Abstract
Delays in language often co-occur among toddlers diagnosed with autism. Despite the high prevalence of language delays, the neurobiology underlying such language challenges remains unclear. Prior research has shown reduced EEG power across multiple frequency bands in 3-to-6-month-old infants with an autistic sibling, followed by accelerated increases in power with age. Here we apply new methods to decompose the power spectra into aperiodic (broad band neural firing) and periodic (oscillations) activity to explore possible links between aperiodic changes in the first year of life and later language outcomes. Combining EEG data across two longitudinal studies of infants with and without autistic siblings, we assessed whether infants with an elevated familial likelihood (EFL) exhibit altered changes in both periodic and aperiodic EEG activity at 3 and 12 months of age, compared to those with a low likelihood (LL), and whether developmental change in activity is associated with language development. At 3-months of age, we observed that EFL infants have significantly lower aperiodic activity from 6.7–55Hz (p<0.05). However, change in aperiodic activity from 3 to 12 months was significantly increased in infants with a later diagnosis of autism, compared to EFL infants without an autism diagnosis. In addition, greater increases in aperiodic offset and slope from 3-to12-months were associated with worse language development measured at 18 months. Findings suggest that early age-dependent changes in EEG aperiodic power may serve as potential indicators of autism and language development in infants with family history of autism.
Introduction
Early delays in language have been shown to have long lasting impacts on behavioral functioning, academic success, vocational outcomes and quality of life(Law et al., 2009; Snowling et al., 2006; Young et al., 2002). Language delays are highly prevalent in young children diagnosed with autism. At the time of diagnosis, as many as 40% of autistic toddlers have co-occurring language delays(Reetzke et al., 2022), and the degree of later gains in language development is variable, with 30% of autistic children remaining minimally verbal(Tager-Flusberg & Kasari, 2013). Notably, younger siblings of autistic children are at elevated likelihood for both autism and language delays, suggesting shared underlying biology (Marrus et al., 2018). Despite the high prevalence of language impairments in autism, our understanding of the neurobiology underlying impairments is limited. Pinpointing neural markers that are specifically associated with language delays in infant-siblings may shed light on neural mechanisms of early language development and facilitate earlier detection and intervention.
Electroencephalography (EEG), which measures network level brain activity at the scalp, has proven to be a promising tool for identifying early neural markers of altered development in infancy. In a number of studies resting (baseline) EEG data have been collected longitudinally in infants with an elevated familial likelihood of developing autism (EFL, infants with an older sibling with autism). These studies have observed a global reduction in frontal EEG power across multiple frequency bands at early ages (3–6 months), as well as differences in EEG trajectories from 6 to 24 months in EFL infants regardless of later autism diagnosis (Huberty et al., 2021; Levin et al., 2017; Tierney et al., 2012; Wilkinson et al., 2020). While EEG power is reduced in early infancy in EFL infants, differences are less pronounced at 12 and 24 months, and studies of older autistic children have observed either no differences, or increases in absolute gamma power(Arutiunian et al., 2024; Huberty et al., 2021; Mukerji et al., 2024; Rojas & Wilson, 2014; van Diessen et al., 2015). This suggests that change in power spectral features early in development may be an important biomarker for autism or other developmental delays.
Studies of EEG power and language development have had mixed findings depending on age and likelihood group. In infancy, lower frequencies (delta, theta, and alpha) have been associated with language development in both low likelihood infants (LL, infants without an older sibling with autism) and EFL infants(Levin et al., 2017; Pierce et al., 2021). In contrast, in toddlers (16–36 months) frontal gamma power has been associated with language development (Benasich et al., 2008; Gou et al., 2011; Wilkinson et al., 2019). Interestingly, the direction of association between brain activity and language often differs between EFL versus LL toddlers. Gamma power is positively associated with language in LL toddlers, yet negatively associated in EFL toddlers, suggesting possible differences in factors impacting language development between groups (Lombardo et al., 2015; Swanson et al., 2017; Wilkinson et al., 2019). The increased gamma power in autistic children has been hypothesized to reflect imbalance in neural excitation/inhibition (E/I)(Brunel & Wang, 2003; Buzsáki & Wang, 2012; Economo & White, 2012). Thus, while higher gamma power in typically developing toddlers and preschoolers may be a beneficial indicator of brain activity for language development, in autistic children it may instead serve as a marker of E/I imbalance impeding processes important for language and cognitive development.
While past EEG studies in autism have largely focused on either absolute or relative measures of power, newer methods utilize parametrization of the spectrum into aperiodic and periodic components to provide a more accurate estimate of oscillatory and non-oscillatory activity(Donoghue et al., 2020; Ostlund et al., 2022). The aperiodic signal is defined by the 1/f power law distribution that underlies the absolute power spectra(Gao et al., 2017; Manning et al., 2009; Miller et al., 2009), and can be described by the offset and slope (defined as the χ in the 1/fχ formula). The aperiodic offset is thought to reflect broad band, non-oscillatory neuronal firing(Manning et al., 2009; Miller, 2010), and, growing evidence suggests that the slope of the aperiodic signal may, in part, reflect the excitatory-inhibitory (E/I) balance of the brain, with a flatter slope associated with increased excitation over inhibition(Chini et al., 2022; Gao et al., 2017; McKeon et al., 2024). Together, these components of aperiodic activity may inherently reflect broader neural dynamics such as cortical excitability or developmental maturation. In addition, modulation in aperiodic activity, such as flatter slopes, can influence absolute power in higher frequencies, like gamma. Thus, previous associations between absolute gamma power and language development could instead reflect associations in aperiodic activity and language development. By accounting for individual differences in aperiodic activity, parametrization of the absolute spectrum also provides more accurate measurement of peak frequency and amplitude of oscillatory, or periodic components of the spectra(Buzsáki et al., 2013; Donoghue et al., 2020; Ostlund et al., 2022). Notably, both aperiodic and periodic components change with development, with dynamic changes occurring during the first year after birth(Cellier et al., 2021; Hill et al., 2022; Rayson et al., 2023; Rico-Picó et al., 2023; Wilkinson et al., 2024).
