Abstract
Objectives
To characterize the serum cytokine profile in myelin oligodendrocyte glycoprotein antibody–associated disease (MOGAD) at onset and during follow-up and assess their utility for predicting relapses and disability.
Methods
This retrospective multicentric cohort study included patients aged 16 years and older meeting MOGAD 2023 criteria, with serum samples collected at baseline (≤3 months from disease onset) and follow-up (≥6 months from the baseline), and age-matched and time to sampling–matched patients with multiple sclerosis (MS). Eleven cytokines were assessed using the ELLA system. Data comparisons and statistical analyses between cytokine levels and clinical outcomes were performed.
Results
Eighty-eight patients with MOGAD and 32 patients with MS were included. Patients with MOGAD showed higher IL6 (p = 0.036), IL8 (p = 0.012), and IL18 (p = 0.026) baseline levels compared with those with MS, in non–optic neuritis (ON) presentations. BAFF values increased over time, especially in patients with MOGAD treated with anti-CD20 (p = 0.002). Baseline BAFF, CXCL10, IL10, and IL8 levels correlated with disease severity at MOGAD onset (all p < 0.05). Finally, higher baseline BAFF levels predicted lower risk of relapses (hazard ratio 0.41 [0.19; 0.89], p = 0.024).
Discussion
This study suggests a proinflammatory Th17-dominant profile in non-ON MOGAD patients, with a novel finding of a potential protective role of BAFF on relapses. These results shed new light on the pathogenesis of MOGAD, potentially guiding therapeutic decisions.
Introduction
Similar to other antibody-mediated conditions, the autoimmune process of myelin oligodendrocyte glycoprotein antibody–associated disease (MOGAD) presumably initiates in the periphery.1 The study of cytokines in serum can help unravel the different pathways and immune cell types involved in the pathogenesis of this disease. It is important to note that they may lead to the discovery of effective therapies as reported with the humanized IL6-receptor antibody, satralizumab, in aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder (AQP4-NMOSD)2 and serve as prognostic biomarkers.
In MOGAD, Th17 (IL6, IL8), Treg (IL10), and B-cell–related (BAFF, APRIL, BLC/CXCL13, CCL19) cytokines are upregulated in both pediatric and adult patients.3-5 However, most studies were conducted in CSF, with small sample sizes and without a prespecified protocol of sample collection. Moreover, robust data on the usefulness of these proteins for prognosis are lacking.
Therefore, our aims were to (1) characterize the serum cytokine profile in patients with MOGAD at onset and during follow-up and (2) assess the usefulness of these cytokines for predicting relapses and disability in MOGAD.
Methods
This is a retrospective multicentric cohort study including patients aged 16 years and older fulfilling MOGAD 2023 criteria,6 with available serum samples obtained at baseline (≤3 months from disease onset) and follow-up (≥6 months from the baseline sample). Age-matched and time to first sampling–matched patients with MS were included as controls. Demographic and clinicoradiologic data at onset and during follow-up were collected.
A panel of 11 cytokines (eTable 1) was assessed in serum using the automated microfluidic analyzer ELLA (BioTechne, Minneapolis, MN) (eMethods). IL12p70 and IL17A were detected in <10% of the patients and were not included in statistical analyses.
Statistical analyses are described in the eMethods.
The study was approved by the Clinical Research Ethics Committee at Vall d’Hebron University Hospital (EPA [AG]57/2013 [3834]) and French ethical committee (Comité de Protection des Personnes [CPP]: reference 2019-A03066-51). All patients signed written informed consents.
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Eighty-eight patients with MOGAD and 32 patients with MS were included. Detailed baseline and follow-up patient characteristics are provided in Table 1.
Table 1.
