ABSTRACT
Background
Lymphoma arises from transformed lymphoid cells. Although surgical excision biopsy is the standard diagnostic tool for patients with lymphoma, image‐guided fine‐needle aspiration (FNA) or core needle biopsy (CNB) is considered an alternative diagnostic option.
Objective
To assess the diagnostic accuracy and safety of ultrasound (US)‐guided core needle biopsy (CNB) in patients with lymphoma.
Methods
A systematic review and meta‐analysis were conducted. A literature search was performed up to January 1, 2024, using the Ovid‐MELIBE and EMBASE databases to identify studies focusing on US‐guided CNB in lymphoma patients. Relevant outcomes, including sensitivity, specificity, and complication rates, were extracted from the included studies. The Der‐Simonian‐Laird random‐effects model was applied to analyze the pooled data.
Results
The pooled sensitivity of US‐guided CNB in lymphoma patients was 94% (95% CI = 89%–96%), and the specificity was 100% (95% CI = 94%–100%). The pooled complication rate was 1% (95% CI = 0%–3%), with self‐limiting complications being the most common.
Conclusion
US‐guided CNB demonstrated high diagnostic accuracy and low complication rates in patients with lymphoma, supporting its use as an alternative diagnostic tool.
Keywords: core needle biopsy, lymphoma, meta‐analysis, systematic review, ultrasound
1. Introduction
Lymphoma, encompassing both Hodgkin lymphoma (HL) and non‐Hodgkin lymphoma (NHL), represents a neoplastic proliferation originating from transformed lymphoid cells [1]. The typical clinical manifestation often includes superficial or peripheral lymphadenopathy across various lymphoma subtypes [2]. Diagnosis relies on an amalgamation of morphological, immunophenotypic, and genetic characteristics, alongside clinical findings [3]. Critical to this evaluation is the assessment of lymph node architecture through tissue biopsy techniques such as fine‐needle aspiration (FNA), core needle biopsy (CNB), and surgical excision biopsy (SEB), with SEB being the preferred method due to its capacity for comprehensive tissue sampling [4]. However, recent advancements have endorsed less invasive techniques like image‐guided FNA and CNB for diagnosis and subclassification of lymphomas [5]. Numerous studies investigating the efficacy and safety of image‐guided CNB have utilized computed tomography (CT) and ultrasound (US) for imaging guidance [2]. Given the distinctive natural history of each lymphoma subtype, tailored management strategies underscore the importance of accurate subclassification [2, 6, 7]. Contemporary diagnostic methodologies enable precise classification even with limited tissue samples [8], with US‐guided CNB demonstrating efficacy in providing adequate tissue for architectural assessment and immunohistochemical analysis [9]. Multiple studies have confirmed the high diagnostic accuracy of US‐guided CNB in diagnosing and subclassifying lymphomas [10, 11, 12, 13, 14, 15], positioning it as a viable alternative diagnostic tool.
Although numerous studies have explored the outcomes of US‐guided CNB in patients with lymphoma, no systematic review or meta‐analysis has been undertaken to date. Hence, our objective was to conduct a systematic review and meta‐analysis to assess the diagnostic accuracy and safety of US‐guided CNB in this patient population.
2. Methods
This systematic review and meta‐analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines (PROSPERO ID: CRD42022341231) [16].
2.1. Literature Search
We conducted a literature search using the Ovid‐MELIBE and EMBASE databases up to January 1, 2024, without date restrictions, to identify studies on the diagnostic performance of US‐guided CNB in patients with lymphoma. Search terms included (“lymphoma” OR “lymphoproliferative” OR “immunoproliferative” OR “malignant lymphoma”) AND (“ultrasonography” OR “US” OR “USG” OR “US‐guided” OR “sonogram” OR “ultrasound”) AND (“biopsy” OR “core needle” OR “needle”) AND (“excision” OR “dissection” OR “lymphadenectomy”). Selected articles underwent further examination to locate additional relevant studies. The literature search was independently conducted by one head and neck radiologist (M. K. L., 9 years of experience) and one training radiologist (M. K. L.). Discrepancies were resolved through consensus.
2.2. Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) Population: patients diagnosed with histologically confirmed lymphoma; (2) Index test: cytopathology via US‐guided CNB; (3) Reference standard: diagnosis confirmed through excision followed by histopathological examination or clinical follow‐up; (4) Outcomes: diagnostic performance metrics (sensitivity, specificity), and complication rates; and (5) Study design: all observational (retrospective or prospective) original articles.
The exclusion criteria were s follows: (1) case reports, review articles, letters, editorials, conference abstracts, systematic reviews, and meta‐analyses; (2) insufficient data to compute diagnostic performance metrics for lymphoma based on true‐positive, true‐negative, false‐positive, and false‐negative rates; and (3) full‐text articles not available in English.
2.3. Data Extraction
The following information was extracted using a standardized form: (1) Study characteristics: first author, year of publication, affiliation, patient enrollment period, and study design (prospective/retrospective); (2) Demographic and clinical characteristics: numbers of total and male patients, mean age or range of included patients, and subtype of lymphoma; (3) Biopsy information: biopsy gun manufacturer, needle size, specimen number and size, biopsy location, number of biopsies performed by physician, and presence of immunohistochemistry (IHC); and (4) Outcomes: diagnostic performance of US‐guided CNB, including sensitivity, specificity, and complication rates.
2.4. Quality Assessment
Two reviewers (M. K. L. independently assessed the quality of the included studies using the revised Quality Assessment of Diagnostic Accuracy Studies‐2 (QUADAS‐2) tool, focusing on risk of bias and applicability. Disagreements were resolved through consensus.
2.5. Data Synthesis and Analysis
The primary outcome of this meta‐analysis was the diagnostic performance of US‐guided CNB in patients with lymphoma. Pooled sensitivity and specificity with 95% confidence intervals (CIs) were calculated using random‐effects modeling in individual studies. Summary receiver operating characteristic (SROC) curves with 95% CIs and predicted regions were graphically constructed. Heterogeneity was assessed using the Higgins I 2 statistic, with values ranging from 0% to 40% indicating insignificant heterogeneity, 30% to 60% indicating moderate heterogeneity, 50% to 90% indicating substantial heterogeneity, and 75% to 100% indicating considerable heterogeneity [17]. Deek's funnel plot was used to evaluate publication bias, and Deek's asymmetry test was used to assess its statistical significance [18]. Subgroup meta‐regression analyses were conducted to explore the sources of heterogeneity across studies, considering the following covariates: (1) needle size (< 18 gauge (G) vs. ≥ 18 G), (2) number of patients (< 100 vs. ≥ 100), and (3) biopsy location (cervical only vs. cervical and other regions). For meta‐analytic pooling of the complication rate, the inverse variance method was used to calculate weights, and the Der‐Simonian‐Laird random‐effects model was used to calculate 95% CIs [19]. Statistical analyses were performed using STATA version 18.0 (StataCorp, College Station, TX, USA), with p values < 0.05 considered statistically significant.
