Abstract
Objectives
To assess the outcome of pediatric pulmonary arterial hypertension (PAH) and to identify the predictors of morbidity and mortality of this progressive disease.
Patients and methods
This prospective observational cohort study was conducted on consecutive pediatric patients with PAH. Medical history was taken with a grading of the WHO functional class as well as the serum N-terminal pro-BNP (NT pro-BNP), 6 min’ walk test (6MWT), and echocardiography at the initial assessment and at follow-up.
Results
The cohort study included 71 patients; 39 patients had idiopathic and heritable PAH, 27 patients had PH secondary to CHD and five patients had miscellaneous causes. Dyspnea was the most common presenting symptom. The mean initial 6MWT distance was 273.3 ± 139.9 m. The median of the initial NT-proBNP level was 1982 pg/ml with IQR from 373.9 to 5472 pg/ml. Death occurred in 17 patients (23.9%) and 16 (22.5%) had evolving morbidity. The predictors of morbidity were NT-proBNP >1032 pg/ml and its Z score > +3.5, WHO functional class 3 and 4, RV failure and low TAPSE. The predictors of mortality were 6MWD ≤210 m, WHO functional class 4, RV failure, syncope, low TAPSE, NT-proBNP >4734 pg/ml and its Z score >4.57.
Conclusion
PAH is a serious disease and reliable predictive outcome is feasible by clinical assessment, echocardiographic parameters. NT pro-BNP is a surrogate biomarker for diagnosis and prognosis of PAH.
Keywords: Pulmonary arterial hypertension, NT pro-BNP, 6MWT, Echocardiography, Mortality
1. Introduction
Pulmonary arterial hypertension (IPAH) is a progressive disease, characterized by escalating pulmonary vascular resistance (PVR), right ventricular remodeling and failure, and ultimately death, unless the provoking cardiopulmonary physiology can be corrected prior to irreversible pulmonary vascular injury [1]. The mean prevalence of PAH in children is 11.6 per million, and the estimated incidence rate is 2.4 per million/year [2]. Based on history, biomarkers, echocardiographic, cardiac catheterization, and MRI findings, children with PAH can be stratified into high-risk or low-risk categories which determine treatment arms [1]. The WHO-FC and NT pro-BNP levels over time are widely used in clinical settings to support treatment decisions and monitor disease progression [3,4]. Moreover, shorter 6MWT combined with lower transcutaneous oxygen saturations correlated with higher WHO-FC and NT pro-BNP levels as well as worse transplant-free outcomes in pediatric PH patient [5]. The value of echocardiographic variables measuring functional properties of both the RV and the LV supports that ventricular interdependency is an important determinant of outcome in PAH [6]. Children with significant PH have marked decreases in their diastolic duration resulting in an increased systolic/diastolic ratio greater than 1.4 inversely correlated with survival in pediatric PH [7]. Monitoring of NT pro-BNP levels may correlate with morbidity and mortality in those with pulmonary hypertension [8]. The aim of this study was to assess the severity, the prognosis and the risk factors of morbidity and mortality of pulmonary arterial hypertension among consecutive pediatric population from a tertiary pediatric referral center.
2. Patients and methods
This is a prospective observational consecutive cohort study that was conducted for all patients who were presented from January 2020 till September 2023 with clinical classes of PAH as proposed by 2018 World Symposium on PH [9]. Patients from 3 months to 18 years of both genders, incident (newly diagnosed) cases and prevalent (referred) cases for management were enrolled in the study. Patients with PH due to operable left to right shunts or secondary to left sided heart lesions or due to lung diseases were excluded. All patients were subjected to the following: clinical data with a special emphasis on: demographic data, onset, course and duration of the disease, the time lags between the symptoms and the diagnosis of PAH. The severity of the disease according to WHO functional classification [10], syncopal attacks, signs of RV failure and Sao2 were reported. 2D transthoracic Doppler echocardiography was performed for the diagnosis of PAH-CHD and for assessment of systolic and diastolic pulmonary artery pressure by application of simplified Bernoulli equation [11]. RVEDD Z score and RV/LV ratio in the parasternal long axis view M mode were used to assess RV dilatation. RA area was estimated by planimetry in the apical 4 chamber view at the end of ventricular systole (largest volume) for tracing the RA cavity. The 2D tricuspid annular plane systolic excursion (TAPSE) was measured by obtaining an apical four-chamber view, then M-mode tracing of the lateral tricuspid valve annulus, then the distance between the end-systole and the end-diastole was measured to assess RV systolic function. RVOT acceleration time was measured by pulsed wave Doppler in the left parasternal short axis view at the level of the pulmonary artery. A mid systolic notching or RVOT time less than 105 milliseconds was considered an indicator of pulmonary hypertension [12]. Signs of RV failure as pericardial effusion, congested IVC with poor collapsibility were elicited. Six-minute walk test (6MWT) which is a submaximal exercise test that entails the measurement of distance walked over a span of 6 min was determined [13]. N-terminal Pro-BNP test using the quantitative sandwich enzyme immunoassay technique (ELISA) was measured [14]. Cardiac catheterization was performed for hemodynamic assessment to measure the following: systolic, diastolic and mean PA pressure, PVR indexed to the body surface area, Rp/Rs, Qp/Qs in cases with left to right shunt lesions, acute vasoreactivity was tested by hyperoxia test or prostacyclin analogues. Thrombophilia panel, immune profile and metabolic screening were performed if indicated. The patients were followed and managed by the following; targeted pulmonary vasodilating agents: Sildenafil from 0.5 to 1 mg/kg every 8 h, Endothelin receptor antagonists such as Bosentan (started with 1 mg/kg every 12 h and then titrated to 2 mg/kg every 12 h). Antifailure measures like Furosemide, Spironolactone and Digoxin were prescribed for some cases. Follow-up after 3–6 months including: History taking and complete physical examination with special emphasis on; vital signs, oxygen saturation, WHO functional class, improvement or worsening of symptoms or appearance of a new symptom, signs of right sided heart failure, follow up 6MWT, follow up echocardiography and measured NT pro-BNP normalized to patient’s age [15]. Liver function tests were performed on a regular basis in all patients receiving Bosentan therapy every 3 months. The study was approved by the scientific research committee of the faculty of medicine, Cairo university (code: MD-228-2020) and performed in accordance with the ethical standards laid down in 1964 Declaration of Helsinki and its later amendments. Written informed consent was obtained from the parents of all the children included in the study.
2.1. Statistical analysis
Data were fed to the computer and analyzed using IBM SPSS software package version 20.0. (Armonk, NY: IBMCorp). Qualitative data were described using number and percent. The Kolmogorov–Smirnov test was used to verify the normality of distribution. Quantitative data were described using range (minimum and maximum), mean, standard deviation, median and interquartile range (IQR). Significance of the comparison was judged at the 5% level. The used tests were: 1-Chi-square test: For categorical variables, to compare between different groups. 2-Monte Carlo correction: Correction for chi-square when more than 20% of the cells have expected count less than 5. 3-F-test (ANOVA): For normally distributed quantitative variables, to compare betweenmore than two groups. 4-Kruskal Wallis test: For non-normally distributed quantitative variables, to compare between more than two studied groups. 5-Marginal Homogeneity Test: Used to analyze the significance between the different stages. 6-Paired t-test: For normally distributed quantitative variables, to compare between two periods. 7-Wilcoxon signed ranks test: For non-normally distributed quantitative variables, to compare between two periods. 8-Student t-test; For normally distributed quantitative variables, to compare between two studied groups. 9-Mann Whitney test: For abnormally distributed quantitative variables, to compare between two studied groups. 10- Receiver operating characteristic curve (ROC): It is generated by plotting sensitivity (TP) on Y axis versus 1-specificity (FP) on X axis at different cut off values. The area under the ROC curve denotes the diagnostic performance of the test. Area more than 50% gives acceptable performance and area about 100% is the best performance for the test. The ROC curve allows also a comparison of performance between two tests. 11- Kaplan–Meier Survival curve was used, and cox regression was done for the significant relation with progression free survival and overall survival.