In this study, we aimed to investigate developmental changes in aperiodic and periodic components in EFL vs LL infants and assess how alterations may be associated with later autism and language outcomes. Leveraging data collected across two infant-sibling studies with 3- and 12- month time points, we first assessed whether there are differences in frontal aperiodic and periodic power in EEG data collected in LL and EFL infants at 3 and 12 months of age. Next, we determined whether EFL-ASD infants exhibit distinctive patterns of change in EEG aperiodic or periodic power from 3 to 12 months of age. Finally, we assessed whether developmental changes in EEG features are associated with language development.
Methods
Participants:
This analysis includes infants who were recruited as part of two consecutive studies in the same lab: The Infant Sibling Project (IRB-X06–08-0374), and the Infant Screening Project (IRB-P00018377). Institutional review board (IRB) approval was obtained from Boston Children’s Hospital and IRB protocol numbers are provided for each study. Secondary analysis for this paper was also approved (IRB-P00037531). Written informed consent was obtained from a parent or guardian prior to each child’s participation in the study. Both studies were prospective, longitudinal studies, enrolling infants with and without first degree family history of ASD starting as early as 3-months of age. However, both studies initially started recruiting at later ages, and added the 3-month enrollment option part way through enrollment. The Infant Screening Project also recruited a group of infants with elevated social communication concerns at 12 months of age, but because of the small sample size, they were excluded from this analysis. Exclusion criteria included gestational age less then 36 weeks, history of prenatal or postnatal medical or neurological problems, as well as identified genetic disorders. Sample characteristics are shown in Table 1.
Table 1:
Sample characteristics
| 3-month | 12-month | |||
|---|---|---|---|---|
| LL N = 59 (11, 48) | EFL N = 51 (26, 25) | LL N = 153 (62, 91) | EFL N = 137 (77, 60) | |
| Sex | 36 M, 23 F | 31 M, 29 F | 81 M, 72 F | 76 M, 61 F |
| Ethnicity, n (%) | ||||
| Not Hispanic or Latino | 57 (97) | 42 (82) | 147 (96) | 126 (92) |
| Hispanic or Latino | 2 (3) | 8 (16) | 4 (3) | 11 (8) |
| Not Answered | 0 (0) | 1 (2) | 2 (1) | 0 (0) |
| Race, n (%) | ||||
| White | 50 (85) | 40 (78) | 123 (80) | 122 (89) |
| Black or African American | 1 (2) | 0 (0) | 4 (3) | 1 (1) |
| Asian | 1 (2) | 2 (4) | 6 (4) | 8 (6) |
| Mixed Race | 7 (12) | 8 (16) | 18 (12) | 6 (4) |
| Not answered | 0 (0) | 1 (2) | 2 (1) | 0 (0) |
| Household income, % (n) | ||||
| <$35,000 | 1 (2) | 3 (6) | 7 (4.5) | 3 (2) |
| $35,000 - $75,000 | 2 (3) | 3 (6) | 7 (4.5) | 15(11) |
| >$75,000 | 54 (92) | 39 (76) | 127 (83) | 99 (72) |
| Not answered | 2 (3) | 6 (12) | 12 (8) | 19 (14) |
| EEG quality metrics | ||||
| Number of Segments | 103.2 ± 39.1 | 97.8 ± 37.4 | 89.3 ± 39.0 | 93 ± 53.1 |
| Percent Good Channels | 92.2 ± 0.05 | 92.8 ± 0.05 | 92.4 ± 0.05 | 92.4 ± 0.04 |
| Percent ICs Rejected | 0.34 ± 0.10 | 0.37 ± 0.13 | 0.39 ± 0.10 | 0.37 ± 0.12 |
| Mean Artifact Probability of Kept ICs. | 0.15 ± 0.05 | 0.14 ± 0.04 | 0.12 ± 0.05 | 0.12 ± 0.05 |
Developmental and autism assessments:
Participants in both studies were seen longitudinally for developmental assessments using the Mullen Scales of Early Learning (MSEL). As the Infant Screening Project spanned the COVID-19 pandemic, in person assessments for a number of participants at 24 and 36 months was not possible and remote evaluations took place. Therefore, to maximize the sample size of analyses related to language development, the MSEL Verbal Developmental Quotient at 18 months (available for all in person) was used instead of later ages. For the Infant Sibling Study, ASD outcomes were determined using the Autism Diagnostic Observation Schedule (ADOS) and parent-child interaction administered at 24 and/or 36 months of age. During the COVID-19 pandemic, remote autism evaluations for the Infant Screening Project included the Brief Observation of Symptoms of Autism (BOSA), parent-child interaction, and Vineland Adaptive Behavioral Scales – Third Edition, Parent Interview Form (Vineland-3). In both studies, for toddlers meeting criteria on the ADOS/BOSA or coming within three points of cutoffs, a licensed clinical psychologist reviewed scores and video recordings of concurrent and previous behavioral assessments and using DSM-5 criteria provided a best estimate of clinical judgement.
EEG data collection:
In both studies, resting-state, non-task-related, EEG data were collected while the infant was held by their seated caregiver in a dimly lit, sound attenuated room with low-electrical signal background. In the Infant Sibling Study, a research assistant ensured the infant remained calm by blowing bubbles and/or showing toys. Continuous EEG data was collected using 64-channel Geodesic Sensor (<10% of data) or a 128-channel Hydrocel Geodesic Sensor Nets (Electrical Geodesics, Inc., Eugene, OR), connected to either a NetAmps 200 or 300 amplifier (Electrical Geodesic Inc.) and sampled at either 250 or 500Hz. In the Infant Screening Project study, a video of abstract moving objects was shown and EEG data was collected with the 128-channel Hydrocel Geodesic Sensor Net, connected to a NetAmps 300 amplifier and sampled at 500Hz. For both studies, data were referenced online to a single vertex electrode (Cz). Electrooculographic electrodes were removed to improve the child’s comfort.
