Abstract
Background
This study aimed to evaluate the efficacy and safety of thermal ablation in the treatment of patients with Bethesda IV thyroid nodules (follicular neoplasms) by analyzing large-scale data on various outcomes.
Materials and methods
Literature searches were conducted in PUBMED, EMBASE, Web of Science, and the Cochrane Library for studies on the use of thermal ablation in patients with Bethesda IV thyroid nodules published from March 1, 2014, to March 1, 2024. Data on volume change at 12 months; the volume reduction rate (VRR) at 1, 3, 6, and 12 months; the complete disappearance rate (CDR); and the complication rate were evaluated. All the data were analyzed with STATA 15.
Results
Five eligible studies were included. The findings indicate that thermal ablation is both effective and safe. The mean change in tumor volume at 12 months postthermal ablation was characterized by a standardized mean difference (SMD) of -1.13 (95% CI: -1.36 - -0.90, p = 0.000). Specifically, the mean changes in tumor volume at 12 months after radiofrequency ablation (RFA) and microwave ablation (MWA) were − 1.19 (95% CI: -1.75 - -0.64) and − 1.26 (95% CI: -1.71 - -0.81), respectively. The VRRs at 1, 3, 6, and 12 months postthermal ablation were 43% (95% CI: 33 − 53%), 47% (95% CI: 20 − 74%), 69% (95% CI: 62 − 76%), and 85% (95% CI: 79 − 90%), respectively. The VRRs at 12 months after RFA and MWA were 84% (95% CI: 76 − 91%) and 85% (95% CI: 75 − 95%), respectively. The VRR at 12 months, stratified by initial nodule size, was 84% (95% CI: 77 − 91%) and 86% (95% CI: 78 − 94%). The CDR at the final follow-up was 88% (95% CI: 80 − 95%). The complication rate was 4.0% (95% CI: 0.0 − 8.0%), with pain and hoarseness being the most frequently reported complications; no life-threatening complications were documented.
Conclusions
Thermal ablation is a reliable treatment for Bethesda IV thyroid nodules, and RFA and MWA are advantageous treatment strategies. However, more prospective, multicenter, and large-sample studies are needed in the future.
Keywords: Thermal ablation, Radiofrequency ablation, Microwave ablation, Bethesda IV, Thyroid nodules
Introduction
Thyroid nodules are common, and the rate of thyroid nodule detection with high-resolution ultrasound (US) is as high as 68%; moreover, most thyroid nodules are benign. However, the incidence of malignancy is 7-15% [1], and fine-needle aspiration cytology (FNAC) is a standard diagnostic tool for evaluating thyroid nodule malignancy. Bethesda IV thyroid nodules (follicular neoplasms), a cytological type with an indeterminate nature, account for 2-25% of all thyroid fine needle puncture samples [2]. The risk of malignancy of Bethesda IV nodules is estimated to be 30% in adults and 50% in children [2]. Because of the high rate of malignancy, surgical resection is recommended for children. Molecular testing and diagnostic lobectomy are used for risk assessment in adults [2–4]. Lobectomy is the definitive diagnostic tool for distinguishing malignant tumors from adenomas based on the presence of vascular or capsular invasion [5]. Molecular testing is helpful for improving the accuracy of diagnosis of indeterminate cases and may guide the scope of surgery [6]. Studies have shown that molecular detection allows 49% of patients with indeterminate nodules to avoid diagnostic surgery [7], but the cost of testing remains the most important problem. With the increasing incidence of Bethesda IV thyroid nodules, the increasing number of elderly patients, and the fact that most nodules tend to be benign, more patients are at high risk of surgery, are not candidates for surgery, or cannot afford the high cost of molecular testing; these patients may also refuse surgery in the hope of undergoing other, more conservative treatments.
Tumor ablation is a minimally invasive technique that is commonly used to treat tumors of the liver, kidney, bone, and lung [8]. All thermal ablation (TA) techniques are based on tissue destruction under extreme hyperthermic conditions. Thermal ablation primarily includes 3 techniques [9]: microwave ablation (MWA), laser ablation (LA), and radiofrequency ablation (RFA). RFA is a nonsurgical, minimally invasive technique that relies on alternating electromagnetic current to cause frictional molecular heating to control tissue mass. Microwave ablation relies on the generation of an electromagnetic field with wavelengths between 0.03 and 30 cm and frequencies between 900 and 2500 MHz to cause the oscillation of polarized ions. Laser thermal ablation is another method of delivering thermal energy that has been applied to thyroid nodules. LTA involves the delivery of a focused beam of light energy through an optical fiber into the target tissue.
Minimally invasive techniques have become alternative options for patients who are considered to be at high surgical risk or who wish to undergo more aggressive treatment than active testing, and thermal ablation has been widely used as a minimally invasive procedure. Thermal ablation has been proven to be effective and safe in the treatment of benign thyroid nodules and autonomously functioning thyroid nodules [10–12]. Recently, thermal ablation has also achieved good results in the treatment of papillary thyroid cancer (PTC) and local recurrent thyroid cancer [13].
The purpose of this meta-analysis was to comprehensively evaluate the efficacy and safety of thermal ablation in the treatment of Bethesda IV thyroid nodules to provide stronger evidence for the treatment of Bethesda IV thyroid nodules.
Materials and methods
Search strategy
This study was conducted according to the PRISMA [14] guidelines. Two reviewers (blind and blind) conducted independent literature searches. Four databases from PubMed, Embase, Web of Science, and the Cochrane Library were retrieved from March 1, 2014, to March 1, 2024, for the keywords “thyroid” and “radiofrequency ablation” or “microwave ablation” or “ablation”.
Inclusion criteria
(1) Bethesda IV thyroid nodules were confirmed by FNA under ultrasound guidance; (2) patients received only one form of ablation, namely, RFA, MWA or LA; (3) the experimental methods used in the study were randomized controlled trials, retrospective analyses or prospective analyses; (4) reported results were detailed enough to assess volume reduction, complete disappearance, complications, and tumor progression.
Exclusion criteria
(1) articles that are not in the field of interest of this research; (2) articles without clear efficacy or safety data; (3) studies with overlapping patient or nodule data; and (4) review articles, editorials, letters, reviews or case series reports.