3. Results
3.1. Literature Search
Figure 1 shows a flow diagram describing the study selection process. Initially, 3459 papers were identified, and 275 duplicate studies were removed. Subsequently, 3184 papers underwent screening on the basis of titles and abstracts, resulting in 178 papers for further evaluation of their eligibility. Among these, the first 152 studies were excluded for being case reports, review articles, letters, editorials, conference abstracts (n = 143), systematic reviews, or meta‐analyses (n = 2), or if the full text was unavailable in English (n = 7). A full‐text review followed, excluding 16 studies due to insufficient data to calculate diagnostic performance (n = 5) [20, 21, 22, 23, 24], or utilizing CNB methods other than US‐guided CNB (n = 8) [25, 26, 27, 28, 29, 30, 31, 32], or covering only patients with lymphoma (n = 3) [5, 33, 34]. Three additional articles were identified as eligible through a manual search [9, 10, 35]. Finally, 13 studies were included in this systematic review and meta‐analysis [9, 10, 11, 12, 13, 14, 15, 35, 36, 37, 38, 39, 40].
FIGURE 1.
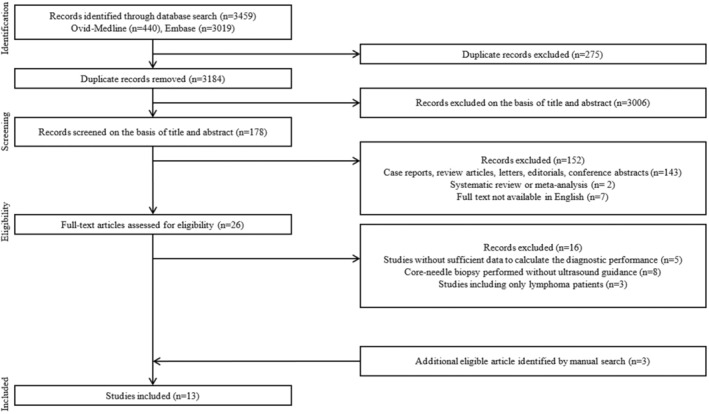
PRISMA flowchart of selecting process of eligible studies.
3.2. Characteristics of the Included Studies
The demographic characteristics of the 13 included studies are shown in Table 1 [9, 10, 11, 12, 13, 14, 15, 35, 36, 37, 38, 39, 40]. Two studies were prospectively designed [14, 39], whereas the other 11 were retrospectively designed [9, 10, 11, 12, 13, 15, 35, 36, 37, 38, 40]. The number of included participants ranged from 24 to 735, with the proportion of male patients ranging from 8% to 64% in nine studies [9, 11, 12, 14, 15, 35, 38, 39, 40]. excluding four studies [10, 13, 36, 37], in which data were not available. The mean or median age of the included patients ranged from 38.0 to 61.1 years in 10 studies [9, 11, 12, 13, 14, 15, 35, 36, 38, 40], except for three studies [10, 37, 39] because of a lack of precise age information. Among the studies that covered lymphoma subtypes [9, 10, 11, 12, 13, 14, 15, 35, 38, 40], more than half resulted in a larger proportion of NHL cases, as proven by US‐guided CNB, than HL [9, 10, 11, 13, 14, 15, 40]. Subtype information was not mentioned in any of the three studies [36, 37, 39]. Table 2 presents the biopsy data from the included studies. Among the included studies, six studies [13, 14, 35, 36, 37, 39] performed US‐guided CNB only in the cervical area, including the cervical node, non‐nodal cervical or facial mass, parotid gland, and submandibular gland, whereas seven studies [9, 10, 11, 12, 15, 38, 40] included biopsy sites in the cervical area and other regions, including the axilla, mediastinum, groin, breast, flank, and abdomen. Most of the included studies performed IHC using US‐guided CNB specimen [9, 10, 11, 12, 13, 14, 15, 35, 38, 39, 40], whereas two studies did not evaluate IHC [36, 37].
TABLE 1.
Demographics and clinical characteristics of the included studies.
| First author (year of publication) | Affiliation | Patient enrollment period | Study design | No. of participants | Male (%) | Mean age (range) | Subtype of lymphoma |
|---|---|---|---|---|---|---|---|
| Adeel (2021) | Royal Hallamshire Hospital, Sheffield Teaching Hospitals, Sheffield, UK | May 2017–Apr 2019 | Retrospective | 287 | NA | 58.1 | NA |
| Allin (2017) | Guy's and Saint Thomas' NHS Foundation Trust, London, UK | Dec 2013–Apr 2015 | Retrospective | 70 | NA | NA | NA |
| Baer a (2021) | Johns Hopkins University School of Medicine, Baltimore, US | Jul 2009–Aug 2018 | Retrospective | 24 | 2 (8%) | NA (18–74) | MZL of MALT |
| Cohen (2021) | University College London Hospitals NHS Foundation Trust, London, UK | 2016–2018 | Retrospective | 512 | NA | NA | HL (17.3%) (cHL, NLPHL) NHL (82.7%) (DLBCL, FL, CLL/SLL, MCL, NMZL, HGBL PTCL, etc.) |
| Elhamdoust (2020) | Golestan Hospital, Ahvaz Jundishapur University of Medicine, Ahvaz, Iran | 2019 | Retrospective | 40 | 18 (45%) | 49.4 | HL (56.5%), NHL (43.5%) |
| Groneck (2016) | Klinik I für Innere Medizin, Universitätsklinik Köln, Köln, Germany | NA | Retrospective | 138 | 88 (64%) | NA (17–82) | HL (23.3%) (cHL, NLPHL) NHL (76.7%) (DLBCL, FL, etc.) |
| Howlett (2006) | Eastbourne District General Hospital, Kings Drive, Eastbourne, UK | NA (over 3.5 years period) | Prospective | 82 | 46 (56%) | NA (24–93) | NA |
| Kiliçarslan (2017) | University of Ankara Yıldırım Beyazıt School of Medicine, Ankara, Turkey | 2010–2016 | Retrospective | 60 | 37 (62%) | 50.8 (19–74) | HL (53%) NHL (47%) (DLBCL, MCL, CLL) |
| Kim (2007) | Sungkyunkwan University School of Medicine, Kangbuk Samsung Hospital, Seoul, Korea | Mar 2000–Sep 2005 | Retrospective | 155 | NA | 38 (13–82) | HL (25%) NHL (75%) (DLBCL, Burkitt lymphoma, ALCL, MCL, Precursor B‐cell lymphoma) |
| Nguyen (2014) | Harbor‐UCLA Medical Center, California, USA | Apr 2008–Jan 2014 | Retrospective | 71 | 31 (44%) | 43.1 (18–71) | HL (32%) (cHL, NLPHL) NHL (68%) (FL, DLBCL, THRBCL, NMZL, Plasmablastic lymphoma, etc.) |
| Pfeiffer (2009) | University of Freiburg, Freiburg, Germany | Apr 2003–Oct 2007 | Prospective | 45 | 27 (60%) | 61.1 (21–91) | HL (7.7%) (cHL) NHL (92.3%) (DLBCL, FL, MCL, Marginal zone lymphoma, etc.) |
| Pugliese (2017) | Department of Clinical Medicine and Surgery, Federico II University Medical School, Naples, Italy | Jan 2009– Dec 2015 | Retrospective | 185 | 86 (47%) | NA (17–76) | HL (30.1%) (cHL, NLPHL) NHL (69.9%) (DLBL, FL, CLL/SLL, MCL, NMZL, ALCL, PTCL, etc.) |
| Wilczynski b , c (2020) | University Hospital Marburg und Giessen, Marburg, Germany | Jan 2006–Jun 2015 | Retrospective | 735 | 430 (59%) | 60 (11–90) | HL (17.8%) NHL (82.2%) (B‐cell lymphoma, T‐cell lymphoma, posttransplantation lymphoma) |
Abbreviations: AILD, angioimmunoblastic T‐NHL; ALCL, anaplastic large cell lymphoma; ALL, acute lymphocytic leukemia; cHL, classic Hodgkin's lymphoma; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large cell B‐cell lymphoma; FL, follicular lymphoma; HGBL, high‐grade B‐cell lymphoma; HL, Hodgkin's lymphoma; MCL, mantle cell lymphoma; NHL, non‐Hodgkin's lymphoma; NLPHL, nodular lymphocyte predominant Hodgkin's lymphoma; NMZL, nodal marginal zone lymphoma; PTCL, peripheral T‐cell lymphoma; SLL, small lymphocytic lymphoma; THRBCL, T‐cell‐rich large B‐cell lymphoma.
