Abstract
Goals:
We assessed satisfaction with and adherence to off-label corticosteroids in patients with eosinophilic esophagitis (EoE) in the United States.
Background:
EoE is a chronic inflammatory disease for which there are currently no US Food and Drug Administration-approved swallowed topical corticosteroids.
Study:
This noninterventional, cross-sectional, web-based survey included caregivers of adolescents (aged 11 to 17 y) and adults (aged 18 years or older) with a self-reported [or caregiver-reported (adolescents)] physician diagnosis of EoE who were receiving corticosteroids. Participants were recruited through 2 nonprofit, patient advocacy groups. The 9-item Treatment Satisfaction Questionnaire for Medication (TSQM-9) was used to assess satisfaction across effectiveness, convenience, and global satisfaction domains (scale: 1 to 100 per domain); higher scores indicated greater satisfaction. The 4-item Morisky Green Levine Medication Adherence Scale (MGL-4) was used to assess adherence; an MGL-4 score of <3 indicated adherence. Participants also reported reasons for nonadherence.
Results:
Overall, 201 participants (caregivers of adolescents, n=98; adults, n=103) were included in this study. Mean TSQM-9 scores indicated low satisfaction with off-label corticosteroids across all 3 satisfaction domains in adolescents (≤61.1) and adults (≤55.7). Slightly fewer adolescents (37.1%) than adults (40.8%) were considered adherent. Forgetfulness was the most frequently reported reason for nonadherence; some patients chose not to take their medications, owing to poor palatability (adolescents), difficulty taking medications at specific times (adults), or feeling depressed/overwhelmed (adolescents and adults).
Conclusions:
Satisfaction with and adherence to off-label corticosteroids were low in this web-based survey of adolescents and adults with EoE in the United States.
Key Words: eosinophilic esophagitis, corticosteroids, satisfaction, adherence, off-label, TSQM-9, MGL-4
Eosinophilic esophagitis (EoE) is an immune-mediated clinicopathologic disease, characterized by eosinophilic infiltration of the esophagus, which can lead to chronic esophageal inflammation and stricture formation.1 A range of risk factors have been associated with the development of EoE in both children and adults.1 EoE is also associated with various comorbidities and can manifest with a variety of signs and symptoms that may vary with age.1 It has been identified as one of the most common causes of feeding difficulties in children, and of dysphagia or food impaction in adults.2
A greater duration of untreated EoE increases the risk of structural alterations to the esophagus in most patients.3 These alterations can lead to esophageal remodeling, increasing the risk of mucosal tears and food impaction.4 Management strategies for EoE typically include use of proton pump inhibitors (PPIs) (which are used off-label), swallowed topical corticosteroids, and dietary modification.5 Current US Food and Drug Administration (FDA)-approved treatments for EoE are swallowed topical corticosteroid budesonide oral suspension (BOS; 12-week use in patients aged 11 years and older)6 and biologic dupilumab (patients aged 1 year and older).7 Endoscopic dilation may also be used, particularly in patients with strictures who are refractory to medical therapy.8
Swallowed topical corticosteroids have been shown to improve histologic, symptomatic, and endoscopic outcomes in patients with EoE.9–12 Furthermore, swallowed topical corticosteroids are recommended over no treatment in guidelines for the management of EoE from the American Gastroenterological Association and the Joint Task Force on Allergy-Immunology Practice Parameters.13 One swallowed topical corticosteroid (budesonide orodispersible tablet) has been approved for the treatment of EoE in Europe, Canada, and Australia14; however, a US FDA-approved swallowed topical corticosteroid was lacking at the time this study was conducted.6,15 Corticosteroids were thus often used off-label and in the case of budesonide, required patients to home-mix or obtain formulations compounded at specialty pharmacies.16 In addition, off-label medications are often not covered by insurance providers, resulting in high out-of-pocket expenses for patients.17,18 A further concern is that long-term treatment is frequently required for EoE because many patients experience relapse upon treatment withdrawal.9,19
Patient satisfaction is an important outcome measure when considering management strategies for EoE.20 Satisfaction with treatment has been linked to increased adherence and thus improved outcomes for patients, including more successful symptom management and increased health-related quality of life, as well as decreased health care resource utilization and overall costs.21
To date, few studies have assessed patient satisfaction with20,22,23 and adherence to24–26 off-label corticosteroids for EoE. In addition, the results of previous studies appear to vary depending on the heterogeneity of the sample population and the survey instruments and methodology used. We therefore aimed to assess satisfaction with and adherence to off-label corticosteroids using validated and generic survey instruments27,28 in a broad representation of adolescents and adults with EoE in the United States.
MATERIALS AND METHODS
Study Design and Participants
This was a noninterventional, cross-sectional, web-based survey that was conducted in the United States in caregivers of adolescents (aged 11 to 17 y) and in adults (aged 18 years or older) with a self-reported [or caregiver-reported (adolescents)] physician diagnosis of EoE. Participants were identified and recruited through 2 nonprofit, patient advocacy groups: the American Partnership for Eosinophilic Disorders (APFED), using a direct e-mail, an e-newsletter, or the APFED website; and the Campaign Urging Research for Eosinophilic Disease (CURED), using a direct e-mail or the CURED private Facebook group. There was a maximum sample size of 300 completed surveys, with minimum targets of at least 50 surveys completed by caregivers of adolescents with EoE and at least 100 surveys completed by adults with EoE. The planned data collection period was 8 weeks; however, this was extended to 13 weeks for APFED and to 15 weeks for CURED, owing to recruitment challenges. Participants were recruited and data were collected from APFED between July 29, 2020 and October 30, 2020, and from CURED between November 17, 2020 and February 28, 2021.
Inclusion Criteria
Eligibility for the study was confirmed during the initial web-based screening process, and those who agreed to participate were required to provide electronic informed consent before completing the survey. Caregivers of adolescents aged 11 to 17 years and adults with EoE, herein referred to as “participants” were aged 18 years or older and were residents in the United States. Eligibility for the study required a caregiver-reported (for adolescents) or self-reported (for adults) physician diagnosis of EoE, as well as current use of any liquid-based, aerosol-based, or tablet-based corticosteroid formulation for their EoE; this included all marketed corticosteroid inhalers and nasal sprays. Participants were required to be able to understand and provide consent for their participation and to be able to read and respond to an online survey in English.
