Abstract
Psoriatic arthritis (PsA) presents various clinical manifestations, including skin lesions, peripheral arthritis, axial involvement, enthesitis, nail involvement, dactylitis, and uveitis. In addition, it causes a high incidence of lifestyle-related diseases and an increase in cerebrovascular and cardiovascular events. As the pathology of PsA has been clarified, molecular-targeted drugs targeting tumor necrosis factor-α, interleukin (IL)-17A, IL-17A/F, IL-17 receptor, IL-12/23(p40), IL-23p19, Cytotoxic T-lymphocyte Antigen-4 (CTLA-4), Janus kinase, and phosphodiesterase-4 have been developed and are widely used in clinical practice. PsA is clinically and molecularly heterogeneous, and it is necessary to improve various clinical symptoms with limited treatment options simultaneously; therefore, rheumatologists sometimes encounter difficult situations in clinical practice. Hence, the development of precision medicine may improve treatment outcomes. Recently, the strategic use of molecular-targeted drugs based on the stratification of patients with PsA by peripheral blood lymphocyte phenotyping and serum cytokine concentrations has been reported to possibly lead to a higher therapeutic response. A randomized controlled trial was initiated to verify the efficacy of this treatment strategy. However, to make precision medicine in PsA feasible, shifting from conventional clinical trials to clinical trials based on biomarker profiles and accumulating further data are necessary.
Keywords: precision medicine, psoriatic arthritis, treatment
Plain language summary
Precision medicine in psoriatic arthritis
Psoriatic arthritis (PsA) can cause a wide range of muscle-skeletal manifestations due to tendons, fingers, toes, joints, and spine inflammation. These findings may be associated with other health manifestations, such as gastrointestinal and eye inflammation, and an increased risk of cardiovascular and dysmetabolic diseases. Scientists have figured out the underlying issues in PsA, leading to the development of drugs that target specific molecules in the body, such as tumor necrosis factor-α, interleukins, and enzymes. Currently, these drugs are commonly prescribed by physicians. However, treating PsA is challenging because it greatly varies individually, and treatment options are limited. Therefore, physicians occasionally struggle to determine the optimal approach. Nevertheless, precision medicine, which tailors treatment for each individual, may be beneficial. Some studies have suggested that matching patients with PsA to the right drugs based on their blood and cytokine levels might be effective. Large-scale trials are currently underway to test the effectiveness of this approach. However, making precision medicine work for PsA requires a shift from traditional clinical trials to trials that focus on individuals’ specific characteristics and gathering more data.
Introduction
Psoriatic arthritis (PsA) is a heterogeneous disease that occurs in patients with overt psoriasis or a familial or genetic predisposition to psoriasis. PsA presents with various clinical manifestations, including skin lesions, peripheral arthritis, axial involvement, enthesitis, nail involvement, dactylitis, and uveitis. In addition, patients with PsA are highly comorbid with lifestyle-related diseases and have an increased risk of developing cerebrovascular and cardiovascular events. 1
A mechanism underlying the pathogenesis of PsA is the induction of enthesitis by triggers, such as mechanical stress and infection, against a background of genetic susceptibility. 2 For example, when triggers, such as mechanical stress, are added to the immune microenvironment during enthesis, the interleukin (IL)-23–IL-17 axis is activated. In addition, prostaglandin E2 (PGE2) is induced, which causes vasodilation, promotes neutrophil migration to the enthesitis site, and activates the IL-23–IL-17 pathway by promoting IL-17 production by T cells. In addition, activation of natural immunity (ILC3s (innate lymphoid cells) and γδT cells) that express IL-23 receptors induces the production of IL-17 and tumor necrosis factor-α (TNF-α). IL-17 production is a vital step in enhancing inflammation at the site of enthesitis. IL-17 promotes neutrophil migration and activation, thereby transitioning inflammation to the effector phase. Neutrophils, which are important effector cells in enthesitis, exacerbate the inflammatory response by releasing proteases and reactive oxygen species. Furthermore, IL-17 and IL-22 activate local mesenchymal stem cells, which induce fibrocartilage calcification via hedgehog signaling and parathyroid hormone-related peptides. Bone formation at the same site is mediated by bone morphogenetic proteins and Wnt signaling. PGE2 may also be related to bone formation as a strong activator of osteoblasts.
With the clarification of such pathology, molecular-targeted drugs targeting TNF-α, IL-17A, IL-17A/F, IL-17 receptor, IL-12/23(p40), IL-23p19, CTLA-4, Janus kinase (JAK), and phosphodiesterase-4 have been developed and are now widely used in clinical practice.3–25 However, PsA is clinically and molecularly heterogeneous, and simultaneously improving various clinical symptoms, such as skin and peripheral arthritis, enthesitis, and nail lesions, with limited treatment options, is necessary. Therefore, rheumatologists occasionally encounter difficult situations. The treatment response rate for peripheral arthritis with TNF-i (inhibitor) and IL-17A-i (inhibitor), representative biological agents of PsA, is only 60%–70%. In addition, the achievement rate of minimal disease activity (MDA), which is a treatment target in large-scale clinical studies or clinical practice, is <20% with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and only approximately 30%–40% with biological/targeted synthetic DMARDs (b/tsDMARDs).3–25 Recently, precision medicine has become increasingly considered a treatment strategy that can solve the aforementioned problems of PsA. Herein, we review the expectations of precision medicine for PsA and the latest trends in clinical practice.