3. Results
Demographic and clinical features of the studied groups of PAH: the total number of patients included in the study was 71. The patients were classified into their different pulmonary hypertension groups as proposed by the 2018 World Symposium on Pulmonary Hypertension [9], 39 patients were in group 1.1 and 1.2 (idiopathic and heritable PAH), 27 patients were in group 1.4.4 (PH secondary to left to right shunt lesions) and 5 patients were in group 5 (miscellaneous causes). In group 1.4.4 which includes cases with pulmonary vascular disease secondary to left to right shunt lesions, 8 patients were secondary to VSD (29.6%), 7 patients were secondary to PDA (25.9%), 3 patients were secondary to CAVC (11.1%), 2 patients were secondary to VSD and PDA (7.4%), 2 patients were secondary to hypertensive Glenn anastomosis (7.4%) and 5 patients were secondary to unrepaired other complex congenital heart disease (18.5%). From the 27 patients in group 1.4.4, 20 were unrepaired (74%); 3 cases had progressive pulmonary vascular disease after PDA closure, 1 patient after CAVC repair, 1 patient after VSD closure, and 2 patients had hypertensive Glenn anastomosis (the first patient had a hypertensive glenn after palliation of DILV, LTGA, and large VSD status post pulmonary artery banding, and the other patient had a palliation of DORV, large VSD, and malposed great vessels status post pulmonary artery banding).
No statistically significant differences between the different groups for the demographic data of the studied cases. Eight patients were syndromic; six of them were down syndrome (4 patients in group 1.4.4 and 2 in group 1.1); one patient had Di-George syndrome; and one patient had mucopolysaccharidosis. Exertional dyspnea was the most common presentation (91.5%) followed by cyanosis (23.9 %) and recurrent syncopal attacks (19.3 %). Syncope occurred only in patients with idiopathic PAH, on the other hand, cyanosis was observed mostly in group 1.4.4. Fifty-three patients (74.6%) did the 6MWT, the median value of the 6MWD was 300 m (IQR 150–390) without statistically significant difference between the different groups (Table 1). Table 2 describes the different echocardiographic parameters in various groups of PAH.
Table 1.
Demographic and clinical data of the studied patients.
| All patients N = 71 |
Groups | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Group 1.1 and 1.2 N = 39 |
Group 1.4.4 N = 27 |
Group 5 N = 5 |
|||||||
| Age | |||||||||
| Minimum–maximum | 5.00–228.00 | 12.00–228.00 | 5.00–216.00 | 24.00–144.00 | 0.75 | ||||
| Mean – SD | 91.04 ± 52.34 | 86.97 ± 47.68 | 99.11 ± 59.58 | 79.20 ± 49.91 | |||||
| Median | 84.00 | 84.00 | 84.00 | 84.00 | |||||
| IQR | 54.00 to 130.50 | 46.50 to 120.00 | 54.50 to 162.00 | 33.00 to 117.00 | |||||
| Gender | No. | % | No. | % | No. | % | No. | % | |
| Male | 29 | 40.8 | 17 | 43.6 | 10 | 37 | 2 | 40 | 0.87 |
| Female | 42 | 59.2 | 22 | 56.4 | 17 | 63 | 3 | 60 | |
| Height | |||||||||
| Minimum–maximum | 62.00–159.00 | 74.00–159.00 | 62.00–147.00 | 81.00–130.00 | 0.78 | ||||
| Mean – SD | 115.21 ± 22.94 | 116.35 ± 22.97 | 113.92 ± 23.75 | 113.00 ± 22.43 | |||||
| Median | 117.00 | 119.00 | 110.00 | 120.50 | |||||
| IQR | 97.50 to 132.00 | 100.00 to 132.00 | 95.00 to 140.00 | 97.50 to 128.50 | |||||
| Weight | |||||||||
| Minimum–maximum | 4.50–60.00 | 8.00–60.00 | 4.50–45.00 | 6.50–33.00 | 0.88 | ||||
| Mean – SD | 22.47 ± 11.88 | 22.72 ± 12.74 | 22.57 ± 11.04 | 19.90 ± 11.15 | |||||
| Median | 20.00 | 20.00 | 22.00 | 21.00 | |||||
| IQR | 13.00 to 29.50 | 13.25 to 27.25 | 13.00 to 30.00 | 9.87 to 29.25 | |||||
| Syncope | No. | % | No. | % | No. | % | No. | % | |
| No syncope | 58 | 81.7 | 26 | 66.7 | 27 | 100 | 5 | 100 | a0.0015 |
| Syncope | 13 | 19.3 | 13 | 33.3 | 0 | 0 | 0 | 0 | |
| Cyanosis | No. | % | No. | % | No. | % | No. | % | |
| No cyanosis | 54 | 76.1 | 37 | 94.9 | 12 | 44.4 | 5 | 100 | a<0.0001 |
| Cyanosis | 17 | 23.9 | 2 | 5.1 | 15 | 55.6 | 0 | 0 | |
| Dyspnea | No. | % | No. | % | No. | % | No. | % | |
| No Dyspnea | 6 | 8.5 | 3 | 8 | 3 | 11.1 | 0 | 0 | 0.69 |
| Dyspnea | 65 | 91.5 | 36 | 92.3 | 24 | 88.9 | 5 | 100 | |
| WHO FC | No. | % | No. | % | No. | % | No. | % | |
| WHO FC 1 | 8 | 11.3 | 5 | 12.8 | 3 | 11.1 | 0 | 0 | 0.06 |
| WHO FC 2 | 39 | 54.9 | 16 | 41 | 21 | 77.8 | 2 | 40 | |
| WHO FC 3 | 14 | 19.7 | 11 | 28.2 | 1 | 3.7 | 2 | 40 | |
| WHO FC 4 | 10 | 14.1 | 7 | 18 | 2 | 7.4 | 1 | 20 | |
| O 2 Sat | |||||||||
| Minimum–maximum | 0.65–1.00 | 0.70–1.00 | 0.65–0.99 | 0.88–0.96 | a0.000159 | ||||
| Mean – SD | 0.92 ± 0.075 | 0.94 ± 0.06 | 0.88 ± 0.08 | 0.93 ± 0.038 | |||||
| Median | 0.94 | 0.96 | 0.89 | 0.94 | |||||
| IQR | 0.88 to 0.97 | 0.94 to 0.98 | 0.86 to 0.91 | 0.89 to 0.96 | |||||
