ABSTRACT
A 21‐year‐old man, known case of the repaired congenital heart disease, developed complete atrioventricular block (AVB) one week after simultaneous bioprosthetic pulmonary and tricuspid valve replacement and atrial septal defect repair. Considering the persistence of the AVB, it was decided to implant a permanent pacemaker. After considering all available options and the issues related to the patient, it was decided to implant a leadless pacemaker (LLP). A Micra pacemaker was implanted successfully, and the patient was discharged in good condition and without any complications. Follow‐up evaluation showed appropriate LLP and bioprosthetic valve functioning. Limited prior experiences and the present report showed that LLP appears to be an ideal option in the patients with bioprosthetic tricuspid valve complicated by conduction disorders.
Keywords: bioprosthetic valve, cardiovascular disease, electrophysiology, leadless pacemaker, Micra, tricuspid valve
Summary.
Leadless pacemaker (LLP) is a new technology validated in real‐world setting with advantages of overcoming some limits of the conventional pacing such as infection, lead malfunction, and lead‐related tricuspid valve regurgitation.
Our case shows LLP is a safe option in patients developing conduction disorders after Bioprosthetic Tricuspid valve replacement.
1. Introduction
Postoperative atrioventricular block (AVB) has been reported in 1%–6% of patients after cardiac surgery and 25%–60% of these patients will finally need a permanent pacemaker (PPM) [1, 2, 3, 4]. To avoid tricuspid valve (TV) malfunction, implantation of conventional pacing leads is generally not preferred in the presence of the tricuspid bioprosthesis [ 5 ].
Leadless pacemakers (LLP) have recently become popular in treatment of heart blocks and bradyarrhythmia due to their proven safety and efficacy [6, 7]. LLPs have advantages of avoiding complications encountered with conventional pacemakers (CPM) including infection, lead malfunction, and tricuspid valve regurgitation [8, 9]. Epicardial pacemaker is the standard recommendation in the setting of prior tricuspid valve surgery. However, prior cardiac surgeries are usually associated with significant pericardial adhesion, and most surgeons prefer not to implant epicardial leads in this setting due to impaired electrical properties of pericardial leads in the setting of pericardial adhesions. Therefore, LLPs can be a safe choice for patients with TV surgeries and postoperative AVB. There is few data about the LLP implantation in the presence of the bioprosthetic TV (BTV) [10, 11, 12]. In this report, we described a case of Micra‐VR implantation across the BTV in a patient with repaired congenital heart disease.
2. Case History
A 21‐year‐old man, known case of Pulmonary Valve (PV) Atresia, Large Atrial Septal Defect (ASD), and Patent Ductus Arteriosus (PDA) who underwent pulmonary valvotomy and PDA closure shortly after his birth, presented with exacerbation of dyspnea and peripheral edema. Right heart catheterization and transesophageal echocardiography revealed moderate LV dysfunction (ejection fraction :35%), severe right atrial(RA) enlargement (RA volume index:68CC/m2), moderate right ventricular (RV) enlargement (RV internal diameter = 3.8 cm), moderate to severe RV dysfunction (RV tricuspid annular plane systolic excursion (TAPSE):16 mm, RV S′ by Tissue Doppler Imaging :8 cm/s), severe pulmonary insufficiency (pulmonary pressure half‐time [PHT]:70 milliseconds), severe secondary tricuspid regurgitation (due to large ASD and RV enlargement), and large secundum ASD (size: 1.7 cm × 1.3 cm) with significant bidirectional shunt. He underwent simultaneous bioprosthetic replacement of PV (Perimount 25), tricuspid valve (Magna Ease 31), and ASD closure.
One week after surgery, he became bradycardic, and electrocardiogram showed complete AVB. Considering the persistence of AVB for more than a week, it was decided to implant a permanent pacemaker. As he had undergone recent BTV replacement, insertion of CPM was not preferred (due to increase risk of valve dysfunction and developing infection by CPM) [8]. So, the options were placement of epicardial pacemaker, coronary sinus (CS) lead, or a LLP. As the patient had undergone multiple cardiac surgery with resultant pericardial adhesion, cardiac surgeon refused to implant an epicardial lead. Implantation of a CS lead was impossible due to the absence of proper cardiac vein. Finally, it was decided to implant a LLP (Micra, Medtronic Inc).