Latest treatment recommendations for PsA and expectations for precision medicine
In 2015, President Barack Obama declared that the government would promote precision medicine under national leadership. Since then, precision medicine has significantly advanced in the cancer treatment field. 26 Various methods, including DNA profiling, immune markers, and proteomic and RNA analyses, have revealed complex and unique biological characteristics related to carcinogenesis, which have been used to optimize anticancer chemotherapy in individual cases. Consequently, the nature of clinical studies has changed from study designs that target cancer or tissue types to those that target biomarker profiles, such as tissue-independent genes, to improve treatment outcomes. 27 In many clinical studies, selective treatment strategies using molecular-targeted drugs that match biomarker profiles are more effective than those that do not. Currently, in clinical practice, drugs are selected based on biomarker profiles (e.g., the presence or absence of epidermal growth factor receptor gene mutations or anaplastic lymphoma kinase fusion genes in non-small-cell lung cancer, and the presence or absence of human epidermal growth factor receptor 2 expression in breast cancer).28,29 In the field of cancer treatment, companion diagnostics are widely deployed in clinical practice to promote personalized medicine. Thus, to achieve precision medicine, two elements are essential: patient stratification using biomarkers and molecular-targeted therapy that specifically inhibits single or multiple therapeutic targets. Patients are more likely to achieve satisfactory outcomes. 30
With the emergence of various molecular-targeted drugs for PsA and the accumulation of new data on the efficacy and safety of JAK-i, the European Alliance of Associations for Rheumatology (EULAR) recommendations will be updated in 2023. 31 A new overarching principle was added to this treatment recommendation, emphasizing the importance of considering patient safety. In phase III trials, biologic DMARDs (bDMARDs) are recommended for patients with peripheral arthritis refractory to one or more csDMARDs (methotrexate, leflunomide, or sulfasalazine). Currently, bDMARDs are preferred over JAK-i because of the balance between efficacy and safety, cost, and long-term efficacy. The same is true from the perspective of the comorbidities commonly observed in PsA. In particular, JAK-i is recommended for use considering the safety in patients with peripheral arthritis who have not responded adequately to or are refractory to at least one biological agent, or in patients for whom the introduction of bDMARDs is considered inappropriate. No priority order was assigned to the selected bDMARDs (TNF-i, IL-17-i, IL-12/23-i, and IL-23p19-i). Similarly, biologics are considered for patients with enthesitis who do not respond adequately to non-steroidal anti-inflammatory drugs (NSAIDs) or local injections of glucocorticoids; however, no priority order has been provided for drug selection because all currently available drugs are effective in treating enthesitis. The same is true for patients with spinal lesions for whom NSAIDs are ineffective. Therefore, IL-17A-i, TNF-i, IL-17A/F-i, or JAK-i are recommended, whereas IL-17A, IL-17A/F, IL-23, or IL-12/23-i are recommended for patients with significant skin lesions, TNF-i for patients with uveitis, and TNF, IL-23, IL-12/23, or JAK-i for patients with inflammatory bowel disease. The 2023 revisions highlighted the recommended drugs for each comorbidity. The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): Updated treatment recommendations for PsA 2021 provide treatment recommendations for each of the six clinical domains (peripheral arthritis, axial disease, enthesitis, dactylitis, psoriasis, and nail disease) (domain-based approach). 32 However, as many patients have diseases across multiple domains, all possible active domains and related conditions should be considered in treatment selection. In addition, if evidence indicates that drugs have different efficacies in different domains, selection may be based on the most severe or most affected disease domain. The first approach is to assess the disease activity of each domain and consider comorbidities, previous treatment, and patient preferences. In cases of Crohn’s disease and ulcerative colitis, the use of TNF inhibitors (non-etanercepts (ETNs)) or IL-12/23-i is strongly recommended, whereas not using IL-17-i is strongly recommended; the same applies to uveitis. TNF inhibitors are recommended, and ETN is considered a conditional recommendation. In addition, treatment has been recommended for different comorbidities as follows: bDMARDs and tsDMARDs for active hepatitis B and C, HIV, tuberculosis, history of recent malignancy, and TNF-i, IL-17-i, IL-12/23-i, IL-23-i, and JAK-i, all of which are considered cautiously. By contrast, only JAK-i is considered with caution for the elevated risk of cardiovascular disease and elevated risk for venous thromboembolism. The GRAPPA recommendations emphasize a domain-based approach and individualization of treatment, considering the heterogeneity of PsA with a focus on comorbidities.