Data extraction
The two researchers independently carried out the literature search, screening, and information extraction. When a problem or dispute arose, consensus was reached after discussion or consultation. The following characteristics were extracted from the included studies: (1) study characteristics: first author, year of publication, unit, study period, study design style, treatment of TA, and sample size; (2) demographic and clinical characteristics of patients: mean age, sex, ablation technique (RFA, MWA, LA), and follow-up interval; (3) therapeutic characteristics: tumor volume change [final tumor volume − initial tumor volume], volume reduction rate, and complete disappearance rate; and (4) safety features: complication rate.
Quality assessment
Because the included studies were not randomized controlled, retrospective, or prospective observational studies, two independent researchers used the methodological index for nonrandomized studies. The MINORS scale assesses the quality of evidence for each study [15]. There are a total of 12 events, each scoring 0–2 for a total of 24. Studies from 9 to 16 were classified as medium quality, and those from 17 to 24 were classified as high quality.
Data synthesis and statistical analysis
We analyzed all the data with STATA 15. We evaluated the efficacy of thermal ablation by analyzing the tumor volume, VRR, and CDR for Bethesda IV thyroid nodules and then evaluated its safety by analyzing the incidence of complications. We used I2 and Q tests to assess heterogeneity. If the heterogeneity test result was p ≥ 0.1 and I2 ≤ 50%, the studies were homogeneous, and the fixed effects model was used for comprehensive analysis. If p < 0.1 and I2 > 50%, heterogeneity was present and sensitivity analysis was performed to determine its source. If the heterogeneity was still high, then we used a random effects model or abandoned the outcome combination and used descriptive analysis. We used funnel plots and Egger tests to assess publication bias.
Results
Literature search results
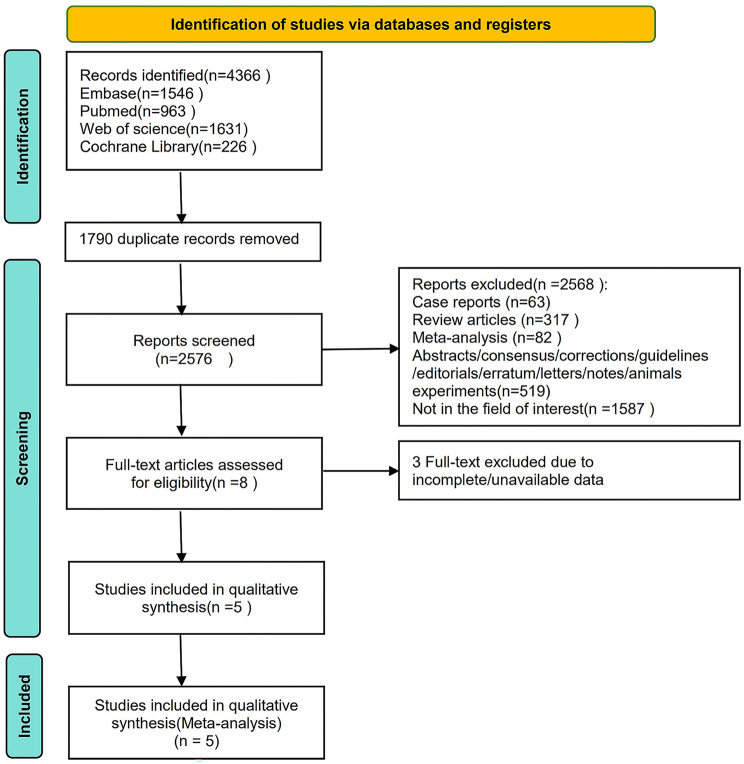
The article selection process is presented in detail in Fig. 1. The initial literature search identified 4366 articles. After removing duplicates, 1790 articles remained. A total of 2568 studies were excluded, including 63 case reports, 317 review articles, 82 meta-analyses, 519 studies in other formats, and 1587 unrelated studies. Eight full-text articles were subsequently assessed for eligibility. After browsing the full-text studies, we obtained 5 studies and excluded any that did not report the outcomes of interest. Finally, we included 5 articles in the meta-analysis (Fig. 1).
Fig. 1.
Flow diagram of the article selection process
Characteristics of the included studies and quality assessment
In total, in this meta-analysis, 5 studies were retrospective studies. The patient sample size totaled 170. Generally, 3 studies included RFA, 1 study included MWA and 1 study included RFA and MWA. Two studies received a score of 21, one study received a score of 20, one study received a score of 13, and one study received a score of 12, indicating that the included studies were of moderate or high quality (Table 1).
Table 1.