Only including patients with Sjögren's syndrome (SS).
Including posttransplantation lymphoma as NHL.
Pooled sensitivity and specificity calculated by number of cases.
TABLE 2.
Biopsy information of the included studies.
| First author (year of publication) | Biopsy gun manufacturer | Needle size | Specimen number | Specimen size | Biopsy location | No. of biopsy performed physician | Immunohistochemistry |
|---|---|---|---|---|---|---|---|
| Adeel (2021) | NA (Temno biopsy needle) | 16 G or 18 G | Average 2–3 | NA | Cervical node, parotid gland, neck‐lump (non‐nodal), submandibular gland | 1 | NA |
| Allin (2017) | Carefusion (Temno biopsy needle) | 16 G | NA | NA | Neck | NA | NA |
| Baer (2021) | INRAD | 18 G | 1–4 (2.29 ± 0.66 per gland) | NA | Submandibular gland, parotid gland | NA (> 1) | Y |
| Cohen (2021) | Argon medicine (Biopince), Cook (Quickcore) | 16 G (major), 18 G | 1–4 (median 3 core) | 1 cm | Lymph nodes (cervical, axillary, inguinal), extra‐nodal | NA (> 1) | Y |
| Elhamdoust (2020) | NA | NA | NA | NA | Superficial (neck, axillary, inguinal, breast, vertical muscle, waist), abdominal | NA | Y |
| Groneck (2016) | NA | 14 G, 16 G, 18 G | NA | NA | Cervical, supraclavicular, axillary, inguinal | NA | Y |
| Howlett (2006) | Franklin Bard (Biopty gun) | 18 or 20 G | NA (mean 2) | NA | Neck (thyroid and salivary gland excluded) | 1 | Y |
| Kiliçarslan (2017) | NA | NA | NA | NA | Cervical, axillary, supraclavicular, abdominal, submandibular, inguinal | NA | Y |
| Kim (2007) | Manan (Pro‐Mag 2.2) TSK Laboratory (Stericut) | 16 G | 2–4 (mean 2.5) | Throw 2.2 cm | Neck | 2 | Y |
| Nguyen (2014) | CareFusion (Achieve core biopsy needle) | 14 G | 8–10 | NA | Cervical, supaclavicular, axillary, inguinal | 1 | Y |
| Pfeiffer (2009) | Bard (Bard Magnum) | 12 G–16 G | 1–4 (mean 2.16) | NA | Cervico‐facial mass | NA | Y |
| Pugliese (2017) | Biomol HS‐Hospital (Modified Menghini needle with automatic aspiration) | 16 G | 1–4 (median 2) | 15–70 mm | Cervical, axillary, mediastinum, inguinal, abdominal | 2 | Y |
| Wilczynski (2020) | NA | 18 G | 1–4 | 9 mm, 19 mm, 29 mm | Peripheral (cervical, supraclavicular, inguinal, axillary), abdominal | 1 | Y |
Abbreviations: NA, not applicable; Y, yes.
3.3. Quality Assessment
Five studies fulfilled all seven domains, four fulfilled six domains, and four fulfilled five domains (Figure 2). Six studies [9, 14, 36, 38, 39, 40] had a low risk of bias in patient selection, whereas the other seven studies had an unclear risk of bias [10, 11, 12, 13, 15, 35, 37]. All studies showed a low risk of bias in the index test domain (Figure 2). One study had an unclear risk of bias in the reference standard domain because there were two patients whose biopsy results were unclassifiable even after SEB [38]. Other 12 studies had a low‐risk bias in the same domain [9, 10, 11, 12, 13, 14, 15, 35, 36, 37, 39, 40]. Three studies resulted in an unclear risk of bias in the flow and timing domains because the specific method for secondary diagnosis was not stated [10, 11, 37], whereas the other 10 studies showed low‐risk bias [9, 12, 13, 14, 15, 35, 36, 38, 39, 40]. Nearly all the studies were categorized as having low concerns regarding applicability in the patient selection, index test, and reference standard domains. Only one study was of high concern for applicability in patient selection because it included only patients with Sjogren's syndrome (SS) [35].
FIGURE 2.
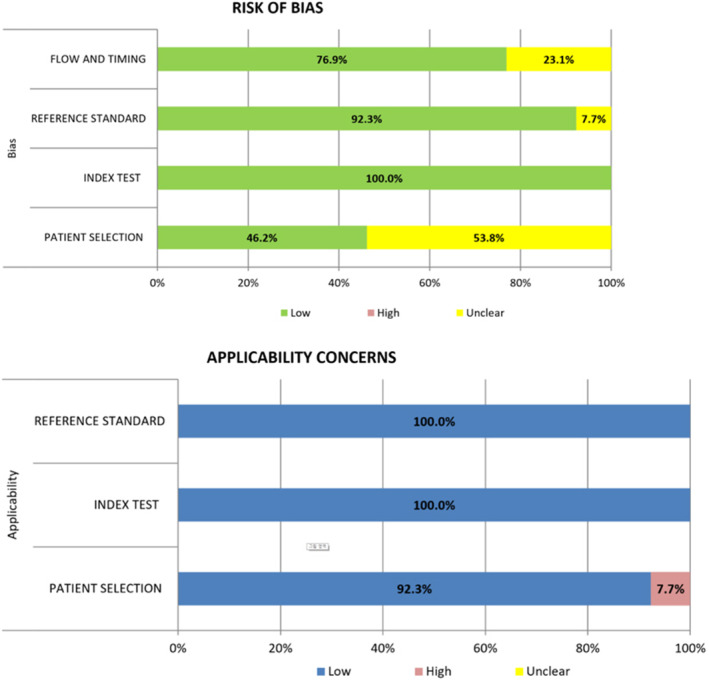
Quality assessment of the included studies according to the QUADAS‐2 criteria.