Demographic and Clinical Characteristics
| Baseline characteristics | Whole cohort (n = 120) | MOGAD (n = 88) | MS (n = 32) | p Value |
| Female; no. (%) | 73 (60.3) | 47 (53.4) | 25 (78.1) | 0.026 |
| Age at onset; y, median (IQR) | 36.2 (27.8–46.2) | 36.6 (27.4–50.5) | 35.8 (28.1–41.4) | 0.211 |
| Topography at onset; no. (%) | ||||
| Optic nerve | 58 (48.3) | 45 (51.1) | 13 (40.6) | 0.417 |
| Spinal cord | 39 (32.5) | 28 (31.8) | 11 (34.4) | 0.965 |
| Encephalic | 5 (4.2) | 5 (5.7) | 0 (0.0) | 0.389 |
| Other | 18 (15.0) | 10 (11.4) | 8 (25.0) | 0.119 |
| EDSS score at onset; median (IQR) | 2.25 [1.0–3.1] | 2.75 [1.5–4.0] | 2.00 [1.0–3.0] | 0.045 |
| CSF-OBs; no. (%) | 41/98 (41.8) | 16/68 (23.5) | 25/30 (83.3) | <0.001 |
| Time from onset to first MRI, median (IQR), d | 12 (3–117) | 9 (2–18) | 97 (19–117) | <0.001 |
| No. of brain lesions, n (%) | <0.001 | |||
| 0 lesions | 39/111 (35.1) | 39/79 (49.4) | 0 (0.0) | |
| 1–8 lesions | 34/111 (30.6) | 26/79 (32.9) | 8/32 (25) | |
| ≥9 lesions | 38/111 (34.2) | 14/79 (17.7) | 24/32 (75) | |
| No. of brain CELs, n (%) | <0.001 | |||
| 0 lesions | 81/109 (74.3) | 69/83 (83.1) | 12/26 (46.2) | |
| ≥1 lesion | 28/109 (25.7) | 14/83 (16.9) | 14/26 (53.9) | |
| No. of spinal lesions, n (%) | 0.024 | |||
| 0 lesions | 39/69 (56.5) | 30/45 (66.7) | 9/24 (37.5) | |
| ≥1 lesion | 30/69 (43.5) | 15/45 (33.3) | 15/24 (62.5) | |
| Time from disease onset to first sampling; mo, median (IQR) | 0.8 (0.3–1.6) | 0.7 (0.2–1.9) | 0.9 (0.7–1.5) | 0.414 |
| Time from disease onset to second sampling; mo, median (IQR) | 10.2 (7.6–20.8) | 9.9 (7.1–16.8) | 49.1 (10.2–96.6) | 0.001 |
| Time between first and second sampling; mo, median (IQR) | 9.2 (6.3–19.5) | 8.9 (6.0–15.5) | 48.0 (9.5–95.2) | 0.001 |
| Acute treatment within one-month before baseline sampling; n (%) | 61 (50.8) | 47 (53.4) | 14 (43.8) | 0.446 |
| Acute treatment within one-month before second sampling; n (%) | 7 (5.8) | 7 (7.9) | 0 (0.0) | 0.187 |
| Disease duration; y, median (IQR) | 4.3 (2.3–7.8) | 3.0 (1.9–6.2) | 8.8 (6.5–13.8) | <0.001 |
| Follow-up characteristics | ||||
| Follow-up; y, median (IQR) | 2.5 (0.8–7.0) | 1.8 (0.8–4.4) | 7.5 (4.7–11.4) | <0.001 |
| Chronic treatment during follow-up*; no. (%) | 69 (57.5) | 48 (54.5) | 21 (65.6) | 0.443 |
| Patients under chronic treatment at first sampling; no. (%) | 6/69 (8.7) | 6/48 (12.5) | 0/21 (0.0) | 0.094 |
| Patients under chronic treatment at second sampling; no. (%) | 54/69 (78.3) | 37/48 (77.1) | 17/21 (81.0) | 1.000 |
| Patients relapsing; no. (%) | 41 (34.7) | 28 (31.8) | 14 (43.8) | 0.320 |
| Time to second relapse; wk, median (IQR) | 20.2 (9.7–68.4) | 15.3 (6.8–37.9) | 44.3 (19.6–132) | 0.016 |
| EDSS score at the last follow-up; median (IQR) | 1.00(0.00–2.00) | 1.00(0.00–2.00) | 1.25(1.00–2.00) | 0.046 |
| EDSS score ≥3.0 at the last follow-up; no (%) | 18 (15.0) | 13 (14.8) | 5 (15.6) | 1.000 |
Abbreviations: CELs = contrast-enhancing lesions; CSF-OBs = cerebrospinal fluid–restricted oligoclonal bands; EDSS = Expanded Disability Status Scale; IQR = interquartile range; MOGAD = myelin oligodendrocyte glycoprotein antibody–associated disease.
Additional missing values: EDSS scores at onset, n = 8 in the MOGAD cohort.