Characteristics of the studies included in this meta-analysis
| Author | Year | Ablation modality | Country | Research type | No. of patients | age | Sex (male/female) | No. of tumors | Initial tumor volume, mean (SD), ml | Follow-up time (month) | MINORS score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yang [5] | 2022 | MWA | China | Prospective | 46 | 46.30 ± 11.54 | 11/35 | 47 | 7.47 ± 6.68 ml | 10.05 ± 3.06 | 21 |
| Lin [16] | 2021 | RFA | Korea | Retrospective | 22 | 39.6 ± 10.5 | 1/21 | NA | 12.60 ± 20.90 | 10.1 (6–12) | 21 |
| Li [17] | 2021 | RFA, MWA | China | Retrospective | 30 | 47.3 ± 13.6 | 4/26 | 30 | 6.56 ± 9.09 | 16.4 ± 5.2 | 20 |
| Su [18] | 2017 | RFA | Korea | Retrospective | 10 | 41.8 ± 11.2 | 0/10 | 10 | 0.60 ± 0.40 | 66.4 ± 5.1 | 13 |
| Dong [19] | 2023 | RFA | China | Retrospective | 62 | 38 ± 10 | 10/52 | 62 | 0.97 ± 0.71 | 40 ± 11 | 12 |
SD, standard deviation; NA, data unavailable
Results of the meta-analysis
Volume changes in tumors after thermal ablation
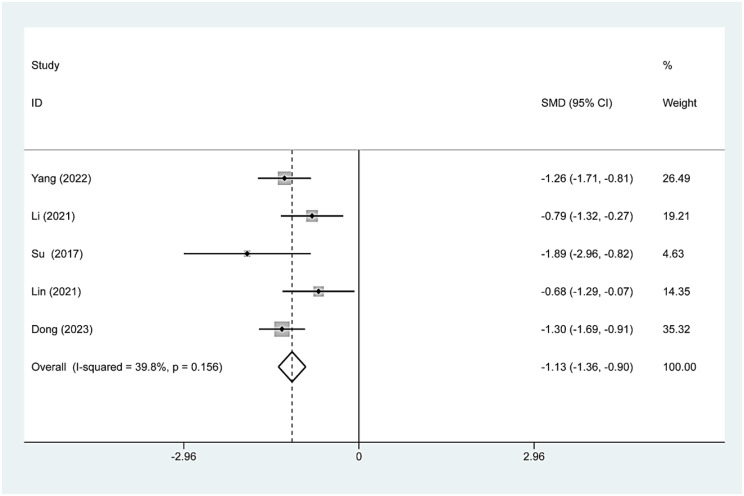
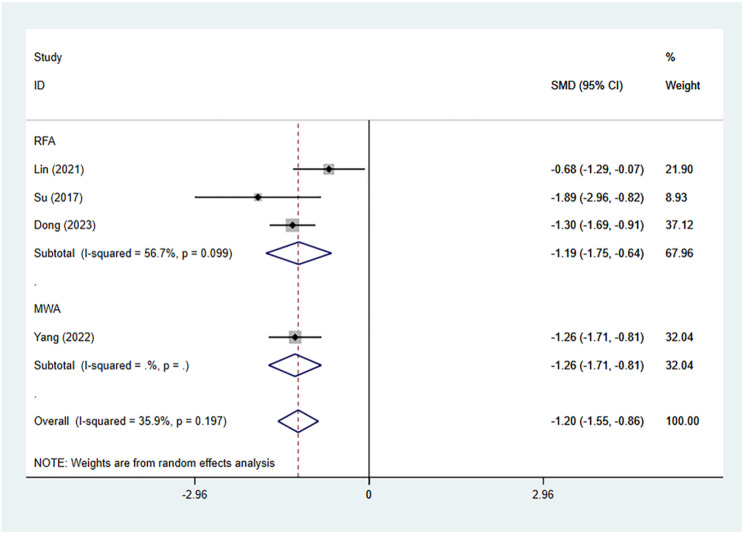
Five studies involving 170 patients reported changes in tumor volume before and after TA treatment. The mean baseline volume of nodules in 170 patients was 5.20 ± 4.08 ml. The changes in tumor volume at the 12-month follow-up after TA, RFA and MWA were as follows: standardized mean difference [SMD]=-1.13, 95% CI:−1.36–0.90, p = 0.156; Fig. 2); ([SMD]=-1.19, 95% CI:−1.75--0.64, p = 0.099; Fig. 3); and ([SMD]=-1.26, 95% CI:−1.71–0.81; Fig. 3).
Fig. 2.
TA’s pooled estimates of tumor volume change at the 12-month follow-up
Fig. 3.
The change in tumor volume after RAF and MWA at the 12-month follow-up
Volume reduction rates
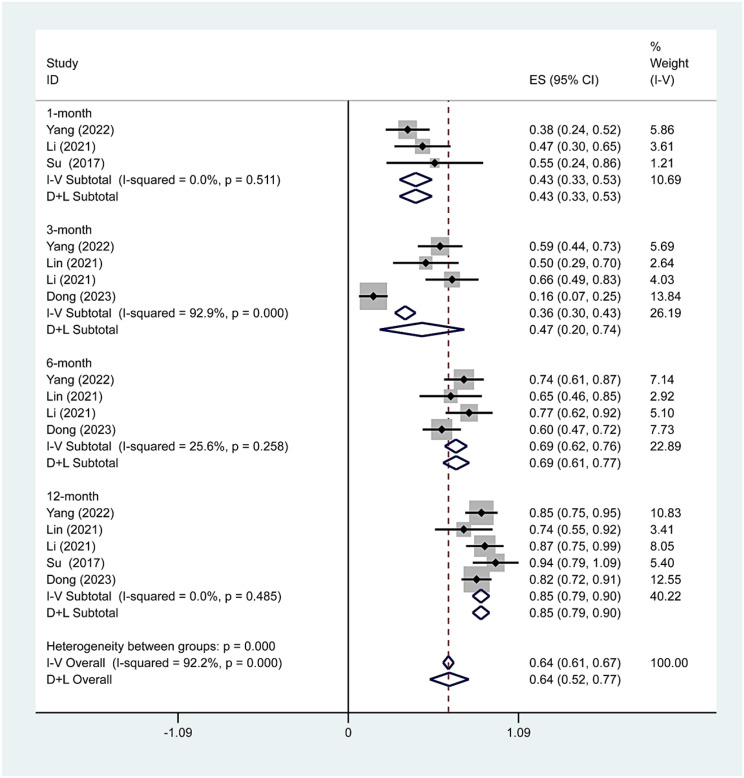
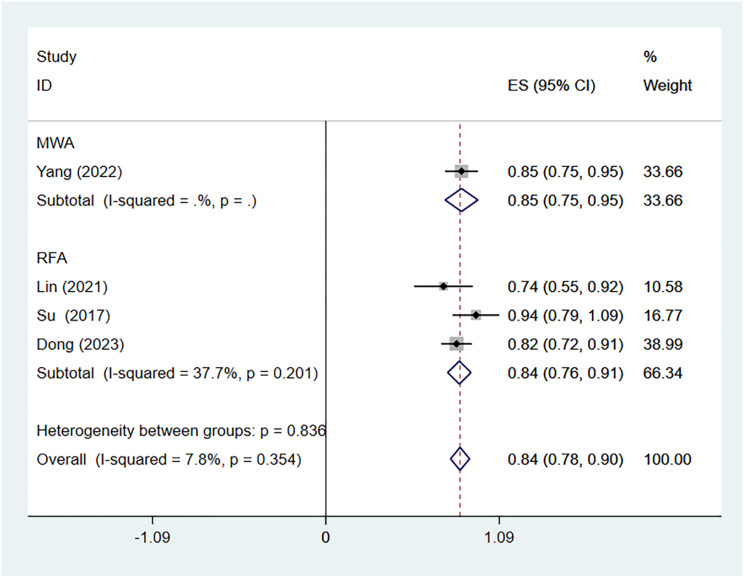
