Abstract
Background and Objectives
Levels of activated complement proteins in the CSF are increased in people with multiple sclerosis (MS) and are associated with clinical disease severity. In this study, we determined whether complement activation profiles track with quantitative MRI metrics and liquid biomarkers indicative of disease activity and progression.
Methods
Complement components and activation products (Factor H and I, C1q, C3, C4, C5, Ba, Bb, C3a, C4a, C5a, and sC5b-9) and liquid biomarkers (neurofilament light chain, glial fibrillary acidic protein [GFAP], CXCL-13, CXCL-9, and IL-12b) were quantified in the CSF of 112 patients with clinically isolated syndromes and 127 patients with MS; longitudinal MRIs according to a standardized protocol of the Swiss MS cohort were assessed. We used multivariable models to analyze associations of the 12 complement parameters as individual independent variables and longitudinal brain volumes, T2-weighted (T2w) lesion volumes, contrast-enhancing (CELs) and paramagnetic rim lesions (PRLs), and molecular biomarkers as dependent variables, respectively.
Results
Strongest associations with accelerated brain atrophy were found for C4a: doubling of C4a CSF levels was associated with an additional brain volume loss of −0.24% (95% CI −0.31% to −0.16%; p < 0.0001) per year, followed by Ba and C3a (−0.22% [−0.29% to −0.15%]) and −0.13% ([−0.21 to −0.06]; both p < 0.001). Doubling of C3a, Ba, and C4a levels correlated with 2.2- (1.6–3.0; p < 0.0001), 2.0- (1.3–3.1; p = 0.0038), and 1.8-fold (1.2–2.6; p = 0.0029) increased longitudinal T2w lesion volumes; C3a and Ba were associated with 2.5- (1.4–4.6; p = 0.0022) and 3.3-fold (1.5–7.2; p = 0.0024) higher odds for CELs and 2.6- (1.7–4.0; p < 0.0001) and 2.3-fold (1.3–4.3; p = 0.006) increased PRL incidence rates. C1q, C3a, and C4a were associated with higher GFAP levels, and CXCL-13, CXCL-9, and IL-12b analyses showed consistent patterns with strongest associations for C1q, followed by Ba, C3a, and C4a.
Discussion
Intrathecal complement activation is consistently associated with MRI metrics and liquid biomarkers indicative for MS disease activity and progression. Our results demonstrate that aberrant complement activation is strongly associated with structural brain damage in MS. Therapeutic targeting of the complement system might limit disability accumulation due to MS.
Introduction
In multiple sclerosis (MS), complex immune mechanisms involving T and B cells, antibodies and other humoral factors, activated macrophages, and microglia along with the vulnerability of the target tissue account for the cascade of events that leads to neurodegenerative processes that manifest clinically as progression.1 Complement components (CCs) and complement activation products (CAPs) in combination with immunoglobulin deposits are present both in the affected gray and white matter of patients with MS, suggesting their pathophysiologic function for tissue damage.2-5
Previously, conflicting results for plasma and serum levels of individual CC and CAP in persons with MS (when compared with healthy controls) and their associations with surrogate markers of disease state were reported.6-9 We have recently shown by use of high sensitivity multiplex assays that mainly early cascade CAPs are increased in the CSF, but not plasma, of persons with clinically isolated syndrome (CIS) and MS. CSF CAP levels (C3a, C4a, Ba, and Bb) were highest in individuals with intrathecal IgM synthesis, in line with the fact that the pentameric IgM is the strongest complement-activating immunoglobulin. Accordingly, CAPs were associated with measures of disease severity and neuroaxonal damage.10
The complement system is increasingly recognized for its multiple physiologic and homeostatic functions, supporting CNS development, maintenance, and tissue remodeling after injury.11 In the context of MS, activated CC might have a “double-edged sword” effect: on the one hand, restoring tissue homeostasis through phagocytic clearance of opsonized myelin degradation products and other debris or on the other hand, promoting tissue injury through amplifying local inflammation and direct complement-mediated damage.12
To better understand whether complement activation in MS is associated with structural CNS tissue damage, we investigated associations of CSF CC and CAP with MRI metrics reflecting disease activity, severity, or progression; molecular biomarkers for CNS injury; and complement activation-associated relevant cytokines.13-15
Methods
Patients, Inclusion Criteria, and Data Collection
CSF samples were obtained from the CSF biobank of the University Hospital Basel, a subset of patients who were prospectively recruited into the Swiss multiple sclerosis cohort (SMSC)16 between 2012 and 2021. We included all eligible CIS/MS patients with a CSF sample without erythrocyte contamination available. Patients were included with a CIS, relapsing remitting multiple sclerosis (RRMS),17 secondary progressive multiple sclerosis (SPMS),18 or primary progressive multiple sclerosis (PPMS).19 First symptoms were defined as neurologic symptoms that lasted for ≥24 hours without fever, infection, or adverse reaction to a prescribed medication.19 RRMS had to have experienced at least 2 relapses before the lumbar puncture (LP).19 Demographic and clinical variables collected included sex, date of birth, patients onset of first symptoms, Expanded Disability Status Scale Score (EDSS) at LP, and disease-modifying treatments history.
Standard Protocol Approvals, Registrations, and Patient Consents
We included patients from the CSF biobank study of the Department of Neurology, University Hospital Basel, which were prospectively recruited into the SMSC. These studies were approved by the local ethical committee, and patients were included after written informed consent.