Thus, various molecular-targeted therapies are available for PsA treatment, and the use of all b/tsDMARDs is recommended. However, drug ordering/response prediction and biomarkers are only listed as high-priority study topics in the Research Agenda, indicating priorities for future studies on PsA, and are considered a future challenge.
Current status and challenges of precision medicine using molecular-targeted drugs in PsA
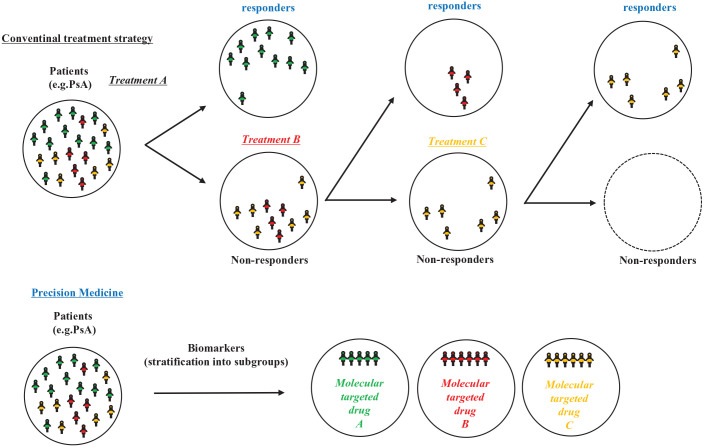
Large-scale clinical studies directly comparing TNF and IL-17A inhibitors for PsA have demonstrated that they are equally effective against peripheral arthritis.33–35 However, some patients resist certain drugs and require a switch to other formulations or drugs with different mechanisms of action. The latest treatment recommendations also require switching to drugs with the same or different mechanisms of action when the drug is ineffective. This suggests diversity not only in clinical phenotypes but also in their treatment targets. Currently, the selection of molecular-targeted drugs for PsA has no priority, and drugs with any mechanism of action are equally positioned. Therefore, drug selection has shown priority for limited clinical phenotypes, such as the presence or absence and severity of skin lesions and the presence or absence of inflammatory bowel disease. For example, a machine-learning approach was used to integrate clinical data from four phase III trials of secukinumab in PsA, and PsA could be classified into seven clusters with various responsiveness trajectories and characteristic clinical phenotypes. 36 Similarly, a study using data from a phase III trial of guselkumab showed that PsA could be classified into eight clusters with various responsiveness trajectories and characteristic clinical phenotypes. 37 However, these are not precision medicines in the strict sense. To practice precision medicine, simply extracting the characteristics and efficacy predictors of each molecular-targeted drug that can be used for PsA is insufficient; however, demonstrating that the selection of molecular-targeted drugs that match the biomarker profile is more effective than nonspecific conventional treatment strategies or treatment selection that does not match the biomarker profile is necessary. Patient stratification using biomarkers and molecular-targeted therapies that specifically inhibit single or multiple therapeutic targets is required for efficient functioning (Figure 1).
Figure 1.
Precision medicine approach in psoriatic arthritis.
Various biomarkers of PsA have been investigated. One study identified candidate biomarkers for distinguishing PsA from rheumatoid arthritis (RA) using three different proteomic platforms as follows: unbiased label-free LC-MS/MS, microsphere bead-based immunoassay (Luminex xMAP), and aptamer-based assay (SOMAscan). 38 Another study has reported that increased ICAM-1, IL-2, IL-6, IL-17A, C5a, and CXCL-9/12 levels in the synovial fluid were characteristic of PsA than of RA. 39 A meta-analysis identifying novel disease biomarkers for PsA has also reported that healthy individuals had significantly higher serum cartilage oligomerization metalloproteinase levels and significantly higher matrix metalloproteinase (MMP) levels than patients with psoriasis, although no genetic biomarkers useful for diagnosing PsA were identified. 40 Studies using synovial fluid have shown that the presence of the JAK1/STAT3/STAT5 transcriptional network in the joint microenvironment and the interaction of IL-6 with IL-23, IL-1β, and γC cytokines, which promote politization and plasticity of Th17 and Treg cells, may contribute to the persistence of arthritis. 41 A serum biomarker panel of C3M, C4M, PRO-C3, PRO-C6, and CPa9-HNE, as well as classification models using several lipids and other metabolites, including lysophosphatidylcholine and sphingomyelin, may predict disease activity.42,43 Metabolites that may be associated with skin disease activity (e.g., eicosanoids with anti- or pro-inflammatory properties, such as 12-hydroxyeicosatetraenoic acid) have also been identified. 44 Regarding treatment response, hyaluronan concentration and mass may be biomarkers predicting disease severity, treatment resistance, and poor outcomes, and a decrease in MMP-3 and an increase in COMP predict the response to TNF-i treatment.45,46 In a cohort study of patients with psoriasis, HLA-C*06:02 status predicted treatment response to ustekinumab, and a meta-analysis showed that high PASI75 response rates were obtained with ustekinumab not only in HLA-C*06:02-positive patients but also in HLA-C*06:02-negative patients. 