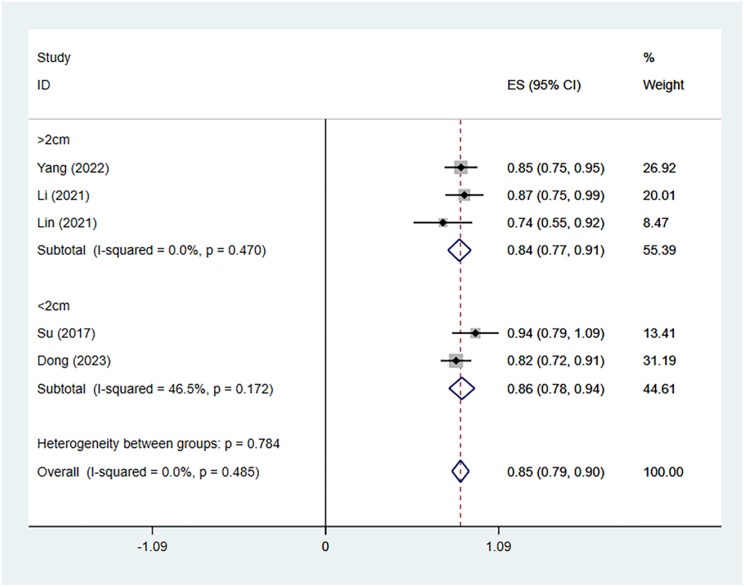
We further analyzed the VRR during different time periods. Three, four, four and five studies including 86, 160, 160 and 170 patients, respectively, reported the VRR after thermal ablation treatment of Bethesda IV thyroid nodules. There was no significant heterogeneity at 1 (I2 = 0.0%, p = 0.511), 6 (I2 = 25.6%, p = 0.258) or 12 (I2 = 0.0%, p = 0.485) months after TA, whereas there was significant heterogeneity at 3 months (I2 = 92.9%, p = 0.000) after TA. The pooled results revealed that the VRRs at 1, 3, 6 and 12 months after TA were 43% (95% CI: 33–53%), 47% (95% CI: 20–74%), 69% (95% CI: 62–76%), and 85% (95% CI: 79–90%, respectively (Fig. 4). The pooled estimates of the VRR based on the ablation method at the 12-month follow-up were 84% (95% CI: 76-91%, I2 = 37.7%; Fig. 5) for RFA and 85% (95% CI:75 − 95% Fig. 5) for MWA. The pooled estimates of the VRR based on initial nodule size at the 12-month follow-up were 84% (95% CI: 77-91%, I2 = 0.0%; Figs. 6) and 86% (95% CI:78 − 94%, I2 = 46.5%; Fig. 6).
Fig. 4.
TA’s pooled estimates of VRR at the 1-, 3-, 6-, and 12-month follow-ups
Fig. 5.
Volume reduction rates after RAF and MWA at the 12-month follow-up
Fig. 6.
Volume reduction rates based on initial nodule size at the 12-month follow-up
Complete disappearance rate
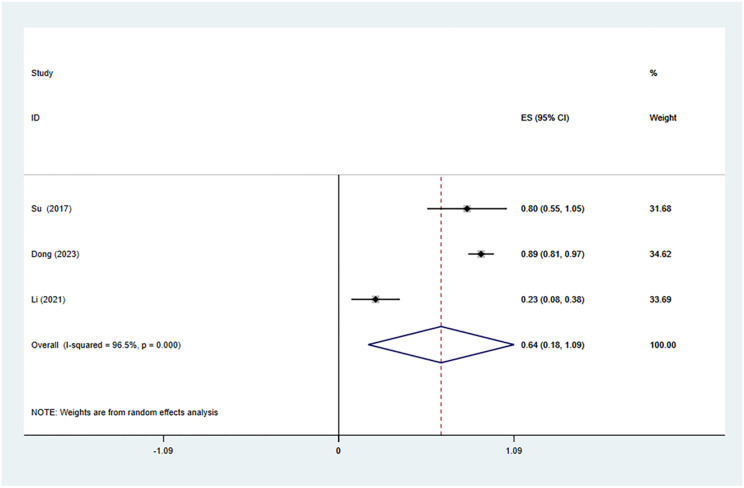
We also analyzed the CDR at the last follow-up period after TA. Three studies with 102 patients reported the CDR after TA treatment of Bethesda IV thyroid nodules. There was significant heterogeneity (I2 = 96.5%, p = 0.000), and we conducted a meta-analysis via a random effects model. The pooled results revealed that the CDR at the last follow-up after TA was 64% (95% CI: 18–109%; Fig. 7).
Fig. 7.