3.4. Pooled Estimation of Diagnostic Performance
The diagnostic performance, represented by sensitivity and specificity, is shown in Figure 3 as an illustration of the coupled forest plots of the pooled data. Among the included studies evaluating the diagnostic performance of US‐guided CNB in patients with lymphoma, the pooled sensitivity was 94% (95% CI: 89%–96%) and specificity was 100% (95% CI: 94%–100%). Considerable heterogeneity was noted in sensitivity (I 2 = 79.12%, p < 0.001) and specificity (I 2 = 81.17%, p < 0.001). The SROC curve showed a large difference between the areas with 95% confidence and predicted regions (Figure S1). There was significant publication bias among the included studies on funnel plots (Figure S2) and Egger's test (p < 0.001).
FIGURE 3.
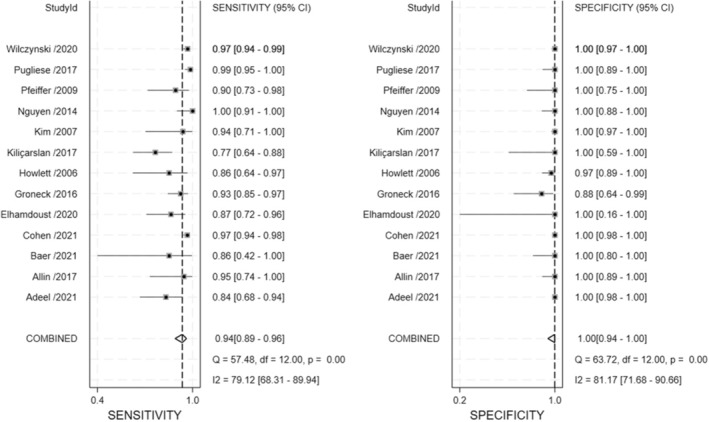
Coupled plots of pooled sensitivity and specificity of US‐guided CNB in diagnosis of patients with lymphoma. Horizontal lines presents 95% CIs of each study. CNB, core needle biopsy; CI, confidence interval; US, ultrasound.
The pooled complication rate of US‐guided CNB is summarized in Figure 4, and two studies were excluded because of the absence of information [12, 37]. The pooled complication rate was 1% (95% CI: 0%–3%), with considerable heterogeneity (I 2=80.32%, p < 0.001). Most complications were self‐limiting; therefore, they were managed conservatively and were not associated with long‐term morbidity. The most common complications were minor bleeding and hematoma, followed by pain (Table S1). Other complications included bruising, vasovagal attack, transient facial weakness related to local anesthesia, self‐limiting lymph fistula, and transient hypoesthesia of the trigeminal nerve. Only one case was reported as a major complication [15], in which active bleeding occurred after biopsy of a cervical lymph node with a venous malformation inside it.
FIGURE 4.
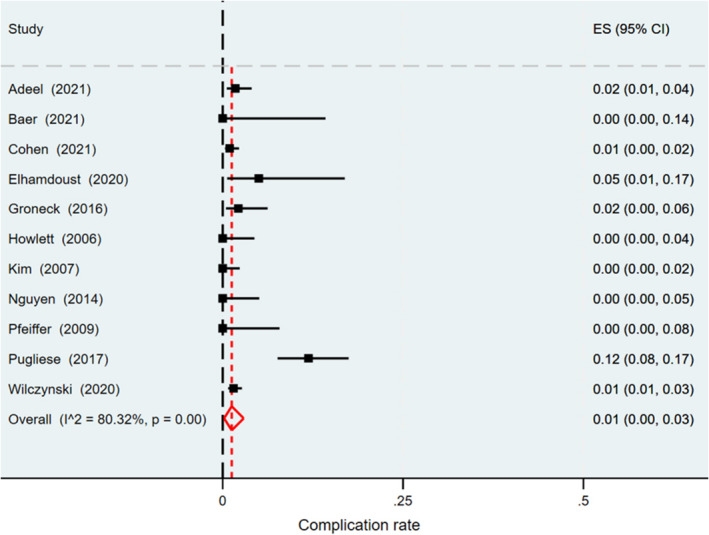
Forest plot of pooled complication rates of US‐guided CNB. Horizontal lines presents 95% CIs of each study. CNB, core needle biopsy; CI, confidence interval; US, ultrasound.
3.5. Subgroup Meta‐Regression Analysis
The subgroup meta‐regression analysis results are outlined in Table 3. We assessed the included studies using three covariates (needle size, number of patients, and biopsy location) to explore heterogeneity sources. Studies utilizing needles with larger circumferences (smaller gauge, < 18 G) for US‐guided CNB exhibited significantly higher pooled sensitivity (97% vs. 94%, p < 0.001) and specificity (100% vs. 95%, p < 0.001) compared with those using smaller circumferences (larger gauge, ≥ 18 G). There were no significant differences in diagnostic performance observed for other variables, including the number of patients and biopsy location.
TABLE 3.
Subgroup meta‐regression analyses for identifying heterogeneity.
| Covariate | No. of studies | Sensitivity | Specificity | p | I 2 |
|---|---|---|---|---|---|
| Needle size (circumferential) | < 0.001 | 89 | |||
| < 18G | 5 | 97 | 100 | ||
| ≥ 18G | 6 | 94 | 100 | ||
| Number of patients | 0.23 | 31 | |||
| < 100 | 7 | 90 | 100 | ||
| ≥ 100 | 6 | 96 | 100 | ||
| Biopsy location | 0.38 | 0 | |||
| Only cervical | 6 | 90 | 100 | ||
| Cervical and other regions | 7 | 95 | 100 |
4. Discussion
This systematic review and meta‐analysis evaluated published studies examining the efficacy and safety of US‐guided CNB for diagnosing lymphoma. Our findings indicate excellent diagnostic performance of US‐guided CNB, with a pooled sensitivity of 94% (95% CI: 89%–96%), specificity of 100% (95% CI: 94%–100%), and an area under the SROC curve of 99% (95% CI: 98%–100%). Complications associated with US‐guided CNB occurred at a pooled rate of 1%, predominantly minor. Traditionally, lymphoma diagnosis relies on SEB, but recent evidence suggests US‐guided CNB as a viable alternative. Notably, this study represents the first systematic review and meta‐analysis investigating the diagnostic accuracy and safety of US‐guided CNB in patients with lymphoma. Our findings strongly support the potential of US‐guided CNB as an alternative diagnostic approach to SEB for lymphoma diagnosis.