Participant-Reported Outcomes
Survey items were formulated using standard survey methodologies and included items related to participant demographics, medical history, current treatments, and satisfaction with and adherence to off-label corticosteroids for the treatment of EoE. Minor changes to the wording of some questions in the survey were made to apply to a caregiver’s perspective, and permission was obtained to apply these word changes, with consent from the authors of the survey, where applicable.
Satisfaction With Off-Label Corticosteroid Treatments
The 9-item Treatment Satisfaction Questionnaire for Medication (TSQM-9) is a general measure of patient satisfaction with medication that has been validated for use in adults.27,29,30 In this study, the TSQM-9 (version 1.4) was used to assess satisfaction with off-label corticosteroids used for the treatment of EoE over the past 2 to 3 weeks.31 Three domains of patient satisfaction were assessed: effectiveness (items 1 to 3), convenience (items 4 to 6), and global satisfaction (items 7 to 9). Scores for each of these domains ranged from 0 to 100, with higher scores indicating greater satisfaction with treatment. Items included in each of these domains and further details of how scores were calculated are provided in Supplementary Table 1 (Supplemental Digital Content 1, http://links.lww.com/JCG/B79).31
Adherence to Off-Label Corticosteroid Treatments
The 4-item Morisky Green Levine Medication Adherence Scale (MGL-4) is a generic, self-reported measure that assesses adherence to medication.28,32 In this study, the MGL-4 was used to assess adherence to off-label corticosteroids over the past 7 days.28 For each of the 4 questions, participants’ responses were captured using a 5-point Likert scale, with response options scored from 0 to 4, where never=0, rarely=1, sometimes=2, often=3, and always=4.28 Total scores were calculated as the sum of the individual scores reported by participants and ranged from 0 to 16, with lower scores indicating greater adherence to treatment. Patients who had a total MGL-4 score of <3 were considered adherent to treatment.28 Items included in this scale and further details of how scores were calculated are provided in Supplementary Table 2 (Supplemental Digital Content 1, http://links.lww.com/JCG/B79).28
Participants were additionally asked whether they forgot or chose not to take their medication for EoE in the past 7 days and were asked to report the number of days that they missed their medication. Adolescents (caregiver-reported) and adults with EoE who chose not to take their medication were asked to report the reasons for doing so.
Statistical Analysis
Descriptive statistics were used to analyze the data; hypothesis testing was not performed. All analyses were performed using SAS software (version 9.4 or higher; SAS Institute Inc., Cary, NC). Patient satisfaction with treatment was reported as the mean of the TSQM-9 scores for each of the satisfaction domains. Adherence to treatment was reported as the proportion of patients who had a total MGL-4 score of <3 and who were therefore considered adherent.28
RESULTS
Participant Demographics
The details of participant enrollment for this study are presented in Supplementary Figure 1 (Supplemental Digital Content 1, http://links.lww.com/JCG/B79). Overall, 201 participants [caregivers of adolescents (n=98) and adults (n=103)] completed the survey. Participant demographics are presented in Table 1. The mean (SD) age of adolescents was 13.4 (2.0) years, and most were male (66.3%). For adults, the mean (SD) age was 36.9 (10.2) years, and most were female (72.8%). Adolescents and adults, respectively, were African American or Black (6.1% and 1.0%), American Indian (3.1% and 1.9%), Asian or Pacific Islander (1.0% and 0.0%), White (90.8% and 94.2%), mixed race (2 or more races) (7.1% and 2.9%), and other (races not included) (1.0% and 1.0%).
TABLE 1.
Demographics of Adolescents (Caregiver-Reported) and Adults (Self-Reported) With EoE
| Demographic | Adolescents (n=98) | Adults (n=103) |
|---|---|---|
| Age, y, mean (SD) | 13.4 (2.0) | 36.9 (10.2) |
| Sex, n (%) | ||
| Male | 65 (66.3) | 23 (22.3) |
| Female | 30 (30.6) | 75 (72.8) |
| Prefer not to say | 3 (3.1) | 5 (4.9) |
| Race,* n (%) | ||
| African American or Black | 6 (6.1) | 1 (1.0) |
| American Indian | 3 (3.1) | 2 (1.9) |
| Asian or Pacific Islander | 1 (1.0) | 0 (0.0) |
| White | 89 (90.8) | 97 (94.2) |
| Mixed race (2 or more races) | 7 (7.1) | 3 (2.9) |
| Other† | 1 (1.0) | 1 (1.0) |
| Prefer not to answer | 2 (2.0) | 3 (2.9) |
| Hispanic, Latino, or Spanish origin/descent, n (%) | 5 (5.1) | 0 (0.0) |
| Employment status,* n (%) | ||
| Employed full time | NA | 38 (36.9) |
| Employed part time | NA | 17 (16.5) |
| Student full time or part time | NA | 10 (9.7) |
| Not employed due to disability | NA | 18 (17.5) |
| Other‡ | NA | 26 (25.2) |
| Highest education level, n (%) | ||
| High school diploma or equivalent (eg, GED) | NA | 36 (35.0) |
| College degree (eg, BA, BS) | NA | 37 (35.9) |
| Professional or graduate degree (eg, MS, PhD, MD, JD) | NA | 20 (19.4) |
| Other§ | NA | 10 (9.7) |
| Medical insurance type,* n (%) | ||
| Private insurance (eg, Blue Cross/Blue Shield, Cigna, Aetna, UnitedHealth Group) or health plan (eg, PPO or HMO) | 72 (73.5) | 67 (65.0) |
| Medicare | 9 (9.2) | 17 (16.5) |
| Medicaid | 20 (20.4) | 20 (19.4) |
| Military-related health care [eg, VA health care, CHAMPVA, TRICARE (formerly CHAMPUS)] | 3 (3.1) | 3 (2.9) |
| Other‖ | 5 (5.1) | 3 (2.9) |
| Region of United States, n (%) | ||
| Midwest | 26 (26.5) | 25 (24.3) |
| Northeast | 14 (14.3) | 17 (16.5) |
| Southeast | 36 (36.7) | 32 (31.1) |
| Southwest | 7 (7.1) | 9 (8.7) |
| West | 15 (15.3) | 20 (19.4) |
| Urban/suburban/rural, n (%) | ||
| Urban | 8 (8.2) | 23 (22.3) |
| Suburban | 56 (57.1) | 57 (55.3) |
| Rural | 24 (24.5) | 15 (14.6) |
| Not sure | 10 (10.2) | 8 (7.8) |
The denominators for percentage calculations were the total n specified for each population.