3. Methods
The procedure was performed according to the standard technique; first we implanted the LLP in the apicoseptal area, however, electrical measures were not acceptable. Acceptable position was obtained in mid‐RV septum. Electrical measurements showed R wave amplitude of 10 mV, pacing impedance of 830 Ω, and pacing threshold of 1.0 V @ 0.24 ms. Pull and hold test was acceptable. Finally, tether was cut; delivery and introducer sheath were removed; and access site was closed using figure‐of‐eight suture. Patient was transferred to ward with good and stable condition.
Fluoroscopic oblique views were essential for a correct engagement of the tricuspid ring without injuries to the BTV. Left anterior oblique (LAO) view 40° was helpful to visualize the tricuspid annulus as a clock to be crossed exactly in the center. Right anterior oblique (RAO) view 30° was used to establish the correct advancement of the Micra delivery system across the tricuspid valve and to evaluate the proper distance of implantation site from the valve (Figure 1).
FIGURE 1.
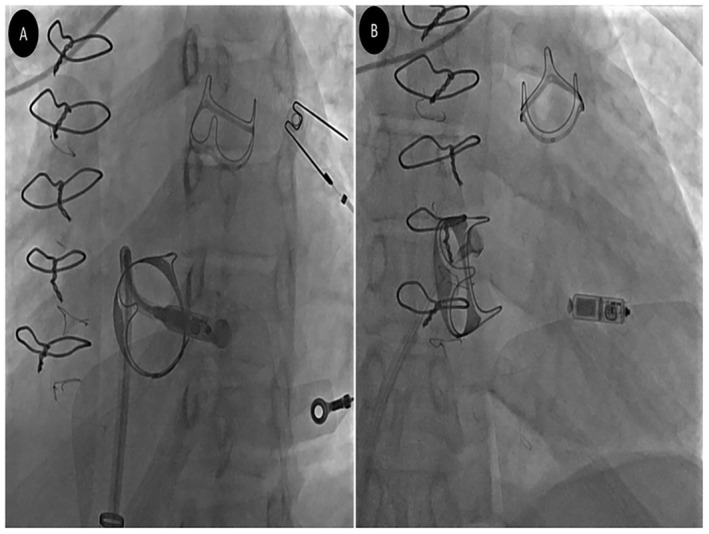
(A) Left anterior oblique (LAO) and (B) right anterior oblique (RAO) views after Micra deployment. LAO view is helpful to visualize the tricuspid annulus as a clock to be crossed exactly in the center and helps to confirm septal orientation of Micra before deployment. RAO view is used to establish the correct advancement of the Micra delivery system across the tricuspid valve and to evaluate the proper distance of implantation site from the valve.
4. Results
The day after the implantation, interrogation of the Micra‐VR revealed satisfactory parameters with a sensed R wave of 11.4 mV, the impedance of 820 Ω, and threshold of 0.63 V @ 0.24 ms. Chest radiography showed proper Micra location in the mid‐RV septum (Figure 2). Transthoracic echocardiography showed no pericardial effusion. During 7‐month follow‐up, the patient was asymptomatic and free of any complications.
FIGURE 2.
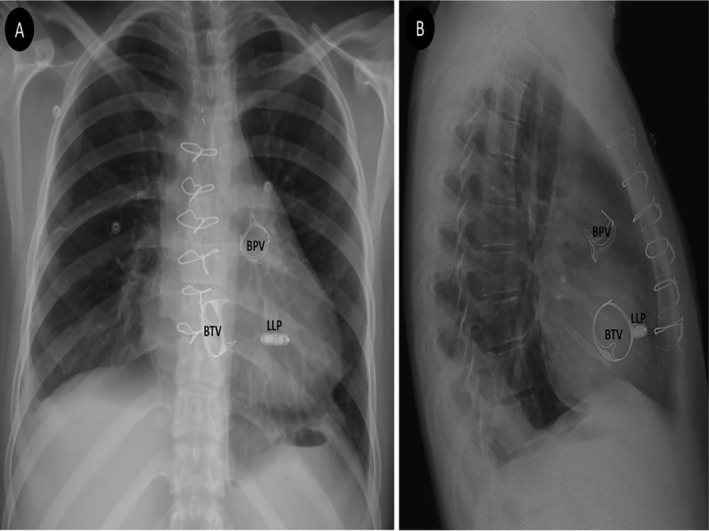
(A) Posteroanterior and (B) lateral chest radiographic views the day after implant. Bioprosthetic tricuspid valve (BTV), bioprosthetic pulmonary valve (BPV), and leadless pacemaker (LLP) are clearly shown in each view.
5. Discussion
In this report, we presented successful Micra implantation through BTV in a patient with repaired congenital heart disease. The procedure was straightforward without any complications. During follow‐up, the patient was asymptomatic and the Micra interrogation showed proper functioning.
