Abstract
Additional cytogenetic abnormalities (ACA) are considered a high risk feature in chronic myeloid leukemia (CML). However, its prognostic significance at the time of diagnosis in the setting of new tyrosine kinase inhibitors (TKIs) is less well understood. Patients with CML in CP with or without ACA at diagnosis treated with frontline TKIs in prospective clinical trials were analyzed for outcomes. Among 603 patients treated, 29 (5%) had ACA. Patients with ACA included 2 of 72 (2.8%) treated with imatinib 400 mg, 9 of 207 (4.3%) with imatinib 800 mg, 10 of 148 (6.7%) with dasatinib, 6 of 126 (4.7%) with nilotinib, and 2 of 50 (4%) with ponatinib. There was a significantly higher rate of complete cytogenetic response (CCyR) at 6 months in patients without ACA (P=.02). However cumulative CCyR and major molecular response (MMR) rates were not different. Similarly, MR4.0 and MR4.5 rates were similar for both groups; two CML-ACA patients maintained MR 4.5 for at least 2 years. At 5 years, ACA at diagnosis did not significantly impact transformation-free, failure-free, event-free, or overall survival expectations. Acknowledging small sample size estimates, response rates and survival outcomes were comparable in CP with ACA irrespective of whether chromosomal abnormalities were “major route” or other. The presence of ACA at diagnosis does not confer worse prognosis for patients with CML treated with TKI. Thus, the presence of ACA at diagnosis should not alter treatment strategies in these patients.
1 | INTRODUCTION
Additional clonal cytogenetic abnormalities (ACA), or cytogenetic clonal evolution (CE) as it is also referred to, are a window to underlying genomic instability and frequently herald an impending progression of disease. The appearance of these purely nonrandom events during the course of therapy portend an adverse prognosis and signal overt disease progression and inferior survival. The frequency of these additional cytogenetic abnormalities increases, across the disease spectrum, from 5–10% at diagnosis in early chronic phase (CP), to 30% and 80% in accelerated (AP) and blast (BP) phase, respectively.1–3 Although the detection of ACAs have been associated with inferior outcomes in AP and BP,4 data on their prognostic implications when present at the time of diagnosis in chronic phase in the context of tyrosine kinase inhibitor (TKI) therapy is still scanty.
Some studies evaluating the role of ACA at the time of diagnosis on patients treated with imatinib suggest a negative prognostic impact on molecular/cytogenetic response rates and overall survival, but data has been conflicting and far from unequivocal.4–7 This has partly to do with the fact that ACAs constitute a heterogeneous collection of karyotypic abnormalities, with widely varying prognostic impact. To this effect, ACAs are broadly classified into “major” and “minor” route chromosomal abnormalities.8,9 The most common ACAs including trisomy 8, a second Ph chromosome, chromosome 17 [isochromosome (17)(q10)], +der(22), are considered “major route changes,” and sporadic, infrequent aberrations such as trisomy 21, t(3;12), t(4;6), t(2;16), and t(1;21) among others are designated as minor ACAs.8,9 It has been recently reported that during disease progression, major, and not minor, route abnormalities confer a negative prognosis.10 In addition, individual, specific chromosomal abnormalities may also carry an adverse prognosis. For instance, chromosome 17, chromosome 3, and complex cytogenetic abnormalities have been associated with worse outcomes, while other abnormalities like deletion of derivative 9 have been shown to have no impact on prognosis when subjected to TKI therapy.11–13 Thus, the minor route ACA are not an entirely homogenous group with certain changes such as those involving 11q23 and 3q26 associated with TKI resistance and inferior outcomes.13,14 Wang and colleagues proposed a new classification system accounting for the impact of these abnormalities.15
The objective of this study was to analyze the impact of ACAs present at the time of diagnosis on outcome of patients with CML-CP receiving frontline TKI therapy.
2 | METHODS
2.1 | Study population
A total of 603 patients with CML in chronic phase were enrolled in consecutive or parallel single-institution clinical trials of frontline TKI therapy with imatinib (standard dose n = 72, high-dose n = 207), dasatinib (n = 148), nilotinib (n = 126), or ponatinib (n = 50). The trials were approved by the institutional review board and were performed according to the declarations of Helsinki. All the patients provided a written informed consent before participation. Patients were divided into two cohorts based on the presence or absence of ACA. Patients with clinical features of accelerated or blast phase were excluded.