TA’s pooled estimates of the complete disappearance rate at the final follow-up
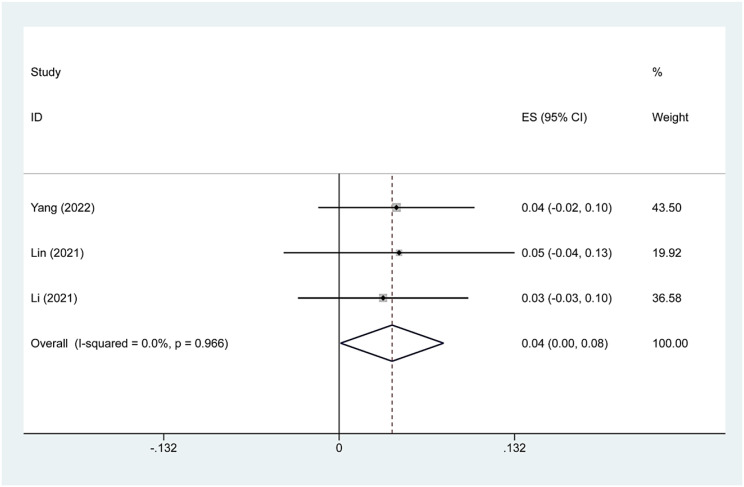
Complication rate
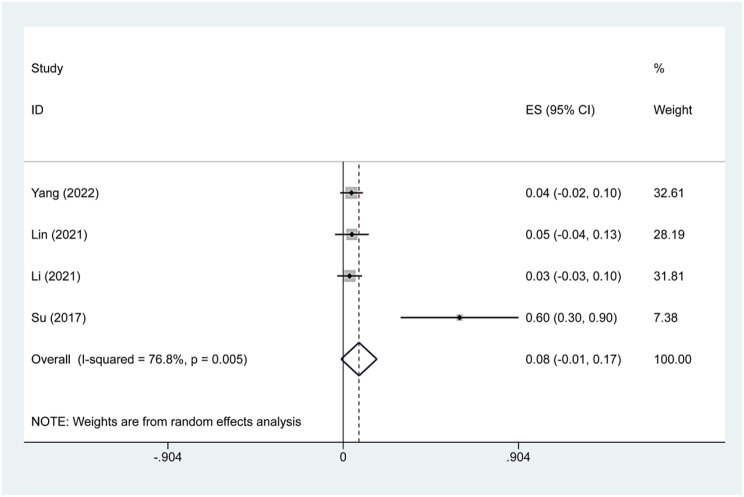
Four studies reported minor complications after TA treatment of Bethesda IV thyroid nodules. We found that the main complications were hoarseness and pain. Because there was significant heterogeneity (I2 = 76.8%, p = 0.005), we conducted a sensitivity analysis and found that Su’s 2017 study had a large impact on the results. Heterogeneity decreased after excluding this study (I2 = 0.0%, p = 0.966), and we conducted a meta-analysis using a fixed effects model. The pooled results revealed that the incidence of minor complications after TA treatment was 4.0% (95% CI:0.0–8.0%; Figs. 8, 9).
Fig. 8.
Pooled estimates of complication rates before sensitivity analysis
Fig. 9.
Pooled estimates of complication rates after sensitivity analysis
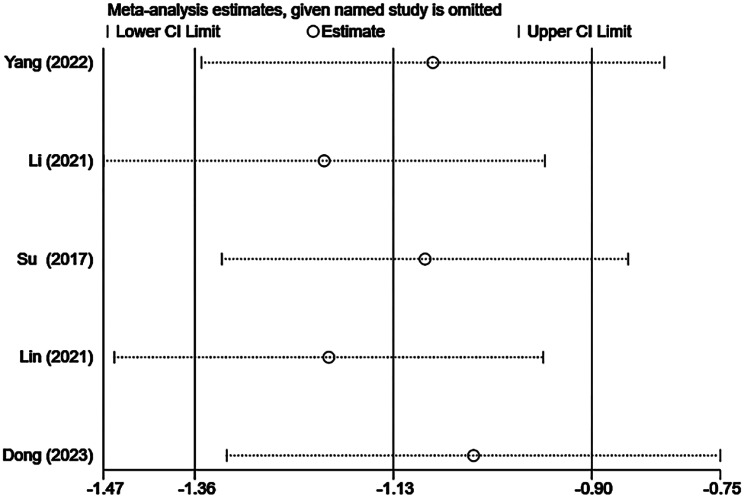
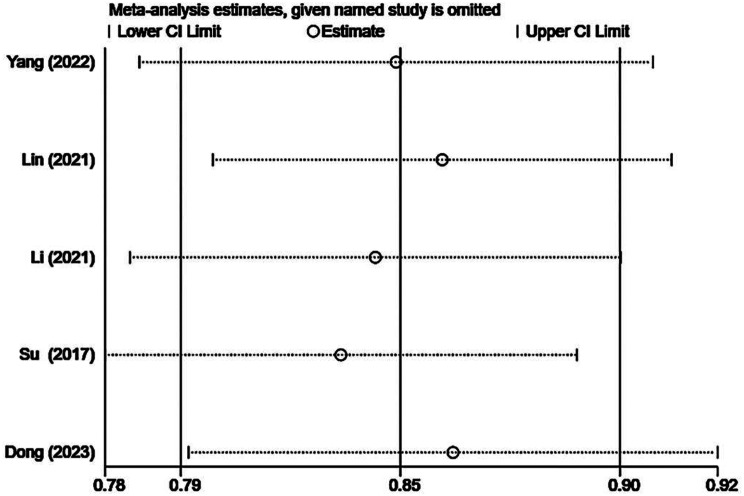
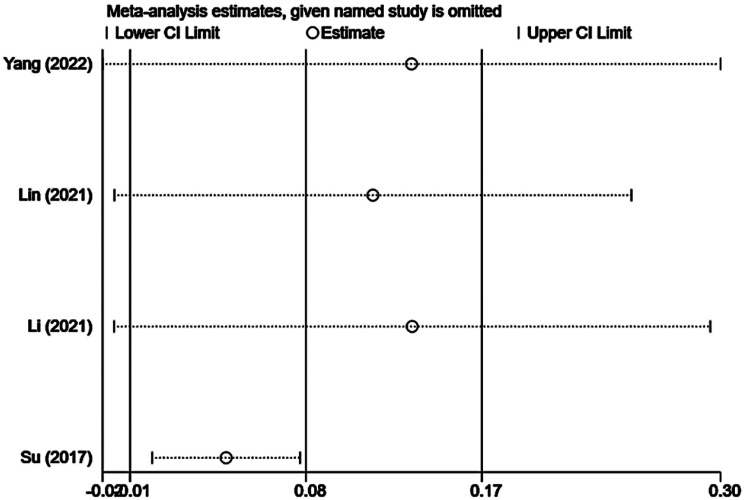
Sensitivity analysis
The results of the sensitivity analysis are presented in Supplementary Figs. 10, 11, 12. We performed sensitivity analysis by individually excluding each included study and conducting a summary analysis of the remaining studies. Our results revealed that Su’s [18] article strongly interfered with complication rates, which may be related to the small sample size, and the remaining studies did not unduly influence the results of the meta-analysis.
Fig. 10.
Sensitivity analysis of the change in tumor volume after TA
Fig. 11.
Sensitivity analysis of the volume reduction rate after TA
Fig. 12.
