Abstract
Background:
This series investigates the efficacy of regenerative endodontic therapy (RET) using various platelet-rich fibrin (PRF) formulations in treating apical periodontitis and necrotic pulp in immature permanent teeth.
Aim:
This study aims to evaluate the effectiveness of different PRF formulations in RET.
Materials and Methods:
Three cases involving patients aged 15–16 with immature teeth and necrotic pulp were treated with RET using PRF, injectable PRF, and advanced PRF. The procedure included inducing bleeding with a Hedstrom file, applying PRF variants, and sealing with mineral trioxide aggregate and composite resin. Patients were followed up at 1, 3, 6, 12 and 18 months.
Results:
Radiographic evidence showed apical closure, root maturation, and healing of periapical tissues in all cases. However, pulp sensibility tests were negative.
Conclusion:
RET using PRF variants promotes root development and apical healing in immature teeth. Further research with larger sample sizes is needed to understand these therapies’ potential and limitations.
Keywords: Advanced platelet-rich fibrin, injectable platelet-rich fibrin, platelet-rich fibrin, regenerative endodontic therapy
INTRODUCTION
Apexification using calcium hydroxide or mineral trioxide aggregate (MTA) barrier has been used for apical periodontitis and necrotic pulp in immature permanent teeth. Calcium hydroxide requires multiple appointments and increases the risk of root fracture, while an MTA plug shortens treatment time with similar outcomes. However, apexification does not revive the tooth or promote root maturation and apical closure.[1]
In 2001, endodontic revascularization was introduced to address chronic apical periodontitis in young secondary teeth. Regenerative endodontic therapy (RET) involved employing tissue engineering, stem cells, biomimetic scaffolds, and bioactive growth factors within the root canal space. The American Association of Endodontists officially recognized and accepted this method in 2007.[2]
This case report uniquely compares the use of platelet-rich fibrin (PRF), injectable PRF (i-PRF), and advanced PRF (A-PRF) in RET for immature permanent teeth with necrotic pulp, providing a comparative analysis of their efficacy.
MATERIALS AND METHODS
This case report has been written according to CARE 2013 guidelines for case reports.
CASE REPORTS
Case report 1
A 16-year-old male patient in good health reported to the department of conservative dentistry and endodontics with the complaint of discolored front teeth of the upper jaw. The patient described an impact trauma 7 years prior, leading to a fracture of the coronal part of both the upper central incisors. Clinical evaluation showed fractured crowns with respect to #11 and #21 without abnormal tooth mobility and sinus tract. With the exception of these two teeth, every tooth in the maxillary arch responded to the Endo-Frost cold test. The radiograph showed a periapical radiolucency along with immature roots in teeth #11 and #21 which was diagnosed as asymptomatic apical periodontitis secondary to pulp necrosis.
The individual undergoing treatment and their legal guardian were fully informed about the procedure and of alternative options before obtaining their informed consent.[3] After access cavity preparation, double antibiotic paste (DAP) was placed as an intracanal medicament [Figure 1].
Figure 1.
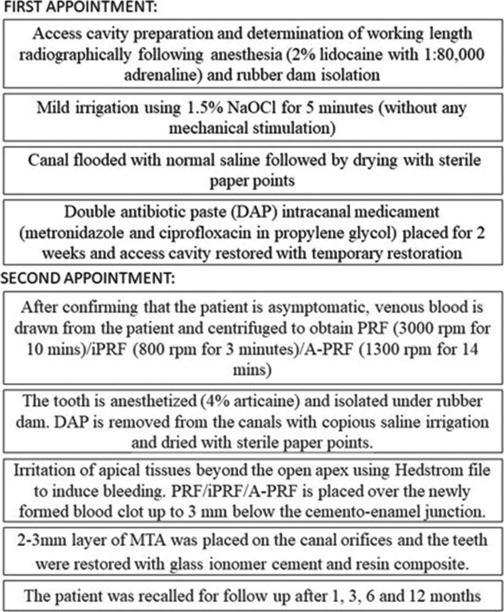
Detailed flowchart of procedure followed
During the second appointment, irritation of apical tissues beyond the apex was performed using #30 Hedstrom file to induce bleeding. PRF (venous blood centrifuged for 10 min at 3000 rpm)[4] was placed in #11 and #21 after which 2–3 mm MTA was placed on both the canal orifices and the teeth were restored with glass ionomer cement (GIC) and resin composite. The patient was recalled for follow-up after 1, 3, 6, 12 and 18 months [Figure 2a-i].
Figure 2.