2.2 | Treatment monitoring
Routine karyotype with G-banding was performed in all patients at baseline and every 3 months for the first 12 months, and every 12 months thereafter with 20 evaluable metaphases required for determination of response. In instances where less than 20 metaphases were evaluable, cytogenetic response was scored by FISH.
Patients were also monitored by real-time PCR performed at baseline, every 3 months for the first 12 months, and every 6 months thereafter, or in case of suspected failure or disease progression. All BCR-ABL measures are reported in the international scale (IS).
2.3 | Definition of responses
Definition of responses were according to the ELN criteria.16 A complete hematologic response (CHR) was defined as a white blood cell count of <10 × 109/L, a platelet count of < 450 × 109/L, no immature cells in the peripheral blood, and the disappearance of all signs and symptoms related to leukemia (including palpable splenomegaly). Cytogenetic response included complete cytogenetic response (CCyR): 0% Ph+ metaphases (or FISH <1% BCR-ABL nuclei of at least 200 nuclei), Partial cytogenetic response (PCyR): 1–35% Ph+ metaphases, Major cytogenetic response (MCyR): 0–35% Ph+ metaphases, minor cytogenetic response (mCyR): 35–95% Ph+ metaphases, and no cytogenetic response (no CyR): >95% Ph+ metaphases. Molecular responses included major molecular response (MMR): ≤0.1% BCR-ABL IS; MR4 ≤0.01% BCR-ABL IS; and MR4.5 ≤0.0032% BCR-ABL IS. An event was defined by the loss of CHR, MCyR, transformation to AP/BP, or death while on study. Failure was defined as any event as defined above, or the lack of achievement of CCyR by 18m or PCyR by 12 m, or treatment discontinuation for any reason other than elective discontinuation for sustained deep molecular response. Transformation was defined as the progression to AP/BP or death while on study. Overall survival included death at any time for any cause.
2.4 | Study end points
All comparisons between the 2 groups of patients were assessed with the Mann Whitney U test and Fisher exact test for continuous and categorical variables, as appropriate. Times to CCyR and MMR were calculated from the date of start of treatment until the achievement of the response. Overall survival (OS), transformation-free survival (PFS), failure-free survival (FFS), and event-free survival (EFS) were calculated from the date of the first TKI dose until the defining event as described above. Probabilities of CCyR, MMR, OS, PFS, FFS, and EFS were estimated using the Kaplan-Meier method. Times to response and survival times were compared with the log-rank test. Classification of CML-ACA patients by different classification (i.e., “major” vs “minor” route10 changes, and “favorable” vs “poor” prognostic15 groups) were based upon published criteria.
3 | RESULTS
A total of 603 consecutive patients were treated in the trials in question. Of the evaluated patients, 557 (92%) had at least 20 evaluable metaphases at diagnosis of whom 29 (5.2%) had ACA (4.8% of all patients treated). Detailed baseline characteristics of patients with and without ACA are outlined in Table 1. The two groups were similar across demographic and clinical parameters. The proportions of patients treated with each of the 4TKI were similar across the two groups. Patients without ACA received initial therapy with imatinib-400 (N = 72), imatinib-800 (N = 207), dasatinib (N = 148), nilotinib (N = 126), or ponatinib (N = 50), and CP patients with ACA received imatinib-400 (N = 2), imatinib-800 (N = 9), dasatinib (N = 10), nilotinib (N = 6), and ponatinib (N = 2).
TABLE 1.