Recently, there have been several papers [10, 11, 12, 13, 14, 15, 38] on the diagnostic performance of US‐guided CNB in patients with lymphoma, such as those included in this study. Although most published studies have not directly compared US‐guided CNB and SEB for the diagnosis of lymphoma, they showed good diagnostic performance. A recent multicenter French study involving 32,285 cases compared US‐guided CNB and SEB for lymphoma diagnosis [41]. They found SEB to have significantly higher diagnostic performance, with 98.1% sensitivity, whereas US‐guided CNB exhibited lower sensitivity at 92.3% (p < 0.0001). Another comparative study yielded similar results, showing SEB with higher diagnostic performance at 98.8% sensitivity compared with US‐guided CNB at 95.9% sensitivity (p = 0.049) [42]. Although US‐guided CNB showed a lower sensitivity than SEB for lymphoma diagnosis, the authors mentioned that considering clinical information and IHC data could improve the diagnostic performance of US‐guided CNB. In addition, a large‐scale, multi‐institutional study evaluated the diagnostic yield of small volume biopsy (SVB), which comprises FNA with or without CNB, across common clinical indications throughout the disease course of follicular lymphoma [43, 44, 45]. Although SVBs may not always allow for complete classification, they usually provide sufficient information for clinical decision‐making, particularly in cases of recurrent or transformed lymphoma. An initial investigation found that for follicular patients with lymphoma with suspected transformation, the time to diagnosis was comparable between initial biopsy methods (SVB or SEB), suggesting that starting with SVB is unlikely to delay diagnosis or treatment [43]. Notably, the subclassification rate increased with CNB at the time of initial follicular lymphoma diagnosis [44]. A follow‐up study reviewed diagnostic discrepancies between the initial SVB and subsequent biopsy performed within 3 months [45]. In this cohort, the initial SVB demonstrated 70% sensitivity for lymphoma diagnosis, with 7% yielding nondiagnostic results. Across all disease stages, SVB showed 100% specificity, with no instances of overdiagnosis or downgrading from malignant to benign. Therefore, the authors suggested that US‐guided CNB could serve as an alternative tool for lymphoma diagnosis. Evaluating the safety of a technique is crucial for assessing the clinical implications of diagnostic tools. A recent study reported a 5.9% complication rate for SEB, primarily consisting of minor complications (84.2%) [46]. Other studies also reported complication rates ranging from 2.5% to 6.5% [25, 47]. Our study demonstrated a lower pooled complication rate of 1%.
The present meta‐analysis showed good performance, with 94% pooled sensitivity and 100% pooled specificity for US‐guided CNB in the diagnosis of lymphoma. In lymphoma diagnosis, IHC results are important for developing a treatment strategy and can be an important factor for improving the diagnostic performance of US‐guided CNB [2, 6, 7, 42]. Whether the specimen obtained through US‐guided CNB is sufficient to evaluate IHC is the most important question for evaluating diagnostic performance. Most of the included studies performed IHC using US‐guided CNB specimens. Our study shows that US‐guided CNB provides enough tissue for lymphoma diagnosis. Elhamdoust et al. showed the supporting results that IHC results can improve the diagnostic performance of US‐guided CNB and that US‐guided CNB provides sufficient tissue for evaluating IHC [38]. Therefore, US‐guided CNB provides an adequate amount of tissue for lymphoma subtyping using IHC, which can contribute to the development of appropriate treatment plans.
Although US‐guided CNB has shown good diagnostic performance and safety in the diagnosis of lymphoma, several issues have been considered. In particular, the diagnostic performance differed according to the lymphoma subtype because it may not be enough to give a final diagnosis solely by US‐guided CNB in HL patients, rather than NHL patients [48]. Groneck et al. showed that HL had a higher false‐negative rate on US‐guided CNB than NHL [11]. All false‐negative US‐guided CNB results for patients with HL were clearly detected with a secondary biopsy, such as SEB or biopsy from other sites, or confirmed by an expert review. The sensitivity for clinically conclusive histopathological diagnosis of US‐guided CNB specimens of lymph nodes or tumors was 96.7% for NHL and only 66.0% for HL. Other studies have also shown similar results; the misdetection rate of HL was higher than that of NHL [49, 50]. The relatively poor diagnostic performance of US‐guided CNB in patients with HL may be due to its complex architecture [11]. Therefore, we recommend repeating US‐guided CNB, consulting an expert review, or performing SEB in patients with HL for an accurate diagnosis.
Moreover, there is no standard US‐guided CNB technique for lymphoma diagnosis. In our subgroup analysis, needle with larger circumferential (smaller gauge, < 18 G) resulted in a better diagnostic performance with a significantly higher pooled sensitivity and similar pooled specificity (< 18 G vs. ≥ 18 G; 97% and 100% vs. 94% and 100%, p < 0.001). These results suggest that more histopathologic information can be obtained from larger specimens, but because no study has addressed the details of needle size in each biopsy case, we could conclude that no definite statistical evidence has been achieved for the advantage of a needle with a smaller gauge. Groneck et al. suggested a slightly better, yet not significant, outcome with the use of 14 G needles than with 16 G and 18 G needles, concluding that needle size is not crucial [11]. However, another study recommended the use of not too small cutting needles (≥ 16 G) [14]. Both studies agree that acquiring multiple cores from different areas of the lymph nodes is important for obtaining good sampling results. Accordingly, to improve the quality of biopsy samples, biopsies should be performed more than once in various regions of the target lymph node using a larger cutting needle, if necessary. The debate between CNB and SEB in lymphoma diagnosis remains ongoing. Although 100% specificity in CNB is commendable, for SEB it may reflect the potential for unnecessary procedures in some cases [51]. However, less invasive techniques such as FNA and CNB, especially when combined with ancillary studies, offer safe, rapid, and accurate diagnoses in most cases [52]. Each method, including SEB, has its own strengths and limitations. One study advice caution against the routine use of CNB, favoring SEB in appropriate cases due to potential diagnostic pitfalls in certain lymphoma subtypes [51]. On the contrary, another study highlights the practicality and accuracy of less invasive methods, particularly when enhanced by advanced ancillary techniques [52]. Ultimately, the choice between these approaches should be guided by individual patient factors, suspected pathology, and available expertise. Although less invasive methods have shown promising results, SEB remains an important option in more complex cases. This ongoing debate underscores the need for continued research and a multidisciplinary approach in lymphoma diagnostics.
