ABSTRACT
Purpose
Upper tract urothelial carcinoma (UTUC) presents a higher incidence rate in Taiwan compared to Western societies. The aim of this study is to investigate the potential of metformin in improving survival outcomes for patients with UTUC in Taiwan.
Material and Methods
This retrospective study included 940 patients with UTUC and type 2 diabetes from the Taiwan UTUC Collaboration Group, spanning 21 hospitals from July 1988 to September 2023. Patients were divided into two groups: those treated with metformin (n = 215) and those without metformin treatment (n = 725). Parameters analyzed included age, BMI, renal function, tumor grade and location, and pathological staging. Oncological outcomes measured were overall survival (OS), cancer‐specific survival (CSS), and bladder recurrence‐free survival (BRFS). Statistical analysis involved the use of Student's t‐test, Mann–Whitney test, Chi‐squared test, Fisher's exact test, and Cox proportional hazard regression.
Results
Significant differences were observed between the two groups in BMI, preoperative creatinine, eGFR, tumor location, tumor laterality, tumor size, and pathological grade and T stage. Patients treated with metformin exhibited a lower risk of CSS (HR = 0.619; p = 0.018) and improved OS (HR = 0.713; p = 0.024), although no significant association was found with BRFS (HR = 1.034; p = 0.791). The protective effect of metformin on OS was particularly significant in patients with advanced T stage, metastasis, and high‐grade tumors.
Conclusion
The study suggests that metformin use in UTUC patients with diabetes is associated with improved OS and CSS but not BRFS. The underlying mechanisms warrant further investigation. Repurposing metformin, a well‐established and safe drug, may develop new therapeutic strategies for UTUC.
Keywords: metformin, Survival outcome, upper tract urothelial carcinoma
1. Introduction
Upper tract urothelial carcinoma (UTUC) has higher incidence rate in Taiwan compared to the western society. It accounts for approximately 40% of urothelial carcinoma (UC) with a higher incidence in females than males [1, 2]. UTUC is characterized by relatively aggressive biological behavior and poorer differentiation compared to bladder cancer. Therefore, it necessitates a precise evaluation of disease progression and tumor invasiveness in each case [3]. Factors such as age, tumor grade, stage, sessile tumor growth, lymphovascular invasion (LVI), lymph node involvement, necrosis, and tumor location have been identified in the literature as associated with the prognosis of UTUC patients [4]. Previous studies demonstrated that smoking, environmental factors including exposure to aristolochic acid in Chinese herbal products and arsenic contamination are related to risk of UTUC. Due to the advanced characteristics of UTUC, it is important to identify factors or intervention that can reduce the progression of cancer.
Metformin is the most widely used oral medication to treat type 2 diabetes mellitus (DM). From previous studies, metformin has potential role to improve survival outcomes in various cancer including urologic cancers [5]. In particular, the anticancer characteristics of metformin have been studied in lung cancer, breast cancer, pancreatic cancer and colon cancer, prostate cancer, bladder cancer [6, 7]. The possible antitumor mechanism may come from direct impact on cancer cells through activation of AMPK pathway and inhibition of the regulatory associated protein of mTORC1. In addition, metformin also present effects on tumor microenvironment, tumor angiogenesis and related to inflammatory factors and immune activation [8].
Because of the extended research and development period for new drugs, there is a growing interest in exploring the use of established and safe drugs already on the market to advance tumor treatment. As such, researchers are investigating the potential of metformin in treating various cancer. However, findings regarding metformin's protective effects in UTUC is lack. Based on the high incidence rate of UTUC in Taiwan, we conducted this research to verify if metformin has similar effect with other cancers.
2. Material and Methods
2.1. Patient Collection
We conducted this retrospective study from the Taiwan UTUC Collaboration Group includes 21 participating hospitals. This research was approved by the Institutional Review Board (KMUHIRB‐E(I)‐20180214). A total of 6208 UTUC patients were included from July 1988 to September 2023. After excluding patients without receiving surgery, without receiving neoadjuvant and adjuvant systemic therapy or loss follow up, or incomplete data and patients without DM comorbidity, we finally included 940 patients. There are 725 DM patients without metformin treatment and 215 DM patients using metformin for controlling sugar. The duration of metformin exposure is at least over 6 months.
In addition to different anti‐diabetic drug interventions, we collected various parameters for analysis, including age, body mass index (BMI), renal function, gender, tumor grade, tumor location, tumor focality, pathological T and N stage who received lymph node dissection, tumor laterality, tumor size, concomitant carcinoma in situ (CIS), lymphovascular invasion (LVI).
2.2. Definitions and Endpoints
The samples obtained from radical surgery were assessed by pathologists using identical criteria. Pathological staging from the 2010 TNM (tumor, lymph node, metastasis) system, and tumor grading adhered to the 2004 World Health Organization/International Society of Urologic Pathology consensus classification. The regular follow‐up program strictly adhered to standard guidelines. The study aimed to compare oncological outcomes between UTUC patients with DM receiving metformin treatment or not. Overall survival (OS), cancer‐specific survival (CSS), and bladder recurrence‐free survival (BRFS) were analyzed. The cause of death was determined by the attending physician or death certificate.