Baseline patient and disease characteristics
| Chronic phase | Clonal evolution | ||
|---|---|---|---|
| N = 574 | N = 29 | ||
| Characteristics | Number (%) or median [range] | P-value | |
| Age, years | 52 [17.5–80.3] | 51.8 [17.2–81.6] | .53 |
| White blood cell, × 109/L | 22.5 [1.4–194] | 17.8 [3.6–130] | .12 |
| Hemoglobin, × 109/L | 12 [7.2–15.9] | 12 [8.8–15.8] | 1.00 |
| Platelets, × 109/L | 345 [109–1769] | 102 [312–2000] | .38 |
| Peripheral blood blasts, % | 0 [0–9] | 0 [0–2] | .87 |
| Peripheral blood basophils, % | 4 [0–14] | 3 [0–14] | .67 |
| Bone marrow blasts, % | 1 [0–6] | 2 [0–6] | .64 |
| Bone marrow basophils, % | 2 [0–13] | 2 [0–6] | .31 |
| Prior therapy | 66 (11) | 5 (17) | .37 |
| TKI therapy | |||
| Imatinib-400 | 70 (12) | 2 (7) | .57 |
| Imatinib-800 | 198 (35) | 9 (31) | .58 |
| Dasatinib | 138 (24) | 10 (34) | .46 |
| Nilotinib | 120 (21) | 6 (21) | .64 |
| Ponatinib | 48 (8) | 2 (7) | .57 |
| Cytogenetic abnormalities | |||
| Major route | NA | 12 (41) | NA |
| Minor route | NA | 17 (59) | NA |
| Distribution by Wang et al classification | |||
| Good risk | NA | 21 (72) | NA |
| Poor risk | NA | 8 (28) | NA |
Abbreviations used: NA-not applicable.
Among the 29 patients with ACA, major route abnormalities were identified in 12 patients (Supporting Information Table S1). These included trisomy 8, N = 3; der(22)t, N = 7; +19, N = 1; and double Ph (N = 1) (Figure 1A). Two of the highest risk cytogenetic abnormalities, chromosome 3 and chromosome 17 abnormalities, were not identified in any of these patients. Three patients had a variant Ph chromosome. Chromosomal abnormalities were also grouped by the classification proposed by Wang et al (Figure 1B). Eight of the 29 (28%) patients were classified as poor risk by this classification.
FIGURE 1.
(A) Clonal chromosomal abnormalities at diagnosis. (B) Chromosomal abnormalities according to classification by Wang et al.15 Foot note: ^- ≥ 2 ACAs: (+8,-Y,der(5)t=> N = 1); (+der(22)t,−7,t(1:13)=> N = 1); (+der (22)t + other rare chromosomal=> N = 4); (+19,+21: N = 1)]
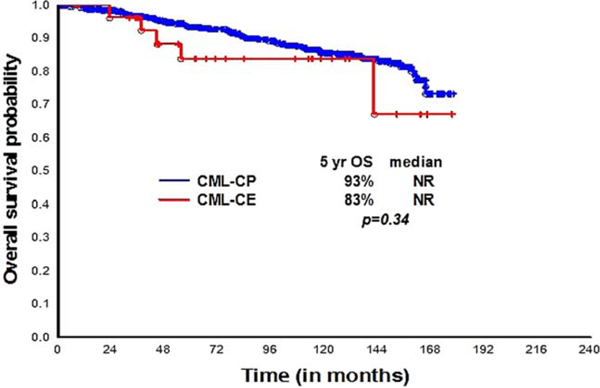
The median follow up for the cohorts with and without ACA were 97.5 (18–168) and 96 (0.5–177) months, respectively. Overall, 25 (86%) patients achieved a major molecular response (MMR) and 20 (69%) an MR4.5. Corresponding responses among those without ACA are 85% and 70%, respectively (Table 2). Two patients with ACA have achieved a sustained MR4.5 (i.e., MR4.5 maintained for 2 years with at least 5 measurements); 78 of 574 (14%) patients without ACA had a sustained MR4.5 (P=.41). The median times to CHR, CCyR, and MMR in CP and CP-ACA patients were 0.8, 3, and 5.9 months, and 0.7, 3, and 5.3 months, respectively. There was a somewhat higher rate of CCyR at 6 months among patients with ACA but no other significant differences were noted. The long-term survival outcomes were similar between the two cohorts. There was a nonsignificant trend for an inferior failure-free (Supporting Information Figure S1) and overall survival (Figure 2) for patients with ACA, with no difference in event-free and transformation-free survival (Supporting Information Figures S3and S4) (Table 2). One patient in the ACA cohort and 22 in the non-ACA cohort transformed to AP or BP (0 AP and 1 BP with ACA, 14 AP and 8 BP without ACA, respectively). The patient in the ACA cohort who transformed had a 46 XX,t(9;22) [15]/47XX,t(9;22),+8[5] karyotype at the time of diagnosis. Sixty nine and five patients have died in each cohort, respectively.
TABLE 2.