| Clubbing | No. | % | No. | % | No. | % | No. | % | |
| No clubbing | 57 | 80.3 | 37 | 94.8 | 16 | 59.3 | 4 | 80 | a0.0055 |
| Grade 1 | 6 | 6 | 2 | 5.1 | 4 | 14.8 | 0 | 0 | |
| Grade 2 | 8 | 11.3 | 0 | 0.0 | 7 | 25.9 | 1 | 20 | |
| RV failure | No. | % | No. | % | No. | % | No. | % | |
| No RV failure | 48 | 67.6 | 24 | 61.5 | 23 | 85.2 | 1 | 20 | *0.0081 |
| RV failure | 23 | 32.4 | 15 | 38.5 | 4 | 14.8 | 4 | 80 | |
| 6MWD | |||||||||
| Minimum–maximum | 40.00–540.00 | 40.00–450.00 | 60.00–540.00 | 100.00–418.00 | 0.17 | ||||
| Mean – SD | 273.32 ± 139.99 | 242.73 ± 136.38 | 316.80 ± 137.05 | 289.33 ± 167.45 | |||||
| Median | 300.00 | 256.00 | 355.00 | 350.00 | |||||
| IQR | 150.00 to 390.00 | 100.00 to 380.00 | 180.00 to 413.00 | 162.50 to 401.00 | |||||
| 6MWD (age adjusted) | |||||||||
| Minimum–maximum | −10.00–0.50 | −10.00–0.50 | −10.00–−0.40 | −9.56–−2.40 | 0.33 | ||||
| Mean – SD | −4.89 ± 2.72 | −5.31 ± 2.65 | −4.17 ± 2.70 | −5.45 ± 3.69 | |||||
| Median | −4.88 | −5.38 | −4.11 | −4.40 | |||||
| IQR | −6.84 to −2.86 | −7.25 to −2.98 | −4.93 to −2.21 | −8.27 to −2.90 | |||||
| 6MWD (height adjusted) | |||||||||
| Minimum–maximum | −9.50–1.48 | −9.28–1.00 | −9.50–1.48 | −7.90–−2.70 | 0.40 | ||||
| Mean – SD | −4.20 ± 2.53 | −4.57 ± 2.40 | −3.59 ± 2.69 | −4.52 ± 2.92 | |||||
| Median | −3.59 | −4.41 | −3.09 | −2.98 | |||||
| IQR | −5.92 to −2.68 | −6.35 to −2.69 | −4.17 to −2.57 | −6.67 to −2.77 | |||||
| NT Pro-BNP | |||||||||
| Minimum–maximum | 20.00–15000.00 | 20.00–15000.00 | 25.20–15000.00 | 479.80–5019.00 | 0.58 | ||||
| Mean – SD | 3553.79 ± 4101.02 | 4101.82 ± 4140.68 | 3107.19 ± 4305.48 | 1910.12 ± 1900.59 | |||||
| Median | 1982.00 | 2405.40 | 1234.10 | 1033.00 | |||||
| IQR | 373.92 to 5472.00 | 304.37 to 6160.40 | 368.37 to 4423.47 | 571.30 to 3067.50 | |||||
| NT Pro-BNP z score | |||||||||
| Minimum–maximum | −1.48–6.99 | −1.48–6.23 | −0.69–6.99 | 2.51–5.18 | 0.51 | ||||
| Mean – SD | 3.57 ± 1.91 | 3.69 ± 2.09 | 3.42 ± 1.82 | 3.50 ± 1.08 | |||||
| Median | 3.94 | 4.39 | 3.33 | 3.00 | |||||
| IQR | 2.42 to 5.07 | 1.84 to 5.38 | 2.60 to 4.76 | 2.77 to 4.25 | |||||
| Outcome | No. | % | No. | % | No. | % | No. | % | |
| Morbidity | 16 | 22.5 | 8 | 20.5 | 5 | 18.5 | 3 | 60 | 0.32 |
| Mortality | 17 | 23.9 | 10 | 25.6 | 6 | 22.2 | 1 | 20 | |
p: p value for comparing between the different studied groups.
Statistically significant at p ≤ 0.05.
Table 2.
Echocardiographic data of the studied patients.
| All patients N = 71 |
Groups | P value | |||
|---|---|---|---|---|---|
|
| |||||
| Idiopathic and heritable pulmonary hypertension (group 1.1 and 1.2) N = 39 |
PH secondary to CHD (group 1.4.4) N = 27 |
PH secondary to miscellaneous causes (group 5) N = 5 |
|||
| TR PG | |||||
| Minimum–maximum | 40.00–160.00 | 40.00–160.00 | 45.00–115.00 | 55.00–154.00 | 0.23 |
| Mean – SD | 84.02 ± 28.71 | 85.71 ± 30.70 | 76.39 ± 19.11 | 99.80 ± 39.50 | |
| Median | 78.00 | 82.00 | 70.00 | 100.00 | |
| IQR | 60.25 to 102.50 | 59.25 to 103.75 | 63.35 to 90.25 | 66.25 to 128.50 | |
| PR PG | |||||
| Minimum–maximum | 2.60–70.00 | 17.00–70.00 | 2.60–60.00 | 19.00–61.00 | 0.91 |
| Mean – SD | 36.28 ± 12.89 | 35.91 ± 12.59 | 36.31 ± 12.98 | 38.60 ± 17.04 | |
| Median | 37.00 | 35.00 | 39.00 | 40.00 | |
| IQR | 25.50 to 44.75 | 25.00 to 43.75 | 27.75 to 44.25 | 23.50 to 51.25 | |
| MPA | |||||
| Minimum–maximum | 14.00–43.00 | 14.00–35.00 | 14.00–43.00 | 17.00–25.00 | 0.06 |
| Mean – SD | 24.22 ± 5.348 | 23.37 ± 4.56 | 26.21 ± 6.33 | 21.60 ± 2.97 | |
| Median | 24.00 | 24.00 | 25.50 | 22.00 | |
| IQR | 21.00 to 27.00 | 20.00 to 26.00 | 21.50 to 29.00 | 20.00 to 23.50 | |
| MPA z score | |||||
| Minimum–maximum | 0.00–5.40 | 0.00–4.39 | 0.20–5.40 | 0.66–4.30 | 0.19 |
| Mean – SD | 2.31 ± 1.28 | 2.11 ± 1.11 | 2.71 ± 1.48 | 2.03 ± 1.35 | |
| Median | 2.20 | 2.16 | 2.50 | 1.74 | |
| IQR | 1.41 to 3.12 | 1.22 to 2.81 | 1.85 to 3.91 | 1.44 to 2.40 | |
| RA area | |||||
| Minimum–maximum | 4.30–40.60 | 5.00–40.60 | 4.30–25.50 | 5.50–11.50 | 0.05 |
| Mean – SD | 13.35 ± 6.97 | 14.87 ± 7.82 | 11.79 ± 5.33 | 8.88 ± 2.19 | |
| Median | 11.65 | 12.90 | 11.30 | 9.00 | |
| IQR | 9.00 to 15.60 | 10.20 to 16.40 | 7.57 to 14.47 | 7.82 to 10.22 | |
| RA area z score | |||||
| Minimum–maximum | −1.00–10.30 | −0.90–10.30 | −1.00–5.00 | 0.00–4.90 | *0.02 |
| Mean – SD | 2.60 ± 2.13 | 3.24 ± 2.28 | 1.80 ± 1.52 | 1.57 ± 2.05 | |
| Median | 2.50 | 2.78 | 1.90 | 0.87 | |
| IQR | 0.97 to 3.59 | 1.83 to 4.54 | 0.42 to 2.95 | 0.00 to 2.80 | |
| RV/LV ratio | |||||
| Minimum–maximum | 0.40–2.40 | 0.40–2.40 | 0.42–1.42 | 0.78–1.40 | *0.001 |
| Mean – SD | 1.13 ± 0.50 | 1.30 ± 0.53 | 0.82 ± 0.30 | 1.08 ± 0.28 | |
| Median | 1.00 | 1.15 | 0.77 | 0.95 | |
| IQR | 0.77 to 1.40 | 0.90 to 1.75 | 0.63 to 0.97 | 0.89 to 1.37 | |