2.3. Statistical Analysis
Student's t‐test and Mann–Whitney test were used for continuous variables that were normally distributed or not, respectively. Continued variables were reported as mean (SD) or median (Q1–Q3) and frequencies (%). We used the Chi‐squared test and Fisher's exact test for categorical variables. The hazard ratio (HR) with its 95% confidence interval (CI) showed the association between each predictor variable and OS, CSS, BRFS. It was calculated with Cox proportional hazard regression. Variables with a p‐value < 0.05 were selected into the adjusted model by stepwise selection in univariate Cox regression. The statistical analysis was performed using SAS software (version 9.4, SAS Institute Inc., Cary, NC), and statistical significance was assigned as p < 0.05.
3. Results
Nine Hundred and Forty patients were included and divided into groups with metformin (n = 215) and without metformin (n = 725). The demographic and pathological characteristics of UTUC patients in the groups with or without metformin were compared in Table 1. There were differences in BMI (p = 0.024), preoperative creatinine (p < 0.001), preoperative eGFR (p < 0.001), tumor location (p = 0.004), tumor laterality (p = 0.014), tumor size (p < 0.001) and tumor pathological grade (p = 0.016) and T stage (p = 0.026) between the two groups.
TABLE 1.
Comparison of clinicopathological data between DM without metformin and with metformin in UTUC patients.
| Variables | Without metformin (n = 725) | With metformin (n = 215) | p |
|---|---|---|---|
| Age (years) | 71.38 (65.28–77.82) | 70.47 (64.32–77.26) | 0.422 |
| BMI (kg/m2) | 24.93 (22.6–27.14) | 25.72 (23.44–28.03) | 0.024* |
| eGFR (mL/min/1.73 m2) before surgery | 39.95 (21.17–58.33) | 56.38 (41.39–74.39) | < 0.001* |
| Gender | 0.058 | ||
| Male | 308 (77.78) | 88 (22.22) | |
| Female | 417 (76.65) | 127 (23.35) | |
| Grade | 0.016* | ||
| Low grade | 86 (70.49) | 36 (29.51) | |
| High grade | 632 (78.22) | 176 (21.78) | |
| Location | 0.004* | ||
| Renal pelvis | 284 (76.34) | 88 (23.66) | |
| Ureter | 290 (76.92) | 87 (23.08) | |
| Synchronous | 149 (78.84) | 40 (21.16) | |
| Multifocality | 0.059 | ||
| No | 449 (77.55) | 130 (22.45) | |
| Yes | 265 (76.37) | 82 (23.63) | |
| Pathological N stage | 0.214 | ||
| pN0/Nx | 14 (82.35) | 3 (17.65) | |
| pN1/N2 | 704 (76.86) | 212 (23.14) | |
| Pathological T stage | 0.026* | ||
| pT < 2 | 348 (75.16) | 115 (24.84) | |
| pT ≥ 2 | 371 (78.77) | 100 (21.23) | |
| Laterality | 0.014* | ||
| Left | 375 (77.64) | 108 (22.36) | |
| Right | 341 (76.29) | 106 (23.71) | |
| Both | 8 (88.89) | 1 (11.11) | |
| Tumor size | < 0.001* | ||
| < 1 cm | 35 (68.63) | 16 (31.37) | |
| < 2 cm | 130 (71.82) | 51 (28.18) | |
| < 3 cm | 111 (69.81) | 48 (30.19) | |
| ≥ 3 cm | 282 (74.02) | 99 (25.98) | |
| Carcinoma in situ | 0.058 | ||
| No | 554 (76.41) | 171 (23.59) | |
| Yes | 164 (78.85) | 44 (21.15) | |
| Lymphovascular invasion | 0.079 | ||
| No | 587 (77.03) | 175 (22.97) | |
| Yes | 128 (78.05) | 36 (21.95) | |
| Metastasis | 0.007* | ||
| No | 543 (79.04) | 144 (20.96) | |
| Yes | 102 (70.34) | 43 (29.66) |
Abbreviation: BMI, body mass index.
p < 0.05.
3.1. Oncological Outcomes
In CSS, pathologic T stage, metastasis, age, and LVI were included in the adjusted model. Patients with metformin had a lower risk of cancer related death (HR = 0.619; 95% CI (0.417–0.920), p = 0.018) than those without using metformin (Table 2). The survival curve in CSS showed no significant difference (p = 0.273) (Figure 1). Older patients, tumor with LVI, metastasis during follow up, advanced T stage have worse CSS. As for OS, the stepwise method selected age, BMI, metastasis, pathologic T stage, and LVI into the adjusted model. The significant protective effect of metformin on OS (HR = 0.713; 95% CI (0.532–0.956), p = 0.024) was observed (Table 3) The survival curve in OS also showed better survival outcome in patients with metformin usage (p = 0.001) (Figure 2). In addition, older patients, patients with low BMI, tumor with LVI, metastasis, advanced T stage have lower OS. In Table 4 and Figure 3, it showed no significant association if patients under metformin usage or not with BRFS (HR = 1.034; 95% CI (0.808–1.322), p = 0.791).