Response options were not mutually exclusive; participants could select any that applied.
Other races not included here.
These included “not employed but looking for employment” (n=9), “not employed and not looking for employment” (n=6), “retired” (n=1), and other employment status not included here (n=7), as well as those who preferred not to answer (n=3).
These included “less than high school” (n=3) and other education levels not included here (n=4), as well as those who preferred not to answer (n=3).
These included “no health insurance” for adults (n=1), and other insurance not included here for adolescents (n=4), as well as those who preferred not to answer (adolescents, n=1; adults, n=2).
CHAMPUS indicates Civilian Health and Medical Program of the Uniformed Services; CHAMPVA, Civilian Health and Medical Program of the Department of Veterans Affairs; EoE, eosinophilic esophagitis; GED, General Educational Developmental Test; HMO, health maintenance organization; NA, not applicable; PPO, preferred provider organization; VA, Veterans Affairs.
Medical History and Current Treatments
Medical history and current off-label corticosteroid treatments reported by participants are presented in Table 2 and Supplementary Table 3 (Supplemental Digital Content 1, http://links.lww.com/JCG/B79). At the time of diagnosis of EoE, the mean (SD) ages of adolescents and adults were 8.4 (4.4) and 30.3 (11.5) years, respectively. From a prespecified list, the most frequently reported signs and symptoms of EoE that patients had experienced over the past month were abdominal pain (42.9%) for adolescents, and difficulty or discomfort in swallowing solid food (68.0%) for adults.
TABLE 2.
Current Treatments Used to Treat EoE in Adolescents (Caregiver-Reported) and Adults (Self-Reported)
| Survey question | Adolescents* (n=98) | Adults (n=103) |
|---|---|---|
| Which of the following medications are you (is your child) currently taking to treat EoE?† | ||
| n | 98 | 103 |
| Budesonide slurry | 43 (43.9) | 33 (32.0) |
| Fluticasone oral inhaler | 39 (39.8) | 41 (39.8) |
| Budesonide liquid (compounded) | 5 (5.1) | 6 (5.8) |
| Prednisone | 4 (4.1) | 11 (10.7) |
| Budesonide oral inhaler | 4 (4.1) | 7 (6.8) |
| Other‡ | 3 (3.1) | 5 (4.9) |
Data are presented as n (%) unless otherwise specified.
Minor changes to the wording were made for the adolescent survey to apply to a caregiver’s perspective; all differences in wording are shown in square brackets.
Participants could select responses from a prespecified list of orally administered corticosteroid medications. Categories were not mutually exclusive.
Other corticosteroid medications included beclomethasone nasal or oral inhaler (adolescents, n=2; adults, n=0), flunisolide nasal or oral inhaler (adolescents, n=1; adults, n=1), mometasone oral inhaler (adolescents, n=0; adults, n=2), and triamcinolone acetonide (adolescents, n=0; adults, n=2).
EoE indicates eosinophilic esophagitis.
In adolescents and adults, the most frequently used off-label corticosteroid treatments were budesonide slurry (43.9% and 32.0%, respectively) and fluticasone oral inhaler (39.8% and 39.8%, respectively). Most adolescents (78.6%) and adults (67.6%) reported using off-label corticosteroids for at least 6 months. In total, 63.3% of adolescents and 75.7% of adults reported taking other medications concomitantly with off-label corticosteroid treatments, of which the most commonly used were PPIs.
Satisfaction With Current Off-Label Corticosteroid Treatments
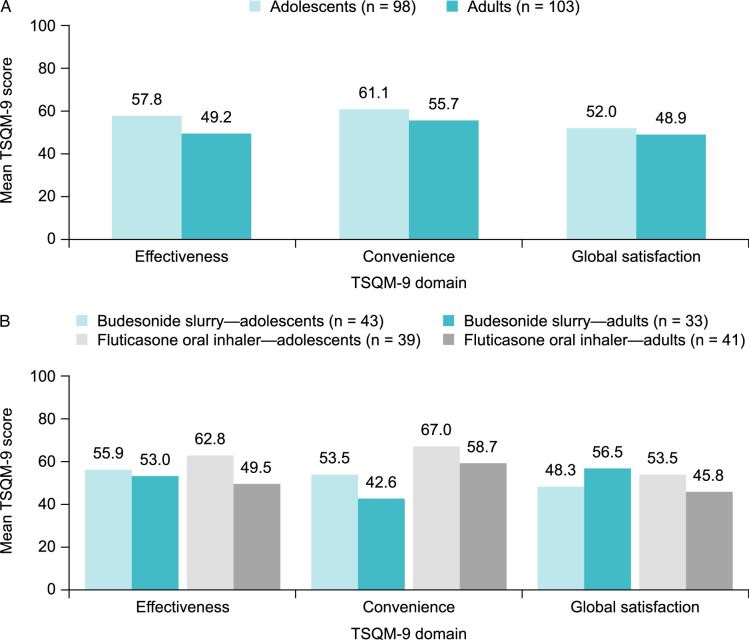
Overall, mean TSQM-9 scores indicated low satisfaction with current off-label corticosteroid treatments across the 3 domains, with slightly higher satisfaction observed in adolescents than adults (Fig. 1A). Mean (SD) TSQM-9 scores for adolescents were 57.8 (22.1) for effectiveness, 61.1 (20.5) for convenience, and 52.0 (20.8) for global satisfaction, out of possible scores of 100 for each domain. Mean (SD) TSQM-9 scores for adults were 49.2 (19.2) for effectiveness, 55.7 (24.3) for convenience, and 48.9 (22.3) for global satisfaction. In adolescents, satisfaction related to effectiveness, convenience, and global satisfaction was slightly better for fluticasone oral inhaler than budesonide slurry (Fig. 1B). In adults, satisfaction was slightly greater for budesonide slurry than fluticasone oral inhaler for the domains of effectiveness and global satisfaction; however, satisfaction for the domain of convenience was slightly better in patients using a fluticasone oral inhaler than in those taking a budesonide slurry (Fig. 1B).
