therefore, flow cytometry can be considered better than other techniques for the detection of clinically significant antibodies, as supported by evidence from previous studies.33,34
In this study, the serum stability was monitored continuously over ten consecutive days, the test results showed a CV = 7.74%, indicating good reproducibility of flow cytometry. To prove the clinical value of flow cytometry, healthy human RBC-O was used to react with positive serum collected from rabbits, and successfully detected anti-RBC-bound IgG antibodies in the samples. Thus, the establishment of this phenomenon may open a new avenue for the treatment of various diseases in clinical settings, highlighting the significant role of flow cytometry in disease diagnosis across laboratories and research centers. The results showed that flow cytometry was more effective and clinically significant for the detection of RBC-bound IgG antibodies, flow cytometry was found to be more sensitive and faster than the traditional Coombs tube method in the detection of antibodies as evidenced by agglutination patterns observed. 35
In the present study, the traditional Coombs tube method used for the detection of RBC-bound IgG was found to be limited by automation and standardization problems, the manual reading, and observation of agglutination titer with naked eyes were unsatisfactory and the chances of false positive results were higher than those of the flow cytometry method. Finally, Coombs testing via flow cytometry proved to be more intuitive and reliable than the traditional Coombs tube method. Furthermore, these findings highlight the dynamic nature of the immune response and emphasize the importance of a prolonged immunization period in achieving higher and more specific antibody titers.
The limitation of this study is the potential impact of residual RBC aggregates on the accuracy of erythrocyte-bound IgG quantification using flow cytometry. The presence of residual agglutination events, known as “microagglutination,” can pose challenges to accurate quantification. Future studies should focus on optimizing sample preparation, refining gating strategies, developing specialized quantification algorithms, conducting validation studies, and promoting collaboration and standardization to address this limitation and improve the accuracy of erythrocyte-bound IgG quantification using flow cytometry. Increasing the sample size in future studies will provide more robust and reliable results, enhancing the accuracy and sensitivity of flow cytometry in detecting clinically significant antibodies.
Conclusion
In this study, we concluded that flow cytometry is a more precise, sensitive, and reliable tool for detecting RBC-bound IgG antibodies. Additionally, the flow cytometry method was found to be more cost-effective and rapid than the traditional Coombs tube method. These advantages highlight its potential to discover new phenomena and provide more opportunities for routine clinical laboratories to use flow cytometry in detecting RBC-bound IgG antibodies. However, further more depth studies are required on flow cytometry for disease diagnosis and detection of specific antibodies in the future.
Footnotes
Author contribution: H.L. designed the experiments. And U.A and X.D analyzed the data and U.A wrote the manuscript. H.L. and X.Q. edited and reviewed the manuscript. The final manuscript has been checked and approved by all authors and take responsibility for its integrity.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Ethical approval for this study was obtained from the Ethics Committee of Dalian Medical University, with the ethical (APPROVAL NUMBER AEE23125). Additionally, all necessary informed consent was obtained from human participants in written form. For the animal studies, the research received approval from the appropriate regulatory body, and all procedures were carried out in compliance with the relevant animal welfare guidelines and regulations.
Data availability: No additional data are available, and all relevant data are provided in the manuscript.
References
- 1. Pamphilon DH, Scott ML. (2007) Robin Coombs: His life and contribution to haematology and transfusion medicine. British Journal of Haematology 137(5): 401–408. [DOI] [PubMed] [Google Scholar]
- 2. Segel GB, Lichtman MA. (2014) Direct antiglobulin (“Coombs”) test-negative autoimmune hemolytic anemia: A review. Blood Cells, Molecules, and Diseases 52(4): 152–160. [DOI] [PubMed] [Google Scholar]
- 3. Theis SR, Hashmi MF. (2019). Coombs Test. Europe PMC. [PubMed] [Google Scholar]
- 4. Matthews J, Newton S. (2010) The Coombs test. Clinical Journal of Oncology Nursing 14(2): 143–145. [DOI] [PubMed] [Google Scholar]
- 5. Wardrop KJ. (2005) The Coombs’ test in veterinary medicine: Past, present, future. Veterinary Clinical Pathology 34(4): 325–334. [DOI] [PubMed] [Google Scholar]
- 6. Radhakrishnan N, Dua S, Arora S. (2020) IgA-mediated autoimmune hemolytic anemia in an infant. Apheresis Science 59(2): 102695. [DOI] [PubMed] [Google Scholar]
- 7. Green RE, Hughes V. (2005) The antiglobulin test. In: Green RE, Hughes (eds) Modern Blood Banking and Transfusion Practices (pp. 93–107). F. A. Davis Company. [Google Scholar]
- 8. Parker V, Tormey CA. (2017) The direct antiglobulin test: Indications, interpretation, and pitfalls. Archives of Pathology & Laboratory Medicine 141(2): 305–310. [DOI] [PubMed] [Google Scholar]
- 9. Keir A, Agpalo M, Lieberman L, et al. (2015) How to use: The direct antiglobulin test in newborns. Archives of Disease in Childhood-Education and Practice 100(4): 198–203. [DOI] [PubMed] [Google Scholar]