Tricuspid valve surgery carries a significant risk of conduction disorders requiring PPM implantation. The implantation rate decreased over time from 13% to 22% before 2000 [13] to 5–11– in the recent years [14]. The PPM implantation after TV surgery involves technical challenges that must be acknowledged by the implanters to select the best technical option in each patient.
Several approaches have been reported: epicardial leads, CPM, His‐bundle pacing, leadless pacing, or coronary sinus leads [15].
-
1
Although epicardial PPMs are proven to provide adequate pacing, the reliability of endocardial leads has been shown to be superior to the epicardial systems [16]. This is especially applicable if patients had previous multiple cardiac surgeries with resultant pericardial adhesion, since surgeons may have a tough time to find a ventricular site with acceptable pacing thresholds.
-
2
CPM can interfere with the function of tricuspid valve, leading to significant morbidity and mortality rates through hemodynamic impairment. The presence of transvenous lead was an independent predictor of tricuspid regurgitation (TR) during follow‐up [17]. Although there is no clear evidence of increased TR after lead implantation in the presence of BTV, most operators prefer to avoid transvenous lead in these patients.
-
3
His‐bundle pacing (HBP) is a more physiologic form of pacing compared to ventricular pacing. This could be an interesting alternative for treating AVBs after TV surgeries, especially as the block site is nodal in most cases. HBP has been described to be feasible in small series (n = 10) of patients after TV repair but none with TV replacement [18]. In these settings, the TV ring may act as a radiographic marker of the his‐bundle and facilitate the implantation.
-
4
Since Cardiac Resynchronization Therapy (CRT) emerged as a cornerstone treatment for advanced heart failure patients, rare data have been published in the literature regarding CS pacing after TV surgery. Only one small series of 17 patients (11 TV repairs and 6 TV replacements) was published [19]. Due to the right atrial dilatation and resulting malposition of the CS ostium, CS catheterization and lead placement may be more challenging in this specific situation compared to typical CRT patients.
-
5
There are currently no large data about the safety and efficacy of leadless pacemakers in patients after TV surgery, current few studies support implanting LLPs in patients with bioprosthetic TV due to less major complications such as lead infection or valve dysfunction [10, 12, 15, 20, 21, 22].
LLP is associated with fewer infectious and lead‐related or pocket‐related complications in compare to CPM [8, 23].
The Micra Transcatheter Pacing Study, a multi‐site, single‐arm clinical trial conducted at nearly 70 centers around the world, assess safety and efficacy of Micra Pacemaker. The study has two primary endpoints: (i) a safety endpoint assessing freedom from major complications and (ii) effectiveness endpoint to evaluate pacing capture thresholds.
According to the results of this trial, Micra patients free from major complications (death, hospitalization, permanent loss of device function, and system revision) are significantly higher than 83% and the percentage of Micra patients with both low and stable thresholds is significantly higher than 80% [20].
Afzal and his colleagues demonstrated on a multicenter experience that implanting Leadless Pacemakers Across Bioprosthetic and Repaired TV is a safe and feasible option without any significant major complications [21].
In another study, a total of 14 patients underwent LLP implantation early after TV surgery. No procedure or device‐related complications happened during or after implantation and the procedure does not affect TV or bioprosthesis function in transthoracic echocardiography. So implantation of an LLP early after TV surgery is a safe option [22].
A retrospective review on complications of LLPs versus CPMs showed that LLPs appear to have a better safety profile than CPMs. There was a low pocket site and lead‐related infections in LLP as compared to CPM. However, LLPs can have twice the risk of pericardial effusion than CPMs, but this was not statistically significant [24]. Thus LLP implantation is an emerging technology validated in clinical studies and real‐world setting with the potential advantage of overcoming some of the limits of the conventional pacing lead such pocket infection. Micra LLP do not need extraction after battery depletion because LLP is endothelialized into ventricle and according to the existing studies, up to 3 LLPs (with battery longevity of 10–12 years) can be placed inside the RV. Therefore, there is no need to remove the previous LLP, and a new one can be implanted into the RV [23], so it prevents further open surgeries and the risk of post operation complications. LLP implantation after BTV might represent an ideal option in this setting by eliminating the risks related to the lead's presence across the bioprosthetic valve, including valve dysfunction and valvular endocarditis [8, 25, 26].