Our study had a few limitations. First, among the 13 articles, very few included a direct comparison of US‐guided CNB with SEB in the diagnosis of lymphoma [12, 13, 38]. Considering the difficulty to proceed meta‐analysis on this subject, if related future papers are more published, further study should be performed to investigate and approve the diagnostic performance of US‐guided CNB in patients with lymphoma, directly compared with SEB. Second, the number of included studies providing details of the specimen number (number of US‐guided CNB passes) [9, 10, 13, 14, 15, 35, 40] and the size of the biopsied lymph nodes [9, 10, 13, 15] were too small for subgroup analysis. Due to a lack of information, the reported specimen number or size in the included studies [9, 10, 13, 14, 15, 35, 40] is limited, making it difficult to draw conclusions about the relationship between the mentioned biopsy information and the diagnostic performance of US‐guided CNB. Third, a challenge lies in conducting a detailed analysis of the subtypes of NHL. Although we listed the subtypes mentioned in the included studies, extracting data specific to each subtype was difficult, making it impractical to perform a thorough subgroup analysis. Similarly, although the subtype of HL was referenced in several reviewed papers, the available data did not allow for in‐depth exploration of these subtypes. This limitation may have influenced the ability to fully assess the nuanced differences in sensitivity and specificity across lymphoma subtypes. Finally, due to limited information provided in the studies we reviewed, it was challenging to conduct a comparative analysis of diagnostic accuracy before and after the application of flow cytometry or molecular studies. Among the five mentioning the performance of molecular biological studies [9, 10, 11, 14, 35], studies reported that CNB provides sufficient material for molecular analyses [9, 10, 11]. Moreover, through literature search, notable studies have been identified that address the importance of flow cytometry in needle biopsy of lymphoma, both in diagnosis and subclassification [53, 54, 55, 56, 57].
In conclusion, this review suggests that US‐guided CNB shows promising diagnostic performance and low complication rates in the diagnosis of lymphoma. Although the results indicate that US‐guided CNB may be a useful diagnostic tool for patients with lymphoma, further research is needed to confirm its effectiveness as an alternative to other diagnostic methods.
Author Contributions
Yongmin Kwon: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), writing – original draft (equal). Min Kyoung Lee: conceptualization (lead), data curation (equal), formal analysis (equal), funding acquisition (lead), investigation (equal), methodology (lead), project administration (lead), resources (lead), software (lead), supervision (lead), validation (lead), visualization (lead), writing – original draft (equal), writing – review and editing (lead).
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Figure S1. Summary receiver operating characteristics (SROC) curves of US‐guided CNB for diagnosis in patients with lymphoma.
Figure S2. Deek’s funnel plot asymmetry test of 13 included studies.
Table S1. Outcomes: complications of USCNB.
Acknowledgments
The authors have nothing to report.
Funding: This work was supported by the financial support of the Catholic Medical Center Research Foundation made in the program year of 2023 and the Nuri‐light Radiological Medicine Research Society.
Data Availability Statement
Data are openly available in a public repository that issues datasets with DOIs.
References
- 1. Morton L. M., Turner J. J., Cerhan J. R., et al., “Proposed Classification of Lymphoid Neoplasms for Epidemiologic Research From the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph),” Blood 110, no. 2 (2007): 695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matasar M. J. and Zelenetz A. D., “Overview of Lymphoma Diagnosis and Management,” Radiologic Clinics of North America 46, no. 2 (2008): 175–198. [DOI] [PubMed] [Google Scholar]
- 3. de Leval L. and Jaffe E. S., “Lymphoma Classification,” Cancer Journal 26, no. 3 (2020): 176–185. [DOI] [PubMed] [Google Scholar]
- 4. Jamil A. and Mukkamalla S. K. R., “Lymphoma,” 2020.
- 5. Burke C., Thomas R., Inglis C., et al., “Ultrasound‐Guided Core Biopsy in the Diagnosis of Lymphoma of the Head and Neck. A 9 Year Experience,” British Journal of Radiology 84, no. 1004 (2011): 727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ansell S. M., “Non‐Hodgkin Lymphoma: Diagnosis and Treatment,” Paper presented at: Mayo Clinic Proceedings 90, no. 8 (2015): 1152–1163. [DOI] [PubMed] [Google Scholar]
- 7. Ansell S. M., “Hodgkin Lymphoma: Diagnosis and Treatment,” Paper presented at: Mayo Clinic Proceedings, 90, no. 11 (2015), 1574–1583. [DOI] [PubMed] [Google Scholar]
- 8. Mutlu Y. G., Aydın B. B., Çakır A., Canöz Ö., Erol C., and Gökmen Sevindik Ö., “Should Core Needle Lymph Node Biopsy Be a Relevant Alternative to Surgical Excisional Biopsy in Diagnostic Work Up of Lymphomas?,” Eurasian Journal of Medicine 55, no. 2 (2023): 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pugliese N., Di Perna M., Cozzolino I., et al., “Randomized Comparison of Power Doppler Ultrasonography‐Guided Core‐Needle Biopsy With Open Surgical Biopsy for the Characterization of Lymphadenopathies in Patients With Suspected Lymphoma,” Annals of Hematology 96 (2017): 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cohen O. C., Brodermann M. H., Dervin A., et al., “Lymph Node Core Biopsies Reliably Permit Diagnosis of Lymphoproliferative Diseases. Real‐World Experience From 554 Sequential Core Biopsies From a Single Centre,” European Journal of Haematology 106, no. 2 (2021): 267–272. [DOI] [PubMed] [Google Scholar]
- 11. Groneck L., Quaas A., Hallek M., Zander T., and Weihrauch M. R., “Ultrasound‐Guided Core Needle Biopsies for Workup of Lymphadenopathy and Lymphoma,” European Journal of Haematology 97, no. 4 (2016): 379–386. [DOI] [PubMed] [Google Scholar]
- 12. Kiliçarslan A., Doğan M., Süngü N., et al., “Can Cutting‐Needle Biopsy Be an Alternative to Excisional Biopsy in Lymph Node Pathologies?,” Turk Patoloji Dergisi 1, no. 1 (2017): 235–239. [DOI] [PubMed] [Google Scholar]