FIGURE 1.
Treatment satisfaction among adolescents (caregiver-reported) and adults (self-reported) with EoE who were receiving off-label corticosteroids overall (A) and among those who were taking a budesonide slurry or using a fluticasone oral inhaler (B), across 3 domains of satisfaction assessed using the TSQM-9. Corticosteroid treatment satisfaction was assessed using the TSQM-9 (version 1.4); scores range from 0 to 100 for each domain, with higher scores indicating greater satisfaction. EoE indicates eosinophilic esophagitis; TSQM-9, 9-item Treatment Satisfaction Questionnaire for Medication.
Adherence to Current Off-Label Corticosteroid Treatments
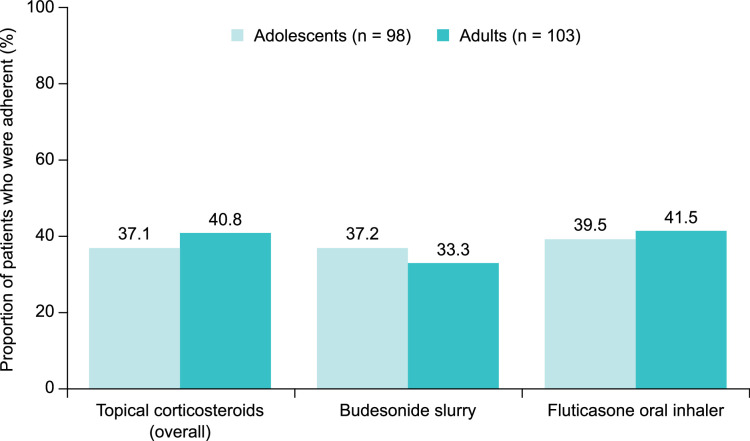
On the basis of an MGL-4 score of <3, only 37.1% of adolescents and 40.8% of adults were considered adherent to their off-label corticosteroid treatments (Fig. 2). Adherence was slightly higher in adolescents and adults who were using a fluticasone oral inhaler [39.5% (15/38) and 41.5% (17/41), respectively] than in those who were taking a budesonide slurry [37.2% (16/43) and 33.3% (11/33), respectively].
FIGURE 2.
Treatment adherence among adolescents (caregiver-reported) and adults (self-reported) with EoE who were receiving off-label corticosteroids overall and among those who were taking budesonide slurry or using a fluticasone oral inhaler. Patients with an MGL-4 score of <3 were considered adherent. EoE indicates eosinophilic esophagitis; MGL-4, 4-item Morisky Green Levine Medication Adherence Scale.
Overall, 43.9% of adolescents (as reported by their caregivers) and 48.5% of adults had not taken their medication every day over the past 7 days. The mean (SD) number of missed days were 2.0 (1.5) and 2.3 (1.6) for adolescents and adults, respectively; thus, participants had not taken their medication 28.6% (adolescents) and 32.9% (adults) of the time during a 7-day period, suggesting that the level of nonadherence was similar for both groups (Table 3). Overall, 37.8% of adolescents and 38.8% of adults forgot to take their off-label corticosteroid treatment at least once over the past 7 days. The respective proportions of adolescents and adults who chose not to take their off-label corticosteroid treatment at least once over the past 7 days were 9.2% and 15.5%. The most commonly reported reasons for adolescents choosing not to take their medications for EoE (as reported by their caregivers) were “the medicine tastes bad” (55.6%), “feeling depressed/overwhelmed” (44.4%), “symptoms have not been bothersome” (33.3%), and “he/she felt okay so did not think it was necessary to take the medicine daily” (33.3%). The most common reasons for adults choosing not to take their medications for EoE were “difficulty taking it at a specific time” (31.3%), “feeling depressed/overwhelmed” (31.3%), and “my EoE symptoms have not been bothersome” (25.0%).
TABLE 3.
Patient Adherence to Current Off-Label Corticosteroid Treatments in Adolescents (Caregiver-Reported) and Adults (Self‑Reported) With EoE.
| Survey question | Adolescents* (n=98) | Adults (n=103) |
|---|---|---|
| Over the past 7 days, did you [your child] at any time forget to take or choose not to take [insert name of treatment] to treat your [his/her] EoE? Please select all that apply. | ||
| Yes, I [he/she] forgot to take my [his/her] treatment | 37 (37.8) | 40 (38.8) |
| Yes, I [he/she] chose not to take my [his/her] treatment | 9 (9.2) | 16 (15.5) |
| No, I [he/she] took my [his/her] treatment every day over the past 7 days | 55 (56.1) | 53 (51.5) |
| Over the past 7 days, how many days did you [your child] forget or choose not to take your [his/her] treatment? | ||
| n | 43 | 50 |
| Number of days, mean (SD) | 2.0 (1.5) | 2.3 (1.6) |
| Proportion of days, % | 28.6 | 32.9 |
| Why did you [your child] choose not to take [insert name of treatment] to treat your [his/her] EoE over the past 7 days? Please select all that apply.† | ||
| n | 9 | 16 |
| [My child thinks the] The medicine tastes bad | 5 (55.6) | 0 (0.0) |
| I [My child] felt depressed/overwhelmed | 4 (44.4) | 5 (31.3) |
| My [His/her] EoE symptoms have not been bothersome | 3 (33.3) | 4 (25.0) |
| I [He/she] felt okay, so I did not think it was necessary to take the medicine daily | 3 (33.3) | 3 (18.8) |
| I have difficulty taking it [giving it to my child] at the specific time I am [he/she is] supposed to take it | 1 (11.1) | 5 (31.3) |
| I [He/she] experienced side effects from the medicine | 2 (22.2) | 3 (18.8) |
| I [He/she] had too many other pills/medications to take | 1 (11.1) | 3 (18.8) |
| It is not effective at controlling my [my child’s] EoE symptoms | 0 (0.0) | 2 (12.5) |
| The medicine is expensive | 0 (0.0) | 2 (12.5) |
| The medicine is difficult or inconvenient to get from the pharmacy in a timely manner | 0 (0.0) | 2 (12.5) |
| I did not have the medicine available (eg, away from home, on vacation) | 0 (0.0) | 2 (12.5) |
| I [My child] just needed a break | 1 (11.1) | 2 (12.5) |
| Other | 3 (33.3) | 3 (18.8) |
Data are presented as n (%) unless otherwise specified. Corticosteroid treatment adherence was assessed using the MGL-4; data presented are responses to items from the MGL-4.