| RVEDD | |||||
| Minimum–maximum | 1.30–5.10 | 1.30–5.10 | 1.60–4.10 | 2.00–3.50 | 0.09 |
| Mean – SD | 3.00 ± 0.79 | 3.18 ± 0.83 | 2.74 ± 0.68 | 2.78 ± 0.68 | |
| Median | 3.00 | 3.20 | 2.60 | 2.80 | |
| IQR | 2.50 to 3.50 | 2.70 to 3.70 | 2.30 to 3.10 | 2.15 to 3.42 | |
| RVEDD z score | |||||
| Minimum–maximum | 1.80–18.60 | 1.80–18.60 | 2.90–12.80 | 5.00–10.00 | 0.07 |
| Mean – SD | 8.35 ± 3.31 | 9.14 ± 3.52 | 7.15 ± 2.80 | 7.63 ± 2.34 | |
| Median | 8.30 | 8.71 | 6.97 | 7.44 | |
| IQR | 5.92 to 10.50 | 7.00 to 11.70 | 5.00 to 9.10 | 5.52 to 10.00 | |
| RVOT acceleration time | |||||
| Minimum–maximum | 40.00–113.00 | 40.00–105.00 | 60.00–113.00 | 46.00–78.00 | 0.30 |
| Mean – SD | 71.33 ± 13.82 | 69.90 ± 14.16 | 74.78 ± 13.26 | 66.60 ± 12.759 | |
| Median | 72.00 | 70.00 | 72.00 | 72.00 | |
| IQR | 61.25 to 80.00 | 61.00 to 79.50 | 66.25 to 80.75 | 58.75 to 75.00 | |
| TAPSE | |||||
| Minimum–maximum | 9.00–28.00 | 9.00–25.00 | 9.00–28.00 | 11.00–19.00 | 0.17 |
| Mean – SD | 16.12 ± 4.73 | 15.49 ± 4.51 | 17.56 ± 5.151 | 14.40 ± 3.21 | |
| Median | 16.00 | 15.00 | 17.00 | 14.00 | |
| IQR | 12.00 to 19.00 | 12.00 to 18.00 | 14.00 to 21.75 | 11.75 to 16.75 | |
TR PG: tricuspid regurge pressure gradient, PR PG: pulmonary regurge pressure gradient, MPA: main pulmonary artery, RV/LV ratio: right ventricle/left ventricle ratio, RVEDD: right ventricular end diastolic diameter, RA area: right atrial area, TAPSE: tricuspid annular plane systolic excursion, RVOT acceleration time: right ventricular outflow tract acceleration time. p: p value for comparing between the different studied groups.
Statistically significant at p ≤ 0.05.
In our study, 44 patients (61.9%) underwent cardiac catheterization, 22 patients from group 1.1 and 1.2 (56.4%) and 22 patients from group 1.4.4 (81.48%), of the 44 patients, 5 patients (11.36%) developed pulmonary hypertensive crises during the procedure.
In our cohort, 3 patients (4 %) received monotherapy (Sildenafil), 65 patients (92%) received dual therapy (Sildenafil and Bosentan).
Outcome of the studied groups are either mortality, morbidity (We defined the morbidity as patients with frequent hospital admissions per year (two times or more), or patients who developed a catastrophic event (as a hypoxic-ischemic insult) during the period of the study) or good outcome without hospital admissions during the period of the study. In our series, we reported 17 mortality (23.9%) and 16 morbidity (22.5%).
Predictors of the morbidity identified by univariate analysis were WHO functional class of PH, RV failure, NT pro-BNP and its z score, TAPSE and 6MWT (Table 3). By analyzing the prognostic performance of the ROC curve, the cut off values for the factors affecting the morbidity were; WHO functional class 3 and 4 (AUC = 0.835, P < 0.001, sensitivity 75%, specificity 92.11%), RV failure (AUC = 0.755, P < 0.001, sensitivity 56.25%, specificity 94.74%), TAPSE ≤15 (AUC = 0.776, P < 0.001, sensitivity 75%, specificity 77.14%), NT-ProBNP >1032 (AUC = 0.755, P < 0.001, sensitivity 80%, specificity 62.16%) and NT-proBNP z score >3.5 (AUC = 0.737, P = 0.001, sensitivity 73.33%, specificity 67.57%) (Table 4).
Table 3.
Univariate and multivariate logistic Regression analysis for Factors affecting the morbidity and the mortality.
| Mortality | Morbidity | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Univariate | #Multivariate | Univariate | #Multivariate | |||||
|
|
|
|
|
|||||
| P | OR (LL – UL 95 %C.I) | p | OR (LL – UL 95% C.I) | p | OR (LL – UL 95% C.I) | p | OR (LL – UL 95% C.I) | |
| Age in months | 0.4261 | 0.9956 (0.9847–1.0066) | 0.0783 | 0.9891 (0.9763–1.0020) | ||||
| Gender | 0.2476 | 0.5229 (0.1739–1.5723) | 0.5099 | 0.6686 (0.2027–2.2057) | ||||
| WHO functional class | *0.0005 | 3.2066 (1.5764–6.5226) | 0.5274 | 0.5243 (0.0708–3.8832) | *< 0.0001 | 13.5533 (3.3358–55.0665) | 0.5426 | 1.9542 (0.2262–16.8859) |
| Syncope | *0.0491 | 3.6623 (1.0256–13.0777) | 0.2879 | 5.4458 (0.2391–124.0372) | 0.9474 | 0.9429 (0.1630–5.4528) | ||
| Seizures | 0.2439 | 3.4667 (0.4497–26.7261) | 0.5385 | 2.4667 (0.1447–42.0521) | ||||
| Cyanosis | 0.5509 | 1.4583 (0.4285–4.9633) | 0.2452 | 0.4000 (0.0770–2.0790) | ||||
| 6MWD | *0.0074 | 0.9930 (0.9875–0.9986) | 0.6449 | 1.0052 (0.9834–1.0274) | *0.0327 | 0.9939 (0.9881–0.9998) | 0.3386 | 0.9959 (0.9876–1.0043) |
| 6MWD (age) | 0.1952 | 0.8486 (0.6593–1.0923) | 0.1542 | 0.8122 (0.6062–1.0880) | ||||
| 6MWD (height) | 0.0598 | 0.7722 (0.5850–1.0194) | 0.4564 | 0.6314 (0.1882–2.1177) | 0.1116 | 0.7862 (0.5796–1.0665) | ||
| O2 Saturation | 0.7885 | 0.3762 (0.0003–449.1007) | 0.9952 | 1.0249 (0.0003–3026.7422) | ||||