Sensitivity analysis of the complication rate after TA
Publication bias
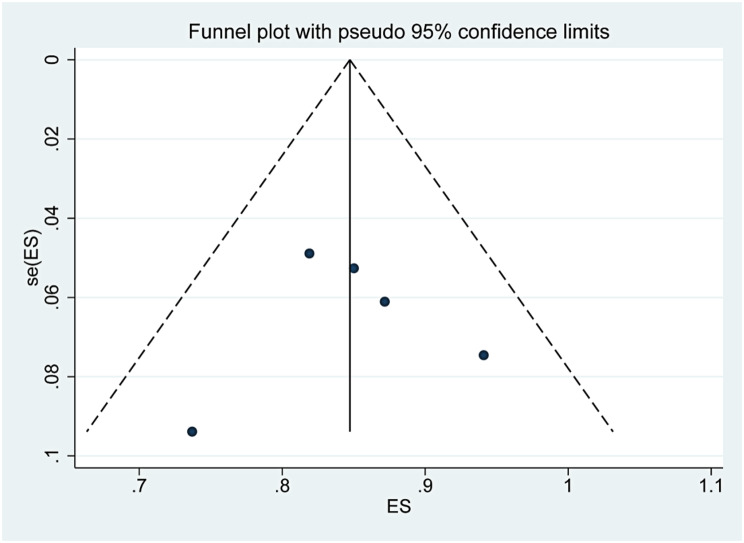
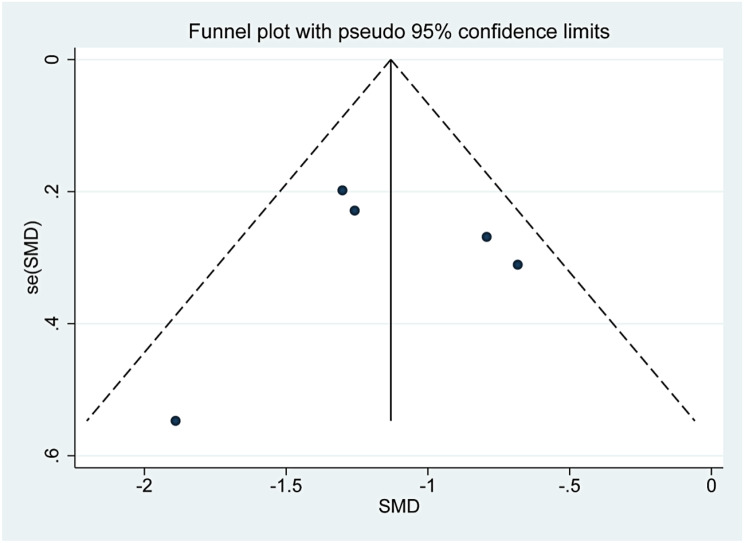
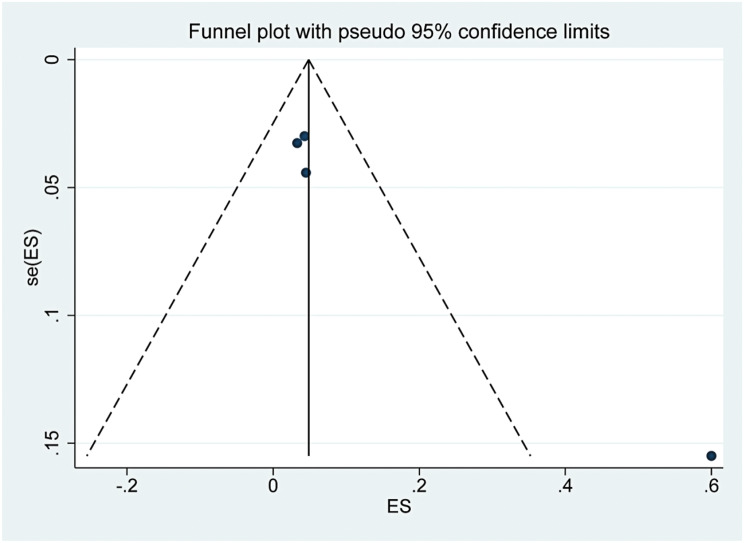
The funnel plots are presented in Supplementary Figs. 13, 14, 15. The three funnel plots were symmetrical, and the p values of Egger’s tests were 0.918, 0.933 and 0.051, which indicated that there was no obvious publication bias in this study.
Fig. 13.
Funnel plot of the change in tumor volume after TA
Fig. 14.
Funnel plot of the volume reduction rate after TA
Fig. 15.
Funnel plot of the complication rate after TA
Discussion
According to data released by the World Health Organization, the incidence of thyroid cancer ranks ninth in the world [18, 19]. In recent years, with the development of ultrasound and other imaging techniques, an increasing number of thyroid nodules have been found, followed by the excessive diagnosis of thyroid cancer, which increases the number of unnecessary surgeries with potential complications and the cost of treatment for individuals and society. Studies have shown that thyroid cancer survivors report greater psychosocial distress than nonthyroid cancer survivors do [20–22]. Therefore, clinicians should try to avoid excessive diagnoses of thyroid nodules. A patient’s perception of their diagnosis and the resulting emotional response can influence treatment decisions and may contribute to decisions that may result in the excessive treatment of low-risk thyroid cancer patients [23].
Fine needle aspiration biopsy is a reliable, commonly used, and widely accepted method for the diagnosis of thyroid cancer [24]. The Bethesda system divides cytology results into six categories; Bethesda III and IV types of lesions are often called indeterminate lesions and are characterized by difficultly of diagnosis [25]. The traditional method for Bethesda IV cytology is diagnostic lobectomy. In the last decade, multiple molecular diagnostic tests for FNA specimens have matured, and these tests can help clinicians make more informed clinical decisions for patients with indeterminate thyroid nodules and may reduce the rate of unnecessary thyroid surgery. A study conducted in Italy [26] noted that repeated thyroid FNAC results for EU-TIRADS 4/5 nodules varied more frequently than did those for EU-TIRADS 2/3 nodules between high-volume and low-volume centers. This finding indicates that repeat FNAC is crucial for accurately determining the cytological nature of indeterminate thyroid nodules, which directly influences the subsequent treatment plan for the nodules.