MRI Data and Analysis
We included all annual brain MRI scans performed prospectively in 1.5 or 3T scanners as part of the SMSC.16 MRI scans were acquired according to a standardized protocol optimized for a homogeneous signal-to-noise ratio and included precontrast and postcontrast 3D T1-weighted, 1 mm isotropic magnetization-prepared rapid gradient-echo, and 3D 1 mm isotropic fluid-attenuated inversion recovery (FLAIR) images. MRI protocol details are reported in eTable 1. T2-weighted (T2w) lesion volume was calculated automatically on FLAIR images using the multidimensional gated recurrent units algorithm, and results were manually reviewed.20 The presence of contrast-enhancing lesions (CELs) on T1 postcontrast images was evaluated manually. Brain volumetric analysis was performed by a SPM12 toolbox using the lesion-filled T1-weighted image.21 Reconstructions were visually assessed for sufficient quality. Brain parenchymal fraction (BPF) is defined as whole-brain volume normalized for total intracranial volume. In those patients with susceptibility-weighted T2* sequences during follow-up, paramagnetic rim lesions (PRLs) were detected as previously described.14
Samples and Measurements of CC and Activation Products
CSF was centrifuged (400 g for 10 minutes) within 30 minutes of collection, and the supernatant frozen at −80°C. The integrity of the blood-CSF barrier was determined by calculating the CSF/serum ratio for albumin (Qalb).22 CSF samples were sent to the University Hospital of Münster (Münster, Germany) on dry ice and CAPs and components determined as described before.10 Briefly, for the quantification of CAP (C4a, C3a, C5a, s-C5b9, Ba, and Bb) and CC (C1q, C4, C3, C5, Factor H and I) levels, samples were thawed on ice and immediately processed. Multiplex ELISAs based on chemiluminescence were performed according to the manufacturer's recommendations (Quidel, San Diego, CA; catalogue number: A900, A917). The mean intra-assay coefficients of variation (CVs) of duplicate determinations for a high-value and low-value quality control (QC) sample, respectively, were as follows: Ba: 1.9%/1.7%; Bb: 2.1%/2.6%; C3a: 0.2%/2.2%; C4a: 5.5%/3.1%; C5a: 4.3%/3.5%; Factor H: 1.1%/2.5%; Factor I: 6.0%/5.2%; s-C5b9: 2.6%/3.6%; C1q: 6.1%/7.9%; C3: 7.4%/3.8%; C4: 5.4%/7.9%; and C5: 5.3%/4.1%. The interassay CVs for the high-value/low-value QC samples, respectively, were for Ba: 5.3%/9.3%; Bb: 2.0%/6.8%; C3a: 18.3%/9.4%; C4a: 6.2%/6.6%; C5a: 4.4%/3.8%; Factor H: 1.9%/9.5%; Factor I: 10.1%/9.9%; s-C5b-9: 5.2%/7.8%; C1q: 13.0%/7.9%; C3: 7.4%/8.8%; C4: 5.2%/9.8%; and C5: 12.8%/8.5%.
Measurements of GFAP Levels
CSF glial fibrillary acidic protein (GFAP) levels were measured in duplicate with the Neurology 2-PLEX B assay (Quanterix, Billerica, MA). The mean intra-assay CV of duplicate determinations for CSF GFAP was 2.3%. The interassay CVs of internal QCs for GFAP were 8.0% (73.6 pg/mL), 6.7% (127.3 pg/mL), and 6.9% (325.9 pg/mL).
Measurements of Cytokines
CXCL-13, CXCL-9, and IL-12b were measured in CSF samples by use of the Octave custom assay, a multiprotein, biomarker assay developed using Olink proximity extension assay methodology.23 The mean intra-assay CV of duplicate determinations for CXCL-13 was 11.4%, for CXCL-9 5.5% and for IL-12b 17.6%. The interassay CVs of 4 internal quality controls (serum) (QC) for CXCL-13 were 8.9% (52.1 pg/mL), 7.6% (43.0 pg/mL), 8.6% (66.4 pg/mL), and 11.0% (49.1 pg/mL); for CXCL-9: 8.6% (29.1 pg/mL), 7.7% (60.2 pg/mL), 8.8% (107.8 pg/mL), and 9.6% (27.3 pg/mL); and for IL-12b were 8.8% (107.8 pg/mL), 7.8% (126.4 pg/mL), 7.5% (121.2 pg/mL), and 10.2% (75.1 pg/mL).
For data points below the lower limit of quantification (LLOQ) (CXCL-13: n = 57 (23.8%); CXCL-9: 25.5%; IL-12b: 0) a random value between zero and LLOQ was imputed to a uniform distribution, no data points were above upper limit of quantification (eTable 2).
Statistics
Data are presented as median and interquartile range (IQR) and as absolute and relative frequencies in case of categorical data. Statistical analyses were performed using R (version 4.1.2).
Distribution of data was inspected using QQplots. All used CSF and blood parameters (CC, CAP, cytokines, GFAP, and Qalb) were logarithm (log)-normally distributed. Accordingly, all these parameters were log-transformed before analysis. If these parameters, as well as BPF, served as dependent variable in a linear regression model, a natural log was used. The estimates were then back-transformed before presentation and thus indicate a multiplicative effect. For CC and CAP functioning as independent variables and for covariables, we used a binary logarithm. Accordingly, the presented estimate corresponds to the expected change in the dependent variable if the CC or CAP was doubled.
Associations of CC and CAP With Different Outcome Parameters
Atrophy
Associations of the log-transformed CSF concentrations of each CC or CAP (separate independent variables) with the log-transformed longitudinal BPF at time point of each scan (dependent variable) were analyzed by separate linear mixed models. Time since first scan (independent variable) was included to quantify atrophy, and the association between each CC or CAP and atrophy was modeled as the interaction between these 2 terms. They were adjusted for age at first scan, sex, MRI magnetic field strength (1.5 vs 3T), log-transformed Qalb, MS subtype (RRMS, SPMS, PPMS vs CIS, respectively), and dominant disease-modifying treatment category during follow-up (platform, orals, monoclonal antibodies vs untreated, respectively, compare eTable 3). Estimates were log-transformed to present them in percentage change, and the resulting multiplicative effect was subtracted −1 and divided by 100.
Longitudinal T2-Weighted Lesion Volumes and CELs
Separate linear mixed-effects models were calculated with the log-transformed CC/CAP levels as individual independent variables and the T2-weighted lesion volumes as dependent variables by including all longitudinal data points, respectively. We adjusted for age, sex, log-transformed Qalb, disease duration, and dominant treatment categories (platform, orals, monoclonal antibodies vs untreated, respectively) between the respective 2 MRIs during follow-up. For analyzing associations with CELs, separate logistic mixed-effects models with the log-transformed CC/CAP levels as individual independent variables and the occurrence of any CEL as dependent variables were used. These were adjusted for age, sex, log-transformed Qalb, and active treatment (platform, orals, monoclonal antibodies vs untreated) at the respective MRI.
PRLs
We used separate negative binomial models with log-transformed CC/CAP levels as individual independent variables and the PRL count as dependent variable with adjustment for age at MRI, sex, log-transformed Qalb, and dominant treatment categories (platform, orals, monoclonal antibodies vs untreated, respectively) during follow-up.