| RV failure | *0.0002 | 9.3818 (2.7266–32.2818) | 0.1738 | 14.9072 (0.3036–732.0509) | *< 0.0001 | 23.1429 (4.0907–130.9280) | 0.0670 | 26.1405 (0.7952–859.2838) |
| NT pro-BNP | *< 0.0001 | 1.0003 (1.0001–1.0005) | 0.8415 | 1.0000 (0.9996–1.0005) | *0.0033 | 1.0003 (1.0001–1.0006) | 0.7580 | 0.9999 (0.9994–1.0005) |
| NT pro-BNP z score | *0.0001 | 2.3149 (1.3764–3.8934) | 0.6701 | 1.3818 (0.3121–6.1182) | *0.0041 | 1.7964 (1.1300–2.8559) | 0.5431 | 1.3759 (0.4920–3.8477) |
| MPA | 0.9717 | 1.0020 (0.8993–1.1163) | 0.4606 | 0.9594 (0.8575–1.0735) | ||||
| MPA z score | 0.2883 | 1.2760 (0.8125–2.0038) | 0.3338 | 1.2556 (0.7899–1.9958) | ||||
| PR PG | 0.8842 | 0.9966 (0.9519–1.0434) | 0.5135 | 1.0169 (0.9667–1.0697) | ||||
| RA area | 0.0634 | 1.0757 (0.9941–1.1640) | 0.2114 | 0.9243 (0.8071–1.0585) | ||||
| RA area z score | *0.0389 | 1.3230 (1.0029–1.7453) | 0.6403 | 1.1693 (0.6068–2.2532) | 0.1137 | 1.3380 (0.9218–1.9421) | ||
| RV/LV ratio | *0.0016 | 6.5538 (1.8511–23.2034) | 0.6227 | 0.2841 (0.0019–42.7284) | 0.7265 | 1.2932 (0.3070–5.4473) | ||
| RVEDD | *0.0011 | 3.8066 (1.5286–9.4797) | 0.3989 | 0.0183 (0.0000–199.6208) | 0.6669 | 1.1991 (0.5241–2.7435) | ||
| RVEDD z score | *0.0003 | 1.4460 (1.1456–1.8252) | 0.2473 | 3.2703 (0.4393–24.3434) | 0.2480 | 1.1306 (0.9156–1.3961) | ||
| TAPSE | *0.0025 | 0.7985 0.6745 to 0.9454 |
0.8317 | 0.9597 (0.6567–1.4025) | *0.0013 | 0.7878 0.6656 to 0.9325 |
0.4878 | 1.1144 (0.8206–1.5135) |
| TR PG | 0.2223 | 0.9868 (0.9650–1.0091) | 0.8754 | 0.9984 (0.9780–1.0192) | ||||
OR: Odd’s ratio C.I: Confidence interval LL: Lower limit UL:Upper Limit.
TR PG: tricuspid regurge pressure gradient, PR PG: pulmonary regurge pressure gradient, MPA: main pulmonary artery diameter, RVEDD: right ventricular end diastolic diameter, RA area: right atrial area, TAPSE: tricuspid annular plane systolic excursion, NT pro-BNP: N terminal pro-brain natriuretic peptide, TAPSE: Tricuspid annular plane systolic excursion.
All variables with p < 0.05 was included in the multivariate analysis
Statistically significant at p ≤ 0.05.
Table 4.
Prognostic performance of the ROC curve to predict the morbidity and mortality.
| AUC | P | 95 % C.I | Cut off value | Sensitivity % | Specificity % | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|
| Mortality | ||||||||
| 6MWD | 0.739 | *0.0102 | 0.600 to 0.850 | ≤210 | 72.73 | 73.81 | 42.1 | 91.2 |
| 6MWD adjusted to height | 0.669 | 0.07 | 0.526 to 0.792 | ≤−5.11 | 54.55 | 76 | 37.5 | 86.5 |
| NT pro BNP | 0.809 | *<0.0001 | 0.696 to 0.894 | >4734 | 76.47 | 80.77 | 56.5 | 91.3 |
| NT pro-BNP z score | 0.802 | *<0.0001 | 0.689 to 0.888 | >4.57 | 76.47 | 73.08 | 48.1 | 90.5 |
| WHO Functional class | 0.716 | *0.0024 | 0.597 to 0.817 | >3 | 47.03 | 96.3 | 80.0 | 85.2 |
| RA area z score | 0.630 | 0.167 | 0.502 to 0.749 | >4.9 | 37.50 | 95.9 | 75.0 | 82.5 |
| RV/LV ratio | 0.733 | *0.0014 | 0.608 to 0.835 | >0.85 | 93.75 | 44.90 | 35.7 | 95.7 |
| TAPSE | 0.733 | *0.0002 | 0.611 to 0.834 | ≤15 | 93.75 | 49.02 | 36.6 | 96.2 |
| RV failure | 0.751 | *<0.0001 | 0.634 to 0.846 | 70.59 | 79.63 | 52.2 | 89.6 | |
| Morbidity | ||||||||
| WHO Functional class | 0.835 | *<0.0001 | 0.709 to 0.922 | >2 | 75.00 | 92.11 | 80 | 89.7 |
| 6MWD | 0.683 | *0.1101 | 0.521 to 0.818 | ≤220 | 70.00 | 84.37 | 58.3 | 90 |
| RV failure | 0.755 | *0.0001 | 0.619 to 0.862 | 56.25 | 94.74 | 81.8 | 83.7 | |
| TAPSE | 0.776 | *0.0003 | 0.637 to 0.881 | ≤15 | 75.00 | 77.14 | 60.0 | 87.1 |
| NT pro-BNP | 0.755 | *0.0008 | 0.616 to 0.864 | >1032 | 80.00 | 62.16 | 46.2 | 88.5 |
| NT pro BNP z score | 0.737 | *0.0019 | 0.596 to 0.849 | >3.5 | 73.33 | 67.57 | 47.8 | 86.2 |
AUC: Area under the curve, 6MWD: 6 min walking distance, NT pro-BNP: N terminal pro-brain natriuretic peptide, RA area: right atrial area, RVEDD: right ventricular end diastolic diameter, TAPSE: Tricuspid annular plane systolic excursion.
Statistically significant at p ≤ 0.05.
Predictors of the mortality identified by univariate analysis were WHO class of PH, syncope, RV failure, 6MWT, TAPSE, RA and RV dilatation, NT pro-BNP and it’s Z score (Table 3). By analyzing the prognostic performance of the ROC curve, the factors that affect the mortality were; 6MWD ≤210 m (AUC = 0.739, P = 0.01, sensitivity 72.73%, specificity 73.81%),WHO functional 4 (AUC = 0.716, P = 0.002, sensitivity 47%, specificity 96.3%), RV failure (AUC = 0.751, P < 0.001, sensitivity 70.59%, specificity 79.63%), TAPSE ≤15 (AUC = 0.733, P < 0.001, sensitivity 93.75%, specificity 49%), NT-ProBNP >4734 (AUC = 0.809, P < 0.001, sensitivity 76.47%, specificity 80.77%) and NT-proBNP z score >4.57 (AUC = 0.802, P = 0.001, sensitivity 76.47%, specificity 73.08%). RV/LV ratio >0.85 and TAPSE less than 15mm had low specificity as a cut off values for mortality prediction (Table 4).