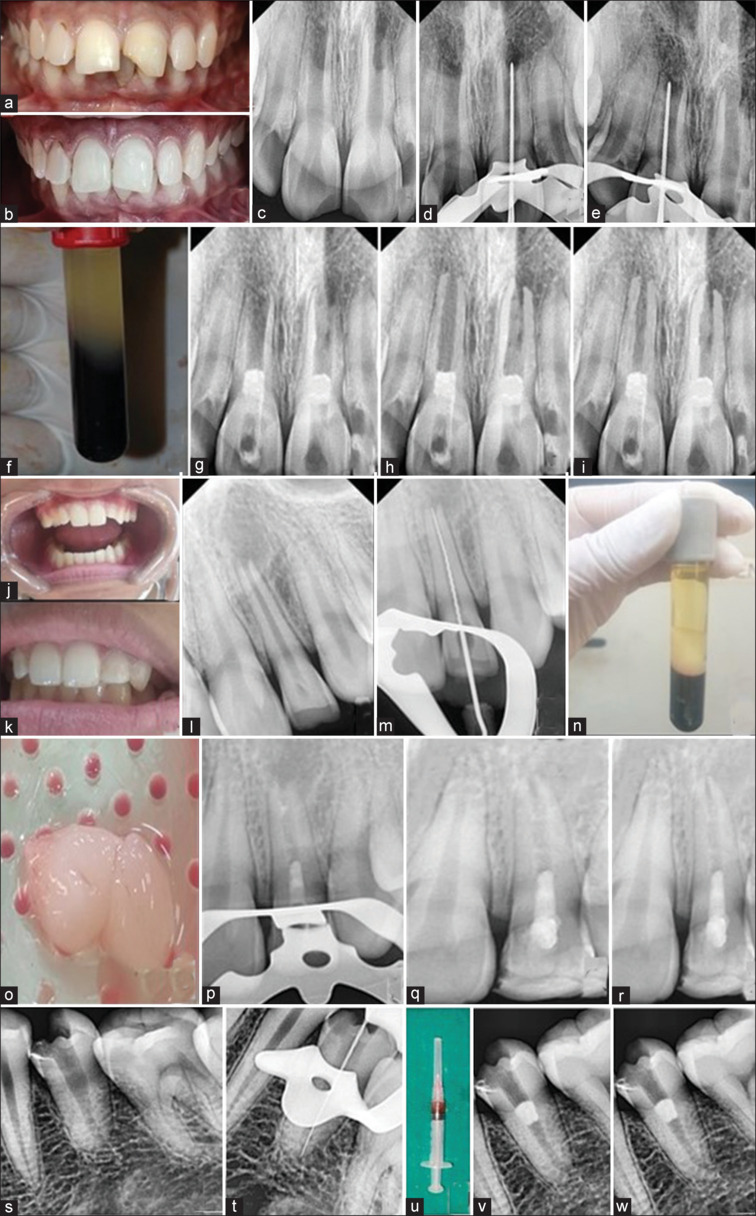
(a) Preoperative clinical picture of case 1 (b) Immediate postoperative clinical picture (c) Preoperative RVG showing immature apices wrt 11 and 21 (d and e) Radiographic working length determination with respect to 11 and 21 (f) platelet-rich fibrin (PRF) prepared using patient’s blood (g) RVG plug showing mineral trioxide aggregate (MTA) plug after inducing periapical bleeding and placement of PRF (h) 12-month follow-up (i) 18-month follow-up (j) Preoperative clinical picture Case 2 (k) Immediate postoperative clinical picture (l) Preoperative RVG showing open apex with respect to 22 (m) Radiographic determination of working length (n and o) A-PRF made from the patient blood (p) MTA placement (q) 12-month follow-up (r) 18-month follow-up (s) Preoperative RVG showing open apex with respect to 35 (t) Radiographic working length determination (u) injectable PRF made from patient blood (v) MTA placement (w) 12-month follow-up
Case report 2
A 15-year-old healthy female patient reported to the department of conservative dentistry and endodontics with the complaint of fractured maxillary left lateral incisor #22 due to associated trauma 6 years ago and wanted to get the tooth restored. Clinical evaluation showed fractured clinical crown with respect to #22 with no sinus tract, abnormal mobility, or tenderness on percussion and gave a negative response when subjected to the Endo-Frost cold test. Through radiographic examination, a diagnosis of an immature apex secondary to pulp necrosis with respect to #22 was made. Treatment options including apexification and RET were comprehensively elucidated to the parents and the patient and RET was initiated after obtaining an informed consent.
The steps followed were the same as shown in Figure 1. Irritation of apical tissues beyond the apex was performed using #30 Hedstrom file to induce bleeding into the canal. The A-PRF clot (venous blood centrifuged at 1300 rpm for 14 min)[5] was removed from the test tube and compressed using a sterile gauge to remove any excess serum. This A-PRF was positioned atop the recently formed blood clot and the orifice was sealed using MTA followed by GIC and composite resin. The patient was kept on follow-up for 1, 3, 6, 12 and 18 months [Figure 2j-r].