GFAP and Complement Activation-Associated Cytokine Levels
We used separate linear regression models with the 12 log-transformed CC/CAP levels as independent variables and the log-transformed cytokine (CXCL-13, CXCL-9, IL-12b) and GFAP levels as dependent variables, respectively, adjusted for age, sex, log-transformed Qalb, and treatment category at LP (vs untreated, respectively): platform (glatirameracetate, interferons), orals (fingolimod, dimethylfumarate), and monoclonal antibodies (natalizumab, ocrelizumab, rituximab) (eTable 3).
Data Availability
Data are available on reasonable request.
Results
Patients and Clinical Data
Two hundred thirty-nine patients with CIS/MS met the inclusion criteria. Patients with SPMS were significantly older, had a longer disease duration and higher EDSS scores at LP than CIS/RRMS patients. MRI follow-up data were available in 134 (56.1%) of patients with a median follow-up of 6.9 years and 7 (IQR 5–9) MRIs per patient (Table 1). Concentrations of uncleaved and activated CCs along with complement-associated cytokines and soluble molecules indicative for CNS tissue injury were determined in CSF samples obtained at baseline.
Table 1.
Demographic, Clinical, and Routine CSF Characteristics at Lumbar Puncture and Follow-Up MRI Data
| CIS | RRMS | SPMS | PPMS | All | |
| n | 112 | 90 | 23 | 14 | 239 |
| Demographic and clinical data | |||||
| Female, n (%) | 80 (71.4) | 64 (71.1) | 13 (56.5) | 8 (57.1) | 165 (69) |
| Age at LP (y) | 34.4 (26.7–43.8) | 38.0 (31.4–48.1) | 53.7 (48.9–59.6) | 48.5 (46.7–57.8) | 38.5 (29.7–49.1) |
| EDSS at LP | 2.0 (1.5–2.5) | 2.5 (2.0–3.5) | 6.0 (4.0–7.0) | 3.5 (3.0–4.0) | 2.5 (2.0–3.5) |
| Untreated, n (%) | 111 (99.1) | 70 (77.8) | 15 (65.2) | 13 (92.9) | 209 (87.4) |
| Disease duration at LP (m) | 0.6 (0.3–1.8) | 56.1 (15.6–91.7) | 253.1 (147.0–332.1) | 50.0 (15.2–81.3) | 7.8 (0.7–67.3) |
| McDonald criteria 2017 met at LP, n (%) | 71 (63.4) | 90 (100) | 23 (100) | 14 (100) | 198 (82.8) |
| CSF data | |||||
| Cell count (cells/mcl) | 4.3 (2.0–9.0) | 3.0 (1.0–6.0) | 1.0 (0.3–2.2) | 1.7 (1.1–2.9) | 3.0 (1.3–6.6) |
| Qalb | 4.5 (3.6–6.4) | 5.4 (3.9–7.3) | 5.5 (3.8–7.7) | 5.0 (4.4–7.2) | 5.0 (3.8–6.8) |
| OCGBa (%) | 90 (80.4) | 73 (82.0) | 17 (85.0) | 11 (78.6) | 191 (81.3) |
| IgGIFa (%) | 66 (58.9) | 53 (59.6) | 9 (45.0) | 8 (57.1) | 136 (57.9) |
| IgMIFa (%) | 31 (27.7) | 24 (27.0) | 2 (10.0) | 3 (21.4) | 60 (25.5) |
| IgAIFa (%) | 5 (4.5) | 7 (7.9) | 1 (5.0) | 1 (7.1) | 14 (6.0) |
| MRI data | |||||
| MRI follow-up available, n (%) | 61 (54.5) | 52 (57.8) | 13 (56.5) | 8 (57.1) | 134 (56.1) |
| Follow-up time MRI (mean, SD, y) | 6.9 (3.2) | 7.0 (3.2) | 7.1 (3.2) | 7.2 (4.3) | 7.0 (3.2) |
| MRIs per patient | 7 (6, 9.25) | 8 (5, 9) | 5 (2.75, 8) | 6.5 (3.5, 7.25) | 7 (5, 9) |
Abbreviations: CIS = clinically isolated syndrome; EDSS = Expanded Disability Status Scale Score; Ig G/M/AIF = immunoglobulin G/M/A intrathecal fraction; IQR = interquartile range; LP = lumbar puncture; OCGB = oligoclonal IgG bands; PPMS = primary progressive MS; Qalb = albumin quotient; RRMS = relapsing-remitting MS; SPMS = secondary progressive MS.
Median and IQR are displayed if not mentioned otherwise.
Presence of OCGB or IgGIF/IgMIF/IgAIF.
Atrophy
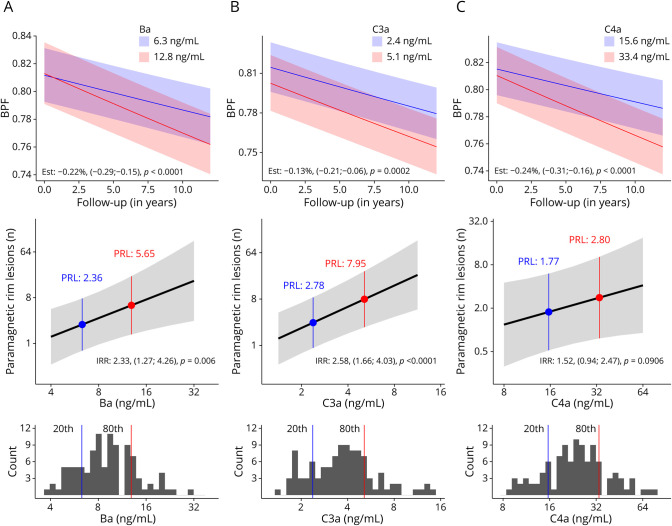
The most prominent effect on accelerated brain atrophy was found for the complement product C4a, which can be generated through activation of both the classical and lectin pathways: doubling of C4a CSF levels was associated with an independent additional annual reduction of BPF by −0.24% (p < 0.0001) per year. Similar results were found for Ba with a reduction by −0.22% (p < 0.0001), followed by C3a (−0.13%; p = 0.0002) and Bb (−0.12%; p < 0.0001) (Table 2; Figure 1).
Table 2.