Comparison of responses in CML-CP patients with and without ACA
| Characteristic | Chronic phase | Clonal evolution | |
|---|---|---|---|
| N = 574 | N = 29 | ||
| Evaluable number | 560, N (%) | 29, N (%) | P-value |
| Response at 3–12 months, N (%) | |||
| CHR at 3 months | 534 (93) | 27 (93) | .98 |
| BCR-ABL ≤ 10% at 3 months | 431 (75) | 20 (69) | .51 |
| CCyR at 6 months | 474 (83) | 19 (65) | .02 |
| BCR-ABL ≤ 1% at 6 months | 339 (59) | 17 (59) | .99 |
| MMR at 12 months | 424 (74) | 21 (73) | .86 |
| Cumulative response, n (%) | |||
| CcyR | 516 (90) | 25 (86) | .22 |
| MMR | 490 (85) | 25 (86) | .90 |
| MR4.0 | 429 (75) | 20 (69) | .48 |
| MR4.5 | 402 (70) | 20 (69) | .90 |
| Sustained MR 4.5a | 78 (14) | 2 (7) | .41 |
| 5-yr survival probability by Kaplan-Meier approach, % (median in months) | |||
| FFS | 70 (NR) | 59 (89) | 0.52 |
| TFS | 93 (NR) | 94 (NR) | 0.56 |
| EFS | 85 (NR) | 86 (NR) | 0.70 |
| OS | 93 (NR) | 83 (NR) | 0.34 |
Sustained MR 4.5 defined by at least 5 measurements all MR4.5 during 2 year period. NR-not reached.
FIGURE 2.
Overall survival according to the presence (N = 29) or absence (N = 574) of ACA at the time of diagnosis. Sixty nine and five deaths occurred in the CML-CP and CML-CE cohorts, respectively. Abbreviations used: NR-not reached, OS-overall survival
Acknowledging the small sample size for subset analysis, response rates and survival outcomes were comparable in CP with ACA irrespective of whether chromosomal abnormalities were “major route” (N = 12) or other (N = 17) [Supporting Information Table S2]. Grouping patients by the Wang classification, only 8 patients were categorized as “poor” prognosis and the remaining 21 patients into the favorable risk category [Supporting Information Table S3]. The small sample size estimates proved inadequate for a reliable survival analysis.
Thirty one of 574 (5.4%) CML-CP patients without ACA at diagnosis developed ACA during the course of therapy (11 (1.9%) were major route abnormalities) while none of the patients with ACA at diagnosis developed additional chromosomal abnormalities during therapy, beyond those present at the time of diagnosis ACA. Over the course of therapy with TKI, 2 CML-ACA patients developed ACA in the Ph- metaphases; in one of them this was the same abnormality (4q deletion) seen with ACA at diagnosis, raising the possibility of a constitutional abnormality. Of the 5 CML-ACA patients with “-Y,” 3 had persistence of “-Y” in Ph negative metaphases after attaining CCyR, suggesting also constitutionality. In the remaining 2 patients, “–Y” was not detected in the Ph- metaphases as they responded to therapy.
4 | DISCUSSION
While inferior survival was more consistently associated with the presence of ACA at the time of diagnosis in CML patients treated with interferon alpha and other therapies,17,18 the prognostic relevance of ACAs in CML-ACA patients treated with imatinib has been controversial. While some reports showed no prognostic significance of the presence of ACA on the response to frontline imatinib,5,6 other studies have suggested lower response rates and inferior overall survival for patients with ACA at the time of diagnosis.4,7 Some of this variability in reported outcomes may be related to the type of ACA present. Fabarius et al recently reported that patients with “major route” ACA, but not those with minor route abnormalities, had an inferior survival compared to those without ACA when treated with imatinib.10 Such adverse prognostic impact of major route abnormalities is reflected in the 2013 ELN recommendations that include the presence of these abnormalities at diagnosis as a “warning” baseline sign that merits close attention.16 It is important however to underscore that both “major” and “minor” route abnormalities constitute heterogeneous entities where certain karyotype abnormalities might be associated with more pronounced TKI resistance and inferior outcomes. In addition, other abnormalities not previously recognized as being major route may also carry an inferior outcome, such as the recently reported effect of chromosome 3 abnormalities. More recently, a prognostically informative risk stratification system was proposed by Wang and colleagues to account for these heterogeneities.15 This proposal classified the more common ACAs into good (trisomy 8, −Y, and an extra copy of Philadelphia chromosome) and poor (i(17)(q10), −7/del7q, and 3q26.2) prognostic groups based on their differential prognostic impact on treatment and survival in the context of TKI therapy. Unfortunately, due to the small numbers of patients, subset analysis for these abnormalities yielded no informative results in our series to determine the impact they may have when present at the time of diagnosis. Of relevance however, none of the patients in our series had i(17)q or 3q26 rearrangements, chromosomal abnormalities associated with the worst survival outcomes. Although firm conclusions cannot be reached in this relatively small series, this could suggest that the highest risk abnormalities are reflective of a clonal evolution process that selects for the most resistant clones, whereas other chromosomal abnormalities may occur earlier in the course of the disease process and have a lesser impact in the biologic progression of the disease. Larger series of patients and further studies to investigate the molecular and biologic genesis and implications of the different abnormalities are required to better understand these phenomena.