- 13. Kim B. M., Kim E.‐K., Kim M. J., Yang W.‐I., Park C. S., and Park S. I., “Sonographically Guided Core Needle Biopsy of Cervical Lymphadenopathy in Patients Without Known Malignancy,” Journal of Ultrasound in Medicine 26, no. 5 (2007): 585–591. [DOI] [PubMed] [Google Scholar]
- 14. Pfeiffer J., Kayser G., and Ridder G. J., “Sonography‐Assisted Cutting Needle Biopsy in the Head and Neck for the Diagnosis of Lymphoma: Can It Replace Lymph Node Extirpation?,” Laryngoscope 119, no. 4 (2009): 689–695. [DOI] [PubMed] [Google Scholar]
- 15. Wilczynski A., Görg C., Timmesfeld N., et al., “Value and Diagnostic Accuracy of Ultrasound‐Guided Full Core Needle Biopsy in the Diagnosis of Lymphadenopathy: A Retrospective Evaluation of 793 Cases,” Journal of Ultrasound in Medicine 39, no. 3 (2020): 559–567. [DOI] [PubMed] [Google Scholar]
- 16. Moher D., Liberati A., Tetzlaff J., Altman D. G., and PRISMA Group , “Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement,” Annals of Internal Medicine 151, no. 4 (2009): 264–269. [DOI] [PubMed] [Google Scholar]
- 17. Kim K. W., Lee J., Choi S. H., Huh J., and Park S. H., “Systematic Review and Meta‐Analysis of Studies Evaluating Diagnostic Test Accuracy: A Practical Review for Clinical Researchers‐Part I. General Guidance and Tips,” Korean Journal of Radiology 16, no. 6 (2015): 1175–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deeks J. J., Macaskill P., and Irwig L., “The Performance of Tests of Publication Bias and Other Sample Size Effects in Systematic Reviews of Diagnostic Test Accuracy was Assessed,” Journal of Clinical Epidemiology 58, no. 9 (2005): 882–893. [DOI] [PubMed] [Google Scholar]
- 19. DerSimonian R. and Laird N., “Meta‐Analysis in Clinical Trials,” Controlled Clinical Trials 7, no. 3 (1986): 177–188. [DOI] [PubMed] [Google Scholar]
- 20. Assaf N., Nassif S., Tamim H., Bazarbachi A., Zaatari G., and Chakhachiro Z., “Diagnosing Lymphoproliferative Disorders Using Core Needle Biopsy Versus Surgical Excisional Biopsy: Three‐Year Experience of a Reference Center in Lebanon,” Clinical Lymphoma, Myeloma & Leukemia 20, no. 8 (2020): e455–e460. [DOI] [PubMed] [Google Scholar]
- 21. Gupta S., Long S. R., Natkunam Y., Kong C. S., Gupta N. K., and Gratzinger D., “Role of FNA With Core Biopsy or Cell Block in Patients With Nodular Lymphocyte‐Predominant Hodgkin Lymphoma,” Cancer Cytopathology 128, no. 8 (2020): 570–579. [DOI] [PubMed] [Google Scholar]
- 22. Lachar W. A., Shahab I., and Saad A. J., “Accuracy and Cost‐Effectiveness of Core Needle Biopsy in the Evaluation of Suspected Lymphoma: A Study of 101 Cases,” Archives of Pathology & Laboratory Medicine 131, no. 7 (2007): 1033–1039. [DOI] [PubMed] [Google Scholar]
- 23. Olubaniyi B., Chow V., Mandalia U., et al., “Evaluation of Biopsy Methods in the Diagnosis of Submandibular Space Pathology,” International Journal of Oral and Maxillofacial Surgery 43, no. 3 (2014): 281–285. [DOI] [PubMed] [Google Scholar]
- 24. Vandervelde C., Kamani T., Varghese A., Ramesar K., Grace R., and Howlett D. C., “A Study to Evaluate the Efficacy of Image‐Guided Core Biopsy in the Diagnosis and Management of Lymphoma—Results in 103 Biopsies,” European Journal of Radiology 66, no. 1 (2008): 107–111. [DOI] [PubMed] [Google Scholar]
- 25. Chatani S., Hasegawa T., Kato S., et al., “Image‐Guided Core Needle Biopsy in the Diagnosis of Malignant Lymphoma: Comparison With Surgical Excision Biopsy,” European Journal of Radiology 127 (2020): 108990. [DOI] [PubMed] [Google Scholar]
- 26. de Kerviler E., de Bazelaire C., Mounier N., et al., “Image‐Guided Core‐Needle Biopsy of Peripheral Lymph Nodes Allows the Diagnosis of Lymphomas,” European Radiology 17 (2007): 843–849. [DOI] [PubMed] [Google Scholar]
- 27. Ensani F., Mehravaran S., Irvanlou G., et al., “Fine‐Needle Aspiration Cytology and Flow Cytometric Immunophenotyping in Diagnosis and Classification of Non‐Hodgkin Lymphoma in Comparison to Histopathology,” Diagnostic Cytopathology 40, no. 4 (2012): 305–310. [DOI] [PubMed] [Google Scholar]
- 28. Ingersoll K. F., Zhao Y., Harrison G. P., Li Y., Yang L.‐H., and Wang E., “Limited Tissue Biopsies and Hematolymphoid Neoplasms: Success Stories and Cautionary Tales,” American Journal of Clinical Pathology 152, no. 6 (2019): 782–798. [DOI] [PubMed] [Google Scholar]
- 29. Johl A., Lengfelder E., Hiddemann W., Klapper W., and German Low‐grade Lymphoma Study Group (GLSG) , “Core Needle Biopsies and Surgical Excision Biopsies in the Diagnosis of Lymphoma—Experience at the Lymph Node Registry Kiel,” Annals of Hematology 95 (2016): 1281–1286. [DOI] [PubMed] [Google Scholar]
- 30. Nyquist G. G., Tom W. D., and Mui S., “Automatic Core Needle Biopsy: A Diagnostic Option for Head and Neck Masses,” Archives of Otolaryngology – Head & Neck Surgery 134, no. 2 (2008): 184–189. [DOI] [PubMed] [Google Scholar]
- 31. Amador‐Ortiz C., Chen L., Hassan A., et al., “Combined Core Needle Biopsy and Fine‐Needle Aspiration With Ancillary Studies Correlate Highly With Traditional Techniques in the Diagnosis of Nodal‐Based Lymphoma,” American Journal of Clinical Pathology 135, no. 4 (2011): 516–524. [DOI] [PubMed] [Google Scholar]
- 32. Krarup Sigaard R., Wennervaldt K., Munksgaard L., Rahbek Gjerdrum L. M., and Homøe P., “Core Needle Biopsy Is an Inferior Tool for Diagnosing Cervical Lymphoma Compared to Lymph Node Excision,” Acta Oncologica 60, no. 7 (2021): 904–910. [DOI] [PubMed] [Google Scholar]
- 33. Cuenca‐Jimenez T., Chia Z., Desai A., et al., “The Diagnostic Performance of Ultrasound‐Guided Core Biopsy in the Diagnosis of Head and Neck Lymphoma: Results in 226 Patients,” International Journal of Oral and Maxillofacial Surgery 50, no. 4 (2021): 431–436. [DOI] [PubMed] [Google Scholar]
- 34. de Larrinoa A. F., del Cura J., Zabala R., Fuertes E., Bilbao F., and Lopez J. I., “Value of Ultrasound‐Guided Core Biopsy in the Diagnosis of Malignant Lymphoma,” Journal of Clinical Ultrasound: JCU 35, no. 6 (2007): 295–301. [DOI] [PubMed] [Google Scholar]
- 35. Baer A. N., Grader‐Beck T., Antiochos B., Birnbaum J., and Fradin J. M., “Ultrasound‐Guided Biopsy of Suspected Salivary Gland Lymphoma in Sjögren's Syndrome,” Arthritis Care & Research 73, no. 6 (2021): 849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Adeel M., Jackson R., Peachey T., and Beasley N., “Ultrasound Core Biopsies of Neck Lumps: An Experience From a Tertiary Head and Neck Cancer Unit,” Journal of Laryngology and Otology 135, no. 9 (2021): 799–803. [DOI] [PubMed] [Google Scholar]