TABLE 2.
Cancer specific‐survival analysis of UTUC with DM patients.
| Crude Cox model | Adj Cox model | |||
|---|---|---|---|---|
| Variables | cHR (95% CI) | p | aHR (95% CI) | p |
| Age | 1.023 (1.005–1.042) | 0.013* | 1.023 (1.004–1.043) | 0.020* |
| BMI | 0.966 (0.919–1.015) | 0.167 | ||
| CIS | ||||
| No | 1 | |||
| Yes | 0.924 (0.626–1.363) | 0.689 | ||
| Grade | ||||
| Low grade | 1 | |||
| High grade | 2.952 (1.506–5.788) | 0.002* | ||
| Location | ||||
| Renal pelvis | 1 | |||
| Ureter | 1.135 (0.788–1.634) | 0.497 | ||
| Synchronous | 1.741 (1.161–2.610) | 0.007* | ||
| LVI | ||||
| No | 1 | 1 | ||
| Yes | 3.389 (2.421–4.744) | < 0.001* | 1.853 (1.290–2.660) | 0.001* |
| Metastasis | ||||
| No | 1 | 1 | ||
| Yes | 22.123 (15.202–32.195) | < 0.001* | 17.236 (11.515–25.801) | < 0.001* |
| Metformin | ||||
| Without metformin | 1 | 1 | ||
| With metformin | 0.811 (0.557–1.181) | 0.274 | 0.619 (0.417–0.920) | 0.018* |
| Multifocality | ||||
| No | 1 | |||
| Yes | 1.72 (1.253–2.361) | < 0.001* | ||
| Pathological N stage | ||||
| pN0/Nx | 1 | |||
| pN1/N2 | 9.283 (4.859–17.735) | < 0.001* | ||
| eGFR (mL/min/1.73 m2) before surgery | 0.993 (0.987–0.999) | 0.035* | ||
| Pathological T stage | ||||
| pT < 2 | 1 | 1 | ||
| pT ≥ 2 | 6.248 (4.122–9.473) | < 0.001* | 2.324 (1.468–3.678) | < 0.001* |
| Gender | ||||
| Male | 1 | |||
| Female | 0.738 (0.539–1.009) | 0.057 | ||
| Laterality | ||||
| Left | 1 | |||
| Right | 1.060 (0.775–1.450) | 0.715 | ||
| Both | — | — | ||
| Size | ||||
| < 1 cm | 1 | |||
| < 2 cm | 1.057 (0.392–2.847) | 0.913 | ||
| < 3 cm | 1.377 (0.517–3.670) | 0.522 | ||
| ≥ 3 cm | 2.786 (1.130–6.869) | 0.026* | ||
Abbreviations: BMI, body mass index; CI, confidence interval; CIS, carcinoma in situ; HR, hazard ratio; LVI, lymphovascular invasion.
p < 0.05.
FIGURE 1.
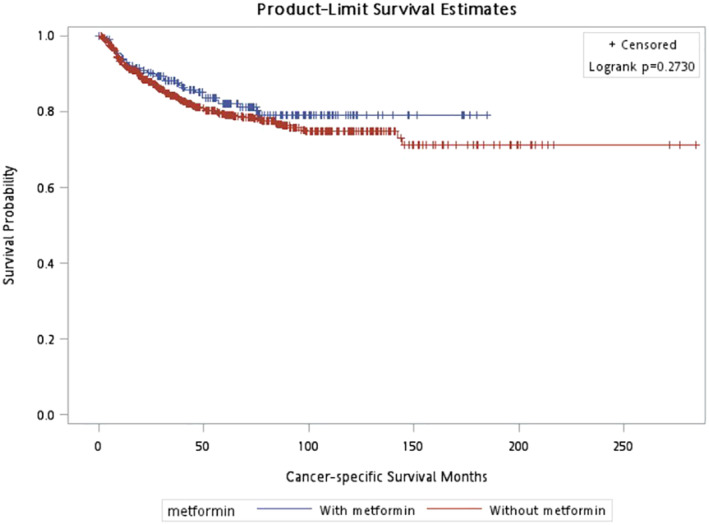
Cancer‐specific survival, p = 0.273.
TABLE 3.