With the use of TKI, the definitions of AP may need to be revised. The presence of cytogenetic clonal evolution was established in 1988 by Kantarjian et al.19 as one of the criteria defining accelerated phase disease. This definition was established based on a multivariate analysis that identified factors associated with a significantly inferior outcome. This definition has remained standard for many years and is the same that has been used in all the major pivotal studies of TKI. The World Health Organization has proposed an alternative definition that has not been validated with prospective large trials using TKI. Our group reported the limited clinical context of the new proposal.6 However, one criterion that seemed to be validated was the presence of “cytogenetic clonal evolution” at the time of diagnosis.6 In that analysis, patients with these criteria had the best overall outcome when treated with imatinib. More recently, it has been reported that patients with criteria for AP at the time of diagnosis had a favorable outcome mimicking that of patients with CP criteria when treated with TKI, particularly if using second generation agents.20 This is much in contrast with the emergence of AP during the course of therapy, whether by hematologic or cytogenetic parameters, which is indeed associated with an inferior response to therapy and long-term survival endpoints.21,22 Thus, with more specific therapy, more biologically oriented criteria for AP should be established. In one such approach, the genetic profile of patients in different stages of the disease were investigated. Patients with clinical CP criteria who had developed resistance to TKI had a genetic signature that resembled that of patients with blast phase.23 Although these results have not been validated, they suggest that in the era of TKI, molecular markers may be more informative indicators of disease stage than the clinical features we have used to date.
Although the proportion of patients achieving CCyR at 6 months was statistically lower in the ACA group as compared to the non-ACA, this transient disadvantage disappeared at 12 months. Cytogenetic testing is performed every 3 months and it is possible that some of the responders might have improved their response to CCyR soon after the 6 month testing, i.e., between the sixth and nine month period. In this context, the achievement of complete cytogenetic remission at 12 months, is a far more powerful predictor for sustained PFS and CCyR status on TKIs than the presence of clonal evolution at diagnosis.24,25 The lack of an impact of ACA on the 12 month CCyR rates suggest that BCR-ABL kinase domain (KD) and ACAs operate via largely independent molecular events, and that KD inhibition and BCR-ABL resistance mechanisms dominate outcomes of TKI therapy in CML-ACA, at least in cases with favorable baseline chromosomal abnormalities.
The current study included a sizeable proportion of CML-ACA patients treated with secondary generation TKIs. On comparing by TKI type, we did not identify statistically significant differences in survival/ event outcomes within CML-ACA based on treatment with imatinib (N = 11) or second generation TKIs (N = 18). However, it is difficult to accurately determine the differential impact of TKI type on outcomes given the small patient numbers and heterogeneity of underlying baseline chromosomal abnormalities present in each TKI group. As to whether second generation TKIs would perform better compared to imatinib in “high risk” patients with baseline major route abnormalities warrants evaluation in a larger study setting.
Sokal et al observed that the impact of ACA on progression and survival are delayed, with effects not apparent until later in the disease course.26 Along these lines, Fabarius et al were only able to demonstrate inferior PFS and OS in their “major route cytogenetic abnormality” group after a prolonged observation period of a median of 6.1 and 6.5 years, respectively.10 Comparably, the median follow up periods in CML-CP and CML-ACA groups in our study were 8.3 and 8 years, respectively. Despite a prolonged median follow up period, we did not observe a difference between CML-CP, CML-ACA minor, or CML-major groups in either PFS or OS. The discrepancies between the Fabarius’ and our study are reconciled by the type of clonal abnormalities constituting the ACA-major route category. Fourteen of the 16 patients in the former study were either trisomy 8 (N = 9) or i(17q (N = 5). In contrast, only 3 of 12 “major route” abnormalities patients in our study had trisomy 8 and none had isochromosome 17q abnormalities.