EEG pre-processing and rejection criteria:
Raw Netstation (Electrical Geodesics, Inc) files were exported to MATLAB (version R2017a) for preprocessing and absolute power calculations using the Batch Automated Processing Platform (BEAPP(Levin et al., 2018)) with integrated Harvard Automated Preprocessing Pipeline for EEG (HAPPE(Gabard-Durnam et al., 2018)). For each EEG, a 1Hz high-pass and 100Hz low-pass filter were applied, data sampled at 500Hz were resampled to 250Hz, and then run through the HAPPE module consisting of 60Hz line noise removal, bad channel rejection, and artifact removal using combined wavelet-enhanced independent component analysis (ICA) and Multiple Artifact Rejection Algorithm (MARA5,6). The following channels, in addition to the 10–20 electrodes, were used for MARA: 64-channel net – 16, 9, 8, 3, 58, 57, 21, 25, 18, 30, 43, 50, 53, 32, 33, 38, 41, 45; and 128-channel net - 28, 19, 4, 117, 13, 112, 41, 47, 37, 55, 87, 103, 98, 65, 67, 77, 90, 75. After artifact removal, channels removed during bad channel rejection were then interpolated, data were referenced to the average reference, detrended to the signal mean, and segmented into 2-second segments. Any segments with retained artifact were rejected using HAPPE’s amplitude and joint probability criteria. EEG recordings were rejected using the following HAPPE data quality measures: Fewer than 20 segments (40 seconds of total EEG), percent good channels < 80%, percent independent components rejected >80%, mean artifact probability of components kept > 0.3, and percent variance retained < 25%. Table 1 shows quality metrics across LL and EFL groups. There were no significant differences between groups.
EEG Power Spectra Analysis:
For each 2-second segment, the power spectral density at each electrode was calculated in the BEAPP Power Spectral Density (PSD) module using a multitaper spectral analysis(Babadi & Brown, 2014) and three orthogonal tapers. For each electrode, the PSD was averaged across segments, and then averaged across all frontal electrodes [4, 11, 19, 24, 28, 117, 124]. The PSD was then further analyzed using a modified version of SpecParam v1.0.0(Donoghue et al., 2020) (https://github.com/fooof-tools/fooof; in Python v3.6.8; modifications described in (Wilkinson et al., 2024) with available code at osf.io/u3gp4. The SpecParam model was used in the fixed mode (no spectral knee) evaluating spectra between 2–55Hz, with peak_width_limits set to [0.5, 18.0], max_n_peaks = 7, and peak_threshold = 2). Mean R2 for the full sample using this modified version of SpecParam was 0.997 (STD 0.009; range 0.875–0.9998).
Here we define aperiodic offset as aperiodic power at 2.5Hz, as there are high levels of error in the SpecParam estimates at frequencies below 2.5Hz. Aperiodic slope, defined by χ in the 1/fχ model fit, is provided by SpecParam. Total aperiodic activity is defined as the integral of the aperiodic spectra from 2.5–55Hz. To characterize the periodic power spectra, the SpecParam estimated aperiodic signal was subtracted from the absolute power spectrum. Periodic gamma power was calculated using the integral of the periodic spectra from 30–45Hz.
Statistical Analyses:
Group differences in the absolute, aperiodic, and periodic power spectra were assessed using a non-parametric clustering method controlling for multiple comparisons using Monte Carlo estimation with 10,000 permutations(Maris & Oostenveld, 2007), employed with MNE-Python(Gramfort et al., 2014). Group differences in EEG measures were statistically compared using Mann Whitney U, or ANCOVA with sex included as covariate.
Study data were collected and managed using REDCap electronic data capture tools hosted at Boston Children’s Hosptial(Harris et al., 2009, 2019).
Results
Table 1 shows sample characteristics for participants providing EEG data either 3-months or 12-months of age. Fewer EEGs were available at 3 months of age for both studies, as the early time point was not included until part-way through each study. Forty-one LL-NoASD, 16 EFL-ASD, and 16 EFL-NoASD infants had EEG data at both 3 and 12 months.
Power spectra differences at 3 months of age.
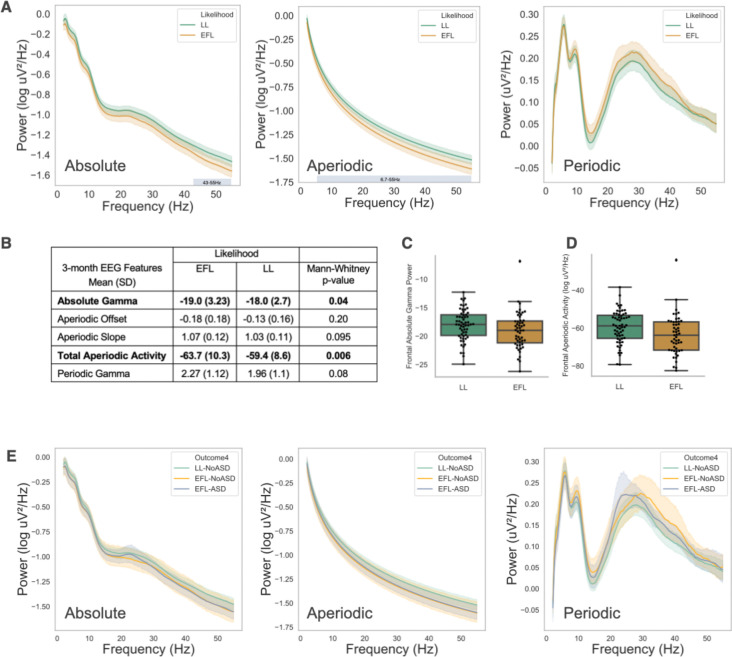
Frontal absolute, aperiodic, and periodic power spectra for LL and EFL infants at 3 months are shown (Figure 1A). We used a non-parametric clustering method, controlling for multiple comparisons, to assess for group differences in the power spectra. Within the 3-month absolute power spectra a significant cluster was identified in the gamma range (43–55Hz, p<0.05). Within the 3-month aperiodic component, a significant cluster spanning alpha through gamma frequencies was identified (6.7–55Hz, p < 0.05). No significant clusters were identified in the 3-month periodic spectra. The combination of these findings suggest that differences observed in absolute gamma power are largely driven by differences in aperiodic activity. Statistically significant group differences were observed for both absolute gamma power and total aperiodic activity, with the EFL group exhibiting reduced activity (Mann Whitney U; Absolute Gamma: U = 1850.0, p < 0.05; Total Aperiodic Activity: U = 1962.0, p < 0.01; Figure 1B, C, D) compared to the LL group. No significant difference was observed for periodic gamma power, aperiodic slope or offset.
Figure 1.