- 10. Jaime-Pérez JC, Almaguer-Gaona C. (2016) Rediscovering the Coombs test. Medicina Universitaria 18(72): 185–186. [Google Scholar]
- 11. Xu L, Li H, Yang S, et al. (2020) Interference in the indirect antiglobulin test and direct antiglobulin test from rheumatoid factor. Journal of International Medical Research 48(3): 0300060519892386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chaudhary R, Das SS, Gupta R, et al. (2006) Application of flow cytometry in detection of red cell bound IgG in Coombs negative AIHA. Hematology 11(4): 295–300. [DOI] [PubMed] [Google Scholar]
- 13. Voulgaridou A, Kalfa TA. (2021) Autoimmune hemolytic anemia in the pediatric setting. Journal of Clinical Medicine 10(2): 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mujahid A, Dickert FL. (2015) Blood group typing: From classical strategies to the application of synthetic antibodies generated by molecular imprinting. Sensors 16(1): 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huet M, Cubizolles M, Buhot A. (2018) Red blood cell agglutination for blood typing within passive microfluidic biochips. High-throughput 7(2): 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gaikwad U, Rajurkar M. (2014) Diagnostic efficacy of Widal slide agglutination test against Widal tube agglutination test in enteric fever. International Journal of Medicine and Public Health 4(3): 227–227. [Google Scholar]
- 17. Mohseni K, Mirnejad R, Piranfar V, et al. (2017) A comparative evaluation of ELISA, PCR, and serum agglutination tests for diagnosis of Brucella using human serum. Iranian Journal of Pathology 12(4): 371. [PMC free article] [PubMed] [Google Scholar]
- 18. Garg S, Saini N, Bedi RK, et al. (2017) Comparison of micro column technology with conventional tube methods for antibody detection. Journal of Laboratory Physicians 9(02): 095–099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fayek MH, Saad AA, Eissa DG, et al. (2012) Role of gel test and flow cytometry in diagnosis of Coombs’ negative autoimmune haemolytic anaemia. International Journal of Laboratory Hematology 34(3): 311–319. [DOI] [PubMed] [Google Scholar]
- 20. Tuchin VV. (2011) Advanced Optical Flow Cytometry: Methods and Disease Diagnoses. John Wiley & Sons. [Google Scholar]
- 21. Wilkerson MJ. (2012) Principles and applications of flow cytometry and cell sorting in companion animal medicine. Veterinary Clinics: Small Animal Practice 42(1): 53–71. [DOI] [PubMed] [Google Scholar]
- 22. Adan A, Alizada G, Kiraz Y, et al. (2017) Flow cytometry: Basic principles and applications. Critical Reviews in Biotechnology 37(2): 163–176. [DOI] [PubMed] [Google Scholar]
- 23. Roback JD, Barclay S, Hillyer CD. (2003) An automatable format for accurate immunohematology testing by flow cytometry. Transfusion 43(7): 918–927. [DOI] [PubMed] [Google Scholar]
- 24. Kang SJ, Lim YA, Baik SY. (2014) Comparison of ABO antibody titers on the basis of the antibody detection method used. Annals of Laboratory Medicine 34(4): 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Won D, Kim BC. (2012) Optimized flow cytometry to measure anti-ABO immunoglobulin G. Laboratory Medicine 43(6): 281–290. [Google Scholar]
- 26. Khunger JM, Pati HP, Mahapatra M, et al. (2019) Utilisation of flow-cytometry in the diagnosis of auto immune haemolytic anaemia. Indian Journal of Hematology and Blood Transfusion 35: 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brown M, Wittwer C. (2000) Flow cytometry: Principles and clinical applications in hematology. Clinical Chemistry 46(8): 1221–1229. [PubMed] [Google Scholar]
- 28. Manohar SM, Shah P, Nair A. (2021) Flow cytometry: principles, applications and recent advances. Bioanalysis 13(3): 181–198. [DOI] [PubMed] [Google Scholar]
- 29. Stroncek DF, Njoroge JM, Procter JL, et al. (2003) A preliminary comparison of flow cytometry and tube agglutination assays in detecting red blood cell-associated C3d. Transfusion Medicine 13(1): 35–42. [DOI] [PubMed] [Google Scholar]
- 30. Chaudhary R, Das SS. (2022) Application of flow cytometry in transfusion medicine: The sanjay gandhi post graduate institute of medical sciences, India experience. Asian Journal of Transfusion Science 16(2): 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin JS, Hao TC, Lyou JY, et al. (2009) Clinical application of a flow cytometric direct antiglobulin test. Transfusion Medicine 49(7): 1335–1346. [DOI] [PubMed] [Google Scholar]
- 32. Ullah A, Ding X, Qi X, Liu H. Establishment of Flow Cytometry-Based Assay for the Quantitative Measurement of Erythrocyte Agglutination in Clinical Laboratory. Indian Journal of Hematology and Blood Transfusion. 2024. Dec 10:1-0. [Google Scholar]
- 33. Wang Z, Shi J, Zhou Y, et al. (2001) Detection of red blood cell—bound immunoglobulin G by flow cytometry and its application in the diagnosis of autoimmune hemolytic anemia. International Journal of Hematology 73: 188–193. [DOI] [PubMed] [Google Scholar]
- 34. Thedsawad A, Taka O, Wanachiwanawin W. (2016) Significances of red cell bound immunoglobulin G as detected by flow cytometry in patients with Coombs-negative immune hemolysis. Transfusion Medicine 26(2): 130–137. [DOI] [PubMed] [Google Scholar]
- 35. Quigley KA, Chelack BJ, Haines DM, et al. (2001) Application of a direct flow cytometric erythrocyte immunofluorescence assay in dogs with immune-mediated hemolytic anemia and comparison to the direct antiglobulin test. Journal of Veterinary Diagnostic Investigation 13(4): 297–300. [DOI] [PubMed] [Google Scholar]