Overall survival analysis of UTUC with DM patients.
| Crude Cox model | Adj Cox model | |||
|---|---|---|---|---|
| Variables | cHR (95% CI) | p | aHR (95% CI) | p |
| Age | 1.045 (1.033–1.058) | < 0.001* | 1.045 (1.029–1.061) | < 0.001* |
| BMI | 0.940 (0.911–0.970) | < 0.001* | 0.954 (0.920–0.989) | 0.010* |
| CIS | ||||
| No | 1 | |||
| Yes | 0.977 (0.771–1.238) | 0.848 | ||
| Grade | ||||
| Low grade | 1 | |||
| High grade | 1.605 (1.169–2.201) | 0.003* | ||
| Location | ||||
| Renal pelvis | 1 | |||
| Ureter | 1.329 (1.067–1.656) | 0.011* | ||
| Synchronous | 1.694 (1.305–2.200) | < 0.001* | ||
| LVI | ||||
| No | 1 | 1 | ||
| Yes | 2.314 (1.846–2.902) | < 0.001* | 1.878 (1.414–2.494) | < 0.001* |
| Metastasis | ||||
| No | 1 | 1 | ||
| Yes | 4.919 (3.916–6.178) | < 0.001* | 4.604 (3.473–6.104) | < 0.001* |
| Metformin | ||||
| Without metformin | 1 | 1 | ||
| With metformin | 0.663 (0.517–0.850) | 0.001* | 0.713 (0.532–0.956) | 0.024* |
| Mutifocality | ||||
| No | 1 | |||
| Yes | 1.459 (1.199–1.775) | < 0.001* | ||
| Pathological N stage | ||||
| pN0/Nx | 1 | |||
| pN1/N2 | 5.490 (3.075–9.802) | < 0.001* | ||
| eGFR (mL/min/1.73 m2) before surgery | 0.988 (0.984–0.992) | < 0.001* | ||
| Pathological T stage | ||||
| pT < 2 | 1 | 1 | ||
| pT ≥ 2 | 2.117 (1.737–2.580) | < 0.001* | 1.489 (1.124–1.972) | 0.006* |
| Gender | ||||
| Male | 1 | |||
| Female | 0.970 (0.798–1.179) | 0.760 | ||
| Laterality | ||||
| Left | 1 | |||
| Right | 1.009 (0.832–1.224) | 0.925 | ||
| Both | 1.660 (0.684–4.029) | 0.263 | ||
| Size | ||||
| < 1 cm | 1 | |||
| < 2 cm | 1.442 (0.827–2.514) | 0.197 | ||
| < 3 cm | 1.473 (0.838–2.590) | 0.178 | ||
| ≥ 3 cm | 2.070 (1.222–3.507) | 0.007* | ||
Abbreviations: BMI, body mass index; CI, confidence interval; CIS, carcinoma in situ; HR, hazard ratio; LVI, lymphovascular invasion.
p < 0.05.
FIGURE 2.
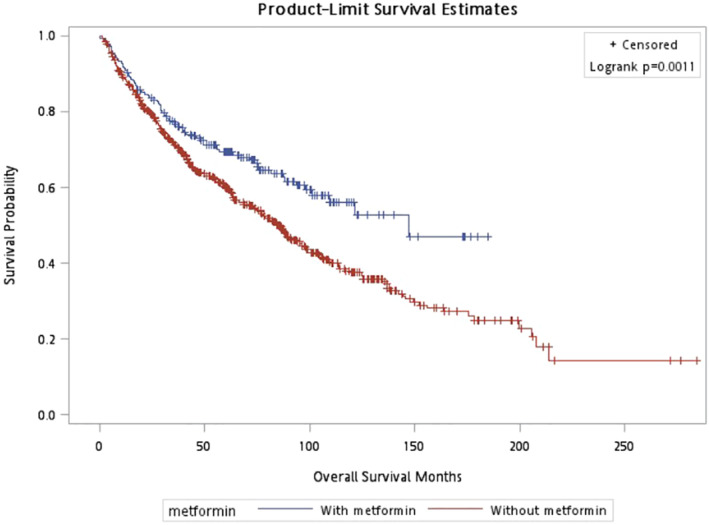
Overall survival, p = 0.001.
TABLE 4.
Bladder recurrence‐free survival analysis of UTUC with DM patients.