47 The association of anti-drug antibody development with HLA-DRB1 residues 9 and 71 within the peptide-binding groove and the association of HLA-DRB1 residues 9 and 71 with adalimumab treatment failure have also been reported. The Rho GTPase pathway can predict the therapeutic response to IL-17A-i based on network and pathway analyses.48,49 As such, biomarkers related to diagnosis, pathology, disease activity, and therapeutic response have been identified using various methods; however, disease-specific problems exist in the identification of biomarker profiles in PsA. The pathogenic mechanisms in each PsA tissue, that is, the immune cell and cytokine signatures that lead to local inflammation, differ. 50 For example, the high expression of IL-20, IL-21, and IL-12 mRNA in psoriatic skin lesions is associated with a favorable therapeutic response to IL-12/23p40 inhibitors. The presence of a specific proteomic signature in the synovial tissue is associated with the efficacy of TNF inhibitor therapy.51,52 Stratifying patients and predicting treatment response based on tissue-based signatures, or for example, focusing on whether bone formation is significant or primarily bone destruction, are also expected. However, unlike with cancer, where biopsies for diagnosis or companion diagnostics are routinely performed, a challenge to overcome with PsA is how and from where to collect tissue samples to clarify the biomarker profile in patients with PsA who can be diagnosed and treated without a histological diagnosis.
Currently, serum cytokine, peripheral blood mononuclear cells, and liquid biopsies are considered more realistic than tissue analyses. Some cancer studies have shown that liquid biopsy is useful for selecting treatment 53 and identifying predictors of treatment efficacy in patients with RA. 54 However, whether the selection of molecular-targeted drugs that match these biomarker profiles is more effective than those that do not remain unverified.
However, we have reported that strategic selection of biological agents based on peripheral blood helper T-cell phenotyping can result in higher efficacy.55,56 This study included 64 patients with PsA resistant to csDMARD treatment. We compared the treatment efficacy for up to 1 year between the strategic treatment group (41 cases), in which biological agents were strategically selected based on peripheral blood lymphocyte phenotyping using eight-color flow cytometry, and the standard treatment group (56 cases), in which TNF inhibitors were introduced based on the EULAR or Group for Research and Assessment of Psoriasis and Psoriatic Arthritis treatment recommendations at the time. Patients with PsA were divided into four groups based on their peripheral blood lymphocyte phenotype (CD3 + CD4 + CXCR3-CCR6 + CD38 + HLA-DR + activated Th17-dominant type, CD3 + CD4 + CXCR3 + CCR6-CD38 + HLA-DR + activated Th1-dominant type, activated Th1/Th17-hybrid type, and activated Th1/Th17-healthy control comparable type). IL-17A inhibitors were administered to activate the Th17-dominant patients, IL-12/223p40 inhibitors to activate the Th1-dominant patients, and TNF inhibitors to activate the Th1/Th17-healthy controls. TNF inhibitors were administered to activated Th1/Th17-hybrid type patients with severe arthritis, and IL-17A inhibitors were administered to patients with severe skin lesions. The Disease Activity Index for Psoriatic Arthritis (DAPSA)-remission achievement rates at 6 and 12 months were significantly higher in the strategic treatment group than in the standard treatment group (85.4% vs 64.8%). The MDA achievement rate at 6 months was also significantly higher in the strategic treatment group than in the standard treatment group (80.5% vs 60.7%). The continuation rate for up to 6 months in the strategic treatment group was significantly higher than that in the standard treatment group (100% vs 87.5%), suggesting that the avoidance of adverse events and primary failure immediately after the start of treatment may have contributed to the favorable therapeutic effect and high continuation rate. The ratio of activated Th1/Th17 cells did not change before and after treatment with TNF- and IL-17A inhibitors; however, the ratio of activated Th1 cells significantly decreased after treatment with IL-12/23p40 inhibitors. Thus, even in the same disease, PsA, differences in the immune phenotype of each patient and the effect of each molecular-targeted drug on the immune phenotype were observed. Harmonizing these two factors may contribute to improved treatment outcomes. Furthermore, the molecular-targeted drug effect varies for each disease in the spondyloarthritis disease group; however, this may be explained by the difference in the immune phenotype for each disease and the effect of each molecular-targeted drug on the immune phenotype for individual diseases. The results suggest not only the possibility of precision medicine but also further elucidation of the pathology of PsA or other diseases that include spondyloarthritis in the future.