Case report 3
A 15-year-old healthy female patient reported to the department of conservative dentistry and endodontics with a chief complaint of blackish discoloration in the lower left back region of the mouth. Clinical evaluation revealed mesio-occlusal caries with respect to #34 with no tenderness on percussion, abnormal mobility, and sinus tract. Radiographic evaluation showed caries along with incomplete root formation. Diagnosis of pulp necrosis was made. After obtaining informed consent, RET was initiated on the tooth. The steps followed were the same as described in Figure 1. In the second appointment, irritation of apical tissues beyond the apex was performed using a Hedstrom file to induce bleeding into the canal. i-PRF (venous blood centrifuged at 800 rpm for 3 min)[6] was obtained from the test tube in a syringe and placed over the recently formed blood clot in a drop wise manner. The orifice was sealed using MTA followed by GIC restoration. The patient was kept on follow-up for 1, 3, 6, and 12 months [Figure 2s-w].
RESULTS
Throughout the course of 12-18 months, radiographic evidence of apical closure, root maturation, and healing of periapical tissues was seen in all three cases. However, when pulp sensibility was tested, the teeth did not show any response.
The objectives of RET are symptomatic relief along with the expression of bone repair (primary), enhanced root width and/or length (secondary) and positive vitality test (tertiary), which, if attained, may point to a better-organized vital pulp tissue.[7] In the above cases, the treatment has been deemed successful since both the primary and secondary goals have been achieved.
DISCUSSION
Scaffolds are crucial to RET as they orchestrate stimulation of stem cells to relocate and multiply. The method of revascularization involving a blood clot is most often utilized. Various investigations on mature necrotic teeth have indicated that blood clot revascularization did not lead to any positive reaction in pulp sensibility after 8–26 months.[8] Research on animals revealed that platelet concentrates create bone-like tissue rather than pulp-like tissue within the root canal. In the absence of histopathological proof, the radiographic widening of the canals as well as continuing root development following RET should not be interpreted as the regeneration of their dentin-pulp system.[9]
Despite the advantages of triple antibiotic paste, it stains teeth due to minocycline, damages stem cells, reduces the micro-hardness of roots, and demineralizes dentin.[10] Thus, DAP consisting of metronidazole along with ciprofloxacin (1:1) was applied in the above cases.
PRF is an autologous biomaterial favored for its minimal blood handling and avoidance of exogenous thrombin, with polymerization occurring naturally. The biomechanical properties of the PRF membrane, notable for its flexibility and elasticity, complement its cost-effectiveness and the simplicity of its single-centrifugation preparation protocol. PRF possesses a significant amount of growth factors which are released up to 7 days or more that promote stem cell migration, proliferation, as well as differentiation.[11]
With the evolution of i-PRF prompt segregation of blood components during centrifugation is possible. It is easier to inject directly into areas of interest, convenient to handle and can be prepared quickly. The liquid consistency may facilitate faster absorption and action of growth factors, potentially leading to quicker therapeutic results in some cases. Comparative analysis of growth factor release has revealed that i-PRF facilitates an expedited release of growth factors, while PRF maintains a sustained release profile, with notable persistence of platelet-derived growth factor – AA, platelet-derived growth factor – AB, epidermal growth factor, insulin-like growth factor-1 up to 10 days.[12]
Compared to L-PRF, A-PRF is superior in terms of the total viable cell population. The augmented presence of these immune cells is posited to influence macrophage differentiation and maturation, which in turn could significantly contribute to bone and soft tissue regeneration, predominantly through the macrophage-mediated liberation of growth factors.[13] In addition, the overall quantum of growth factors discharged was substantially elevated in A-PRF in comparison to L-PRF.[14]
Research has shown that A-PRF serves as a scaffold, a reservoir and demonstrated superior increase in root thickness while PRF showed better increase in root length.[15] All patients reported satisfaction with the treatment outcomes and appreciated the minimally invasive regenerative procedures.
The study’s comparison of PRF, i-PRF, and A-PRF in RET offers valuable insights into their efficacy. The 12-month follow-up enhances the findings’ reliability, and detailed procedural descriptions provide practical guidance for clinicians.
The small sample size limits the findings’ generalizability, and the absence of histological analysis prevents confirmation of the regenerated tissue’s nature. Negative pulp sensibility tests suggest incomplete functional regeneration, and potential biases in a case series design could affect the results.