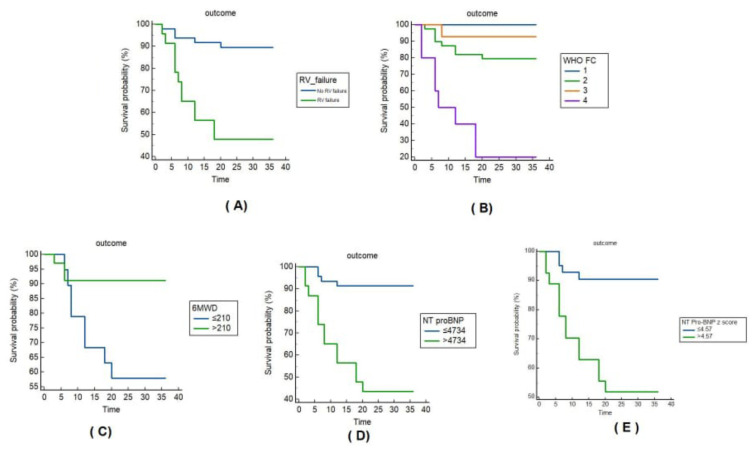
Survival probability of the different groups using Kaplan–Meier survival curves are shown in Fig. 1. Figure 1A shows the survival probability related to the presence of RV failure; the mean survival was 33.2 months for the patients without RV failure (the 3-yerar survival rate was 89.6%) and 21.8 months in patients complaining of RV failure (the 3-year survival rate was 47.8%), log-rank chi-squared = 15.936, P < 0.001. Figure 1B shows the survival probability of the different WHO functional classes; mean survival was 36 months for class 1 (the 3-yerar survival rate was 100%) and 14 months for class IV (the 3-yerar survival rate was 20%), log-rank chi-squared = 28.322, P < 0.001. Figure 1C shows Kaplan–Meier survival curves related to the 6MWD, the mean survival was 33.3 months (the 3-yerar survival rate was 91.2%) in patients with 6MWD>210 m and 25.6 months (the 3-yerar survival rate was 57.9%) in patients with 6MWD <210 m, log-rank chi-squared 7.447, P = 0.006. Figure 1D shows Kaplan–Meier survival curve related to the level of NT pro-BNP test, the mean survival was 33.5 months (the 3-year survival rate was 91.3%) for the patients with serum levels <4734 pg/ml and 20.9 months (the 3-yerar survival rate was 43.5%) in patients with serum levels above 4734 pg/ml, log-rank chi-squared = 20.280, P < 0.001. Figure 1E shows Kaplan–Meier survival curve related to the z score of the NT pro-BNP, the mean survival was 33.3 months (the 3-yerar survival rate was 90.48%) for the patients with z score <4.57 and 23.1 months (the 3-yerar survival rate was 51.85%) in patients with z score above 4.57, log-rank chi-squared = 13.536, P < 0.001.
Fig. 1.
Kaplan–Meier survival curves studied group. A: Survival curves in relation to the RV failure, B: Survival curves in relation to the WHO functional classes, C: survival curves in relation to the 6MWD, D: survival curves related to the level of N terminal pro-BNP test, E: survival curves related to the z score of the N terminal pro-BNP.
4. Discussion
This study is peculiar by the inclusion of large number of patients of idiopathic pulmonary hypertension in the pediatric age within short duration. Moreover, this registry sheds light on the morbidity and mortality related outcomes associated with pediatric PAH and the influence of clinical assessment, 6MWD and NT pro-BNP on the prognosis of this serious disease. As our unit became a referral center for PH in Egypt, our registry included 71 patients with PH; 39 (54.9%) of them were IPAH presented within relatively very short duration (20 months) with close follow-up for a period of 36 months. However, The TOPP (Tracking Outcomes and Practice in Pediatric Pulmonary Hypertension) registry which is a prospective multicenter study involving 55 centers in 19 countries between 2008 and 2010, showed that more than half of patients were diagnosed with idiopathic and hereditary pulmonary hypertension (243/317; 57%) [16]. Also, the Registry of Evaluate Early and Long-term PAH Disease Management (REVEAL registry), a multicenter study conducted in the United States showed that IPAH, HPAH were diagnosed in 122 of 216 (56%) patients [17]. Also, in Beijing Anzhen Hospital study, 42 out of 82 patients were diagnosed as HPAH from September 2008 to December 2018 [18]. Genetic testing was not performed routinely in our institute and in the majority of the published reports, which might be that HPAH was underestimated and misclassified as IPAH.
In our cohort, the median age of our patients was 7 years which was consistent with previous publications of various ethnicities, such as in the TOPP registry [16]. However, older ages were reported in the Polish and Dutch national registries of pediatric PAH [2,4]. As regards the presenting symptoms, dyspnea on exertion was the most common which is consistent finding with most of the registries [4,16]. Regarding the WHO functional class of pulmonary hypertension, the majority of our cohort (66.2%) had class I–II which is in harmony with the previous publications [16,17]. In contrary to The Dutch national registry reported more patients (66%) falling into class III–IV [4]. In our cohort, the mean value of 6MWT for all patients was 273.3 ± 139.9 m, which was lower than other registries. TOPP, REVEAL, and the Polish registries reported a 6MWT above 400 m [2,16,17]. Shorter 6MWD combined with lower transcutaneous oxygen saturations during the 6MWT correlated with higher WHO-FC and NT pro-BNP levels and worse transplant-free outcomes in pediatric PH patients [5].
Our patients had higher levels of NT pro-BNP, with a median level of 1982 pg/ml compared to the Dutch National Network of Pulmonary Hypertension and the Polish registry where the median level were 606 and 272 pg/ml respectively [2,4]. It was reported that NT pro-BNP may be a useful adjunct for the diagnosis of PAH and a value > 389 pg/ml being 87% specific for the presence of a mean pulmonary artery pressure >20 mmHg by cardiac catheterization and become 93% specific by addition of pulmonary artery acceleration time. In addition, echo measures of TAPSE z score, pulmonary artery acceleration time, and RV fractional area change are abnormal in patients with pulmonary hypertension and has negative correlations with NT pro-BNP values [19].
Regarding the treatment of pulmonary hypertension in our cohort, the quality of life and survival have improved significantly. Targeted pulmonary vasodilator therapies, including Endothelin receptor antagonists and Phosphodiesterase type 5 Inhibitors have resulted in hemodynamic and functional improvement in children. The management of pediatric PAH remains challenging as treatment decisions depend largely on results from evidence-based adult studies and the clinical experience of pediatric experts [20]. In our series, the main line of treatment was dual pulmonary vasodilating therapy as 65/71 (91%) patients received Sildenafil and Bosentan. By revising the studies of pediatric PH, the use of dual and triple pulmonary vasodilating agents had increased in the last years. In the analysis of incident PAH patients enrolled in the multinational TOPP registry, at the time of diagnosis, the main line of treatment was the monotherapy, and the double and triple combination therapies were applied to only 18% and 2.3% of patients, respectively [21]. On the other hand, more recent data from the Netherlands (June 2013–March 2016) showed an increased use of double and triple combination therapies in PAH children [15 (50%) and five (17%), respectively] [22]. Early combination therapy with two oral PAH-targeted drugs in newly diagnosed (treatment-naïve) children with PAH in WHO functional class II–III is reasonable [20,23,24]. It was reported that WHO-FC deterioration, PAH-related hospitalization, occurrence/worsening of ≥2 PAH symptoms may be important for risk assessment during clinical management [25]. Unlike what is seen among adult patients, pediatric PAH is intrinsically linked to lung growth and development. Because of this, the young child with pulmonary hypertension has greater potential for pulmonary vascular disease reversal, particularly in the population of patients with PH secondary to lung disease [26].