Gene expression assays using FNAC materials may have high predictive value for cytologically indeterminate thyroid nodules [27]. In a prospective study, the polygenic GC test (ThyroSeqv3) helped potentially avoid diagnostic surgery in up to 61% of patients with Bethesda III or IV indeterminate nodules and in up to 82% of patients with indeterminate benign cytology nodules [28]. It has also been reported that the majority of indeterminate thyroid nodules with benign molecular test results are stable over 3 years of follow-up [29]. However, a previous study [30] reported that ultrasound combined with isolated RAS mutations does not distinguish between benign and malignant nodules. Minimally invasive techniques have emerged as alternative options for patients who are considered to be at high surgical risk or who wish to receive a more aggressive treatment approach than does AS. Radiofrequency ablation reportedly plays a positive role in the treatment of benign nonfunctional thyroid nodules, autonomously functional thyroid nodules, low-risk papillary thyroid carcinoma, and recurrent thyroid carcinoma, while the treatment of follicular thyroid neoplasms is controversial [31]. According to international guidelines [2], Bethesda IV nodules should currently be treated as malignant tumors. Since thermal ablation cannot completely remove large nodules, it is not recommended for treating Bethesda IV nodules. Issa’s [32] study noted that RFA was comparable in terms of ablation effectiveness for indeterminate thyroid nodules and benign thyroid nodules. These findings suggest that radiofrequency ablation is an effective method for the treatment of indeterminate thyroid nodules. A study [33] in China reported that preoperative CEUS was helpful for determining the ablation range of thyroid follicular adenoma by microwave ablation to more accurately evaluate the efficacy of ablation.
In this study, we analyzed the short-term efficacy of thermal ablation in the treatment of Bethesda IV thyroid nodules by aggregating 5 studies (including 170 patients) that reported the use of ultrasound-guided thermal ablation for Bethesda IV thyroid nodules to guide clinical treatment. Dobrinja’s [34] study revealed that a single RFA does not affect subsequent thyroid surgery or histological diagnosis. Given the rapid growth of follicular carcinoma, if no recurrence of nodules is found during follow-up, we may wish to perform a secondary ablation for complete ablation. This controversy needs to be resolved by more clinical studies in the future.
With respect to age, the patients who were included in the study were not considered advanced in years and were predominantly middle-aged with a long life expectancy. Given the high risk of malignancy of Bethesda IV thyroid nodules and the short follow-up time of this study, thermal ablation is not recommended for middle-aged patients diagnosed with Bethesda IV nodules.
In terms of safety, we analyzed the incidence of minor complications. No life-threatening events were reported. The primary complications identified included hoarseness and postoperative pain. After sensitivity analysis, the incidence of complications after thermal ablation was determined to be 4.0% (95% CI: 0.0–8.0%). In terms of tumor progression, no instances of recurrence, metastatic lymph nodes, or distant metastasis were observed during follow-up.
There are several limitations to our study. Owing to the short follow-up time of the included studies and the small number of patients ultimately enrolled in the cohort, there is insufficient evidence on the efficacy and safety of thermal ablation for the treatment of Bethesda IV nodules. The lack of long-term follow-up data makes the recurrence rate difficult to assess.
Conclusion
In summary, this study revealed that ultrasound-guided thermal ablation was technically feasible, safe, and effective during the average follow-up period of 12 months, with a high tumor volume reduction rate and a low complication rate. Surgical treatment is preferred, but thermal ablation may be an option for elderly patients with large nodules and symptomatic nodules who refuse surgery. In this meta-analysis, the persistence and reproducibility of these findings need to be demonstrated with larger populations and longer follow-up periods.
Acknowledgements
Thanks to Xi han Zhang and Zi geng Li for their contributions in our study.
Author contributions
Yu Xi and Jia-shan Yao wrote the main manuscript text, Xi-han Zhang prepared Fig. 1 and Table 1, Zi-geng Li prepared Figs. 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14. All authors reviewed the manuscript.
Funding
XPCC guiding science and technology plan project.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agree to publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid Cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid Cancer. Thyroid. 2016;26(1):1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali SZ, Baloch ZW, Cochand-Priollet B, et al. The 2023 Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2023;33(9):1039–44. [DOI] [PubMed] [Google Scholar]
- 3.Carty SE, Ohori NP, Hilko DA, et al. The Clinical Utility of Molecular Testing in the Management of Thyroid Follicular Neoplasms (Bethesda IV Nodules). Ann Surg. 2020;272(4):621–7. [DOI] [PubMed] [Google Scholar]
- 4.Roth MY, Witt RL, Steward DL. Molecular testing for thyroid nodules: Review and current state. Cancer. 2018;124(5):888–98. [DOI] [PubMed] [Google Scholar]
- 5.Yang J, Zhang Y, Li X, Zhao Y, Han X, Chen G, et al. Efficacy and safety of ultrasound-guided microwave ablation versus surgical resection for bethesda category IV thyroid nodules: a retrospective comparative study. Front Endocrinol (Lausanne). 2022;13:924993. [DOI] [PMC free article] [PubMed]
- 6.Livhits MJ, Zhu CY, Kuo EJ, et al. Effectiveness of Molecular Testing Techniques for Diagnosis of Indeterminate Thyroid Nodules: A Randomized Clinical Trial. JAMA Oncol. 2021;7(1):70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papini E, Monpeyssen H, Frasoldati A, Hegedüs L. 2020 European Thyroid Association Clinical Practice Guideline for the Use of Image-Guided Ablation in Benign Thyroid Nodules. Eur Thyroid J. 2020;9(4):172–185. [DOI] [PMC free article] [PubMed]