-
6
Thus LLP implantation is an emerging technology validated in clinical studies and real‐world setting with the potential advantage of overcoming some of the limits of the conventional pacing lead such as need for extraction after battery depletion. LLPs overcome this limit and do not need extraction after battery depletion because LLP is endothelialized into ventricle and according to the existing studies, up to 3 LLPs (with battery longevity of 10–12 years) can be placed inside the RV. Therefore, there is no need to remove the previous LLP, and a new one can be implanted into the RV [23], so it prevents further open surgeries and the risk of post operation complications. LLP implantation after BTV might represent an ideal option in this setting by eliminating the risks related to the lead's presence across the bioprosthetic valve, including valve dysfunction and valvular endocarditis [8, 25, 26].
Author Contributions
Majid Haghjoo: conceptualization, data curation, supervision, writing – review and editing. Mahsa Mohammadi: conceptualization, data curation, supervision, writing – original draft, writing – review and editing. Mohammadreza Iranian, Amirfarjam Fazelifar, and Sedigheh Saedi: data curation; writing – review and editing. Yaser Toloueitabar: data curation; writing – original draft, writing – review and editing.
Consent
Written informed consent was obtained from the patient to publish this report in accordance with the patient consent policy of the Clinical Case Reports journal.
Funding: The authors received no specific funding for this work.
Data Availability Statement
The authors have nothing to report.
References
- 1. Romer A. J., Tabbutt S., Etheridge S. P., et al., “Atrioventricular Block After Congenital Heart Surgery: Analysis From the Pediatric Cardiac Critical Care Consortium,” Journal of Thoracic and Cardiovascular Surgery 157, no. 3 (2019): 1168–1177.e2. [DOI] [PubMed] [Google Scholar]
- 2. Del D. F., Nishimura S. C., Lau C. C., Sever J., and Goldman B. S., “Cardiac Pacing Following Surgery for Acquired Heart Disease,” Cardiac Pacing Following Surgery for Acquired Heart Disease 11, no. 5 (1996): 332–340. [DOI] [PubMed] [Google Scholar]
- 3. Ayyildiz P., Kasar T., Ozturk E., et al., “Evaluation of Permanent or Transient Complete Heart Block After Open Heart Surgery for Congenital Heart Disease,” Pacing and Clinical Electrophysiology 39, no. 2 (2015): 160–165. [DOI] [PubMed] [Google Scholar]
- 4. Ashida Y., Ohgi S., Kuroda H., et al., “Permanent Cardiac Pacing Following Surgery for Acquired Valvular Disease,” Annals of Thoracic and Cardiovascular Surgery 6, no. 3 (2000): 161–166. [PubMed] [Google Scholar]
- 5. Guella E.‐H., Devereux F., Ahmed F., Scott P., Cunnington C., and Zaidi A., “Novel Atrioventricular Sequential Pacing Approach Using a Transvenous Atrial Pacemaker and a Leadless Pacemaker: A Case Report,” 5, no. 7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lancellotti P., Gach O., and Marechal P., “Pacemaker Miniature Sans Sonde de Type Micra®,” Revue Médicale de Liège 74, no. S1 (2019): S104–S108. [PubMed] [Google Scholar]
- 7. Fichtner S., Estner H. L., Näbauer M., and Hausleiter J., “Percutaneous Extraction of a Leadless Micra Pacemaker After Dislocation: A Case Report,” European Heart Journal ‐ Case Reports 3, no. 3 (2019): ytz113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. El‐Chami M. F., Bonner M., Holbrook R., et al., “Leadless Pacemakers Reduce Risk of Device‐Related Infection: Review of the Potential Mechanisms,” Heart Rhythm 17, no. 8 (2020): 1393–1397. [DOI] [PubMed] [Google Scholar]
- 9. Bertelli M., Toniolo S., Ziacchi M., et al., “Is Less Always More? A Prospective Two‐Centre Study Addressing Clinical Outcomes in Leadless Versus Transvenous Single‐Chamber Pacemaker Recipients,” Journal of Clinical Medicine 11, no. 20 (2022): 6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kerwin S. A., Mayotte M. J., and Gornick C. C., “Transcatheter Pacemaker Implantation in a Patient With a Bioprosthetic Tricuspid Valve,” Journal of Interventional Cardiac Electrophysiology 44, no. 1 (2015): 89–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boveda S., Durand P., Combes S., and Mariottini C. J., “Leadless Pacemaker Surrounded by Three Valvular Prostheses,” Heart Rhythm 14, no. 9 (2017): 1421. [DOI] [PubMed] [Google Scholar]