(A) Absolute, Aperiodic, and Periodic spectra at 3 months by Likelihood group. Colored shading around spectra represents 95% confidence intervals. Identified statistically significant clusters are shown as horizontal bars defined by the cluster’s frequency band. (B) Mean comparison of EEG measures by Likelihood group. (C, D) Box plot of group differences in absolute gamma power and total aperiodic activity. (E) Absolute, aperiodic, and periodic spectra at 3 months by Outcome group. Shading around spectra represents 95% confidence intervals.
Next, we assessed whether differences in aperiodic activity at 3 months were driven by those infants later diagnosed with autism. Power spectra from EEG collected at 3 months of age are shown in Figure 1E based on later autism outcome (LL-noASD n = 48, EFL-noASD n = 23, EFL-ASD n = 22). No significant differences were observed between outcome groups on non-parametric clustering methods across the spectrum and no significant differences were observed for specific aperiodic or gamma power measures (ANCOVA with sex included as covariate, Supplemental Table 1)
Power spectra differences at 12 months.
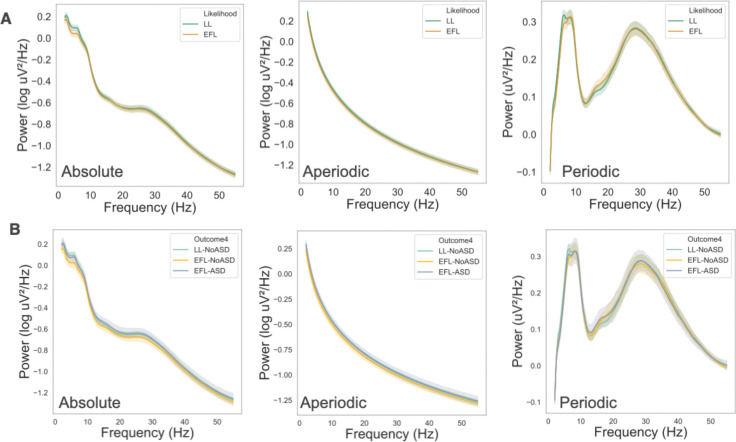
Power spectra for LL and EFL infants at 12 months are shown in Figure 2A, and 12-month spectra group by later autism outcomes are shown in Figure 2B. Unlike at 3 months, there were no significant differences between groups on any measures.
Figure 2.
Absolute, Aperiodic, and Periodic spectra at 12 months groups by (A) Likelihood and (B) Outcome groups. Shading represent 95% confidence intervals.
Change in EEG aperiodic and periodic activity from 3 to 12 months.
Our 3- and 12-month-old findings suggests that EFL infants with significantly lower aperiodic activity early in infancy have greater increases aperiodic activity between 3 and 12 months, leading to similar EEG aperiodic spectra at 12 months. This finding might reflect greater developmental increases in aperiodic activity exhibited by the EFL group, regardless of ASD outcome. Alternatively, it could be primarily driven by more pronounced changes in aperiodic activity among the EFL-ASD infants. To test this, we limited our analysis to those infants with both 3- and 12-month-old data, and confirmed that outcome groups were similar in the number of days between visits. 41 LL-NoASD, 16 EFL-ASD, and 16 EFL-NoASD infants had EEG data at both 3 and 12 months.
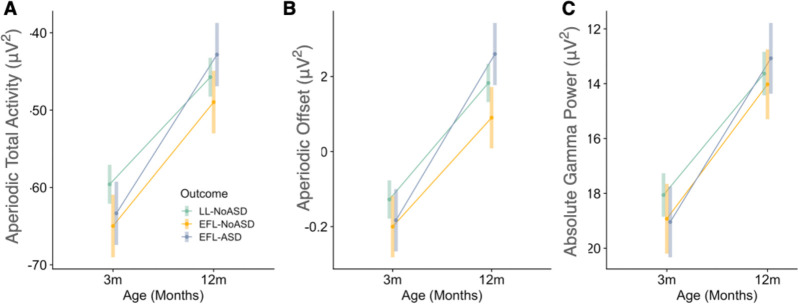
Mixed-effects linear regression models were performed to test whether change in aperiodic features (slope, offset, total aperiodic activity) were different between outcome groups (LL-noASD, EFL-noASD, EFL-ASD). Age (months) was treated as a within-subjects factor and outcome groups were included as between-subjects factor. Sex was included as a covariate in analyses as there were differences in sex distribution between outcome groups (with the EFL-ASD group having higher proportion of males). A priori pairwise comparisons revealed significant outcome group differences in the change in EEG measures from 3 and 12 months (Table 2 and Figure 3; see Supplementary Table 2 for full model outputs). Specifically, EFL-ASD infants exhibited greater change in aperiodic total activity when compared to LL-NoASD infants, and greater change in aperiodic offset when compared to both EFL-noASD and LL-noASD infants. Given prior literature on absolute gamma activity in autism, we also tested whether outcome groups differed in change of absolute gamma power. No significant differences were observed.
Table 2:
A priori pairwise comparisons of EEG changes between outcome groups (3-to-12 months)
| Outcome Comparison | estimate | SE | df | t.ratio | p.value | |
| Aperiodic Total Activity | (EFL-ASD) - (EFL-NoASD) | −4.50 | 3.93 | 70 | −1.147 | 0.255 |
| (EFL-ASD) - (LL-NoASD) | −6.67 | 3.27 | 70 | −2.037 | 0.046 | |
| (EFL-NoASD) - (LL-NoASD) | −2.17 | 3.27 | 70 | −0.661 | 0.511 | |
| Aperiodic Offset | (EFL-ASD) - (EFL-NoASD) | −0.153 | 0.077 | 70 | −1.997 | 0.049 |
| (EFL-ASD) - (LL-NoASD) | −0.133 | 0.064 | 70 | −2.079 | 0.041 | |
| (EFL-NoASD) - (LL-NoASD) | 0.020 | 0.064 | 70 | 0.316 | 0.753 | |
| Absolute Gamma Power | (EFL-ASD) - (EFL-NoASD) | −1.065 | 1.165 | 70 | −0.914 | 0.364 |
| (EFL-ASD) - (LL-NoASD) | −1.539 | 0.971 | 70 | −1.585 | 0.118 | |
| (EFL-NoASD) - (LL-NoASD) | −0.474 | 0.971 | 70 | −0.488 | 0.627 |
Figure 3.
Change in EEG measures from 3 to 12 months. (A) Aperiodic Total Activity, (B) Aperiodic Offset, and (C) Absolute Gamma Power.