Minor changes to the wording of the MGL-4 were made for the adolescent survey to apply to a caregiver’s perspective; all differences in wording are shown in square brackets.
Included only participants who reported that they/their child chose not to take the medication.
EoE indicates eosinophilic esophagitis; MGL-4, 4-item Morisky Green Levine Medication Adherence Scale.
DISCUSSION
The prevalence and incidence of EoE are increasing for both children and adults in the United States and Europe.33 Although the treatment landscape of EoE is constantly evolving,34 there is only one US FDA-approved swallowed topical corticosteroid (BOS) for the treatment of EoE57.6 In this noninterventional, cross-sectional, web-based survey, we investigated satisfaction with and adherence to off-label corticosteroids in adolescents and adults with EoE in the United States. Most patients were either taking a budesonide slurry or using a fluticasone oral inhaler to manage their EoE symptoms, and most had been receiving their treatment for at least 6 months. Satisfaction with and adherence to off-label corticosteroids were generally poor. Mean scores for global satisfaction, effectiveness, and convenience, measured using the TSQM-9, were generally low, but slightly higher for adolescents than adults. Adherence was also low, but slightly higher in adults (40.8%) than adolescents (37.1%). Forgetfulness was the most frequently reported reason for nonadherence in both populations. The most common reasons for choosing not to take their medications for EoE was poor palatability in adolescents, taking medications for EoE at specific times in adults, and feeling depressed/overwhelmed in both populations.
The slightly higher satisfaction observed in adolescents than adults in our study was reflected in a cross-sectional survey of adults (aged 18 years or older) and caregivers of pediatric patients (aged 11 to 17 y) receiving treatments for EoE, including PPIs, topical corticosteroids, and dietary restriction.20 More caregivers reported that they were “satisfied” with their child’s current treatments than adult patients reported for their own current treatments (54.3% vs. 38.1%).20 These findings suggest that younger patients, who are likely to be at the early stage of a progressive disease such as EoE35 and are more likely to have their treatment supervised by a parent or caregiver, may be more responsive to, and accepting of, treatment, thus positively affecting their satisfaction. Conversely, adults who may experience severe symptoms due to fibrotic complications, which are often refractory to treatment,36,37 may be less likely to consider their treatment satisfactory. Another possible explanation is that caregiver-reported findings may not accurately reflect the experience of adolescent patients. The low satisfaction scores reported in our study for off-label corticosteroids in adult patients with EoE were consistent with the findings of a 2018 survey conducted in adults with EoE and dysphagia.22 The 2018 survey reported a mean TSQM-9 score of 61.4 for global satisfaction in patients receiving swallowed inhaled corticosteroids, and mean scores of 57.1 and 64.2 for the domains of effectiveness and convenience, respectively.22
The duration of treatment may also influence satisfaction with corticosteroids in patients with EoE.23 Although most patients in our study had been using their corticosteroids for at least 6 months, the exact length of treatment was unknown, which may partly explain the lower patient satisfaction scores (mean TSQM-9 score of 48.9) compared with another study of adult patients with EoE (median TSQM-14 score of 78.6).23 In this other population of adult patients, the relatively high overall satisfaction scores were attributed to a long median duration of treatment (at least 5 y).23 Moreover, patients in that study reported high satisfaction with swallowed topical corticosteroids in terms of effectiveness and convenience, indicated by median TSQM-14 scores of 83.3 for both domains.23 However, direct comparison of these data with ours is challenging owing to the variation in the number of questions posed in the different versions of the TSQM.23
Although a validated consensus for the definition of adequate adherence to medications has not yet been established,38 patients who take at least 80% of their medication are typically considered adherent.39 Despite this, it is challenging to assign adherence thresholds consistently because rates can vary depending on the disease, treatments, and individual patients.39 During our study, fewer than 50% of adolescents and adults were considered adherent (MGL-4 score <3; 37.1% and 40.8%, respectively). Nevertheless, nonadherence was higher in our adult patients (MGL-4 score of ≥3; 59.2%) than in another adult population with EoE (43.8%);24 however, that study used the validated Medication Adherence Rating Scale, with poor adherence to corticosteroids defined as a score of <21 on a scale of 5 to 25.24 A prospective study noted adherence of 59.2% in adult patients with severe erosive esophagitis when adherence was defined based on patients taking medications for at least 6 months and undergoing a follow-up endoscopy.40 In a cross-sectional study that assessed adherence to PPIs in adult patients with gastroesophageal reflux disease, adherence was 52.5% based on the Morisky questionnaire (score of ≥3 on a scale of 0 to 4),41 further emphasizing the comparatively poorer adherence in our population.
Poor adherence to medication during adolescence is evident in chronic conditions such as EoE,25,42 asthma,43 and inflammatory bowel disease.44 Similar to the adherence rate in adolescents with EoE during our study (37.1%), the adherence rate in an adolescent population with asthma was 37.9%, as defined by patients taking medication for at least 5 days during the past 7 days.45 However, another study reported comparatively higher adherence in adolescents with inflammatory bowel disease (66.8%; defined as ≥80% intake of their prescribed weekly medication) than in adolescents with EoE in our study population (37.1%).44 In addition, Mehta et al42 reported that adolescents aged 13 to 18 years with EoE who were receiving swallowed topical corticosteroids had higher adherence (76.2%; determined using the Medication-Taking Checklist) than our adolescent population.42 These data were self-reported by adolescents42 and, thus, may align more with their own experiences, whereas caregivers’ subjective experiences may have affected their reports for adolescents in our study. Direct comparisons between data are challenging, owing to discordance between outcome measurements and a lack of standardization for monitoring adherence.