| Variables | Crude Cox model | Adj Cox model | ||
|---|---|---|---|---|
| cHR (95% CI) | p | aHR (95% CI) | p | |
| Age | 1.029 (1.017–1.041) | < 0.001* | 1.027 (1.015–1.040) | < 0.001* |
| BMI | 0.974 (0.947–1.002) | 0.072 | ||
| CIS | ||||
| No | 1 | |||
| Yes | 1.202 (0.959–1.506) | 0.110 | ||
| Grade | ||||
| Low grade | 1 | |||
| High grade | 1.272 (0.945–1.711) | 0.112 | ||
| Location | ||||
| Renal pelvis | 1 | 1 | ||
| Ureter | 1.201 (0.959–1.503) | 0.110 | 1.238 (0.975–1.572) | 0.080 |
| Synchronous | 1.615 (1.246–2.092) | < 0.001* | 1.473 (1.117–1.942) | 0.006* |
| LVI | ||||
| No | 1 | |||
| Yes | 1.689 (1.329–2.145) | < 0.001* | ||
| Metastasis | ||||
| No | 1 | 1 | ||
| Yes | 2.009 (1.557–2.591) | < 0.001* | 1.645 (1.254–2.158) | < 0.001* |
| Metformin | ||||
| Without metformin | 1 | 1 | ||
| With metformin | 0.993 (0.79–1.248) | 0.953 | 1.034 (0.808–1.322) | 0.791 |
| Mutifocality | ||||
| No | 1 | |||
| Yes | 1.409 (1.154–1.72) | < 0.001* | ||
| Pathological N stage | ||||
| pN0/Nx | 1 | |||
| pN1/N2 | 2.152 (1.112–4.167) | 0.023* | ||
| eGFR (mL/min/1.73 m2) before surgery | 0.995 (0.991–0.999) | 0.009* | ||
| Pathological T stage | ||||
| pT < 2 | 1 | 1 | ||
| pT ≥ 2 | 1.724 (1.413–2.102) | < 0.001* | 1.429 (1.146–1.782) | 0.002* |
| Gender | ||||
| Male | 1 | 1 | ||
| Female | 0.645 (0.53–0.784) | < 0.001* | 0.639 (0.516–0.791) | < 0.001* |
| Laterality | ||||
| Left | 1 | |||
| Right | 1.005 (0.826–1.223) | 0.960 | ||
| Both | 1.125 (0.360–3.519) | 0.839 | ||
| Size | ||||
| < 1 cm | 1 | |||
| < 2 cm | 1.057 (0.643–1.738) | 0.826 | ||
| < 3 cm | 1.419 (0.868–2.318) | 0.163 | ||
| ≥ 3 cm | 1.500 (0.944–2.383) | 0.086 | ||
Abbreviations: BMI, body mass index; CI, confidence interval; CIS, carcinoma in situ; HR, hazard ratio; LVI, lymphovascular invasion.
p < 0.05.
FIGURE 3.
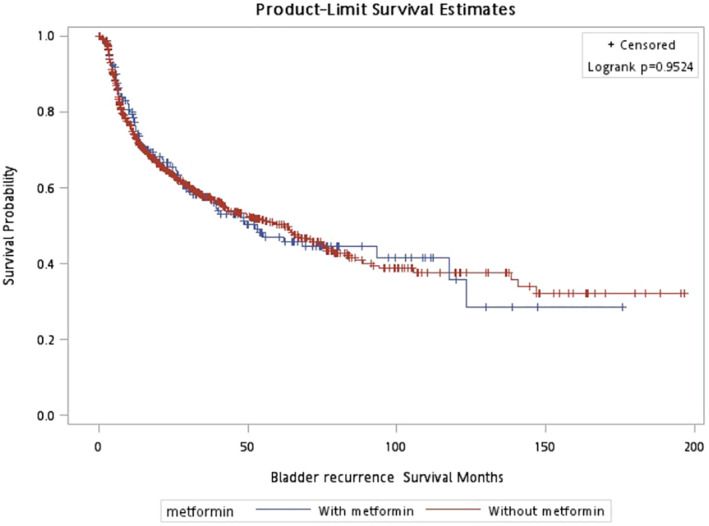
Bladder recurrence‐free survival, p = 0.952.
In the stratified analysis, the protective effect of metformin on OS showed significant in patients with any pathological T stage, metastasis during follow up, tumors with LVI or not, high grade tumor, excluding low grade tumor. The relationship between metformin usage and CSS was observed in those with high grade tumor and metastasis. The significance association between metformin usage and BRFS was not seen in stratifications (Table 5).
TABLE 5.
Stratified survival analysis of UTUC with DM patients.
| Stratification | OS | CSS | BRFS | |||
|---|---|---|---|---|---|---|
| aHR (95% CI) | p | aHR (95% CI) | p | aHR (95% CI) | p | |
| Pathological T stage | ||||||
| ≥ T2 | 0.698 (0.500–0.975) | 0.035* | 0.676 (0.439–1.040) | 0.075 | 1.158 (0.835–1.607) | 0.379 |
| < T2 | 0.449 (0.278–0.722) | 0.001* | 0.432 (0.152–1.227) | 0.115 | 0.772 (0.524–1.138) | 0.192 |
| Metastasis | ||||||
| No | 0.630 (0.444–0.894) | 0.010* | 0.813 (0.356–1.852) | 0.621 | 1.080 (0.814–1.433) | 0.595 |
| Yes | 0.537 (0.348–0.827) | 0.005* | 0.578 (0.368–0.907) | 0.017* | 0.756 (0.456–1.254) | 0.279 |
| Grade | ||||||
| High grade | 0.566 (0.421–0.760) | < 0.001* | 0.608 (0.404–0.913) | 0.017* | 0.959 (0.730–1.26) | 0.765 |
| Low grade | 0.693 (0.306–1.568) | 0.378 | 1.525 (0.094–24.618) | 0.766 | 1.192 (0.615–2.311) | 0.603 |
| LVI | ||||||
| LVI | 0.483 (0.281–0.829) | 0.008* | 0.555 (0.280–1.101) | 0.092 | 0.728 (0.419–1.265) | 0.260 |
| Non‐LVI | 0.677 (0.495–0.926) | 0.015* | 0.695 (0.429–1.127) | 0.140 | 1.056 (0.799–1.396) | 0.702 |
Note: Compare Kaplan–Meier curves between patients with and without metformin by log‐rank test. Adjusted age, Metastasis, pT, and LVI.