Multivariable Associations of CSF Complement Components and Activation Products With MRI Parameters
| Longitudinal brain atrophy (BPF) n = 829 MRIs/120 pat | Longitudinal total T2-weighted lesion volume n = 930 MRIs/128 pat | CE lesions n = 996 MRIs/133 pat | PRLs n = 101 MRIs/pat | |||||||||
| Est (%) | CI | p Value | ME | CI | p Value | OR | CI | p Value | IRR | CI | p Value | |
| C4a | −0.24 | −0.31 to −0.16 | <1e-04 | 1.79 | 1.23–2.60 | 0.00292 | 1.81 | 0.97–3.36 | 0.0623 | 1.52 | 0.94–2.47 | 0.0906 |
| C4 | −0.05 | −0.15 to 0.05 | 0.3607 | 0.93 | 0.59–1.46 | 0.75055 | 1.46 | 0.59–3.60 | 0.4126 | 0.75 | 0.44–1.28 | 0.2896 |
| C3a | −0.13 | −0.21 to −0.06 | 0.00024 | 2.19 | 1.58–3.04 | <1e-04 | 2.54 | 1.40–4.61 | 0.00224 | 2.58 | 1.66–4.03 | <0.01 |
| C3 | −0.12 | −0.16 to −0.07 | <1e-04 | 1.14 | 0.92–1.41 | 0.22440 | 1.26 | 0.88–1.82 | 0.2041 | 1.16 | 0.90–1.49 | 0.2605 |
| C5a | −0.07 | −0.11 to −0.04 | <1e-04 | 1.16 | 0.98–1.38 | 0.07889 | 1.40 | 1.03–1.91 | 0.03323 | 1.25 | 1.03–1.52 | 0.0264 |
| s-C5b9 | −0.06 | −0.09 to −0.03 | <1e-04 | 1.20 | 1.04–1.38 | 0.01415 | 1.23 | 0.92–1.64 | 0.1550 | 1.17 | 0.98–1.40 | 0.0863 |
| C5 | −0.07 | −0.13 to −0.01 | 0.0332 | 0.82 | 0.56–1.21 | 0.31617 | 0.68 | 0.31–1.49 | 0.3360 | 0.59 | 0.35–1.00 | 0.0518 |
| Ba | −0.22 | −0.29 to −0.15 | <1e-04 | 1.97 | 1.26–3.08 | 0.00376 | 3.32 | 1.53–7.21 | 0.00240 | 2.33 | 1.27–4.26 | <0.01 |
| Bb | −0.12 | −0.17 to −0.07 | <1e-04 | 1.15 | 0.85–1.56 | 0.35397 | 1.51 | 0.89–2.57 | 0.1263 | 1.30 | 0.90–1.86 | 0.1609 |
| C1q | +0.05 | 0.10 to 0.19 | 0.5141 | 0.78 | 0.42–1.47 | 0.44894 | 0.91 | 0.31–2.66 | 0.8674 | 0.51 | 0.24–1.10 | 0.0878 |
| Factor H | −0.13 | −0.31 to 0.06 | 0.1714 | 1.00 | 0.35–2.87 | 0.99970 | 0.64 | 0.08–5.28 | 0.6809 | 2.85 | 0.63–12.86 | 0.173 |
| Factor I | −0.05 | −0.13 to 0.03 | 0.24133 | 1.17 | 0.72–1.90 | 0.51989 | 0.95 | 0.41–2.23 | 0.9121 | 1.72 | 0.93–3.19 | 0.0826 |
Abbreviations: BPF = brain parenchymal fraction; CAP = complement activation product; CC = complement component; CE = contrast-enhancing; Est. = estimate; IRR = incidence rate ratio; ME = multiplicative effect; OR = odds ratio; pat = patients; PRLs = paramagnetic rim lesions.
Reading example: effect of CC/CAP concentration doubling: per doubling of CSF C3a levels longitudinal total T2-weighted lesion volume is 119% higher.
Figure 1. Multivariable Associations of CSF Ba, C3a, and C4a Levels With Brain Volume Loss and PRL Counts.
Higher CSF levels of complement activation products Ba, C3a, and C4a in persons with CIS/MS are associated with an accelerated brain volume loss and higher PRL counts: (A) Top: The additional brain volume loss in a person with a Ba level corresponding to the 80th percentile (12.8 ng/mL; red line) within the CIS/MS cohort is −0.22% (p < 0.0001) as compared with a patient with a CSF Ba level corresponding to the 20th percentile (6.3 ng/mL; blue line). Middle: A patient with a Ba level (6.3 ng/mL) corresponding to the 20th percentile would have an estimated PRL count of 2.4 (blue), while one with an 80th percentile value (12.8 ng/mL) would correspond to 5.6 PRLs (red) (p < 0.0001). Bottom: The distribution of the percentiles within the CIS/MS cohort for the Ba concentration is expressed in this figure: the 20th percentile value is represented by the blue line and the 80th percentile level by the red line. (B) CSF C3a levels corresponding to the 80th percentile (5.1 ng/mL; red line) are associated with −0.13% (p = 0.0002) increased brain volume loss as compared with the 20th percentile (2.4 ng/mL; blue line) and correspond to estimated 7.9 for the 80th vs 2.8 PRLs (p < 0.0001) for the 20th percentile. (C) An 80th percentile C4a level (33.4 ng/mL) is associated with −0.24% (p < 0.0001) increased brain atrophy in comparison with a 20th percentile value (15.6 ng/mL) and with estimated 2.8 vs 1.8 PRLs (p = 0.0906). CIS = clinically isolated syndrome; MS = multiple sclerosis; PRL = paramagnetic rim lesion.
However, also for later cascade activation products, less pronounced effects on brain atrophy were seen: doubling of C5a levels showed a BPF reduction by −0.07%, and similarly for the terminal complement complex (s-C5b9), we observed an accelerated brain volume loss by −0.06% (both p < 0.0001) per year.
From the covariables used in the multivariable analysis older age, male (vs female) sex, MRI magnetic field strength (3 vs 1.5T), and disease subtype SPMS (vs CIS) were associated with a higher degree of brain volume loss (eTable 4).
Longitudinal T2w Lesion Volumes and CELs
Doubling of C4a levels was associated with 1.8-fold (p = 0.0029) higher T2w lesion volumes during follow-up; likewise, C3a and Ba correlated with 2.2- (p < 0.0001) and 2.0-fold (p = 0.0038) increased T2w lesion volumes and 2.5- (p = 0.0022) and 3.3-fold (p = 0.0024) higher odds for the occurrence of CEL. The later cascade activation product s-C5b9 was associated with 1.2-fold (p = 0.0142) increased T2-weighted lesion volumes, and C5a showed a 1.4-fold (p = 0.0332) higher probability for the occurrence of CELs (Table 2; eTables 5 and 6).