During our study, patients using a fluticasone oral inhaler reported slightly better adherence and satisfaction related to convenience than those taking a budesonide slurry. Poor satisfaction may be associated with low-adherence behaviors and, thus, low effectiveness. The low adherence observed in our study was primarily driven by patients forgetting to take their medications, which may be linked to the inconvenience of self-mixing formulations16 and the recommendation to take their treatment twice daily.46 Forgetfulness has also been reported as a major contributor to suboptimal adherence in patients with other chronic diseases such as hypertension47 and diabetes.48
In our adolescent population, the most common reason for choosing not to take medication was its undesirable taste. This was not unexpected given that home-mixed preparations may lack palatability, which compromises compliance in children and adolescents.49 “Feeling depressed/overwhelmed” was another frequently reported reason for selective nonadherence. Signs of depression are common in patients with EoE regardless of age.50 A significant association between depressive symptoms and nonadherence to medication has been noted in patients aged 2.5 to 18 years with eosinophilic gastrointestinal disease, when statistically controlling for the influence of anxiety and somatization.26 Patients with eosinophilic gastrointestinal disease are susceptible to developing associated comorbidities, such as depression, as a result of dietary and lifestyle changes, treatment regimens, impaired social relationships, and internalized and perceived illness stigma.26,50 This can affect motivation and, therefore, adherence to medications.26
There are substantial challenges associated with the management of EoE, particularly because patients typically require long-term maintenance therapy to control inflammation and avoid symptom recurrence.51 For long-term intervention, doses of corticosteroids are often reduced in clinical settings.52,53 High prevalence of poor adherence to maintenance treatment has been reported in adult patients with EoE, a recent study indicated that more than 40% of patients had poor adherence to prescribed medications (measured using the Medication Adherence Rating Scale).24 These practices can result in artificial lowering of corticosteroid doses, causing a loss of response and increased risk of relapse,52,54 potentially leading to greater frequency of and/or recurrent esophageal stricture formation.52
Limitations of this study include potential responder bias that could, as with all voluntary surveys, lead to an underestimation or overestimation of satisfaction and adherence. Corroboration of adherence was not possible with either medical records or physician reports. In addition, data were not adjusted to account for any shared decision-making models that patients and their health care providers may have been using. The survey could only be completed online, limiting participation to only those with internet access. Participants were recruited through patient advocacy groups and, therefore, may represent a more engaged patient population in which greater compliance may be expected compared with a less engaged population. In addition, most adults who completed the survey were female (72.8%), deviating from the well-documented male predominance in EoE.55 The interpretation of these study results should therefore be undertaken in the context of our population potentially not fully aligning with the real-world epidemiology of EoE for adolescents and adults. Furthermore, caregivers’ personal experiences or perceptions may have influenced the responses provided on behalf of their child. Given that existing studies use a variety of tools to measure medication adherence in patients with EoE,24,42 directly comparing adherence data between our study and other populations is challenging. In our study, most participants were identified as White, this aligns with a recent prevalence study which reports a substantially increased prevalence of EoE amongst White patients compared with patients of other races.56 However, other racial groups only represented <10% of the patient population in our study and therefore our population may not be reflective of the real-world epidemiology of EoE for adolescents or adults.56 Also, this study did not examine patient satisfaction with and adherence to treatments other than off-label corticosteroids, and it did not adjust for factors such as dietary modification or the presence of comorbidities. Lastly, the surveys are not validated for caregiver reporting in adolescents, which may have impacted the results.
A major strength of this study is that we present real-world data on the impact of off-label corticosteroid use in patients with EoE in the United States, using a large population of 201 participants recruited from 5 different census regions. Participants were recruited from 2 patient advocacy groups for EoE in the United States, with limited inclusion and exclusion criteria to ensure a heterogeneous population and to allow generalizability of the data. This study captured the patient perspective on off-label corticosteroids used for the treatment of EoE, including the reasons for nonadherence to these medications across 2 distinct age groups, with the aid of a validated 9-item TSQM27 and a widely recognized, self-reported (or caregiver-reported), 4-item MGL.28,32
CONCLUSIONS
Patient satisfaction with and adherence to a prescribed schedule of off-label corticosteroids were low in adolescents and adults with EoE in the United States. These data demonstrate the unmet medical need for a standardized, palatable, and convenient corticosteroid treatment option for these patients and provide important insights into areas to target to improve adherence to treatment.
Supplementary Material
ACKNOWLEDGMENTS
Medical writing support was provided by Sandra Cheriyamkunnel (MSc) and Jessica Boles (PhD) of PharmaGenesis London, London, UK, and funded by Takeda Pharmaceuticals USA Inc.
Footnotes
B.D., K.D., C.S., R.Z., J.J., T.F., M.B., and S.-T.C: concept and design. K.D., C.S., and R.Z.: acquisition of data. K.D., C.S., and R.Z.: statistical analysis and provision of study materials or patients. B.G., J.J., T.F., M.B., and S.-T.C.: obtaining funding. B.G., K.D., C.S., R.Z., J.J., T.F., M.B., and S.-T.C.: administrative, technical, or logistical support. B.G., K.D., C.S., R.Z., J.J., T.F., M.B., and S.-T.C.: supervision. Analysis and interpretation of data, drafting of the manuscript, and critical revision of paper for important intellectual content done by all authors.
This study was funded by Takeda Pharmaceutical Company Ltd. The funder/sponsor funded the study and medical writing support. The analysis, interpretation, preparation, and decision to submit the manuscript for publication was the responsibility of all authors.
B.D.G. provided consultancy and participates in continuing medical education activities for DiaSorin Molecular LLC, Evolve BioSystems Inc., Johnson & Johnson (Janssen), Mead Johnson Nutrition, Nestlé USA, Nutricia North America, and Takeda Pharmaceutical Company Limited. B.G. and S.-T.C. are employees of Takeda Development Center Americas Inc., and are stockholders of Takeda Pharmaceutical Company Limited. K.D. is an employee of Takeda Development Center Americas Inc., is a stockholder of Takeda Pharmaceutical Company Limited, and at the time of the analysis was an employee of RTI Health Solutions and received funding from Takeda Pharmaceutical Company Limited to conduct this study. C.S. and R.Z. are employees of RTI Health Solutions and were funded by Takeda Pharmaceutical Company Limited to conduct this study. J.J., T.F., and M.B. are employees of Takeda Pharmaceuticals USA Inc., and stockholders of Takeda Pharmaceutical Company Limited. D.A.K. has received research funding from Shire, a Takeda company, and has provided consultancy for Receptos/Celgene.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.jcge.com
Contributor Information
Benjamin D. Gold, Email: bgold@gicareforkids.com.