In our series, we reported 17 mortality cases (23.9%) which are lower compared to the Dutch National Network of Pulmonary Hypertension with 38% mortality [4]. The predictors of 16 (22.5%) morbidity cohort were WHO functional class of PH, RV failure, NT pro-BNP, TAPSE and 6MWT. On the other hand, it was previously published that WHO-FC at diagnosis was not the predictive of mortality, but the response to therapy as assessed by change in FC over time and FC at last visit was associated with morbidity and mortality [26]. It was reported that children with syncope were older at diagnosis and had a higher incidence of chest pain and fatigue [27]. Syncope is thought to occur due to RV dysfunction and inability to augment cardiac output particularly during exertion. Based on these findings, studies of children with PAH list syncope as a high risk factor, with significant implications for functional classification and treatment selection [28]. In the current work, WHO class of PH, syncope, RV failure, 6MWT, TAPSE, RV dilatation, NT pro-BNP and its Z score were found as predictors of mortality. However, in Dutch National Network of PH, WHO functional class IV, NT pro-BNP >1200 pg/ml and TAPSE less than 12 mm were the predictors of worse outcome. In addition, independent of its absolute value, an increase of >10% in serially measured NT pro-BNP serum levels over time predicted an adverse outcome [4]. Large registry studies have not shown 6MWD to be predictor of survival [29,30]. In particular, a combination of functional class, 6MWD and brain natriuretic peptide (BNP) or NT pro-BNP was found to have strong prognostic value, both at the time of diagnosis and even more during follow-up, i.e. after initiation of targeted therapies [31,32]. In children, as in adults, clinical risk stratification tools composed of various clinical and biochemical variables (including NT pro-BNP) as proposed in guidelines based on expert opinion are of clinical value in risk stratification and prognostication both at diagnosis and at follow-up [33].
4.1. Limitations of the study
Being a single center study, the limited data for the pediatric age group of pulmonary hypertension regarding the guidelines for the use of new targeted pulmonary vasodilating agents. The COVID-19 pandemic at the start of the study limited the number of patients and hindered the follow-up of the diagnosed cases during this period.
5. Conclusion
Predictors of morbidity in pediatric PAH are the functional class 3 and 4, RV failure, NT pro-BNP >1032 and its z score >3.5, TAPSE less than 15 mm. WHO class 4, syncope, RV failure, 6MWT ≤210, NT pro-BNP >4734 pg/ml and its Z score >4.57 are the predictors of mortality in pediatric PAH. Through this registry, the national database of PAH is feasible for the benefit of understanding epidemiology, burdens to treatment and survival, and knowledge base of PHN in Egypt.
Acknowledgment
The authors thank the great efforts of Mr Amgad Aly Hamza for his contribution in the statistical analysis of our data.
Abbreviations
- CHD
Congenital Heart Disease
- CT
Computed Tomography
- IPAH
Idiopathic pulmonary arterial hypertension
- LV
Left ventricle
- MRI
Magnetic resonance imaging
- MSCT
Multislices Computed Tomography
- NT
pro-BNP N-terminal pro-Brain Natriuretic Peptides
- PAH
Pulmonary arterial hypertension
- PAH-CHD
Pulmonary arterial hypertension related to Congenital Heart Disease
- PVR
pulmonary vascular resistance
- Qp/Qs
Pulmonary to systemic flow
- Rp/Rs
Ratio of pulmonary resistance to systemic resistance
- RA
Right atrium
- RHF
Right heart failure
- RV
Right ventricle
- RVEDD
Right ventricular end-diastolic dimension
- RVF
Right ventricular functions
- RVOT
Right ventricular outflow tract
- Sao2
Saturation of O2
- 6MWD
Six minutes-walk test
- TAPSE
Tricuspid annular plane systolic excursion
- WHO-FC
World health organization-functional class
Footnotes
Ethical approval: The study was approved by the ethical scientific research committee of the faculty of medicine, Cairo university (code: MD-228-2020).
Author contribution: Study conception and design: HA, RH.
Data collection: HA, RH, AG, AB, NA, RE, DAEA, AA, AA, NS.
Analysis and interpretation of results: HA, AG, ND.
Hala Agha and Ahmed Gamal wrote the main manuscript.
All authors reviewed the results and approved the final version of the manuscript.
Conflict of interest: There are no conflicts of interest to declare.
Funding: This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
References
- 1. Condon DF, Nickel NP, Anderson R, Mirza S, de Jesus Perez VA. The 6th World Symposium on Pulmonary Hypertension: what’s old is new. F1000Res. 2019 Jun 19;8:F1000 Faculty Rev-888. doi: 10.12688/f1000research.18811.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kwiatkowska J, Zuk M, Migdal A, Kusa J, Skiba E, Zygielo K, et al. Children and adolescents with pulmonary arterial hypertension: baseline and follow-up data from the polish registry of pulmonary hypertension (BNP-pl) J Clin Med. 2020;9(6):1717. doi: 10.3390/jcm9061717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. White RJ, Jerjes-Sanchez C, Bohns Meyer GM, Pulido T, Sepulveda P, Wang KY, et al. Combination therapy with oral treprostinil for pulmonary arterial hypertension. A double-blind placebo-controlled clinical trial. Am J Respir Crit Care Med. 2020;201(6):707–17. doi: 10.1164/rccm.201908-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Said F, Haarman MG, Roofthooft MTR, Hillege HL, Ploegstra MJ, Berger RMF. Serial measurements of N-terminal pro-B-type natriuretic peptide serum level for monitoring pulmonary arterial hypertension in children. J Pediatr. 2020 May;220:139–45. doi: 10.1016/j.jpeds.2020.01.001. . Epub 2020 Feb 27. [DOI] [PubMed] [Google Scholar]
- 5. Choi JH, Shin MJ, Lee BJ, Park JH. Exercise-induced desaturation during a six-minute walk test is associated with poor clinical outcomes inpatients with pulmonary arterialhypertension. Clin Hypertens. 2023 Dec 1;29(1):33. doi: 10.1186/s40885-023-00256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koestenberger M, Hansmann G. Echocardiographic estimation of elevated right ventricular afterload in preterm infants at risk for pulmonary hypertension: next steps. J Pediatr. 2018 Nov;202:335–6. doi: 10.1016/j.jpeds.2018.07.054. . Epub 2018 Aug 23. [DOI] [PubMed] [Google Scholar]
- 7.Jone PN.Echocardiographic assessment of pulmonary hypertension. Echocardiography in pediatric and congenital heart disease: from fetus to adult ed wiley. 2021. pp. 992–1010. [DOI]
- 8. Amdani SM, Mian MUM, Thomas RL, Ross RD. NT-pro BNP-A marker for worsening respiratory status and mortality in infants and young children with pulmonary hypertension. Congenit Heart Dis. 2018 Jul;13(4):499–505. doi: 10.1111/chd.12601. . Epub 2018 Mar 25. [DOI] [PubMed] [Google Scholar]