Here, it is noteworthy that our series with CML-ACA included 11 imatinib-treated patients, 9 of whom were treated with imatinib-800 and two with imatinib-400 mg. Our previous experience4 reporting on ACA at diagnosis and its impact on survival had only evaluated CML-CP patients treated on imatinib 400 mg daily dose. The Luatti et al group, which also reported inferior outcomes in CML-CP-ACA patients treated with imatinib 400/800 mg, did not explore the effect of imatinib dosing on response rates. Studies suggest that higher doses of imatinib are able induce earlier and deeper cytogenetic and molecular responses compared with imatinib 400 mg daily.27,28 Notably, all 11 imatinib-treated CML-ACA patients in our study had swift responses, achieving a major cytogenetic response by their third month ofcytogenetic evaluation. This is particularly relevant since achieving major cytogenetic response at 3 months has superior prognostic impact over the presence/absence of baseline cytogenetic evolution, on survival.4 It still remains to be clarified if higher doses of imatinib would be more beneficial in CML-ACA, as compared to the standard 400 mg dose, by their ability to induce earlier responses thereby positively impacting survival outcomes.
In conclusion, the presence of clonal evolution per se does not signal worse prognosis at least in the chronic phase. The type of abnormality appears to have a minimal role on outcome, although the highest risk abnormalities (i.e., abnormalities in chromosomes 3 and i17q) are rarely if ever detected at diagnosis. In this context, it could be questioned whether cytogenetic evaluation at diagnosis carries value. For the purposes of establishing a baseline that may help assess ACA that may occur later in the disease as pre-existent of newly occurring, such assessment may still have some, albeit modest, value.
Supplementary Material
Funding information
University of Texas MD Anderson Cancer Center, Grant/Award Number: P30 CA16672, P01 CA049639 (PI, Dr. Richard Champlin); National Cancer Institute (NCI). Jorge Cortes is the recipient of a grant from the NCI (P01 CA049639)
DISCLOSURES
Jorge Cortes: Consultant for Ariad, BMS, Novartis, and Pfizer; Research support from Ariad, BMS, Novartis, Pfizer, And Teva.
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- [1].Anastasi J, et al. The relationship between secondary chromosomal abnormalities and blast transformation in chronic myelogenous leukemia. Leukemia. 1995;9(4):628–633. [PubMed] [Google Scholar]
- [2].Swolin B, Weinfeld A, Westin J, et al. Karyotypic evolution in Ph- positive chronic myeloid leukemia in relation to management and disease progression. Cancer Genet Cytogenet. 1985;18(1):65–79. [DOI] [PubMed] [Google Scholar]
- [3].Zaccaria A, Testoni N, Valenti AM, et al. Chromosome abnormalities additional to the Philadelphia chromosome at the diagnosis of chronic myelogenous leukemia: pathogenetic and prognostic implications. Cancer Genet Cytogenet. 2010;199(2):76–80. [DOI] [PubMed] [Google Scholar]
- [4].Cortes JE. Prognostic significance of cytogenetic clonal evolution in patients with chronic myelogenous leukemia on imatinib mesylate therapy. Blood. 2003;101(10):3794–3800. [DOI] [PubMed] [Google Scholar]
- [5].Schoch C, Haferlach T, Kern W, et al. Occurrence of additional chromosome aberrations in chronic myeloid leukemia patients treated with imatinib mesylate. Leukemia. 2003;17(2):461–463. [DOI] [PubMed] [Google Scholar]
- [6].Cortes JE, Talpaz M, O’brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106(6):1306–1315. [DOI] [PubMed] [Google Scholar]
- [7].Luatti S, Castagnetti F, Marzocchi G, et al. Additional chromosomal abnormalities in Philadelphia-positive clone: adverse prognostic influence on frontline imatinib therapy: a GIMEMA Working Party on CML analysis. Blood. 2012;120(4):761–767. [DOI] [PubMed] [Google Scholar]
- [8].Mitelman F, Levan G, Nilsson PG, et al. Non-random karyotypic evolution in chronic myeloid leukemia. Int J Cancer. 1976;18(1):24–30. [DOI] [PubMed] [Google Scholar]