Bridgett Goodwin, Email: bridgett.goodwin@takeda.com.
Kimberly Davis, Email: kimberly.davis@takeda.com.
Carolyn Sweeney, Email: csweeney@rti.org.
Ryan Ziemiecki, Email: rziemiecki@rti.org.
Jeanne Jiang, Email: jeanne.jiang@takeda.com.
Tao Fan, Email: tao.fan@takeda.com.
Mena Boules, Email: menaboules@hotmail.com.
Szu-Ta Chen, Email: starr.chen@takeda.com.
David A. Katzka, Email: dak2178@cumc.columbia.edu.
REFERENCES
- 1. Khan S, Guo X, Liu T, et al. An update on eosinophilic esophagitis: etiological factors, coexisting diseases, and complications. Digestion. 2021;102:342–356. [DOI] [PubMed] [Google Scholar]
- 2. Furuta GT, Katzka DA. Eosinophilic esophagitis. N Engl J Med. 2015;373:1640–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schoepfer AM, Safroneeva E, Bussmann C, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013;145:1230–1236; e1231–1232. [DOI] [PubMed] [Google Scholar]
- 4. Straumann A, Bussmann C, Zuber M, et al. Eosinophilic esophagitis: analysis of food impaction and perforation in 251 adolescent and adult patients. Clin Gastroenterol Hepatol. 2008;6:598–600. [DOI] [PubMed] [Google Scholar]
- 5. Główczewski A, Krogulska A. Formulations of topical steroids in eosinophilic esophagitis-current treatment and emerging possibilities. J Clin Med. 2022;11:1454–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EOHILIA. Prescribing Information.https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/213976s000lbl.pdf Accessed April 5, 2024.
- 7. Dupilumab. Prescribing Information. 2024. Accessed April 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761055s057lbl.pdf
- 8. Strossman N, Donovan K, Trovato A, et al. Exploring treatment options for eosinophilic esophagitis. Gastroenterol Insights. 2022;13:228–237. [Google Scholar]
- 9. Dellon ES, Collins MH, Katzka DA, et al. Long-term treatment of eosinophilic esophagitis with budesonide oral suspension. Clin Gastroenterol Hepatol. 2022;20:1488–1498.e1411. [DOI] [PubMed] [Google Scholar]
- 10. Ketchem CJ, Reed CC, Stefanadis Z, et al. Treatment with compounded fluticasone suspension improves the clinical, endoscopic, and histologic features of eosinophilic esophagitis. Dis Esophagus. 2021;34:doaa120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miehlke S, Hruz P, Vieth M, et al. A randomised, double-blind trial comparing budesonide formulations and dosages for short-term treatment of eosinophilic oesophagitis. Gut. 2016;65:390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dellon ES, Katzka DA, Collins MH, et al. Budesonide oral suspension improves symptomatic, endoscopic, and histologic parameters compared with placebo in patients with eosinophilic esophagitis. Gastroenterology. 2017;152:776–786.e775. [DOI] [PubMed] [Google Scholar]
- 13. Hirano I, Chan ES, Rank MA, et al. AGA institute and the joint task force on allergy-immunology practice parameters clinical guidelines for the management of eosinophilic esophagitis. Gastroenterology. 2020;158:1776–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Uchida AM, Burk CM, Rothenberg ME, et al. Recent advances in the treatment of eosinophilic esophagitis. J Allergy Clin Immunol Pract. 2023;11:2654–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muir A, Falk GW. Eosinophilic esophagitis: a review. JAMA. 2021;326:1310–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reed CC, Fan C, Koutlas N, et al. Compounded oral viscous budesonide is effective and provides a durable response in eosinophilic esophagitis. HSOA J Gastroenterol Hepatol Res. 2018;7:2509–2515. [PMC free article] [PubMed] [Google Scholar]
- 17. Pokrzywinski RM, Harding G, Brooks A, et al. Documenting the journey of patients with eosinophilic esophagitis and the impact of the disease on patients and their caregivers: a cross-sectional, qualitative research study. Adv Ther. 2020;37:4458–4478. [DOI] [PubMed] [Google Scholar]
- 18. Hiremath G, Kodroff E, Strobel MJ, et al. Individuals affected by eosinophilic gastrointestinal disorders have complex unmet needs and frequently experience unique barriers to care. Clin Res Hepatol Gastroenterol. 2018;42:483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dellon ES, Woosley JT, Arrington A, et al. Rapid recurrence of eosinophilic esophagitis activity after successful treatment in the observation phase of a randomized, double-blind, double-dummy trial. Clin Gastroenterol Hepatol. 2020;18:1483–1492.e1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chang JW, Rubenstein JH, Mellinger JL, et al. Motivations, barriers, and outcomes of patient-reported shared decision making in eosinophilic esophagitis. Dig Dis Sci. 2021;66:1808–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mäkelä MJ, Backer V, Hedegaard M, et al. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med. 2013;107:1481–1490. [DOI] [PubMed] [Google Scholar]
- 22. Adkins C, Takakura W, Spiegel BMR, et al. Prevalence and characteristics of dysphagia based on a population-based survey. Clin Gastroenterol Hepatol. 2020;18:1970–1979.e1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Safroneeva E, Hafner D, Kuehni CE, et al. Systematic assessment of adult patients’ satisfaction with various eosinophilic esophagitis therapies. Int Arch Allergy Immunol. 2020;181:211–220. [DOI] [PubMed] [Google Scholar]
- 24. Haasnoot ML, Safi S, Bredenoord AJ. Poor adherence to medical and dietary treatments in adult patients with eosinophilic esophagitis. Am J Gastroenterol. 2022;117:1412–1418. [DOI] [PubMed] [Google Scholar]
- 25. Hommel KA, Franciosi JP, Hente EA, et al. Treatment adherence in pediatric eosinophilic gastrointestinal disorders. J Pediatr Psychol. 2012;37:533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hommel KA, Franciosi JP, Gray WN, et al. Behavioral functioning and treatment adherence in pediatric eosinophilic gastrointestinal disorders. Pediatr Allergy Immunol. 2012;23:494–499. [DOI] [PubMed] [Google Scholar]
- 27. Bharmal M, Payne K, Atkinson MJ, et al. Validation of an abbreviated treatment satisfaction questionnaire for medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes. 2009;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vik SA, Maxwell CJ, Hogan DB, et al. Assessing medication adherence among older persons in community settings. Can J Clin Pharmacol. 2005;12:e152–e164. [Google Scholar]
- 29. Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the treatment satisfaction questionnaire for medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Atkinson MJ, Kumar R, Cappelleri JC, et al. Hierarchical construct validity of the treatment satisfaction questionnaire for medication (TSQM version II) among outpatient pharmacy consumers. Value Health. 2005;8(suppl 1):S9–S24. [DOI] [PubMed] [Google Scholar]
- 31.https://www.iqvia.com/landing/treatment-satisfaction-questionnaire-for-medication-tsqm IQVIA. IQVIA Treatment Satisfaction Questionnaire for Medication (TSQM). Accessed February 10, 2023.