PRLs
Complement activation products C3a, Ba, and C5a were associated with higher PRL counts: for doubling of C3a, CSF levels we found an incidence rate ratio of 2.58 (p < 0.0001), followed by Ba with 2.33 (p = 0.006) and C5a with 1.25 (p = 0.0264) (Table 2; eTable 7; Figure 1).
GFAP
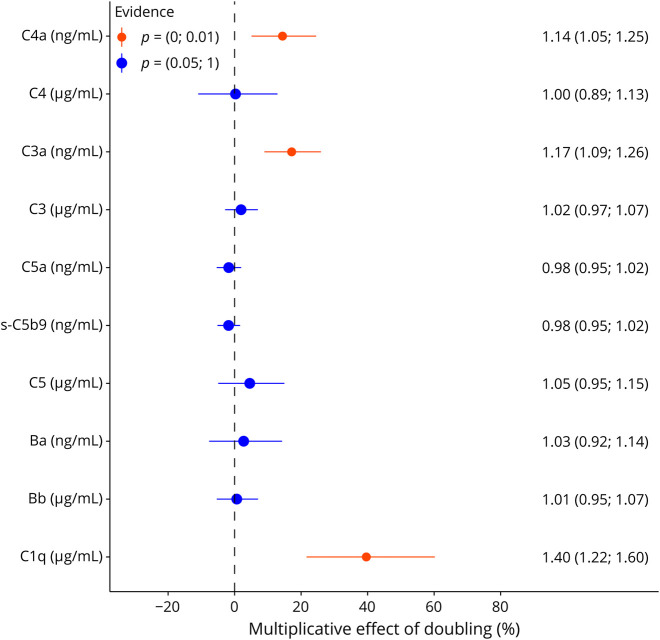
GFAP is associated with disease progression in MS and structural CNS damage, especially of gray matter.24 CSF levels of C4a and C3a were associated with 14% (p = 0.0021) and 17% (p < 0.0001) increased GFAP concentrations, and for component C1q 40%, higher GFAP levels were found (p < 0.0001) (Table 3; eTable 8; Figure 2).
Table 3.
Multivariable Associations of CSF Complement Components and Activation Products With GFAP and Cytokine Levels in CSF
| n = 235 | GFAP | CXCL-13 | CXCL-9 | IL-12b | ||||||||
| ME | CI | p Value | ME | CI | p Value | ME | CI | p Value | ME | CI | p Value | |
| C4a | 1.14 | 1.05–1.25 | 0.0021 | 1.63 | 1.21–2.19 | 0.0014 | 1.50 | 1.23–1.82 | <1e-04 | 1.38 | 1.17–1.64 | 0.0003 |
| C4 | 1.00 | 0.89–1.13 | 0.9629 | 0.60 | 0.40–0.90 | 0.0146 | 0.81 | 0.62–1.07 | 0.1422 | 0.72 | 0.57–0.92 | 0.0083 |
| C3a | 1.17 | 1.09–1.26 | <1e-04 | 2.12 | 1.65–2.70 | <1e-04 | 1.85 | 1.59–2.16 | <1e-04 | 1.79 | 1.57–2.04 | <1e-04 |
| C3 | 1.02 | 0.97–1.07 | 0.4313 | 1.21 | 1.03–1.43 | 0.0252 | 1.11 | 0.99–1.24 | 0.0628 | 1.05 | 0.95–1.16 | 0.3036 |
| C5a | 0.98 | 0.95–1.02 | 0.3536 | 1.21 | 1.06–1.38 | 0.0042 | 1.12 | 1.03–1.22 | 0.0086 | 1.09 | 1.01–1.18 | 0.0235 |
| s-C5b9 | 0.98 | 0.95–1.02 | 0.3029 | 1.14 | 1.01–1.29 | 0.0328 | 1.11 | 1.02–1.20 | 0.0114 | 1.04 | 0.97–1.12 | 0.2488 |
| C5 | 1.05 | 0.95–1.15 | 0.3584 | 0.98 | 0.70–1.36 | 0.8840 | 1.33 | 1.07–1.66 | 0.0101 | 1.17 | 0.97–1.42 | 0.1062 |
| Ba | 1.03 | 0.92–1.14 | 0.6203 | 2.29 | 1.60–3.28 | <1e-04 | 2.07 | 1.65–2.60 | <1e-04 | 1.73 | 1.41–2.12 | <1e-04 |
| Bb | 1.01 | 0.95–1.07 | 0.8348 | 1.42 | 1.15–1.75 | 0.0013 | 1.36 | 1.18–1.56 | <1e-04 | 1.27 | 1.12–1.43 | 0.0002 |
| C1q | 1.40 | 1.22–1.60 | <1e-04 | 2.43 | 1.49–3.98 | 0.0005 | 2.13 | 1.55–2.94 | <1e-04 | 1.77 | 1.33–2.35 | 0.0001 |
| Factor H | 1.23 | 0.97–1.55 | 0.0824 | 1.03 | 0.45–2.35 | 0.9454 | 1.26 | 0.73–2.17 | 0.4067 | 0.95 | 0.59–1.54 | 0.8429 |
| Factor I | 1.11 | 0.99–1.23 | 0.0663 | 1.02 | 0.70–1.49 | 0.9204 | 1.30 | 1.01–1.66 | 0.0424 | 1.12 | 0.89–1.39 | 0.3325 |
Abbreviations: CAP = complement activation product; CC = complement component; DMT = disease-modifying treatment; GFAP = glial fibrillary acidic protein; LP = lumbar puncture; ME = multiplicative effect; Qalb = albumin-quotient.
Reading example: effect of CC/CAP concentration doubling: per doubling of CSF C4a levels CXCL-13 levels are increased by 63% (after adjustment for age, sex, Qalb, and DMT category at LP).
Figure 2. Multivariable Associations of CSF CC or CAP With GFAP Levels.
In CIS/MS (n = 235), doubling of CSF C3a levels was associated with by 17%, C4a 14% and C1q 40% increased GFAP CSF levels (compare Table 3). CAP = complement activation product; CC = complement component; CIS = clinically isolated syndrome; GFAP = glial fibrillary acidic protein; MS = multiple sclerosis.