- [9].Mitelman F. The cytogenetic scenario of chronic myeloid leukemia. Leuk Lymphoma. 1993;11(Suppl 1):11–15. [DOI] [PubMed] [Google Scholar]
- [10].Fabarius A, Leitner A, Hochhaus A, et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118(26):6760–6768. [DOI] [PubMed] [Google Scholar]
- [11].Reid AG, Nacheva EP. A potential role for PRDM12 in the pathogenesis of chronic myeloid leukaemia with derivative chromosome 9 deletion. Leukemia. 2004;18(1):178–180. [DOI] [PubMed] [Google Scholar]
- [12].Verma D, et al. Survival outcomes for clonal evolution in chronic myeloid leukemia patients on second generation tyrosine kinase inhibitor therapy. Cancer. 2010;116(11):2673–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang W, Cortes JE, Lin P, et al. Clinical and prognostic significance of 3q26.2 and other chromosome 3 abnormalities in CML in the era of tyrosine kinase inhibitors. Blood. 2015;126(14):1699–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang W, et al. Chromosomal rearrangement involving 11q23 locus in chronic myelogenous leukemia: a rare phenomenon frequently associated with disease progression and poor prognosis. J Hematol Oncol. 2015;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang W, Cortes JE, Tang G, et al. Risk stratification of chromosomal abnormalities in chronic myelogenous leukemia in the era of tyrosine kinase inhibitor therapy. Blood. 2016;127(22):2742–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kantarjian HM, et al. Chronic myelogenous leukemia: a multivariate analysis of the associations of patient characteristics and therapy with survival. Blood. 1985;66(6):1326–1335. [PubMed] [Google Scholar]
- [18].Farag SS, et al. Prognostic significance of additional cytogenetic abnormalities in newly diagnosed patients with Philadelphia chromosome-positive chronic myelogenous leukemia treated with interferon-alpha: a Cancer and Leukemia Group B study. Int J Oncol. 2004;25(1):143–151. [PubMed] [Google Scholar]
- [19].Kantarjian HM, Dixon D, Keating MJ, et al. Characteristics of accelerated disease in chronic myelogenous leukemia. Cancer. 1988;61 (7):1441–1446. [DOI] [PubMed] [Google Scholar]
- [20].Ohanian M, et al. Tyrosine kinase inhibitors as initial therapy for patients with chronic myeloid leukemia in accelerated phase. Clin Lymphoma Myeloma Leuk. 2014;14(2):155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].O’Dwyer ME, et al. Clonal evolution and lack of cytogenetic response are adverse prognostic factors for hematologic relapse of chronic phase CML patients treated with imatinib mesylate. Blood. 2004;103(2):451–455. [DOI] [PubMed] [Google Scholar]
- [22].Cortes J, O’dwyer ME. Clonal evolution in chronic myelogenous leukemia. Hematol Oncol Clin North Am. 2004;18(3):671–684, x. [DOI] [PubMed] [Google Scholar]
- [23].Radich JP, Dai H, Mao M, et al. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc Natl Acad Sci U S A. 2006;103(8):2794–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].de Lavallade H, Apperley JF, Khorashad JS, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008;26(20):3358–3363. [DOI] [PubMed] [Google Scholar]
- [25].Druker BJ, Guilhot F, O’brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. [DOI] [PubMed] [Google Scholar]
- [26].Sokal JE, et al. Prognostic significance of additional cytogenetic abnormalities at diagnosis of Philadelphia chromosome-positive chronic granulocytic leukemia. Blood. 1988;72(1):294–298. [PubMed] [Google Scholar]
- [27].Cortes JE, Kantarjian HM, Goldberg SL, et al. High-dose imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: high rates of rapid cytogenetic and molecular responses. J Clin Oncol. 2009;27 (28):4754–4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Deininger MW, Kopecky KJ, Radich JP, et al. Imatinib 800 mg daily induces deeper molecular responses than imatinib 400 mg daily: results of SWOG S0325, an intergroup randomized PHASE II trial in newly diagnosed chronic phase chronic myeloid leukaemia. Br J Haematol. 2014;164(2):223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.