Exploratory analysis of EEG features associated with language development.
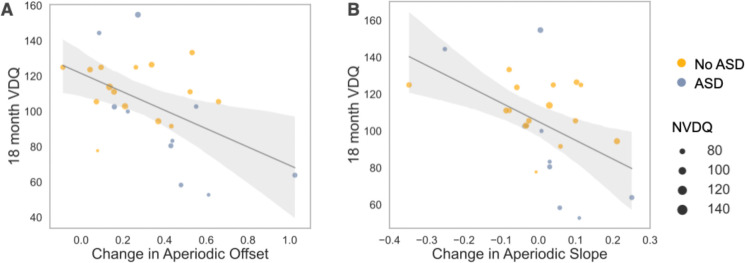
Language and cognitive impairments often co-occur in autistic toddlers, and therefore EEG findings associated with autism outcomes may not be specific to the diagnosis, but rather reflective of developmental delays. In addition, while we have previously found that increased absolute gamma power measured at both 18 and 24 months is associated with reduced language scores in EFL infants, our current analyses, now parametrizing the power spectra into aperiodic and periodic components, suggest that associations between absolute gamma power and language in autism may instead be driven by alterations in underlying aperiodic activity. Given our above observation that increased change in aperiodic offset and total aperiodic activity are associated later ASD diagnosis, we next explored whether change in aperiodic activity is associated with future language impairment across the EFL group (n = 25) when controlling for either autism outcome or nonverbal cognitive ability. Linear regressions were performed with 18-month verbal developmental quotients as the dependent variable, developmental change from 3-to 12-months in aperiodic measures as the independent variable (Model 1), and either 18m nonverbal developmental quotients (Model 2) or ASD diagnosis included as a covariate (Model 3). Results are shown in Table 3. Increased changes in aperiodic offset and slope were significantly associated with reduced 18 month verbal developmental quotients (Figure 4). Notably, associations remained significant when accounting for differences in 18-month nonverbal developmental quotient or for autism diagnosis. No significant associations were observed for aperiodic total activity or absolute gamma power.
Table 3:
Linear regressions assessing associations between developmental change EEG features and 18-month MSEL VDQ
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Adjusted R2 | 0.223 | 0.325 | |
| Variables [B coefficient (SE)] | |||
| Δ Aperiodic Offset | −52.1 (18.5) * | −44.4 (17.7) * | −45.6 (19.8) * |
| 18m NVDQ | 0.71 (0.34) * | ||
| ASD | −9.3 (9.8) | ||
| Adjusted R2 | 0.246 | 0.385 | 0.272 |
| Variables [B coefficient (SE)] | |||
| Δ Aperiodic Slope | −101.6 (37.1) * | −98.9 (32.8) ** | −96.4 (35.8) * |
| 18m NVDQ | 0.85 (0.31) * | ||
| ASD | −15.1 (8.9) | ||
| Adjusted R2 | 0.058 | 0.181 | 0.078 |
| Variables [B coefficient (SE)] | |||
| Δ Aperiodic Total Activity | −0.71 (0.45) | −0.51 (0.43) | −0.50 (0.48) |
| 18m NVDQ | 0.78 (0.37) | ||
| ASD | −13.1 (10.6) | ||
| Adjusted R2 | 0.044 | 0.164 | 0.057 |
| Variables [B coefficient (SE)] | |||
| Δ Absolute Gamma Power | −2.6 (1.8) | −1.66 (1.76) | −1.60 (2.0) |
| 18m NVDQ | 0.78 (0.38) | ||
| ASD | −13.1 (11.3) | ||
p<0.05
p<0.10
p<0.005
Figure 4.
Regression plots from Model 1 comparing (A) change in aperiodic offset and 18-month MSEL verbal developmental quotient (VDQ) and (B) change in aperiodic slope and 18-month MSEL VDQ. Size of dots indicated individual’s MSEL nonverbal developmental quotient (NVDQ). Color represents autism outcome.
Discussion
Leveraging two longitudinal data sets with a prospective infant-sibling design, we compared early developmental changes in EEG power spectra from 3 to 12 months between infants with low and elevated likelihood based on family history of autism. To do this, we both used traditional assessment of absolute EEG power, but also separately analyzed aperiodic and periodic activity. We observe two major findings: First we observe early differences in aperiodic activity in infants with family history of autism regardless of later autism outcome. Second, we observe that greater developmental increases in aperiodic activity are associated with both later autism diagnosis and reduced language development in EFL infants.
Early differences in EEG activity at 3 months associated with autism family history.
At 3-months of age, EFL infants exhibited significantly reduced absolute gamma power as well as overall reduced aperiodic activity compared to LL infants. Interestingly, these differences were not driven by those infants with later diagnoses of autism, suggesting that while early differences in EEG activity may be driven by genetic/familial factors associated with autism, reduced 3-month aperiodic activity alone is not predictive of later diagnosis. We, and others have previously reported reduced EEG power in EFL infants early in infancy(Huberty et al., 2021; Levin et al., 2017; Tierney et al., 2012; Wilkinson et al., 2020), but until now, studies have not examined whether this is due differences in aperiodic or periodic activity. Our observations suggest that prior reported differences in absolute power, could be largely driven by reduced aperiodic activity in EFL infants.
Aperiodic activity represents non-oscillatory, broad band neuronal spiking activity(Manning et al., 2009). The most dramatic increased in aperiodic activity occur in the first months after birth, likely reflecting increases in neuronal number, neuronal connections and synaptogenesis(Wilkinson et al., 2024). In addition, growing evidence suggests that aperiodic slope is an indirect measurement of network excitatory and inhibitory (E/I) balance(Chini et al., 2022; Gao et al., 2017; McKeon et al., 2024). While it is hypothesized that alterations in E/I balance play a role in the development of autism and other neurodevelopmental disorders, we did not observe significant differences in aperiodic slope based on familial likelihood or later outcomes. Thus, reductions in aperiodic activity observed in EFL infants may reflect early hypoconnectivity and/or delays in synaptogenesis.
Change in aperiodic EEG activity associated with later autism diagnosis.