Complement Activation-Associated Cytokines
CSF cytokines previously associated with MS disease activity and severity such as CXCL-13, CXCL-9, and IL-12b25,26 are produced by complement-receptor–positive cells of the innate immune system and can amplify B-cell and T-cell autoreactivity.27-30Doubling of C4a, C3a, and C5a levels was associated with higher CXCL-13 (C4a: 63%, p = 0.0014; C3a: 112%, p < 0.0001; C5a: 21%, p = 0.0042 increase), CXCL-9 (C4a: 50%, p < 0.0001; C3a: 85%, p < 0.0001; C5a: 12%, p = 0.0086), and IL-12b levels (C4a: 38%, p = 0.003; C3a: 79%, p < 0.0001; C5a: 9%, p = 0.0235), respectively. In return for nonactivated complement component C4, 40% lower CXCL-13 (p = 0.0146) and 28% lower IL-12b (p = 0.0083) levels were found, while there were no clear patterns for C3 and C5 (Table 3).
Regarding the alternative pathway, doubling of activation product Ba and Bb CSF levels was associated with 129% (p < 0.0001)/42% (p = 0.0013) higher CXCL-13, 107% (p < 0.0001)/36% (p < 0.0001) CXCL-9, and 73% (p < 0.0001)/27% (p = 0.0002) higher IL-12b levels, respectively. Most pronounced effects were observed for complement component C1q: per doubling of C1q levels, CXCL-13 concentrations were increased by 143% (p = 0.0005): CXCL-9 113% (p <0.0001) and IL-12b by 77% (p = 0.0001) (Table 3; eTable 9; Figure 3).
Figure 3. Multivariable Associations of CSF C4a, C3a, Ba, and C1q Levels With Cytokines.
Strong multivariable associations of C4a, C3a, Ba, and C1q with higher IL-12b, CXCL-9, and CXCL-13 CSF levels in a homogenous and consistent pattern are displayed. Reading example: doubling of C4a CSF levels was associated with by 38% higher IL-12b levels (red bar), by 50% increased CXCL-9 levels (orange bar), and 63% higher CXCL13 levels (yellow bar) (compare Table 3).
Discussion
Our study demonstrates that CSF complement activation in MS is consistently associated with MRI endpoints reflecting structural tissue damage, focal inflammation, and neurodegeneration: Earlier (C3a, Ba, C4a) and to a lesser extent later cascade CAP (C5a, s-C5b9) in the CSF were associated with longitudinal total T2 lesion volumes, atrophy, CELs, and PRLs. We consistently found strong associations of CSF C1q levels with earlier cascade (Ba, C3a, C4a, Bb) and, also to a lesser extent, later cascade CAP (C5a, s-C5b9) with cytokine levels of CXCL-13, CXCL-9, and Il-12b and with GFAP levels.
These results are in line with our previous study reporting that earlier cascade CAP (C3a, C4a, Ba, Bb) are associated with outcomes reflecting disease severity such as the MS Severity Score, EDSS score, and neurofilament light chain (NfL) levels, reflecting neuroaxonal damage.10 Moreover, several neuropathologic studies are consistent with our findings: lesion-associated complement activation was observed in a large subgroup of patients with early MS.31 IgG and IgM colocalizing with C3b on demyelinated axons and oligodendrocytes and antibody-antigen immunocomplexes were detected in foamy macrophages in active lesion areas.32 In patients with longer disease duration, perivascular inflammatory infiltrates, astrocytic gliosis, diffuse axonal injury, and microglial activation as well as clusters of microglia around damaged axons coated with complement, especially C3 activation products, were consistently present in the normal-appearing white matter or in the periplaque of MS brains.2,3,33 In addition, in the cortical gray matter of progressive MS, C1q depositions and complement activation fragments (Bb, C3b, s-C5b9) were increased, especially in those areas with elevated numbers of complement-receptor–positive microglia.5 These data are in line with the observation that increased immunoreactivity for C1q, C4d, Bb, and C3b occurs in thalamic lesions with an active inflammatory pathology in patients with progressive MS.34
The complement system not only opsonizes targets for phagocytosis but also activates the immune system through soluble anaphylatoxins.4,12 Activated complement proteins link innate with adaptive immune responses by acting directly on receptors expressed on T cells, B cells, or myeloid immune cells.35-38 We found a homogenous association pattern of mainly proximal CC and CAP with cytokines such as CXCL-13, CXCL-9, and IL-12b. CXCL-9 and IL-12b are in general secreted by complement-receptor–positive cells such as macrophages and dendritic cells and regulate migration, differentiation, and activation of TH1-lymphocytes and natural killer cells.29,27 CXCL-13 is produced by complement-receptor rich follicular dendritic cells39 in B-cell follicles and is the only known ligand of the CXCR5-receptor. This “B-cell–attracting chemokine” is supposed to be one of the key players involved in the organization of lymphoid tissue and regulates homing of B cells and subsets of T cells to the follicles.28 Formation of leptomeningeal ectopic lymphoid follicles comprising germinal centers (GC) and chronic active lesions with activated microglia is supposed to be involved in clinical deterioration in MS.13,40,41 Signaling of activated complement through complement receptors C3aR/C5aR is crucial for GC formation and function because it promotes affinity maturation, plasma cell and memory B-cell production.42,43
We found that C1q, and to a lesser extent C3a and C4a were associated with GFAP levels. NfL as a marker of neuroaxonal damage was strongly associated with CAP (C4a, C3a, Ba, Bb) but in contrast to GFAP not with C1q levels. GFAP is supposed to indicate either activation or damage of inflamed astrocytes and is associated with progression in MS and accelerated gray matter loss.24 Our results are in line with a recent study postulating that C1q is upregulated and expressed by inflamed microglia in MS, whereas inflamed astrocytes were found to have 20-fold upregulation (relative to nonreactive astrocytes) of C1q-complex activators and C1q-receptors as well as an upregulation of complement component C3. Microglia expressed C3 receptors, implicating early complement compounds as mediators of crosstalk between inflamed astrocytes and microglia.44 In another neuropathologic study, C1q expression and C3 activation were increased in hippocampal neurons of patients with MS, showing a marked decrease of synaptic density; also, these authors concluded that the C1q-C3 complement axis might play an important role in MS.45
Mechanisms how complement activation contributes to CNS tissue damage and disability accumulation in human MS remain to be clarified, but there are results from experimental autoimmune encephalomyelitis (EAE) models: it was shown that a C1q-blocking antibody efficiently reduced C1q intensity staining in the brain of an EAE model and microglia reflecting reactive gliosis in the white matter were significantly reduced. This suggested that pharmacologic inhibition especially of C1q might be a potential therapeutic avenue to address chronic inflammation in the white matter.44 Local intraparenchymal inhibition of C3 in MOG35-55-induced EAE mice models resulted in synaptic preservation, which means that these mice were protected from complement-mediated synaptic pruning by microglia. It also blocked loss of visual acuity.46 Treatment with recombinant soluble human complement receptor-1 (CR1), blocking C3- and C5-convertase, significantly reduced clinical disease severity, inhibited CNS inflammation, almost blocked demyelination, and markedly reduced tissue deposition of C1, C3, and C9 in an antibody-mediated demyelinating EAE rat model.47 Focusing specifically on the later cascade, C6-deficient rats developed significantly milder acute EAE after myelin basic protein immunization48 and no demyelination, decreased T-cell and macrophage infiltration and no C9 detection was found.49 In line with this study, mice deficient in terminal complement complex regulator CD59a developed a more severe disease with substantial demyelination in the acute phase of EAE MOG35-55.50 However, in the chronic phase, C5 deficiency was reported to be associated with more inflammatory demyelination and Wallerian degeneration, while in C5-sufficient mice, axonal sparing and extensive remyelination were found.51 Overall, these data suggest that activation of the distal complement cascade might represent a double-edge sword with effects both on damage and repair, also depending on the timing. From a translational perspective, these data suggest that treatment with inhibitors of the early complement cascade might have a higher therapeutic potential in MS, while conventional C5-inhibitors could even worsen disease severity and progression in MS.