- 32. Beyhaghi H, Reeve BB, Rodgers JE, et al. Psychometric properties of the four-item Morisky Green Levine Medication Adherence Scale among atherosclerosis risk in communities (ARIC) study participants. Value Health. 2016;19:996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Navarro P, Arias A, Arias-Gonzalez L, et al. Systematic review with meta-analysis: the growing incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther. 2019;49:1116–1125. [DOI] [PubMed] [Google Scholar]
- 34. Greuter T, Hirano I, Dellon ES. Emerging therapies for eosinophilic esophagitis. J Allergy Clin Immunol. 2020;145:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dellon ES, Hirano I. Epidemiology and natural history of eosinophilic esophagitis. Gastroenterology. 2018;154:319–332.e313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Muir AB, Brown-Whitehorn T, Godwin B, et al. Eosinophilic esophagitis: early diagnosis is the key. Clin Exp Gastroenterol. 2019;12:391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eluri S, Runge TM, Cotton CC, et al. The extremely narrow-caliber esophagus is a treatment-resistant subphenotype of eosinophilic esophagitis. Gastrointest Endosc. 2016;83:1142–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chan W, Chen A, Tiao D, et al. Medication adherence in inflammatory bowel disease. Intest Res. 2017;15:434–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baumgartner PC, Haynes RB, Hersberger KE, et al. A systematic review of medication adherence thresholds dependent of clinical outcomes. Front Pharmacol. 2018;9:1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mari A, Na’amnih W, Gahshan A, et al. Comparison in adherence to treatment between patients with mild-moderate and severe reflux esophagitis: a prospective study. J Clin Med. 2022;11:3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dal-Paz K, Moraes-Filho JP, Navarro-Rodriguez T, et al. Low levels of adherence with proton pump inhibitor therapy contribute to therapeutic failure in gastroesophageal reflux disease. Dis Esophagus. 2012;25:107–113. [DOI] [PubMed] [Google Scholar]
- 42. Mehta P, Pan Z, Skirka S, et al. Medication adherence aligns with age and a behavioral checklist but not symptoms or quality of life for patients with eosinophilic esophagitis. J Pediatr. 2021;235:246–252.e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kaplan A, Price D. Treatment adherence in adolescents with asthma. J Asthma Allergy. 2020;13:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lim JK, Lee YJ, Park JH. Medication-related knowledge and medication adherence in pediatric and adolescent patients with inflammatory bowel disease. J Korean Med Sci. 2020;35:e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guglani L, Havstad SL, Ownby DR, et al. Exploring the impact of elevated depressive symptoms on the ability of a tailored asthma intervention to improve medication adherence among urban adolescents with asthma. Allergy Asthma Clin Immunol. 2013;9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu X, Xiao X, Liu D, et al. A meta-analysis on randomized controlled trials of treating eosinophilic esophagitis with budesonide. Ann Med. 2022;54:2078–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gebreyohannes EA, Bhagavathula AS, Abebe TB, et al. Adverse effects and non-adherence to antihypertensive medications in University of Gondar Comprehensive Specialized Hospital. Clin Hypertens. 2019;25:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Adisa R, Alutundu MB, Fakeye TO. Factors contributing to nonadherence to oral hypoglycemic medications among ambulatory type 2 diabetes patients in southwestern Nigeria. Pharm Pract (Granada). 2009;7:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bai S, Dormer N, Shoults C, et al. Palatability of a novel oral formulation of prednisone in healthy young adults. J Pharm Pharmacol. 2017;69:489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Taft TH, Guadagnoli L, Edlynn E. Anxiety and depression in eosinophilic esophagitis: a scoping review and recommendations for future research. J Asthma Allergy. 2019;12:389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nennstiel S, Schlag C. Treatment of eosinophlic esophagitis with swallowed topical corticosteroids. World J Gastroenterol. 2020;26:5395–5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Eluri S, Runge TM, Hansen J, et al. Diminishing effectiveness of long-term maintenance topical steroid therapy in PPI non-responsive eosinophilic esophagitis. Clin Transl Gastroenterol. 2017;8:e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Straumann A, Conus S, Degen L, et al. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2011;9:400–409.e401. [DOI] [PubMed] [Google Scholar]
- 54. Greuter T, Godat A, Ringel A, et al. Effectiveness and safety of high- vs low-dose swallowed topical steroids for maintenance treatment of eosinophilic esophagitis: a multicenter observational study. Clin Gastroenterol Hepatol. 2021;19:2514–2523 e2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mansoor E, Cooper GS. The 2010-2015 prevalence of eosinophilic esophagitis in the USA: a population-based study. Dig Dis Sci. 2016;61:2928–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hahn JW, Lee K, Shin JI, et al. Global incidence and prevalence of eosinophilic esophagitis, 1976-2022: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2023;21:3270–3284.e77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.