However, because peripheral blood lymphocyte phenotyping using eight-color flow cytometry is complex, we explored the possibility of using precision medicine for PsA based on serum cytokine concentrations. 57 In this study, we explored the possibility of a strategic treatment using TNF- and IL-17A inhibitors, which have demonstrated equivalent efficacy against arthritis in head-to-head studies. Baseline cytokines predicting the response to treatment 1 year later were extracted from 24 patients treated with TNF inhibitors and 23 patients treated with IL-17A inhibitors. Serum IL-22 levels were extracted as predictors of DAPSA remission 1 year after IL-17A inhibitor therapy. No cytokines that predicted MDA or Psoriasis Area and Severity Index response rates were identified. No cytokines predicting the response to treatment 1 year after TNF inhibitor therapy were identified. Focusing on baseline serum cytokine concentrations, baseline IL-22 concentrations were significantly lower in cases that achieved remission 1 year after the introduction of IL-17A inhibitors than in cases that did not. In addition, comparing cases with high and low serum IL-22 concentrations and healthy participants showed that elevated IL-17A concentrations were common in cases with both high and low IL-22 levels, although serum TNF-α was a characteristic observed only in cases with high serum IL-22 levels. Thus, although increased serum IL-17A level is a common feature of all patients with PsA, a group of cases in which TNF-α level is also elevated. Therefore, by focusing on such differences in cytokine profiles, we derived a new treatment strategy of introducing IL-17A inhibitors to IL-22 low cases and TNF-α to IL-22 high cases and verified its effectiveness in another cohort. The treatment strategy (strategic treatment group) had a significantly higher DAPSA remission achievement (strategic treatment group, 58.8% vs conventional treatment group, 25.3%) and MDA achievement (strategic treatment group, 82.3% vs conventional treatment group, 41.2%) than the group that did not match the treatment strategy (conventional treatment group) rates. Thus, in addition to extracting the characteristics and efficacy predictors of each molecular-targeted drug that can be used for PsA, the use of molecular-targeted drugs that match a biomarker profile is more effective than nonspecific conventional treatment strategies or treatment selection that does not match the biomarker profile. These results need to be verified in multicenter or large-scale clinical studies to make these treatment strategies feasible in clinical practice.
An open-label multicenter, parallel-group, two-arm randomized controlled study (OPTIMISE) was recently planned to verify the efficacy of strategic treatment based on peripheral blood lymphocyte phenotype (sample size: 240 participants; relative interaction effect: 0.2; type I error rate: 0.05; and 90% power). The trial was opened for recruitment in January 2022, and the estimated completion date was December 2024. In this study, patients were randomly assigned to receive either a TNF or IL-17A inhibitor, and the primary endpoint will be whether immunological markers can predict the achievement of MDA after 24 weeks. Furthermore, we examined whether the surface or intracellular signatures of activated Th17 cells differ between treatment responders and non-responders and whether immune subset-specific transcriptomic signatures can be used as predictors of the efficacy of IL-17A and TNF inhibitors, either independently or in combination with the Th17 signature. Other strategies include the identification of transcriptomic biomarkers and pathways involved in the resistance to biologics. The first powerful randomized controlled trial to test a precision medicine approach using biologics for PsA is currently underway, and the results are expected to be promising. 58
Unlike cancer, systemic autoimmune diseases, such as PsA, have a long (chronic) clinical course. However, temporal factors, such as how biomarker profiles or immune phenotypes change over time in relapsed patients; whether a biomarker profile predicts treatment resistance at the time of onset; and whether treatment resistance is acquired during the disease remain unclear. Elucidating this point may provide important insights into which b/tsDMARDs should be selected and predict whether patients will need to be treated with b/tsDMARDS in the future. Biomarker profiles may change during and after the disease onset. Clarifying temporal changes, that is, the natural molecular and cellular history of the disease, may help establish precision medicine that considers temporal factors specific to systemic autoimmune diseases with a chronic course.
Conclusion
The emergence of highly effective molecular-targeted drugs has dramatically improved the outcomes of PsA treatment. PsA is highly heterogeneous, and various clinical symptoms require simultaneous improvement. However, in some cases of PsA, treatment can be challenging. Although new drugs will emerge, the expectations for the establishment of appropriate drug selection strategies based on precision medicine are high. Patient stratification based on clinical phenotypes and biomarkers alone is insufficient to make precision medicine feasible in clinical practice. The use of selective molecular-targeted drugs based on biomarker-based patient stratification produces higher therapeutic effects (or higher safety) needs to be proven. Therefore, shifting from clinical trials that have been conducted based on each disease (e.g., PsA, systemic lupus erythematosus, and rheumatic arthritis) and their main organ disorders (e.g., arthritis and nephritis) to clinical trials based on biomarker profiles is necessary. 59 Various candidate biomarkers have been identified for patient stratification and the prediction of treatment efficacy. However, only a few studies have reported that the use of selective molecular-targeted drugs based on these results contributes to the improvement of clinical outcomes. Further studies are required to make precision medicine feasible for treating PsA.