- 12. Morani G., Bolzan B., Pepe A., and Ribichini F. L., “Leadless Pacemaker Through Tricuspid Bioprosthetic Valve: Early Experience,” Journal of Arrhythmia 37, no. 2 (2021): 414–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koplan B. A., Stevenson W. G., Epstein L. M., Aranki S. F., and Maisel W. H., “Development and Validation of a Simple Risk Score to Predict the Need for Permanent Pacing After Cardiac Valve Surgery,” Journal of the American College of Cardiology 12, no. 3 (2003): 88. [DOI] [PubMed] [Google Scholar]
- 14. Jokinen J. J., Turpeinen A. K., Pitkänen O., Hippeläinen M. J., and Hartikainen J. E. K., “Pacemaker Therapy After Tricuspid Valve Operations: Implications on Mortality, Morbidity, and Quality of Life,” Annals of Thoracic Surgery 87, no. 6 (2009): 1806–1814. [DOI] [PubMed] [Google Scholar]
- 15. Martins R. P., Galand V., Leclercq C., and Daubert J. C., “Cardiac Electronic Implantable Devices After Tricuspid Valve Surgery,” Heart Rhythm 15, no. 7 (2018): 1081–1088. [DOI] [PubMed] [Google Scholar]
- 16. McLeod C. J., Attenhofer Jost C. H., Warnes C. A., et al., “Epicardial Versus Endocardial Permanent Pacing in Adults With Congenital Heart Disease,” Journal of Interventional Cardiac Electrophysiology 28, no. 3 (2010): 235–243. [DOI] [PubMed] [Google Scholar]
- 17. Mazine A., Bouchard D., Moss E., et al., “Transvalvular Pacemaker Leads Increase the Recurrence of Regurgitation After Tricuspid Valve Repair,” Annals of Thoracic Surgery 96, no. 3 (2013): 816–822. [DOI] [PubMed] [Google Scholar]
- 18. Sharma P. S., Subzposh F. A., Ellenbogen K. A., and Vijayaraman P., “Permanent his‐Bundle Pacing in Patients With Prosthetic Cardiac Valves,” Heart Rhythm 14, no. 1 (2017): 59–64. [DOI] [PubMed] [Google Scholar]
- 19. Sideris S., Drakopoulou M., Oikonomopoulos G., et al., “Left Ventricular Pacing Through Coronary Sinus Is Feasible and Safe for Patients With Prior Tricuspid Valve Intervention: Ventricular Pacing Through Coronary Sinus,” Pacing and Clinical Electrophysiology 39, no. 4 (2016): 378–381. [DOI] [PubMed] [Google Scholar]
- 20. Ritter P., Duray G. Z., Zhang S., et al., “The Rationale and Design of the Micra Transcatheter Pacing Study: Safety and Efficacy of a Novel Miniaturized Pacemaker,” Europace 17, no. 5 (2015): 807–813. [DOI] [PubMed] [Google Scholar]
- 21. Afzal M. R., Daoud E. G., Hussain S., et al., “Multicenter Experience of Feasibility and Safety of Leadless Pacemakers Across Bioprosthetic and Repaired Tricuspid Valves,” JACC Clinical Electrophysiology 5, no. 9 (2019): 1093–1094. [DOI] [PubMed] [Google Scholar]
- 22. Theis C., Huber C., Kaesemann P., et al., “Implantation of Leadless Pacing Systems in Patients Early After Tricuspid Valve Surgery: A Feasible Option,” Pacing and Clinical Electrophysiology 43, no. 12 (2020): 1486–1490. [DOI] [PubMed] [Google Scholar]
- 23. Beurskens N. E., Tjong F. V., and Knops R. E., “End‐Of‐Life Management of Leadless Cardiac Pacemaker Therapy,” Arrhythmia & Electrophysiology Review 6, no. 3 (2017): 129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sattar Y., Ullah W., Roomi S., et al., “Complications of Leadless vs Conventional (Lead) Artificial Pacemakers – A Retrospective Review,” Journal of Community Hospital Internal Medicine Perspectives 10, no. 4 (2020): 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Steinwender C., Blessberger H., Kiblböck D., Saleh K., and Kammler J., “Sondenloser Schrittmacher Micra™: Klinische Erfahrungen und Perspektiven,” Herzschrittmachertherapie & Elektrophysiologie 29, no. 4 (2018): 334–339. [DOI] [PubMed] [Google Scholar]
- 26. Bernardes‐Souza B., Mori S., Hingorany S., Boyle N. G., and Do D. H., “Late‐Onset Infection in a Leadless Pacemaker,” JACC Case Reports 4, no. 24 (2022): 101645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors have nothing to report.