CONCLUSION
Currently, RET allows tissue generation that resembles bone within the canal rather than actual pulpal tissue; however, further investigation into stem cell-based pulp bioengineering could provide complete regeneration and better treatment results. This case report demonstrates that RET using PRF, i-PRF, and A-PRF is effective in promoting apical closure, root maturation, and healing in immature teeth with necrotic pulp. While structural outcomes are positive, the negative pulp sensibility tests indicate that complete functional regeneration may not be achieved and thus further research including larger-scale and long-term studies are needed.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Conflicts of interest
There are no conflicts of interest.
Funding Statement
Nil.
REFERENCES
- 1.Abdellatif D, Iandolo A, De Benedetto G, Giordano F, Mancino D, Euvrard E, et al. Pulp regeneration treatment using different bioactive materials in permanent teeth of pediatric subjects. J Conserv Dent Endod. 2024;27:458–84. doi: 10.4103/JCDE.JCDE_140_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: A review of current status and a call for action. J Endod. 2007;33:377–90. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 3.American Association of Endodontists AAE Clinical Considerations for a Regenerative Procedure. Available from: https://www.aae.org/specialty/wp-content/uploads/sites/2/2017/06/currentregenerative endodonticconsiderations.pdf . [Last accessed on 2016 Dec 02] [Google Scholar]
- 4.Pavlovic V, Ciric M, Jovanovic V, Trandafilovic M, Stojanovic P. Platelet-rich fibrin: Basics of biological actions and protocol modifications. Open Med (Wars) 2021;16:446–54. doi: 10.1515/med-2021-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujioka-Kobayashi M, Miron RJ, Hernandez M, Kandalam U, Zhang Y, Choukroun J. Optimized platelet-rich fibrin with the low-speed concept: Growth factor release, biocompatibility, and cellular response. J Periodontol. 2017;88:112–21. doi: 10.1902/jop.2016.160443. [DOI] [PubMed] [Google Scholar]
- 6.Mourão CF, Valiense H, Melo ER, Mourão NB, Maia MD. Obtention of injectable platelets rich-fibrin (i-PRF) and its polymerization with bone graft: Technical note. Rev Col Bras Cir. 2015;42:421–3. doi: 10.1590/0100-69912015006013. [DOI] [PubMed] [Google Scholar]
- 7.Nangia D, Saini A, Sharma S, Kumar V, Chawla A, Perumal V, et al. Treatment outcome of regenerative endodontic procedures in mature permanent teeth compared to nonsurgical endodontic treatment: A systematic review and meta-analysis. J Conserv Dent. 2021;24:530–8. doi: 10.4103/jcd.jcd_535_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nageh M, Ahmed GM, El-Baz AA. Assessment of regaining pulp sensibility in mature necrotic teeth using a modified revascularization technique with platelet-rich fibrin: A clinical study. J Endod. 2018;44:1526–33. doi: 10.1016/j.joen.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Lin LM, Kahler B. A review of regenerative endodontics: Current protocols and future directions. J Istanb Univ Fac Dent. 2017;51:S41–51. doi: 10.17096/jiufd.53911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vijayaraghavan R, Mathian VM, Sundaram AM, Karunakaran R, Vinodh S. Triple antibiotic paste in root canal therapy. J Pharm Bioallied Sci. 2012;4:S230–3. doi: 10.4103/0975-7406.100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saluja H, Dehane V, Mahindra U. Platelet-rich fibrin: A second generation platelet concentrate and a new friend of oral and maxillofacial surgeons. Ann Maxillofac Surg. 2011;1:53–7. doi: 10.4103/2231-0746.83158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miron RJ, Fujioka-Kobayashi M, Hernandez M, Kandalam U, Zhang Y, Ghanaati S, et al. Injectable platelet rich fibrin (i-PRF): Opportunities in regenerative dentistry? Clin Oral Investig. 2017;21:2619–27. doi: 10.1007/s00784-017-2063-9. [DOI] [PubMed] [Google Scholar]
- 13.Ghanaati S, Booms P, Orlowska A, Kubesch A, Lorenz J, Rutkowski J, et al. Advanced platelet-rich fibrin: A new concept for cell-based tissue engineering by means of inflammatory cells. J Oral Implantol. 2014;40:679–89. doi: 10.1563/aaid-joi-D-14-00138. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi E, Flückiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, et al. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig. 2016;20:2353–60. doi: 10.1007/s00784-016-1719-1. [DOI] [PubMed] [Google Scholar]
- 15.Jayadevan V, Gehlot PM, Manjunath V, Madhunapantula SV, Lakshmikanth JS. A comparative evaluation of Advanced platelet-rich fibrin (A-PRF) and Platelet-rich fibrin (PRF) as a scaffold in regenerative endodontic treatment of traumatized immature non-vital permanent anterior teeth: A prospective clinical study. J Clin Exp Dent. 2021;13:e463–72. doi: 10.4317/jced.57902. [DOI] [PMC free article] [PubMed] [Google Scholar]