Acknowledgments
The authors thank all the medical staff at our institution, without whom this study would have never been accomplished, and the investigators for their participation.
Footnotes
ORCID iD: Ippei Miyagawa  https://orcid.org/0000-0002-9071-145X
https://orcid.org/0000-0002-9071-145X
Contributor Information
Ippei Miyagawa, The First Department of Internal Medicine, School of Medicine, University of Occupational and Environmental Health, Japan, Kitakyushu, Japan.
Yoshiya Tanaka, The First Department of Internal Medicine, School of Medicine, University of Occupational and Environmental Health, Japan, 1-1 Iseigaoka, Yahata-nishi, Kitakyushu 807-8555, Japan.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Ippei Miyagawa: Conceptualization; Writing – original draft; Writing – review & editing.
Yoshiya Tanaka: Conceptualization; Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Y.T. has received consulting fees, speaking fees, and honoraria from Behringer-Ingelheim, Eli Lilly, Abbvie, Gilead, AstraZeneca, Bristol-Myers, Chugai, Daiichi-Sankyo, Eisai, Pfizer, Mitsubishi-Tanabe, and GlaxoSmithKline and research grants from Asahi-Kasei, Abbvie, Chugai, Eisai, Takeda, Daiichi-Sankyo, and Behringer-Ingelheim. I.M.: None.
Availability of data and materials: Not applicable.
References
- 1. FitzGerald O, Ogdie A, Chandran V, et al. Psoriatic arthritis. Nat Rev Dis Primers 2021; 7: 59. [DOI] [PubMed] [Google Scholar]
- 2. Schett G, Lories RJ, D’Agostino MA, et al. Enthesitis: from pathophysiology to treatment. Nat Rev Rheumatol 2017; 13: 731–741. [DOI] [PubMed] [Google Scholar]
- 3. Roubille C, Richer V, Starnino T, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2015; 74: 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mease PJ, Ory P, Sharp JT, et al. Adalimumab for long-term treatment of psoriatic arthritis: 2-year data from the Adalimumab Effectiveness in Psoriatic Arthritis Trial (ADEPT). Ann Rheum Dis 2009; 68: 702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Antoni CE, Kavanaugh A, van der Heijde D, et al. Two-year efficacy and safety of infliximab treatment in patients with active psoriatic arthritis: findings of the Infliximab Multinational Psoriatic Arthritis Controlled Trial (IMPACT). J Rheumatol 200; 35: 869–876. [PubMed] [Google Scholar]
- 6. Kavanaugh A, Krueger GG, Beutler A, et al. Infliximab maintains a high degree of clinical response in patients with active psoriatic arthritis through 1 year of treatment: results from the IMPACT 2 trial. Ann Rheum Dis 2007; 66: 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kavanaugh A, McInnes IB, Mease P, et al. Clinical efficacy, radiographic and safety findings through 5 years of subcutaneous golimumab treatment in patients with active psoriatic arthritis: results from a long-term extension of a randomised, placebo-controlled trial (the GO-REVEAL study). Ann Rheum Dis 2014; 73: 1689–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mease PJ, Fleischmann R, Deodhar AA, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis 2014; 73: 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McInnes IB, Kavanaugh A, Gottlieb AB, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 2013; 382: 780–789. [DOI] [PubMed] [Google Scholar]
- 10. Ritchlin C, Rahman P, Kavanaugh A, et al.; PSUMMIT 2 Study Group. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis 2014; 73: 990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kavanaugh A, Puig L, Gottlieb AB, et al. Efficacy and safety of ustekinumab in psoriatic arthritis patients with peripheral arthritis and physician-reported spondylitis: post-hoc analyses from two phase III, multicentre, double-blind, placebo-controlled studies (PSUMMIT-1/PSUMMIT-2). Ann Rheum Dis 2016; 75: 1984–1988. [DOI] [PubMed] [Google Scholar]
- 12. Deodhar A, Gottlieb AB, Boehncke WH, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet 2018; 391: 2213–2224. [DOI] [PubMed] [Google Scholar]
- 13. McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015; 386: 1137–1146. [DOI] [PubMed] [Google Scholar]
- 14. Strand V, Mease P, Gossec L, et al. Secukinumab improves patient-reported outcomes in subjects with active psoriatic arthritis: results from a randomised phase III trial (FUTURE 1). Ann Rheum Dis 2017; 76: 203–207. [DOI] [PubMed] [Google Scholar]
- 15. Kavanaugh A, McInnes IB, Mease PJ, et al. Efficacy of subcutaneous secukinumab in patients with active psoriatic arthritis stratified by prior tumor necrosis factor inhibitor use: results from the randomized placebo-controlled FUTURE 2 study. J Rheumatol 2016; 43: 1713–1717. [DOI] [PubMed] [Google Scholar]
- 16. Mease PJ, van der Heijde D, Ritchlin CT, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naïve patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis 2017; 76: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nash P, Kirkham B, Okada M, et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet 2017; 389: 2317–2327. [DOI] [PubMed] [Google Scholar]