*In patients with MOGAD, the number of chronic treatments included the following: anti-CD20, 19; azathioprine, 11; mycophenolate mofetil, 5; prednisone, 8; IV immunoglobulin, 1; interferon, 2; glatiramer acetate, 1; cladribine, 1; teriflunomide, 1.
*In patients with MS, chronic treatments included the following: alemtuzumab, 1; cladribine, 1; dimethyl fumarate, 1; fingolimod, 1; interferon, 3; glatiramer acetate, 11; clinical trial, 1; natalizumab, 1.
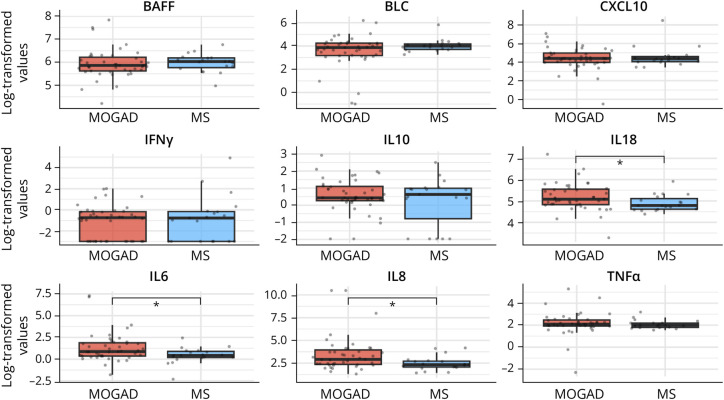
All the 9 cytokines had comparable baseline serum values between MOGAD and MS cohorts. IL6 values were higher in MOGAD than in MS, but the difference was not statistically significant (p = 0.058) (eFigure 1). Within non–optic neuritis (ON) presentations, patients with MOGAD (n = 43) displayed higher median (interquartile range [IQR]) baseline values of IL6 (2.40 pg/mL [1.42–6.60] vs 1.54 [1.21–2.46], p = 0.036), IL8 (18.5 [10.7–52.8] vs 10.3 [7.89–15.3], p = 0.012), and IL18 (161 [125–258] vs 120 [101–166], p = 0.026) compared with patients with MS (n = 19) (Figure 1).
Figure 1. Baseline Serum Cytokines Between Patients With MOGAD and MS With Non–Optic Neuritis Presentations.
Boxplots depict the distribution of baseline serum log-transformed values of the 9 cytokines between patients with MOGAD and MS with non–optic neuritis presentations. Median values are represented by the horizontal bar, IQR by hinges, 1.5 × IQR by whiskers, and individual values by dots. p Values are represented by asterisks as follows: * <0.05. IQR = interquartile range; MOGAD = myelin oligodendrocyte glycoprotein antibody–associated disease; MS = multiple sclerosis.
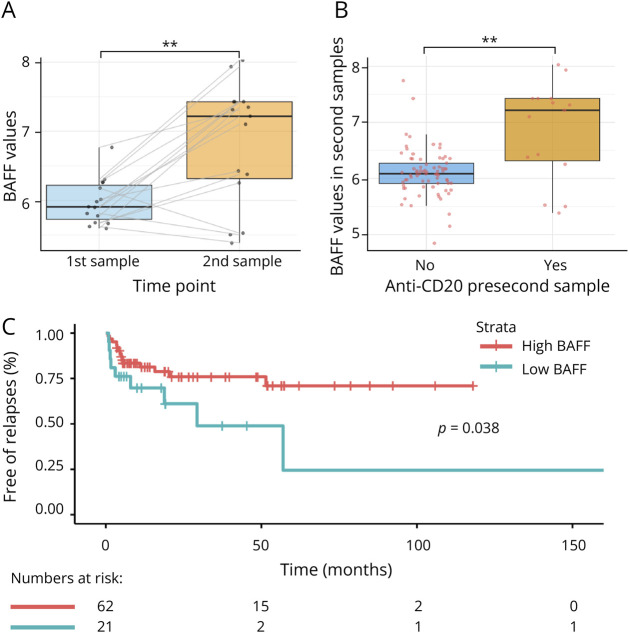
Regarding cytokine dynamics, BAFF values were increased in the second sample compared with the baseline sample in both MOGAD (p = 0.002) and MS (p = 0.049) cohorts while the remaining cytokine values were stable. In patients with MOGAD treated with anti-CD20 before second sampling (n = 15), BAFF notably increased in the second sample compared with the first sample (p = 0.002) (Figure 2A), but not in the remaining patients (treated with other therapies and nontreated [n = 73]) (p = 0.063). Similarly, second samples from patients with MOGAD under anti-CD20 had higher BAFF values compared with the remaining (p = 0.002) (Figure 2B).