One limitation of our study is the lack of longitudinal CSF complement and cytokine data. In particular, sC5b-9 had a high percentage of samples below the sensitivity of the assay used, which necessitated imputation to include low levels of respective analytes in the analysis. Another limitation was a relatively small number of patients with progressive MS in this data set.
Taken together, aberrant complement activation in the CSF is prominently associated with biomarkers indicative for structural brain damage relevant to disability accumulation in MS. Although mechanistic underpinnings of these associations remain to be further investigated and clarified, our data suggest that complement inhibition is a potential future therapeutic avenue to address disease progression and disability accumulation in MS.
Acknowledgment
The authors express their deep thankfulness to the patients and their relatives for their participation and support, to the study nurses for their motivated collaboration and recruitment efforts, and to the administrative personnel of the CSF databank study and the Swiss Multiple Sclerosis Cohort study.
Glossary
- CIS
clinically isolated syndrome
- EDSS
Expanded Disability Status Scale Score
- Est
estimate
- FLAIR
fluid-attenuated inversion recovery
- GC
germinal center
- GFAP
glial fibrillary acidic protein
- IQR
interquartile range
- LLOQ
lower limit of quantification
- LP
lumbar puncture
- MS
multiple sclerosis
- NfL
neurofilament light chain
- PPMS
primary progressive multiple sclerosis
- PRL
paramagnetic rim lesion
- QC
quality control
- RRMS
relapsing-remitting multiple sclerosis
- SMSC
Swiss multiple sclerosis cohort
- SPMS
secondary progressive multiple sclerosis
- T2w
T2-weighted
Author Contributions
J. Oechtering: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data. S.A. Schaedelin: drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data. K. Stein: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. A. Maleska Maceski: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. L. Melie-Garcia: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. P. Benkert: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. A. Cagol: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. S. Leber: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. R. Galbusera: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. E. Ruberte: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. W. Hu: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. F. Qureshi: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. A. Orleth: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. L. Demuth: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. E. Willemse: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. I. Heijnen: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. A. Regeniter: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. T.J. Derfuss: drafting/revision of the manuscript for content, including medical writing for content. B. Fischer-Barnicol: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. L. Achtnichts: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. S. Mueller: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. R. Hoepner: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. P.H. Lalive: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. C. Bridel: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. M. D'Souza: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. C. Pot: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. R.A. Du Pasquier: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. C. Gobbi: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. C. Zecca: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. H. Wiendl: drafting/revision of the manuscript for content, including medical writing for content. J.M. Lieb: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. C. Lamers: drafting/revision of the manuscript for content, including medical writing for content. L. Kappos: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. M. Trendelenburg: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. D. Leppert: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. C. Granziera: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. J. Kuhle: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data. J.D. Lünemann: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data.
Study Funding
This investigation was supported by the Swiss Multiple Sclerosis Society (research grant 2021/10). The Swiss MS Cohort study received funding from the Swiss Multiple Sclerosis Society and grant funding from Biogen, Bristol Myers Squibb, Celgene, Merck, Novartis, Roche, and Sanofi. The authors acknowledge support by the German Research Foundation (LU 900/3-1 to J.D. Lünemann. and through the Collaborative Research Centre SFB-TRR128 “Initiating/Effector vs Regulatory Mechanisms in Multiple Sclerosis – Progress towards Tackling the Disease” SFB/TR128 to H. Wiendl and J.D. Lünemann).