- 18. van der Heijde D, Gladman DD, Kishimoto M, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52-week results from a phase III study (SPIRIT-P1). J Rheumatol 2018; 45: 367–377. [DOI] [PubMed] [Google Scholar]
- 19. Coates LC, Kishimoto M, Gottlieb A, et al. Ixekizumab efficacy and safety with and without concomitant conventional disease-modifying antirheumatic drugs (cDMARDs) in biologic DMARD (bDMARD)-naïve patients with active psoriatic arthritis (PsA): results from SPIRIT-P1. RMD Open 2017; 3: e000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McInnes IB, Asahina A, Coates LC, et al. Bimekizumab in patients with psoriatic arthritis, naive to biologic treatment: a randomised, double-blind, placebo-controlled, phase 3 trial (BE OPTIMAL). Lancet 2023; 401: 25–37. [DOI] [PubMed] [Google Scholar]
- 21. Merola JF, Landewé R, McInnes IB, et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-α inhibitors: a randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE). Lancet 2023; 401: 38–48. [DOI] [PubMed] [Google Scholar]
- 22. Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med 2014; 370: 2295–2306. [DOI] [PubMed] [Google Scholar]
- 23. Mease PJ, Lertratanakul A, Anderson JK, et al. Upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PsA 2. Ann Rheum Dis 2021; 80: 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McInnes IB, Anderson JK, Magrey M, et al. Trial of upadacitinib and adalimumab for psoriatic arthritis. N Engl J Med 2021; 384: 1227–1239. [DOI] [PubMed] [Google Scholar]
- 25. Mease PJ, Gottlieb AB, van der Heijde D, et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis 2017; 76(9): 1550–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haendel MA, Chute CG, Robinson PN. Classification, ontology, and precision medicine. N Engl J Med 2018; 379: 1452–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsimberidou AM, Fountzilas E, Nikanjam M, et al. Review of precision cancer medicine: evolution of the treatment paradigm. Cancer Treat Rev 2020; 86: 102019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sholl LM, Awad M, Basu Roy U, et al. Programmed death ligand-1 and tumor mutation burden testing of patients with lung cancer for selection of immune checkpoint inhibitor therapies: guideline from the College of American Pathologists, Association for Molecular Pathology, International Association for the Study of Lung Cancer, Pulmonary Pathology Society, and LUNGevity Foundation. Arch Pathol Lab Med 2024; 148(7): 757–774. [DOI] [PubMed] [Google Scholar]
- 29. Honma N, Yoshida M, Kinowaki K, et al. The Japanese breast cancer society clinical practice guidelines for pathological diagnosis of breast cancer, 2022 edition. Breast Cancer 2024; 31: 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miyagawa I, Kubo S, Tanaka Y. A wide perspective of targeted therapies for precision medicine in autoimmune diseases. Expert Rev Precis Med Drug Dev 2020; 5: 447–453. [Google Scholar]
- 31. Kerschbaumer A, Smolen JS, Ferreira RJO, et al. Efficacy and safety of pharmacological treatment of psoriatic arthritis: a systematic literature research informing the 2023 update of the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 2024; 83(6): 760–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Coates LC, Soriano ER, Corp N, et al. Group for research and assessment of psoriasis and psoriatic arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol 2022; 18: 465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mease PJ, Smolen JS, Behrens F, et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis 2020; 79: 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smolen JS, Mease P, Tahir H, et al. Multicentre, randomised, open-label, parallel-group study evaluating the efficacy and safety of ixekizumab versus adalimumab in patients with psoriatic arthritis naïve to biological disease-modifying antirheumatic drug: final results by week 52. Ann Rheum Dis 2020; 79: 1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McInnes IB, Behrens F, Mease PJ, et al. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3b trial. Lancet 2020; 395: 1496–1505. [DOI] [PubMed] [Google Scholar]
- 36. Pournara E, Kormaksson M, Nash P, et al. Clinically relevant patient clusters identified by machine learning from the clinical development programme of secukinumab in psoriatic arthritis. RMD Open 2021; 7: e001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Richette P, Vis M, Ohrndorf S, et al. Identification of PsA phenotypes with machine learning analytics using data from two phase III clinical trials of guselkumab in a bio-naïve population of patients with PsA. RMD Open 2023; 9: e002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McArdle A, Qasim Butt A, Szentpetery A, et al. Developing clinically relevant biomarkers in inflammatory arthritis: a multiplatform approach for serum candidate protein discovery. Proteomics Clin Appl 2016; 10: 691–698. [DOI] [PubMed] [Google Scholar]