Abbreviations: BRFS, bladder recurrence‐free survival; CI, confidence interval; CSS, cancer‐specific survival; HR, hazard ratio; LVI, Lymphovascular invasion; OS, overall survival.
p < 0.05.
4. Discussion
Metformin is a safe and effective oral antidiabetic drug in glycemic control which emerging as a promising candidate for the prevention and treatment of malignant tumors. Recent years have seen encouraging results from the use of metformin in managing various types of cancer. However, clinical outcomes have varied [9, 10, 11]. Our database showed that metformin usage might be associated with a significant improvement in the OS and CSS of UTUC.
UTUC in Taiwan exhibits distinct characteristics and high incidence rate compared to Western countries, which may be attributed to genetic, environmental, and dietary factors specific to the region [12, 13]. Chang YH et al. indicated that there was a high occurrence in women, particularly in renal pelvis cancer between 1985 and 2019 [2]. Additionally, it showed increasing trend in the incidence of renal pelvis cancer in women from 1985 to 1999. Furthermore, UTUC in Taiwanese patients often presents with aggressive behavior and a high rate of recurrence. Chen CH et al. demonstrated that people living in Taiwan may exposure both arsenic and aristolochic acid carcinogens, the risk of developing UTUC may additive [14]. Improving long‐term survival rates and quality of life for UTUC patients remains a significant challenge.
The anti‐tumor effects of metformin have attracted increasing attention recently. Previous research highlighted the mechanism involving the activation of tumor cell cycle and associated signaling pathways [15]. These encompass the stimulation of AMPK‐associated pathways, the enhancement of apoptosis in cancer cells, and the suppression of mitochondrial metabolism [16, 17, 18]. Such studies have illuminated the crucial function of metformin in cancer treatment and the pathways that are regulated. The insights gained from these studies offer considerable potential for improving clinical practices and drug development in cancer therapy. Chen et al. [19] utilized MR analysis to reveal the genetic connections between metformin use and the risk of prevalent cancers. Shen Z. et al. [20] conducted bladder cancer cell line experiments to explore the possible molecular mechanisms of metformin. They discovered metformin can inhibit cancer cell migration and proliferation in terms of inhibiting bladder cancer progression.
Some previous studies have revealed that the PI3K/AKT/mTOR was dysregulated in over 40% of patients with urothelial carcinoma [21]. This finding can be used to infer that metformin can inhibit the growth of bladder cancer cell through the PI3K pathway which was identified with in vitro study [20]. Furthermore, combination therapy may also have additive effect on survival of cancer. Almaimani RA et al. demonstrated that combinations of metformin with 5‐fluorouracil or even triple therapy regimens effectively induced anticancer activity, which included cell cycle arrest and apoptosis, compared to single drug treatments. This enhanced anticancer effect is likely due to the attenuation of the PI3K/AKT/mTOR oncogenic pathway [22].
Our current study unveiled the potential role of metformin in improving OS and CSS of UTUC. From previous systematic review, the use of metformin may be related to improvement in recurrence, CSS and OS of prostate cancer and the progression of kidney cancer [5]. In prostate cancer, the beneficial impact especially on patients who received radical radiotherapy. This effect may be due to the involvement of the AMPK pathway in managing how cells react to radiation treatment [23]. On the contrary, several research did not show significant positive correlation between metformin and survival outcomes [24, 25]. However, the meta‐analysis of Liu et al. demonstrated that metformin emerged as a substantial protective factor in reducing the risk of bladder cancer instead of improving survival outcomes after including six studies altogether for analysis [7]. Despite this, Malte Rieken et al. have demonstrated that diabetic patients who do not use metformin seem to face a higher risk of CSS and OS compared to patients without DM [26]. Regarding the impact of metformin on UTUC, previous study has also found positive effects. M. Rieken et al. [27] found DM patients with UTUC who did not use metformin had a worse CSS and higher disease recurrence rate. The definition of recurrence is tumor progression in operative field, regional lymph nodes and/or distant metastasis.
There are some limitations in our study. First, it is a retrospective design research, however it is the largest collaboration study of UTUC. Second, the timing of metformin exposure, whether before or after the diagnosis of UTUC is unknown. Further prospective study warrants to clarify if the timing of metformin usage can influence the prognosis of UTUC. Third, we must clarify whether the severity of DM in this group of patients or the use of metformin itself affects the outcomes of UTUC.