Consistent with previous studies of absolute EEG power, at 12-months, we observed no significant group differences (likelihood or outcome group) for either aperiodic or periodic activity. However, group differences were observed when assessing the change in aperiodic activity from 3- to 12-months of age. Here we found infants with later ASD diagnoses exhibited greater developmental increases in aperiodic total power and offset. These findings are aligned with longitudinal MRI studies of infant-sibling design which find that EFL-ASD infants exhibit increased growth rates in cortical surface area during the first year of life and increased total brain volume growth rates in the second year compared to both EFL-noASD and LL infants(Hazlett et al., 2017).
Again, changes in aperiodic slope were not different between likelihood or outcome groups. However, it is important to note that these findings do not directly conflict with prior literature finding altered E/I balance in children or adults with autism. Our observations are specific to EEG activity across the first year after birth, when networks are being established and some of the most significant changes in aperiodic and periodic activity occur. It is still unclear whether aperiodic slope measured during the first year of life is an accurate proxy for E/I balance.
Greater early developmental change in aperiodic activity associated with reduced language development.
Finally, exploratory analysis found that for EFL infants, increased change in aperiodic offset and slope from 3-to-12months was associated with lower language ability at 18 months. Importantly this association remained significant after accounting for either differences in nonverbal development or autism diagnosis.
This is the first study to examine change in EEG activity between these specific ages. Levin et al 2017, focusing on 3-month EEG data (using an overlapping data set to this paper), showed that increased high alpha power at 3 months was associated with better 12-month expressive language skills. Huberty et al. (2023), specifically investigated whether change in absolute EEG spectral power from 6 to 36 months is associated with language development in infant-siblings. In Huberty et al., significant associations were limited to 6-month alpha power and concurrent language ability, with increased 6-month alpha power associated with better expressive language skills at 6-months, while controlling for concurrent nonverbal skills. In contrast to our findings, there were no significant associations between change in EEG power from 6 to 36 months and later language abilities. Differences in both the age range analyzed, and EEG processing methods (absolute vs aperiodic activity) may explain the difference in findings.
A recent study by Piazza et al 2023(Piazza et al., 2023), evaluated EEG data collected at 6 and 12 month of age in infants with either elevated likelihood for autism or language impairment. They observed reduced absolute power in low frequency bands (delta, theta, low alpha) at both ages in infants with elevated autism likelihood compared to those with low autism likelihood. Evaluation based on later outcomes observed that infants with later autism diagnosis also showed reduced delta and theta power at 6 months, and in theta at 12 months, and infants with later language delays showed increased high frequency power at 12 months of age. However, authors did not specifically assess within subject change in EEG power, and it is possible that the observed differences at 6 and 12 months, reflect greater changes in aperiodic activity in those infants with later autism diagnoses.
Why might greater changes in aperiodic offset and slope be associated with reduced language development in infants with family history of autism? EFL infants as a group also exhibited significantly lower aperiodic activity at 3 months compared to LL infants. While it is still unclear what neurobiological mechanisms underly this reduced activity, it is possible that EFL infants who overcompensate for these early differences have altered processing of sensory information critical for language development. For example, infants undergoing a rapid shift in connectivity or synaptogenesis in response to underlying aberrant circuitry may not make appropriate connections in response to the sensory environment. In addition, early alterations in aperiodic activity maybe impact the development of neural circuits important for rhythmic activity that play a critical role in language processing and therefore language acquisition.
Limitations
While we combined data from two longitudinal studies, increasing our 3- and 12-month samples sizes, fewer than 73 infants provided data at both time points. Larger prospective longitudinal studies starting early in infancy are required to replicate our findings. Our sample was also largely comprised of infants from white families with higher levels of income, reducing generalizability. We have previously observed that lower household income is also associated with reduced EEG absolute power at 2–3 months of age, with greater increases in power from 3 to 9 months(Wilkinson et al., 2023). Larger datasets with greater variability in SES measures are needed to test whether SES factors such as income, parental education, and stress, moderate our observed associations between EEG aperiodic activity and autism or language outcomes.
Conclusions
Leveraging data from two longitudinal infant sibling studies with data collected as early as 3 months of age, we found that EFL infants regardless of later autism diagnosis have reduced aperiodic activity compared to LL infants at 3 months, but not at 12 months of age. Further, EFL infants with later ASD diagnosis exhibited significantly greater increases in aperiodic activity from 3- to 12-months compared those without ASD diagnosis, and change in aperiodic slope and offset were associated with language development at 18 months of age. Given the close association between ASD and language development, larger longitudinal studies that include EEG data in early infancy are needed to further tease apart the complex interactions between brain and behavior associations.
Supplementary Material
Acknowledgements
We thank all the children and families who generously participated in this research. We thank all the research staff involved in participant recruitment, data collection, and database administration. Funding Statement: This research was supported by the National Institutes of Health (R01-DC010290 to C.A.N. and HTF, K23DC07983 and T32MH112510 to C.L.W.)
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Data availability:
Consents obtained from human participants at our institution for the studies described prohibit sharing of identifiable and de-identified individual data without a data use agreement in place. Please contact the corresponding author with data requests.