Figure 2. BAFF Increases After Rituximab Treatment and Predicts Relapses During Follow-Up.
Boxplots depict the distribution of serum BAFF log-transformed values between first and second samples in the 15 patients with MOGAD who received anti-CD20 therapy between both time points (A) and the distribution of serum BAFF log-transformed values in second samples between those patients under anti-CD20 treatment (N = 15) and the remaining patients (N = 73) at the time of second sampling (B). Median values are represented by the horizontal bar, IQR by hinges, 1.5 × IQR by whiskers, and individual values by dots joined by gray lines between time points. p Values are represented by asterisks as follows: ** <0.01. (C) The representation of the Kaplan-Meier curves for the time to first relapse between patients with high (≥5.71) and low (<5.71) baseline BAFF values in the MOGAD cohort. The cutoff 5.71 is the 25th percentile value of BAFF in the MOGAD cohort. *Note: five patients were excluded from the Kaplan-Meier and Cox analyses because their baseline samples were obtained after the first relapse. IQR = interquartile range; MOGAD = myelin oligodendrocyte glycoprotein antibody–associated disease.
Baseline BAFF (β 0.06 95% CI [0.01–0.11], p = 0.030), CXCL10 (0.10 [0.01–0.20], p = 0.036), IL10 (0.11 [0.01– 0.21], p = 0.040), and IL8 (−0.21 [−0.41 to −0.02], p = 0.033) values were associated with EDSS level at onset. Within non-ON presentations, BAFF (0.08 [0.01–0.16], p = 0.047), CXCL10 (0.16 [0.01–0.31], p = 0.037), IL10 (0.17 [0.06–0.28], p = 0.004), and IL6 (0.14 [0.01–0.28], p = 0.046) were associated with EDSS scores at onset. In addition, BAFF values were associated with length of the myelitis on MRI (0.05 [0.01–0.09], p = 0.012). No other significant associations were found (eTable 2).
None of the cytokines was associated with a EDSS score ≥ 3.0 at the last follow-up in the whole MOGAD cohort. In non-ON presentations, IL6 (OR 1.51 [1.01–2.54]) and IL8 (1.42 [1.01–2.21]) were associated with this outcome but did not reach statistical significance (p = 0.064 and p = 0.059, respectively).
Regarding relapses, higher baseline BAFF independently reduced the risk of first relapse after adjustment by proportion of time under chronic treatment (HR 0.41 [0.19–0.89], p = 0.024).
Figure 2C shows the Kaplan-Meier survival curve for time to relapse between patients with high and low levels of BAFF (log-rank p value = 0.038).
Discussion
In this multicentric study of adult patients with MOGAD, we conducted a longitudinal analysis of 11 cytokines in serum using an ultrasensitive immunoassay. We confirmed the presence of a proinflammatory Th17-dominant profile in MOGAD with non-ON presentations and the association of cytokines involving different pathways (Th1, Th17, Treg, and B-cell response) with clinical and radiologic severity at onset. The novel and intriguing finding is the association between baseline BAFF levels and risk of relapses during disease course in MOGAD.
Several studies have demonstrated a common profile of cytokines in serum and/or CSF in both MOGAD and AQP4-NMOSD, characterized by an upregulated Th17 (IL6, IL8, IL17) and Treg (IL10) signature, compared with MS, in both adult and pediatric cohorts.3-5,7-9 Among them, IL6 has attracted special interest because of its pleiotropic effects such as promoting Th17 cell differentiation, producing autoantibodies by plasmablasts, and increasing the blood-brain barrier permeability.8 The approval of satralizumab for AQP4-NMOSD,2 with an ongoing trial for patients with MOGAD as well, and the efficacy of tocilizumab in some patients with refractory MOGAD10 highlight the relevance of this cytokine. In our cohort, focusing on non-ON presentations, IL6, IL8, and IL18 showed higher baseline levels in MOGAD than in MS. This aligns with some studies reporting higher IL6 levels in non-ON MOGAD phenotypes, especially with brain involvement.4,8 In addition, in non-ON MOGAD patients, baseline levels of IL6, BAFF, CXCL10, and IL10 correlated with disease severity at onset, reflecting a more inflammatory component and compensatory regulatory mechanisms in severe presentations. A lesser extent of damage in ON compared with other phenotypes with a less robust inflammatory response could influence the lack of differences in cytokines between MOGAD and MS, and the absence of correlation with clinical status in ON presentations and our total cohort.