5. Conclusions
UTUC patients with DM who use metformin appear to be lower risk of CSS and OS but not bladder recurrence than patients without usage of metformin in our study. However, the underlying mechanisms and possible effects of metformin on UTUC require further clarification. Nevertheless, repurposing old drugs is a potential candidate for development of new therapeutic role for UTUC.
Author Contributions
Hsiang Ying Lee: data curation (lead), writing – original draft (lead). Po‐Hung Lin: resources (equal), supervision (equal). See‐Tong Pang: resources (equal), supervision (equal). Jen‐Kai Fang: resources (equal), supervision (equal). Chung‐You Tsai: resources (equal), supervision (equal). Yao‐Chou Tsai: conceptualization (equal), resources (equal), supervision (equal). Yung‐Tai Chen: resources (equal), supervision (equal). Wei‐Chieh Chen: resources (equal), supervision (equal). Hsin‐Chih Yeh: supervision (equal), writing – review and editing (equal). Wei‐Ming Li: conceptualization (equal), supervision (equal), writing – review and editing (equal).
Consent
A waiver was granted by the IRB/Ethics Committee for this purpose. This research was approved by the Institutional Review Board (KMUHIRB‐E(I)‐20180214).
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
This study was partially supported by the Ministry of Science and Technology (MOST‐111‐2314‐B‐037‐100‐MY2), Kaohsiung Medical University Hospital (KMUH‐111‐1R55, KMUH‐112‐2R57), and Regenerative Medicine and Cell Therapy Research Center (KMU‐TC112A02).
Funding: The authors received no specific funding for this work.
Contributor Information
Hsin‐Chih Yeh, Email: patrick1201.tw@yahoo.com.tw.
Wei‐Ming Li, Email: u8401067@yahoo.com.tw.
Data Availability Statement
The authors confirms that all data generated or analyzed during this study are included in this published article.
References
- 1. Han J., Xian Z., Zhang Y., Liu J., and Liang A., “Systematic Overview of Aristolochic Acids: Nephrotoxicity, Carcinogenicity, and Underlying Mechanisms,” Frontiers in Pharmacology 10 (2019): 648, 10.3389/fphar.2019.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang Y.‐H., Hsu W.‐L., Lee Y.‐K., et al., “Trends and Sex‐Specific Incidence of Upper Urinary Tract Cancer in Taiwan: A Birth Cohort Study,” Cancer Medicine 12, no. 14 (2023): 15350–15357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Margulis V., Shariat S. F., Matin S. F., et al., “Outcomes of Radical Nephroureterectomy: A Series From the Upper Tract Urothelial Carcinoma Collaboration,” Cancer 115 (2009): 1224–1233, 10.1002/cncr.24135. [DOI] [PubMed] [Google Scholar]
- 4. Rouprêt M., Babjuk M., Burger M., et al., “European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update,” European Urology 79, no. 1 (2021): 62–79, 10.1016/j.eururo.2020.05.042. [DOI] [PubMed] [Google Scholar]
- 5. Yao X., Liu H., and Hua X., “The Impact of Metformin Use With Survival Outcomes in Urologic Cancers: A Systematic Review and Meta‐Analysis,” BioMed Research International 2021 (2021): 5311828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yin M., Zhou J., Gorak E. J., and Quddus F., “Metformin Is Associated With Survival Benefit in Cancer Patients With Concurrent Type 2 Diabetes: A Systematic Review and Meta‐Analysis,” Oncologist 18, no. 12 (2013): 1248–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu C.‐Q., Sun J.‐X., Jin‐Zhou X., et al., “Metformin Use on Incidence and Oncologic Outcomes of Bladder Cancer Patients With T2DM: An Updated Meta‐Analysis,” Frontiers in Pharmacology 7, no. 13 (2022): 865988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feng Y., Jia B., and Shen Z., “Metformin and Bladder Cancer: Drug Repurposing as a Potential Tool for Novel Therapy: A Review,” Medicine (Baltimore) 101, no. 45 (2022): e31635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barakat H. E., Hussein R. R. S., Elberry A. A., Zaki M. A., and Ramadan M. E., “The Impact of Metformin Use on the Outcomes of Locally Advanced Breast Cancer Patients Receiving Neoadjuvant Chemotherapy: An Open‐Labelled Randomized Controlled Trial,” Scientific Reports 12, no. 1 (2022): 7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang B. Y., Gulinazi Y., du Y., et al., “Metformin Plus Megestrol Acetate Compared With Megestrol Acetate Alone as Fertility‐Sparing Treatment in Patients With Atypical Endometrial Hyperplasia and Well‐Differentiated Endometrial Cancer: A Randomised Controlled Trial,” BJOG: An International Journal of Obstetrics and Gynaecology 127, no. 7 (2020): 848–857. [DOI] [PubMed] [Google Scholar]