References
- Arutiunian V., Santhosh M., Neuhaus E., Borland H., Tompkins C., Bernier R. A., Bookheimer S. Y., Dapretto M., Gupta A. R., Jack A., Jeste S., McPartland J. C., Naples A., Van Horn J. D., Pelphrey K. A., & Webb S. J. (2024). The relationship between gamma-band neural oscillations and language skills in youth with Autism Spectrum Disorder and their first-degree relatives. Molecular Autism, 15(1), 19. 10.1186/s13229-024-00598-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babadi B., & Brown E. N. (2014). A review of multitaper spectral analysis. IEEE Transactions on Biomedical Engineering, 61(5), 1555–1564. 10.1109/TBME.2014.2311996 [DOI] [PubMed] [Google Scholar]
- Benasich A. a., Gou Z., Choudhury N., & Harris K. D. (2008). Early cognitive and language skills are linked to resting frontal gamma power across the first 3 years. Behavioural Brain Research, 195, 215–222. 10.1016/j.bbr.2008.08.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunel N., & Wang X.-J. (2003). What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation-inhibition balance. Journal of Neurophysiology, 90(1), 415–430. 10.1152/jn.01095.2002 [DOI] [PubMed] [Google Scholar]
- Buzsáki G., Logothetis N., & Singer W. (2013). Scaling brain size, keeping timing: Evolutionary preservation of brain rhythms. Neuron, 80(3), 751–764. 10.1016/j.neuron.2013.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G., & Wang X.-J. (2012). Mechanisms of Gamma Oscillations. Annual Review of Neuroscience, 35(1), 203–225. 10.1146/annurev-neuro-062111-150444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellier D., Riddle J., Petersen I., & Hwang K. (2021). The development of theta and alpha neural oscillations from ages 3 to 24 years. Developmental Cognitive Neuroscience, 50, 100969. 10.1016/J.DCN.2021.100969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini M., Pfeffer T., & Hanganu-Opatz I. (2022). An increase of inhibition drives the developmental decorrelation of neural activity. eLife, 11, e78811. 10.7554/eLife.78811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue T., Haller M., Peterson E. J., Varma P., Sebastian P., Gao R., Noto T., Lara A. H., Wallis J. D., Knight R. T., Shestyuk A., & Voytek B. (2020). Parameterizing neural power spectra into periodic and aperiodic components. Nature Neuroscience, 23(12), 1655–1665. 10.1038/s41593-020-00744-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economo M. N., & White J. A. (2012). Membrane properties and the balance between excitation and inhibition control gamma-frequency oscillations arising from feedback inhibition. PLoS Computational Biology, 8(1), e1002354. 10.1371/journal.pcbi.1002354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam L. J., Mendez Leal A. S., Wilkinson C. L., & Levin A. R. (2018). The Harvard Automated Processing Pipeline for Electroencephalography (HAPPE): Standardized processing software for developmental and high-artifact data. Frontiers in Neuroscience, 12, 97. 10.3389/FNINS.2018.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R., Peterson E. J., & Voytek B. (2017). Inferring synaptic excitation/inhibition balance from field potentials. NeuroImage, 158, 70–78. 10.1016/j.neuroimage.2017.06.078 [DOI] [PubMed] [Google Scholar]
- Gou Z., Choudhury N., & Benasich A. A. (2011). Resting frontal gamma power at 16, 24 and 36 months predicts individual differences in language and cognition at 4 and 5 years. Behavioural Brain Research, 220(2), 263–270. 10.1016/j.bbr.2011.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramfort A., Luessi M., Larson E., Engemann D. A., Strohmeier D., Brodbeck C., Parkkonen L., & Hämäläinen M. S. (2014). MNE software for processing MEG and EEG data. NeuroImage, 86, 446–460. 10.1016/j.neuroimage.2013.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P. A., Taylor R., Minor B. L., Elliott V., Fernandez M., O’Neal L., McLeod L., Delacqua G., Delacqua F., Kirby J., & Duda S. N. (2019). The REDCap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics, 95, 103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P. A., Taylor R., Thielke R., Payne J., Gonzalez N., & Conde J. G. (2009). Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett H. C., Gu H., Munsell B. C., Kim S. H., Styner M., Wolff J. J., Elison J. T., Swanson M. R., Zhu H., Botteron K. N., Collins D. L., Constantino J. N., Dager S. R., Estes A. M., Evans A. C., Fonov V. S., Gerig G., Kostopoulos P., McKinstry R. C., … Piven J. (2017). Early brain development in infants at high risk for autism spectrum disorder. Nature, 542(7641), 348–351. 10.1038/nature21369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. T., Clark G. M., Bigelow F. J., Lum J. A. G., & Enticott P. G. (2022). Periodic and aperiodic neural activity displays age-dependent changes across early-to-middle childhood. Developmental Cognitive Neuroscience, 54, 101076. 10.1016/J.DCN.2022.101076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberty S., Carter Leno V., van Noordt S. J. R., Bedford R., Pickles A., Desjardins J. A., Webb S. J., & Elsabbagh M. (2021). Association between spectral electroencephalography power and autism risk and diagnosis in early development. Autism Research, 14(7), 1390–1403. 10.1002/aur.2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law J., Rush R., Schoon I., & Parsons S. (2009). Modeling developmental language difficulties from school entry into adulthood: Literacy, mental health, and employment outcomes. Journal of Speech, Language, and Hearing Research: JSLHR, 52(6), 1401–1416. 10.1044/1092-4388(2009/08-0142) [DOI] [PubMed] [Google Scholar]
- Levin A. R., Méndez Leal A. S., Gabard-Durnam L. J., & O’Leary H. M. (2018). BEAPP: The Batch Electroencephalography Automated Processing Platform. Frontiers in Neuroscience, 12, 513. 10.3389/fnins.2018.00513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin A. R., Varcin K. J., O’Leary H. M., Tager-Flusberg H., & Nelson C. A. (2017). EEG power at 3 months in infants at high familial risk for autism. Journal of Neurodevelopmental Disorders, 9(1). 10.1186/s11689-017-9214-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo M. V., Pierce K., Eyler L. T., Carter Barnes C., Ahrens-Barbeau C., Solso S., Campbell K., & Courchesne E. (2015). Different Functional Neural Substrates for Good and Poor Language Outcome in Autism. Neuron, 86(2), 567–577. 10.1016/j.neuron.2015.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning J. R., Jacobs J., Fried I., & Kahana M. J. (2009). Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. Journal of Neuroscience, 29(43), 13613–13620. 10.1523/JNEUROSCI.2041-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E., & Oostenveld R. (2007). Nonparametric statistical testing of EEG-and MEG-data. Journal of Neuroscience Methods, 164, 177–190. 10.1016/j.jneumeth.2007.03.024 [DOI] [PubMed] [Google Scholar]