- 8.Knavel EM, Brace CL. Tumor ablation: common modalities and general practices. Tech Vasc Interv Radiol. 2013;16(4):192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angelos P, Baek JH, Brumund KT, et al. Radiofrequency ablation and related ultrasound-guided ablation technologies for treatment of benign and malignant thyroid disease: An international multidisciplinary consensus statement of the American Head and Neck Society Endocrine Surgery Section with the Asia Pacific Society of Thyroid Surgery, Associazione Medici Endocrinologi, British Association of Endocrine and Thyroid Surgeons, European Thyroid Association, Italian Society of Endocrine Surgery Units, Korean Society of Thyroid Radiology, Latin American Thyroid Society, and Thyroid Nodules Therapies Association. Head Neck. 2022;44(3):633–60. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Cho SJ, Baek JH, Suh CH. Efficacy and safety of thermal ablation for autonomously functioning thyroid nodules: a systematic review and meta-analysis. Eur Radiol. 2021;31(2):605–15. [DOI] [PubMed] [Google Scholar]
- 11.Cui T, Jin C, Jiao D, Teng D, Sui G. Safety and efficacy of microwave ablation for benign thyroid nodules and papillary thyroid microcarcinomas: A systematic review and meta-analysis. Eur J Radiol. 2019;118:58–64. [DOI] [PubMed] [Google Scholar]
- 12.Yan L, Zhang M, Song Q, Luo Y. Ultrasound-Guided-Radiofrequency Ablation Versus Thyroid Lobectomy for Low-Risk Papillary Thyroid Microcarcinoma: A Propensity-Matched Cohort Study of 884 Patients. Thyroid. 2021;31(11):1662–72. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, Yoo WS, Park YJ, et al. Efficacy and Safety of Radiofrequency Ablation for Treatment of Locally Recurrent Thyroid Cancers Smaller than 2 cm. Radiology. 2015;276(3):909–18. [DOI] [PubMed] [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–6. [DOI] [PubMed] [Google Scholar]
- 16.Lin WC, Tung YC, Chang YH, Luo SD, Chiang PL, Huang SC, et al. Radiofrequency ablation for treatment of thyroid follicular neoplasm with low SUV in PET/CT study. Int J Hyperthermia. 2021;38(1):963–9. [DOI] [PubMed]
- 17.Li X, Lan Y, Li N, Yan L, Xiao J, Zhang M, Luo Y. Ultrasound-Guided Thermal Ablation of Bethesda IV Thyroid Nodules: A Pilot Study. Front Endocrinol (Lausanne). 2021;12:674970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ha SM, Sung JY, Baek JH, Na DG, Kim JH, Yoo H, Lee D, Whan Choi D. Radiofrequency ablation of small follicular neoplasms: initial clinical outcomes. Int J Hyperth. 2017;33(8):931–7. [DOI] [PubMed] [Google Scholar]
- 19.Dong Y, Zhan W, Zhou J, Li N, Liu Z, Xia S, Ni X, Liu J, Zhang J, Xu S, Yang Z, Hua Q. Volume reduction rate of radiofrequency ablation in ≤ 2 cm Bethesda IV thyroid nodules. Eur Radiol. 2024;34(3):1597–604. [DOI] [PubMed] [Google Scholar]
- 20.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 21.Pizzato M, Li M, Vignat J, Laversanne M, Singh D, La Vecchia C, Vaccarella S. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol. 2022;10(4):264–72. [DOI] [PubMed] [Google Scholar]
- 22.Barrows CE, Belle JM, Fleishman A, Lubitz CC, James BC. Financial burden of thyroid cancer in the United States: An estimate of economic and psychological hardship among thyroid cancer survivors. Surgery. 2020;167(2):378–84. [DOI] [PubMed] [Google Scholar]
- 23.Jensen CBa, Pitt. Susan C.b,c. Patient perception of receiving a thyroid cancer diagnosis. Curr Opin Endocrinol Diabetes Obes. October 2021;28(5):533–9. [DOI] [PMC free article] [PubMed]
- 24.Bible KC, Kebebew E, Brierley J et al. 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid. 2021;31(3):337–386. [DOI] [PMC free article] [PubMed]
- 25.Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. J Am Soc Cytopathol. 2017 Nov-Dec;6(6):217–22. [DOI] [PubMed]
- 26.Scappaticcio L, Trimboli P, Iorio S, et al. Repeat thyroid FNAC: Inter-observer agreement among high- and low-volume centers in Naples metropolitan area and correlation with the EU-TIRADS. Front Endocrinol (Lausanne). 2022;13:1001728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yaprak Bayrak B, Eruyar AT. Malignancy rates for Bethesda III and IV thyroid nodules: a retrospective study of the correlation between fine-needle aspiration cytology and histopathology. BMC Endocr Disord. 2020;20(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steward DL, Carty SE, Sippel RS, et al. Performance of a Multigene Genomic Classifier in Thyroid Nodules With Indeterminate Cytology: A Prospective Blinded Multicenter Study. JAMA Oncol. 2019;5(2):204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim NE, Raghunathan RS, Hughes EG, et al. Bethesda III and IV Thyroid Nodules Managed Nonoperatively After Molecular Testing With Afirma GSC or Thyroseq v3. J Clin Endocrinol Metab. 2023;108(9):e698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scappaticcio L, Di Martino N, Caruso P, et al. The value of ACR, European, Korean, and ATA ultrasound risk stratification systems combined with RAS mutations for detecting thyroid carcinoma in cytologically indeterminate and suspicious for malignancy thyroid nodules. Horm (Athens). 2024 Jun;17. 10.1007/s42000-024-00573-8. [DOI] [PMC free article] [PubMed]
- 31.Tufano RP, Pace-Asciak P, Russell JO, et al. Update of Radiofrequency Ablation for Treating Benign and Malignant Thyroid Nodules. The Future Is Now. Front Endocrinol (Lausanne). 2021;12:698689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Issa PP, Omar M, Issa CP, et al. Radiofrequency Ablation of Indeterminate Thyroid Nodules: The First North American Comparative Analysis. Int J Mol Sci. 2022;23(19):11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shuai-nan SHI, Ling-xiao LIU, Dan ZUO, Qi ZHANG, Yi-jie QIU, Yi DONG. WANG Wen-ping. Application of contrast enhanced ultrasound in microwave ablation of thyroid nodules[J]. Fudan Univ J Med Sci. 2022;49(1):10–5. [Google Scholar]
- 34.Dobrinja C, Bernardi S, Fabris B, et al. Surgical and Pathological Changes after Radiofrequency Ablation of Thyroid Nodules. Int J Endocrinol. 2015;2015:576576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.