- 11. Skuli S. J., Alomari S., Gaitsch H., Bakayoko A.’., Skuli N., and Tyler B. M., “Metformin and Cancer, an Ambiguanidous Relationship,” Pharmaceuticals (Basel) 15, no. 5 (2022): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen C. H., Dickman K. G., Moriya M., et al., “Aristolochic Acid‐Associated Urothelial Cancer in Taiwan,” Proceedings of the National Academy of Sciences of the United States of America 109, no. 21 (2012): 8241–8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li C. C., Chang T. H., Wu W. J., et al., “Significant Predictive Factors for Prognosis of Primary Upper Urinary Tract Cancer After Radical Nephroureterectomy in Taiwanese Patients,” European Urology 54, no. 5 (2008): 1127–1134. [DOI] [PubMed] [Google Scholar]
- 14. Chen C. H., Grollman A. P., Huang C. Y., et al., “Additive Effects of Arsenic and Aristolochic Acid in Chemical Carcinogenesis of Upper Urinary Tract Urothelium,” Cancer Epidemiology, Biomarkers & Prevention 30, no. 2 (2021): 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zheng Z., Bian Y., Zhang Y., Ren G., and Li G., “Metformin Activates AMPK/SIRT1/NF‐κB Pathway and Induces Mitochondrial Dysfunction to Drive caspase3/GSDME‐Mediated Cancer Cell Pyroptosis,” Cell Cycle 19, no. 10 (2020): 1089–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou G., Myers R., Li Y., et al., “Role of AMP‐Activated Protein Kinase in Mechanism of Metformin Action,” Journal of Clinical Investigation 108, no. 8 (2001): 1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klose K., Packeiser E. M., Müller P., et al., “Metformin and Sodium Dichloroacetate Effects on Proliferation, Apoptosis, and Metabolic Activity Tested Alone and in Combination in a Canine Prostate and a Bladder Cancer Cell Line,” PLoS One 16, no. 9 (2021): e0257403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vasan K., Werner M., and Chandel N. S., “Mitochondrial Metabolism as a Target for Cancer Therapy,” Cell Metabolism 32, no. 3 (2020): 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen Y., Bai B., Ye S., et al., “Genetic Effect of Metformin Use on Risk of Cancers: Evidence From Mendelian Randomization Analysis,” Diabetology and Metabolic Syndrome 15, no. 1 (2023): 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shen Z., Xue D., Wang K., et al., “Metformin Exerts an Antitumor Effect by Inhibiting Bladder Cancer Cell Migration and Growth, and Promoting Apoptosis Through the PI3K/AKT/mTOR Pathway,” BMC Urology 22, no. 1 (2022): 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Houédé N. and Pourquier P., “Targeting the Genetic Alterations of the PI3K‐AKT‐mTOR Pathway: Its Potential Use in the Treatment of Bladder Cancers,” Pharmacology & Therapeutics 145 (2015): 1–18. [DOI] [PubMed] [Google Scholar]
- 22. Almaimani R. A., Aslam A., Ahmad J., et al., “In Vivo and In Vitro Enhanced Tumoricidal Effects of Metformin, Active Vitamin D3, and 5‐Fluorouracil Triple Therapy Against Colon Cancer by Modulating the PI3K/Akt/PTEN/mTOR Network,” Cancers (Basel) 14, no. 6 (2022): 1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zannella V. E., Cojocari D., Hilgendorf S., et al., “AMPK Regulates Metabolism and Survival in Response to Ionizing Radiation,” Radiotherapy and Oncology 99, no. 3 (2011): 293–299. [DOI] [PubMed] [Google Scholar]
- 24. Wissing M. D., O'Flaherty A., Dragomir A., Tanguay S., Kassouf W., and Aprikian A. G., “Chronic Prednisone, Metformin, and Nonsteroidal Anti‐Inflammatory Drug Use and Clinical Outcome in a Cohort of Bladder Cancer Patients Undergoing Radical Cystectomy in Québec, Canada,” BMC Urology 23, no. 1 (2023): 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fiala O., Buti S., Takeshita H., et al., “Use of Concomitant Proton Pump Inhibitors, Statins or Metformin in Patients Treated With Pembrolizumab for Metastatic Urothelial Carcinoma: Data From the ARON‐2 Retrospective Study,” Cancer Immunology, Immunotherapy 72, no. 11 (2023): 3665–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rieken M., Xylinas E., Kluth L., et al., “Effect of Diabetes Mellitus and Metformin Use on Oncologic Outcomes of Patients Treated With Radical Cystectomy for Urothelial Carcinoma,” Urologic Oncology 32, no. 1 (2014): 49.e7–49.e14. [DOI] [PubMed] [Google Scholar]
- 27. Rieken M., Xylinas E., Kluth L., et al., “Diabetes Mellitus Without Metformin Intake Is Associated With Worse Oncologic Outcomes After Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma,” European Journal of Surgical Oncology 40, no. 1 (2014): 113–120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirms that all data generated or analyzed during this study are included in this published article.