Besides the T-cell dominant profile, B cells also play a significant role in MOGAD pathogenesis.1 However, few studies have characterized B-cell–related cytokines/chemokines in these patients.5,7 Moreover, the possible implication of these molecules on clinical prognosis has not been addressed. In this study, higher baseline values of BAFF predicted lower risk of relapse in patients with MOGAD after adjustment by chronic treatment. This finding provides evidence of the potential protective role of BAFF in MOGAD. In MS, BAFF has shown controversial results.11,12 Of interest, a recent study demonstrated that BAFF protects against demyelination and neurodegeneration in an experimental autoimmune encephalomyelitis model and in patients with MS treated with anti-CD20 therapy.13 Indeed, blocking BAFF by atacicept in patients with MS led to exacerbated inflammatory disease activity and the interruption of the clinical trial.14 In our study, BAFF values increased in patients with MOGAD after anti-CD20 treatment, as reported in several autoimmune diseases, including MS and AQP4-NMOSD.15 Whether the BAFF dynamics influence the remarkably different clinic-biological responses to anti-CD20 in MOGAD compared with AQP4-NMOSD and MS16 remains unknown.
Some limitations included the retrospective design, the variability in time of follow-up sampling, and the potential influence of treatment especially on cytokine dynamics. Further studies with longer follow-up and larger comparator groups are needed to confirm our results and analyze the association of treatment-dependent BAFF increase with anti-CD20 efficacy in MOGAD.
In conclusion, our results confirm a proinflammatory Th17-dominant profile in non-ON MOGAD patients, with the novel finding of the protective role of baseline BAFF on relapses. These results shed light on the pathogenesis and prognosis of MOGAD, potentially guiding therapeutic decisions.
Author Contributions
J. Villacieros-Álvarez: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data. C. Espejo: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data. G. Arrambide: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data. A. Dinoto: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data. P. Mulero: drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data. L. Rubio-Flores: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. P. Nieto: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. C. Alcalá: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. J.E. Meca-Lallana: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. J. Millan-Pascual: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. P. Martínez-García: drafting/revision of the manuscript for content, including medical writing for content. R. Bernard-Valnet: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. I. González-Suárez: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. A. Orviz: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. R. Téllez: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. L. Navarro Cantó: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. S. Presas-Rodríguez: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. S. Martínez-Yélamos: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. J.P. Cuello: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. A. Alonso: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. R. Piñar Morales: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. G. Álvarez Bravo: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. L. Benyahya: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data. S. Trouillet-Assant: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data. V. Dyon-Tafan: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data. C. Froment Tilikete: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. A. Ruet: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. B. Bourre: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. R. Deschamps: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. C. Papeix: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. E. Maillart: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. P. Kerschen: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. X. Ayrignac: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. À. Rovira: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. C. Auger: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. B. Audoin: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. X. Montalban: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data. M. Tintore: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data. S. Mariotto: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data. A. Cobo-Calvo: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data. R. Marignier: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data.
Study Funding
This study has been funded by Instituto Carlos III through the projects PI20/00800 granted to A.C.-C. and Fondation pour l'aide à la recherche sur la sclérose en plaques (ARSEP) (ARSEP-1276). A. Cobo-Calvo is supported by Joan Rodes contract JR19/00007 and Javier Villacieros-Álvarez by P-FIS grant FI21/00282.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/NN for full disclosures.