- 9. Rosenzweig EB, Abman SH, Adatia I, Beghetti M, Bonnet D, Haworth S, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019 Jan 24;53(1):1801916. doi: 10.1183/13993003.01916-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fuso L, Baldi F, Di Perna A. Therapeutic strategies in pulmonary hypertension. Front Pharmacol. 2011 Apr 20;2:21. doi: 10.3389/fphar.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010 Jul;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. . quiz 786–8. [DOI] [PubMed] [Google Scholar]
- 12. Gaulton JS, Mercer-Rosa LM, Glatz AC, Jensen EA, Capone V, Scott C, et al. Relationship between pulmonary artery acceleration time and pulmonary artery pressures in infants. Echocardiography. 2019 Aug;36(8):1524–31. doi: 10.1111/echo.14430. . Epub 2019 Jul 1. [DOI] [PubMed] [Google Scholar]
- 13. Joseph P, Oliveira RKF, Eslam RB, Agarwal M, Waxman AB, Systrom DM. Fick principle and exercise pulmonary hemodynamic determinants of the six-minute walk distance in pulmonary hypertension. Pulm Circ. 2020 Sep 11;10(3):2045894020957576. doi: 10.1177/2045894020957576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berghaus TM, Kutsch J, Faul C, von Scheidt W, Schwaiblmair M. The association of N-terminal pro-brain-type natriuretic peptide with hemodynamics and functional capacity in therapy-naive precapillary pulmonary hypertension: results from a cohort study. BMC Pulm Med. 2017 Dec 4;17(1):167. doi: 10.1186/s12890-017-0521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McNeal-Davidson A, Fournier A, Spigelblatt L, Saint-Cyr C, Mir TS, Nir A, et al. Value of amino-terminal pro B-natriuretic peptide in diagnosing Kawasaki disease. Pediatr Int. 2012 Oct;54(5):627–33. doi: 10.1111/j.1442-200X.2012.03609.x. . Epub 2012 May 30. [DOI] [PubMed] [Google Scholar]
- 16. Berger RM, Beghetti M, Humpl T, Raskob GE, Ivy DD, Jing ZC, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012 Feb 11;379(9815):537–46. doi: 10.1016/S0140-6736(11)61621-8. . Epub 2012 Jan 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barst RJ, McGoon MD, Elliott CG, Foreman AJ, Miller DP, Ivy DD. Survival in childhood pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Circulation. 2012 Jan 3;125(1):113–22. doi: 10.1161/CIRCULATIONAHA.111.026591. . Epub 2011 Nov 15. [DOI] [PubMed] [Google Scholar]
- 18. Zhang H, Kan J, Zhang C, Yang Z, Gu H, Fan F, et al. Long-term mortality after pulmonary artery denervation stratified by baseline functional class in patients with pulmonary arterial hypertension: long-term mortality after PADN stratified by functional class. Asia Interven. 2022 Mar;8(1):58–68. doi: 10.4244/AIJ-D-21-00033. . Epub 2022 Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dasgupta S, Bettermann E, Kelleman M, Kanaan U, Sachdeva R, Petit C, et al. N-terminal pro-B-type-natriuretic peptide as a screening tool for pulmonary hypertension in the paediatric population. Cardiol Young. 2021 Oct;31(10):1595–607. doi: 10.1017/S1047951121000585. . Epub 2021 Mar 2. [DOI] [PubMed] [Google Scholar]
- 20. Jone PN, Schäfer M, Pan Z, Ivy DD. Right ventricular-arterial coupling ratio derived from 3-dimensional echocardiography predicts outcomes in pediatric pulmonary hypertension. Circ Cardiovasc Imaging. 2019 Dec;12(1):e008176. doi: 10.1161/CIRCIMAGING.118.008176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Avitabile CM, Vorhies EE, Ivy DD. Drug treatment of pulmonary hypertension in children. Paediatr Drugs. 2020 Apr;22(2):123–47. doi: 10.1007/s40272-019-00374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Humpl T, Berger RMF, Austin ED, Fasnacht Boillat MS, Bonnet D, Ivy DD, et al. Treatment initiation in paediatric pulmonary hypertension: insights from a multinational registry. Cardiol Young. 2017 Aug;27(6):1123–32. doi: 10.1017/S1047951116002493. . Epub 2016 Dec 20. [DOI] [PubMed] [Google Scholar]
- 23. Douwes JM, Berger RMF. Pediatric pulmonary arterial hypertension: on the eve of growing up. Curr Opin Pulm Med. 2017 Sep;23(5):398–403. doi: 10.1097/MCP.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 24. Hansmann G. Pulmonary hypertension in infants, children, and young adults. J Am Coll Cardiol. 2017 May 23;69(20):2551–69. doi: 10.1016/j.jacc.2017.03.575. [DOI] [PubMed] [Google Scholar]
- 25. Beghetti M, Brand M, Berger RMF, Humpl T, Wheeler JG, Ivy DD, et al. Meaningful and feasible composite clinical worsening definitions in paediatric pulmonary arterial hypertension: an analysis of the TOPP registry. Int J Cardiol. 2019 Aug 15;289:110–5. doi: 10.1016/j.ijcard.2019.04.062. . Epub 2019 Apr 25. [DOI] [PubMed] [Google Scholar]
- 26. Beghetti M, Channick RN, Chin KM, Di Scala L, Gaine S, Ghofrani HA, et al. Selexipag treatment for pulmonary arterial hypertension associated with congenital heart disease after defect correction: insights from the randomised controlled GRIPHON study. Eur J Heart Fail. 2019 Mar;21(3):352–9. doi: 10.1002/ejhf.1375. . Epub 2019 Jan 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cerro MJ, Abman S, Diaz G, Freudenthal AH, Freudenthal F, Harikrishnan S, et al. A consensus approach to the classification of pediatric pulmonary hypertensive vascular disease: report from the PVRI Pediatric Taskforce, Panama 2011. Pulm Circ. 2011;1(2):286–98. doi: 10.4103/2045-8932.83456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Balkin EM, Olson ED, Robertson L, Adatia I, Fineman JR, Keller RL. Change in pediatric functional classification during treatment and morbidity and mortality in children with pulmonary hypertension. Pediatr Cardiol. 2016 Apr;37(4):756–64. doi: 10.1007/s00246-016-1347-1. . Epub 2016 Feb 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Linder AN, Hsia J, Krishnan SV, Rosenzweig EB, Krishnan US. Vasoreactive phenotype in children with pulmonary arterial hypertension and syncope. ERJ Open Res. 2022 Oct 10;8(4):223–2022. doi: 10.1183/23120541.00223-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moledina S, Hislop AA, Foster H, Schulze-Neick I, Haworth SG. Childhood idiopathic pulmonary arterial hypertension: a national cohort study. Heart. 2010 Sep;96(17):1401–6. doi: 10.1136/hrt.2009.182378. . Epub 2010 Apr 20. [DOI] [PubMed] [Google Scholar]
- 31. van Loon RL, Roofthooft MT, Hillege HL, ten Harkel AD, van Osch-Gevers M, Delhaas T, et al. Pediatric pulmonary hypertension in The Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation. 2011 Oct 18;124(16):1755–64. doi: 10.1161/CIRCULATIONAHA.110.969584. . Epub 2011 Sep 26. [DOI] [PubMed] [Google Scholar]
- 32. Boucly A, Weatherald J, Savale L, Jaïs X, Cottin V, Prevot G, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J. 2017 Aug 3;50(2):1700889. doi: 10.1183/13993003.00889-2017. [DOI] [PubMed] [Google Scholar]
- 33. Hoeper MM, Kramer T, Pan Z, Eichstaedt CA, Spiesshoefer J, Benjamin N, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J. 2017 Aug 3;50(2):1700740. doi: 10.1183/13993003.00740-2017. [DOI] [PubMed] [Google Scholar]