- Marrus N., Hall L. P., Paterson S. J., Elison J. T., Wolff J. J., Swanson M. R., Parish-Morris J., Eggebrecht A. T., Pruett J. R., Hazlett H. C., Zwaigenbaum L., Dager S., Estes A. M., Schultz R. T., Botteron K. N., Piven J., Constantino J. N., IBIS Network, J. N., & Network, for the I. (2018). Language delay aggregates in toddler siblings of children with autism spectrum disorder. Journal of Neurodevelopmental Disorders, 10(1), 29. 10.1186/s11689-018-9247-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon S. D., Perica M. I., Parr A. C., Calabro F. J., Foran W., Hetherington H., Moon C.-H., & Luna B. (2024). Aperiodic EEG and 7T MRSI evidence for maturation of E/I balance supporting the development of working memory through adolescence. Developmental Cognitive Neuroscience, 66, 101373. 10.1016/j.dcn.2024.101373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. J. (2010). Broadband spectral change: Evidence for a macroscale correlate of population firing rate? Journal of Neuroscience, 30(19), 6477–6479. 10.1523/JNEUROSCI.6401-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. J., Sorensen L. B., Ojemann J. G., & Nijs M. den. (2009). Power-Law Scaling in the Brain Surface Electric Potential. PLOS Computational Biology, 5(12), e1000609. 10.1371/journal.pcbi.1000609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerji C. E., Wilson J. S., Wilkinson C. L., Krol M. A., Nelson C. A., & Tager-Flusberg H. (2024). Resting Frontal Gamma Power is Associated with Both Expressive Language and Non-verbal Cognitive Abilities in Young Autistic Children. Journal of Autism and Developmental Disorders. 10.1007/s10803-024-06308-3 [DOI] [PubMed] [Google Scholar]
- Ostlund B., Donoghue T., Anaya B., Gunther K. E., Karalunas S. L., Voytek B., & Pérez-Edgar K. E. (2022). Spectral parameterization for studying neurodevelopment: How and why. Developmental Cognitive Neuroscience, 54, 101073. 10.1016/j.dcn.2022.101073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza C., Dondena C., Riboldi E. M., Riva V., & Cantiani C. (2023). Baseline EEG in the first year of life: Preliminary insights into the development of autism spectrum disorder and language impairments. iScience, 26(7), 106987. 10.1016/j.isci.2023.106987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce L. J., Reilly E., & Nelson C. A. (2021). Associations Between Maternal Stress, Early Language Behaviors, and Infant Electroencephalography During the First Year of Life. Journal of Child Language, 48(4), 737–764. 10.1017/S0305000920000501 [DOI] [PubMed] [Google Scholar]
- Rayson H., Szul M. J., El-Khoueiry P., Debnath R., Gautier-Martins M., Ferrari P. F., Fox N., & Bonaiuto J. J. (2023). Bursting with Potential: How Sensorimotor Beta Bursts Develop from Infancy to Adulthood. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 43(49), 8487–8503. 10.1523/JNEUROSCI.0886-23.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reetzke R., Singh V., Hong J. S., Holingue C. B., Kalb L. G., Ludwig N. N., Menon D., Pfeiffer D. L., & Landa R. J. (2022). Profiles and correlates of language and social communication differences among young autistic children. Frontiers in Psychology, 13. https://www.frontiersin.org/articles/10.3389/fpsyg.2022.936392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico-Picó J., Moyano S., Conejero Á., Hoyo Á., Ballesteros-Duperón M. Á., & Rueda M. R. (2023). Early development of electrophysiological activity: Contribution of periodic and aperiodic components of the EEG signal. Psychophysiology, 60(11), e14360. 10.1111/psyp.14360 [DOI] [PubMed] [Google Scholar]
- Rojas D. C., & Wilson L. B. (2014). γ-band abnormalities as markers of autism spectrum disorders. Biomarkers in Medicine, 8(3), 353–368. 10.2217/bmm.14.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowling M. J., Bishop D. V. M., Stothard S. E., Chipchase B., & Kaplan C. (2006). Psychosocial outcomes at 15 years of children with a preschool history of speech-language impairment. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 47(8), 759–765. 10.1111/j.1469-7610.2006.01631.x [DOI] [PubMed] [Google Scholar]
- Swanson M. R., Shen M. D., Wolff J. J., Elison J. T., Emerson R. W., Styner M. A., Hazlett H. C., Truong K., Watson L. R., Paterson S., Marrus N., Botteron K. N., Pandey J., Schultz R. T., Dager S. R., Zwaigenbaum L., Estes A. M., Piven J., Piven J., … Gu H. (2017). Subcortical Brain and Behavior Phenotypes Differentiate Infants With Autism Versus Language Delay. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(8), 664–672. 10.1016/j.bpsc.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H., & Kasari C. (2013). Minimally Verbal School-Aged Children with Autism Spectrum Disorder: The Neglected End of the Spectrum. Autism Research, 6(6), 468–478. 10.1002/aur.1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney A. L., Gabard-Durnam L., Vogel-Farley V., Tager-Flusberg H., & Nelson C. A. (2012). Developmental trajectories of resting eeg power: An endophenotype of autism spectrum disorder. PLoS ONE, 7(6). 10.1371/journal.pone.0039127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Diessen E., Senders J., Jansen F. E., Boersma M., & Bruining H. (2015). Increased power of resting-state gamma oscillations in autism spectrum disorder detected by routine electroencephalography. European Archives of Psychiatry and Clinical Neuroscience, 265(6), 537–540. 10.1007/s00406-014-0527-3 [DOI] [PubMed] [Google Scholar]
- Wilkinson C. L., Gabard-Durnam L. J., Kapur K., Tager-Flusberg H., Levin A. R., & Nelson C. A. (2020). Use of Longitudinal EEG Measures in Estimating Language Development in Infants With and Without Familial Risk for Autism Spectrum Disorder. Neurobiology of Language, 1(1), 33–53. 10.1162/nol_a_00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson C. L., Levin A. R., Gabard-Durnam L. J., Tager-Flusberg H., & Nelson C. A. (2019). Reduced frontal gamma power at 24 months is associated with better expressive language in toddlers at risk for autism. Autism Research, aur.2131. 10.1002/aur.2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson C. L., Pierce L. J., Sideridis G., Wade M., & Nelson C. A. (2023). Associations between EEG trajectories, family income, and cognitive abilities over the first two years of life. Developmental Cognitive Neuroscience, 61, 101260. 10.1016/j.dcn.2023.101260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson C. L., Yankowitz L. D., Chao J. Y., Gutiérrez R., Rhoades J. L., Shinnar S., Purdon P. L., & Nelson C. A. (2024). Developmental trajectories of EEG aperiodic and periodic components in children 2–44 months of age. Nature Communications, 15(1), 5788. 10.1038/s41467-024-50204-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A. R., Beitchman J. H., Johnson C., Douglas L., Atkinson L., Escobar M., & Wilson B. (2002). Young adult academic outcomes in a longitudinal sample of early identified language impaired and control children. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 43(5), 635–645. 10.1111/1469-7610.00052 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Consents obtained from human participants at our institution for the studies described prohibit sharing of identifiable and de-identified individual data without a data use agreement in place. Please contact the corresponding author with data requests.