- 39. Caso F, Saviano A, Tasso M, et al. Analysis of rheumatoid- vs psoriatic arthritis synovial fluid reveals differential macrophage (CCR2) and T helper subsets (STAT3/4 and FOXP3) activation. Autoimmun Rev 2022; 21: 103207. [DOI] [PubMed] [Google Scholar]
- 40. Fiocco U, Martini V, Accordi B, et al. Transcriptional network profile on synovial fluid T cells in psoriatic arthritis. Clin Rheumatol 2015; 34(9): 1571–1580. [DOI] [PubMed] [Google Scholar]
- 41. Wirth T, Balandraud N, Boyer L, et al. Biomarkers in psoriatic arthritis: a meta-analysis and systematic review. Front Immunol 2022; 13: 1054539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Groen SS, Nielsen SH, Bay-Jensen AC, et al. Investigating protease-mediated peptides of inflammation and tissue remodeling as biomarkers associated with flares in psoriatic arthritis. Arthritis Res Ther 2024; 26: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Koussiouris J, Looby N, Kotlyar M, et al. Classifying patients with psoriatic arthritis according to their disease activity status using serum metabolites and machine learning. Metabolomics 2024; 20: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Choksi H, Li S, Looby N, et al. Identifying serum metabolomic markers associated with skin disease activity in patients with psoriatic arthritis. Int J Mol Sci 2023; 24: 15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hellman U, Engström-Laurent A, Larsson A, et al. Hyaluronan concentration and molecular mass in psoriatic arthritis: biomarkers of disease severity, resistance to treatment, and outcome. Scand J Rheumatol 2019; 48: 284–293. [DOI] [PubMed] [Google Scholar]
- 46. Chandran V, Shen H, Pollock RA, et al. Soluble biomarkers associated with response to treatment with tumor necrosis factor inhibitors in psoriatic arthritis. J Rheumatol 2013; 40: 866–871. [DOI] [PubMed] [Google Scholar]
- 47. van Vugt LJ, van den Reek JMPA, Hannink G, et al. Association of HLA-C*06:02 status with differential response to ustekinumab in patients with psoriasis: a systematic review and meta-analysis. JAMA Dermatol 2019; 155: 708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tsakok T, Saklatvala J, Rispens T, et al. Development of antidrug antibodies against adalimumab maps to variation within the HLA-DR peptide-binding groove. JCI Insight 2023; 8: e156643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rahmati S, O’Rielly DD, Li Q, et al. Rho-GTPase pathways may differentiate treatment response to TNF-alpha and IL-17A inhibitors in psoriatic arthritis. Sci Rep 2020; 10: 21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Najm A, Goodyear CS, McInnes IB, et al. Phenotypic heterogeneity in psoriatic arthritis: towards tissue pathology-based therapy. Nat Rev Rheumatol 2023; 19: 153–165. [DOI] [PubMed] [Google Scholar]
- 51. Gedebjerg A, Johansen C, Kragballe K, et al. IL-20, IL-21 and p40: potential biomarkers of treatment response for ustekinumab. Acta Derm Venereol 2013; 93: 150–155. [DOI] [PubMed] [Google Scholar]
- 52. Ademowo OS, Hernandez B, Collins E, et al. Discovery and confirmation of a protein biomarker panel with potential to predict response to biological therapy in psoriatic arthritis. Ann Rheum Dis 2016; 75: 234–241 [DOI] [PubMed] [Google Scholar]
- 53. Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol 2017; 14: 531–548. [DOI] [PubMed] [Google Scholar]
- 54. Daraghmeh DN, King C, Wiese MD. A review of liquid biopsy as a tool to assess epigenetic, cfDNA and miRNA variability as methotrexate response predictors in patients with rheumatoid arthritis. Pharmacol Res 2021; 173: 105887. [DOI] [PubMed] [Google Scholar]
- 55. Miyagawa I, Nakayamada S, Ueno M, et al. Precision medicine based on the phenotypic differences in peripheral T helper cells in patients with psoriatic arthritis: one year follow-up outcomes. Front Med (Lausanne) 2022; 9: 934937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Miyagawa I, Nakayamada S, Nakano K, et al. Precision medicine using different biological DMARDs based on characteristic phenotypes of peripheral T helper cells in psoriatic arthritis. Rheumatology (Oxford) 2019; 58: 336–344. [DOI] [PubMed] [Google Scholar]
- 57. Miyagawa I, Nakayamada S, Ueno M, et al. Impact of serum interleukin-22 as a biomarker for the differential use of molecular targeted drugs in psoriatic arthritis: a retrospective study. Arthritis Res Ther 2022; 24: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ooms A, Al-Mossawi H, Bennett L, et al. Optimising psoriatic arthritis therapy with immunological methods to increase standard evaluation: the protocol of an open-label multicentre, parallel-group, two-arm randomised controlled study evaluation precision medicine approach in the treatment of psoriatic arthritis. BMJ Open 2023; 13: e078539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Duan XP, Qin BD, Jiao XD, et al. New clinical trial design in precision medicine: discovery, development and direction. Signal Transduct Target Ther 2024; 9: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]