References
- 1.Moseley CE, Virupakshaiah A, Forsthuber TG, Steinman L, Waubant E, Zamvil SS. MOG CNS autoimmunity and MOGAD. Neurol Neuroimmunol Neuroinflamm. 2024;11(5):e200275. doi: 10.1212/NXI.0000000000200275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Traboulsee A, Greenberg BM, Bennett JL, et al. Safety and efficacy of satralizumab monotherapy in neuromyelitis optica spectrum disorder: a randomised, double-blind, multicentre, placebo-controlled phase 3 trial. Lancet Neurol. 2020;19(5):402-412. doi: 10.1016/S1474-4422(20)30078-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kothur K, Wienholt L, Tantsis EM, et al. B Cell, Th17, and neutrophil related cerebrospinal fluid cytokine/chemokines are elevated in MOG antibody associated demyelination. PLoS ONE. 2016;11(2):e0149411. doi: 10.1371/journal.pone.0149411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaneko K, Sato DK, Nakashima I, et al. CSF cytokine profile in MOG-IgG+ neurological disease is similar to AQP4-IgG+ NMOSD but distinct from MS: a cross-sectional study and potential therapeutic implications. J Neurol Neurosurg Psychiatry. 2018;89(9):927-936. doi: 10.1136/jnnp-2018-317969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofer LS, Mariotto S, Wurth S, et al. Distinct serum and cerebrospinal fluid cytokine and chemokine profiles in autoantibody-associated demyelinating diseases. Mult Scler J Exp Transl Clin. 2019;5(2):2055217319848463. doi: 10.1177/2055217319848463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banwell B, Bennett JL, Marignier R, et al. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD panel proposed criteria. Lancet Neurol. 2023;22(3):268-282. doi: 10.1016/S1474-4422(22)00431-8 [DOI] [PubMed] [Google Scholar]
- 7.Bauer A, Rudzki D, Berek K, et al. Increased peripheral inflammatory responses in myelin oligodendrocyte glycoprotein associated disease and aquaporin-4 antibody positive neuromyelitis optica spectrum disorder. Front Immunol. 2022;13:1037812. doi: 10.3389/fimmu.2022.1037812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uzawa A, Mori M, Masuda H, et al. Contributions of CSF interleukin-6 elevation to the pathogenesis of myelin oligodendrocyte glycoprotein antibody-associated disease. Mult Scler. 2024;30(8):977-982. doi: 10.1177/13524585241254731 [DOI] [PubMed] [Google Scholar]
- 9.Horellou P, Wang M, Keo V, et al. Increased interleukin-6 correlates with myelin oligodendrocyte glycoprotein antibodies in pediatric monophasic demyelinating diseases and multiple sclerosis. J Neuroimmunol. 2015;289:1-7. doi: 10.1016/j.jneuroim.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 10.Ringelstein M, Ayzenberg I, Lindenblatt G, et al. Interleukin-6 receptor blockade in treatment-refractory MOG-IgG-associated disease and neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflamm. 2022;9(1):e1100. doi: 10.1212/NXI.0000000000001100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steri M, Orrù V, Idda ML, et al. Overexpression of the cytokine BAFF and autoimmunity risk. N Engl J Med. 2017;376(17):1615-1626. doi: 10.1056/NEJMoa1610528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kannel K, Alnek K, Vahter L, Gross-Paju K, Uibo R, Kisand KV. Changes in blood B cell-activating factor (BAFF) levels in multiple sclerosis: a sign of treatment outcome. PLoS ONE. 2015;10(11):e0143393. doi: 10.1371/journal.pone.0143393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang AA, Luessi F, Neziraj T, et al. B cell depletion with anti-CD20 promotes neuroprotection in a BAFF-dependent manner in mice and humans. Sci Transl Med. 2024;16(737):eadi0295. doi: 10.1126/scitranslmed.adi0295 [DOI] [PubMed] [Google Scholar]
- 14.Kappos L, Hartung HP, Freedman MS, et al. Atacicept in multiple sclerosis (ATAMS): a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Neurol. 2014;13(4):353-363. doi: 10.1016/S1474-4422(14)70028-6 [DOI] [PubMed] [Google Scholar]
- 15.Nakashima I, Takahashi T, Cree BAC, et al. Transient increases in anti-aquaporin-4 antibody titers following rituximab treatment in neuromyelitis optica, in association with elevated serum BAFF levels. J Clin Neurosci. 2011;18(7):997-998. doi: 10.1016/j.jocn.2010.12.011 [DOI] [PubMed] [Google Scholar]
- 16.Gawde S, Siebert N, Ruprecht K, et al. Serum proteomics distinguish subtypes of NMO spectrum disorder and MOG antibody-associated disease and highlight effects of B-cell depletion. Neurol Neuroimmunol Neuroinflamm. 2024;11(4):e200268. doi: 10.1212/NXI.0000000000200268 [DOI] [PMC free article] [PubMed] [Google Scholar]