- 37. Allin D., David S., Jacob A., Mir N., Giles A., and Gibbins N., “Use of Core Biopsy in Diagnosing Cervical Lymphadenopathy: A Viable Alternative to Surgical Excisional Biopsy of Lymph Nodes?,” Annals of the Royal College of Surgeons of England 99, no. 3 (2017): 242–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Elhamdoust E., Motamedfar A., Gharibvand M. M., and Jazayeri S. N., “Investigation of the Value of Ultrasound‐Guided Core Needle Biopsy From Pathologic Lymph Nodes to the Diagnosis of Lymphoma,” Journal of Family Medicine and Primary Care 9, no. 6 (2020): 2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Howlett D., Menezes L., Bell D., et al., “Ultrasound‐Guided Core Biopsy for the Diagnosis of Lumps in the Neck: Results in 82 Patients,” British Journal of Oral & Maxillofacial Surgery 44, no. 1 (2006): 34–37. [DOI] [PubMed] [Google Scholar]
- 40. Nguyen B. M., Halprin C., Olimpiadi Y., Traum P., Yeh J. J., and Dauphine C., “Core Needle Biopsy Is a Safe and Accurate Initial Diagnostic Procedure for Suspected Lymphoma,” American Journal of Surgery 208, no. 6 (2014): 1003–1008. [DOI] [PubMed] [Google Scholar]
- 41. Syrykh C., Chaouat C., Poullot E., et al., “Lymph Node Excisions Provide More Precise Lymphoma Diagnoses Than Core Biopsies: A French Lymphopath Network Survey,” Blood 140, no. 24 (2022): 2573–2583. [DOI] [PubMed] [Google Scholar]
- 42. Gonçalves M. C., de Oliveira C. R. G., Sandes A. F., et al., “Core Needle Biopsy in Lymphoma Diagnosis: The Diagnostic Performance and the Role of the Multidisciplinary Approach in the Optimization of Results,” American Journal of Surgical Pathology 47, no. 1 (2023): 111–123. [DOI] [PubMed] [Google Scholar]
- 43. Mou E., Falchi L., Sundaram V., et al., “Impact of Initial Biopsy Type on the Time to Final Diagnostic Biopsy in Patients With Follicular Lymphoma and Suspected Histologic Transformation,” Leukemia & Lymphoma 62, no. 12 (2021): 2864–2872. [DOI] [PubMed] [Google Scholar]
- 44. Fitzpatrick M. J., Sundaram V., Ly A., et al., “Small Volume Biopsy Diagnostic Yield at Initial Diagnosis Versus Recurrence/Transformation of Follicular Lymphoma: A Retrospective Cyto‐Heme Interinstitutional Collaborative Study,” Cancer Cytopathology 131, no. 5 (2023): 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Volaric A. K., Lin O., Balassanian R., et al., “Diagnostic Discrepancies in Small‐Volume Biopsy for the Initial Diagnosis, Recurrence, and Transformation of Follicular Lymphoma: A Multi‐Institutional Collaborative Study,” American Journal of Surgical Pathology 47, no. 2 (2023): 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rehell M., Atula T., Tapiovaara L. K., et al., “Complications in Lymph Node Excision in the Head and Neck Area,” Acta Oto‐Laryngologica 142, no. 9–12 (2022): 738–742. [DOI] [PubMed] [Google Scholar]
- 47. Bassiouni M., Kang G., Olze H., Dommerich S., and Arens P., “The Diagnostic Yield of Excisional Biopsy in Cervical Lymphadenopathy: A Retrospective Analysis of 158 Biopsies in Adults,” Ear, Nose & Throat Journal 102 (2021): 01455613211023009. [DOI] [PubMed] [Google Scholar]
- 48. Das D. K., Francis I. M., Sharma P. N., et al., “Hodgkin's Lymphoma: Diagnostic Difficulties in Fine‐Needle Aspiration Cytology,” Diagnostic Cytopathology 37, no. 8 (2009): 564–573. [DOI] [PubMed] [Google Scholar]
- 49. Proctor I. E., McNamara C., Rodriguez‐Justo M., Isaacson P. G., and Ramsay A., “Importance of Expert Central Review in the Diagnosis of Lymphoid Malignancies in a Regional Cancer Network,” Journal of Clinical Oncology 29, no. 11 (2011): 1431–1435. [DOI] [PubMed] [Google Scholar]
- 50. Bröckelmann P. J., Goergen H., Fuchs M., et al., “Impact of Centralized Diagnostic Review on Quality of Initial Staging in Hodgkin Lymphoma: Experience of the German Hodgkin Study Group,” British Journal of Haematology 171, no. 4 (2015): 547–556. [DOI] [PubMed] [Google Scholar]
- 51. Pizzi M., Agostinelli C., Santoro L., et al., “Lymph Node Core Needle Biopsy for the Diagnosis of Lymphoproliferative Disorders: A Word of Caution,” European Journal of Haematology 106, no. 5 (2021): 737–739. [DOI] [PubMed] [Google Scholar]
- 52. Al‐Abbadi M., Barroca H., Bode‐Lesniewska B., et al., “Fine‐Needle Aspiration Cytology and Core‐Needle Biopsy in the Diagnosis of Lymphadenopathies: Words of Endorsement,” European Journal of Haematology 107, no. 2 (2021): 295–296. [DOI] [PubMed] [Google Scholar]
- 53. Young N. A., Al‐Saleem T. I., Ehya H., and Smith M. R., “Utilization of Fine‐Needle Aspiration Cytology and Flow Cytometry in the Diagnosis and Subclassification of Primary and Recurrent Lymphoma,” Cancer Cytopathology 84, no. 4 (1998): 252–261. [PubMed] [Google Scholar]
- 54. Zeppa P., Marino G., Troncone G., et al., “Fine‐Needle Cytology and Flow Cytometry Immunophenotyping and Subclassification of Non‐Hodgkin Lymphoma: A Critical Review of 307 Cases With Technical Suggestions,” Cancer Cytopathology 102, no. 1 (2004): 55–65. [DOI] [PubMed] [Google Scholar]
- 55. Dong H. Y., Harris N. L., Preffer F. I., and Pitman M. B., “Fine‐Needle Aspiration Biopsy in the Diagnosis and Classification of Primary and Recurrent Lymphoma: A Retrospective Analysis of the Utility of Cytomorphology and Flow Cytometry,” Modern Pathology 14, no. 5 (2001): 472–481. [DOI] [PubMed] [Google Scholar]
- 56. Mayall F., Darlington A., and Harrison B., “Fine Needle Aspiration Cytology in the Diagnosis of Uncommon Types of Lymphoma,” Journal of Clinical Pathology 56, no. 11 (2003): 821–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Meda B. A., Buss D. H., Woodruff R. D., et al., “Diagnosis and Subclassification of Primary and Recurrent Lymphoma: The Usefulness and Limitations of Combined Fine‐Needle Aspiration Cytomorphology and Flow Cytometry,” American Journal of Clinical Pathology 113, no. 5 (2000): 688–699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Summary receiver operating characteristics (SROC) curves of US‐guided CNB for diagnosis in patients with lymphoma.
Figure S2. Deek’s funnel plot asymmetry test of 13 included studies.
Table S1. Outcomes: complications of USCNB.
Data Availability Statement
Data are openly available in a public repository that issues datasets with DOIs.