Disclosure
S.A. Schaedelin, K. Stein, A. Maleska Maceski, P. Benkert, S. Leber, E. Ruberte, A. Orleth, L. Demuth, I. Heijnen, A. Regeniter, E. Willemse, C. Lamers, L. Melie-Garcia, A. Cagol, R. Galbusera, J.D. Lünemann, and R. Hoepner report no conflicts of interest. J. Oechtering received research support by the Swiss MS Society and served on advisory boards for Roche and Merck. W. Hu and F. Qureshi are employees of Octave Bioscience, Inc., T.J. Derfuss received speaker fees, research support, travel support, and/or served on Advisory Boards, data safety monitoring boards, or Steering Committees of Alexion, Celgene, Polyneuron, Novartis Pharma, Merck Serono, Biogen, Teva, GeNeuro, Roche, and Sanofi Genzyme. B. Fischer-Barnicol served on an advisory board for Biogen. L. Achtnichts served on scientific advisory boards for Celgene, Novartis Pharmaceuticals, Merck, Biogen, Sanofi Genzyme, Roche, and Bayer; received funding for travel and/or speaker honoraria from Celgene, Biogen, Sanofi Genzyme, Novartis, Merck Serono, Roche, Teva, and the Swiss MS Society; and research support from Biogen, Sanofi, Genzyme, and Novartis. SM received speaker fees, research support, travel support, and/or served on advisory boards by Almirall, Alexion, Bayer, Biogen, Bristol Myers Squibb, Celgene, Genzyme, Merck-Serono, Teva, Novartis, and Roche. P.H. Lalive received honoraria for speaking and/or travel expenses from Biogen, Merck, Novartis, and Roche; consulting fees from Biogen, GeNeuro, Merck, Novartis, and Roche; research support from Biogen, Merck, and Novartis. None were related to this work. C. Bridel served on scientific advisory boards for Biogen, Novartis, and BMS. M.D'Souza has received travel support from Bayer AG, Biogen, Teva Pharmaceuticals, and Sanofi Genzyme and research support from the University Hospital Basel. C. Pot received consulting fees and/or travel compensation, used exclusively for research support, for activities with Biogen, Merck, Novartis, Roche, and Sanofi Genzyme. R.A. Du Pasquierhas served on scientific advisory boards for Biogen, Celgene, Merck, Novartis, Roche, and Sanofi. He has received funding for travel or speaker honoraria from Roche. C. Gobbi Ente Ospedaliero Cantonale (employer) received compensation for C. Gobbi's speaking activities, consulting fees, or grants from Abbvie, Almirall, Biogen, Bristol Meyer Squibb, Lundbeck, Merck, Novartis, Sandoz, Sanofi, Teva Pharma, and Roche. C. Zecca Ente Ospedaliero Cantonale (employer) received compensation for C. Zecca's speaking activities, consulting fees, or grants from Abbvie, Almirall, Biogen, Bristol Meyer Squibb, Lundbeck, Merck, Novartis, Sandoz, Sanofi, Teva Pharma, and Roche. C. Zecca is a recipient of a grant for senior researchers provided by AFRI (Area Formazione accademica, Ricerca e Innovazione), EOC. H. Wiendl receives honoraria for acting as a member of Scientific Advisory Boards for Janssen, Merck, and Novartis as well as speaker honoraria and travel support from Alexion, Amicus Therapeuticus, Biogen, Biologix, Bristol Myers Squibb, Cognomed, F. Hoffmann-La Roche Ltd., Gemeinnützige Hertie-Stiftung, Medison, Merck, Novartis, Roche Pharma AG, Genzyme, TEVA, and WebMD Global. Prof. Wiendl is acting as a paid consultant for Biogen, Bristol Myers Squibb, EMD Serono, Idorsia, Immunic, Novartis, Roche, Sanofi, the Swiss MS Society, and UCB. His research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgesellschaft (DFG), Deutsche Myasthenie Gesellschaft e.V., Alexion, Amicus Therapeutics Inc., Argenx, Biogen, CSL Behring, F. Hoffmann—La Roche, Genzyme, Merck KgaA, Novartis Pharma, Roche Pharma, and UCB Biopharma. L. Kappos's: employer (University Hospital Basel) has received and dedicated to research support steering committee, advisory board, and consultancy fees (Abbvie, Actelion, Almirall, Auriga Vison AG, Bayer HealthCare, Biogen, Eisai, EMD Derono Inc., Genzyme, Genentech Inc., F. Hoffmann-La Roche, Japan Tobacco, Janssen Pharmaceuticals Inc., Merck, Minoryx Therapeutics SL, Novartis, Sanofi, Santhera, Senda Biosciences, Shionogi BV, and TG Therapeutics); speaker fees (Bayer HealthCare, Biogen, Celgene, Genzyme, Janssen Pharmaceuticals Inc., Merck, Novartis, Roche, and Sanofi); support of educational activities (Allergan, Bayer HealthCare, Biogen, CSL Behring, Genzyme, Merck, Novartis, Roche, Pfizer, Sanofi, Shire, and Teva); license fees for Neurostatus products; and grants (Bayer HealthCare, Biogen, European Union, Innosuisse, Merck, Novartis, Roche Research Foundation, Swiss MS Society, and Swiss National Research Foundation). M. Trendelenburg has research collaborations with Roche, Novartis, and Idorsia (all Switzerland). D. Leppert is a Chief Medical Officer of GeNeuro. C. Granziera: The University Hospital Basel (USB), as the employer of Cristina Granziera has received the following fees which were used exclusively for research support: (1) advisory board and consultancy fees from Actelion, Novartis, Genzyme, and F. Hoffmann-La Roche; (2) speaker fees from Biogen and Genzyme-Sanofi; (3) research support from F. Hoffmann-La Roche Ltd.; Before her employment at USB, she has also received speaker honoraria and travel funding by Novartis. J. Kuhle received speaker fees, research support, travel support, and/or served on advisory boards by Swiss MS Society, Swiss National Research Foundation (320030_189140/1), University of Basel, Progressive MS Alliance, Bayer, Biogen, Bristol Myers Squibb, Celgene, Merck, Novartis, Octave Bioscience, Roche, and Sanofi. JDL received speaker fees, research support, travel support, and/or served on advisory boards by the EU Framework Programme Horizon Europe, The German Research Foundation (LU 9001-1; LU 900-4; and the Collaborative Research Centre SFB-TRR128 “Initiating/Effector vs Regulatory Mechanisms in MS—Progress towards Tackling the Disease”), Abbvie, Alexion, Argenx, Biogen, Merck, Novartis, Roche, Sanofi, and Takeda. Go to Neurology.org/N for full disclosures.
References
- 1.Attfield KE, Jensen LT, Kaufmann M, Friese MA, Fugger L. The immunology of multiple sclerosis. Nat Rev Immunol. 2022;22(12):734-750. doi: 10.1038/s41577-022-00718-z [DOI] [PubMed] [Google Scholar]
- 2.Breij EC, Brink BP, Veerhuis R, et al. Homogeneity of active demyelinating lesions in established multiple sclerosis. Ann Neurol. 2008;63(1):16-25. doi: 10.1002/ana.21311 [DOI] [PubMed] [Google Scholar]
- 3.Ingram G, Loveless S, Howell OW, et al. Complement activation in multiple sclerosis plaques: an immunohistochemical analysis. Acta Neuropathol Commun. 2014;2:53. doi: 10.1186/2051-5960-2-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan BP, Gommerman JL, Ramaglia V. An “Outside-In” and “Inside-Out” consideration of complement in the multiple sclerosis brain: lessons from development and neurodegenerative diseases. Front Cel Neurosci. 2020;14:600656. doi: 10.3389/fncel.2020.600656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watkins LM, Neal JW, Loveless S, et al. Complement is activated in progressive multiple sclerosis cortical grey matter lesions. J Neuroinflammation. 2016;13(1):161. doi: 10.1186/s12974-016-0611-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ingram G, Hakobyan S, Hirst CL, et al. Systemic complement profiling in multiple sclerosis as a biomarker of disease state. Mult Scler. 2012;18(10):1401-1411. doi: 10.1177/1352458512438238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ingram G, Hakobyan S, Hirst CL, et al. Complement regulator factor H as a serum biomarker of multiple sclerosis disease state. Brain. 2010;133(Pt 6):1602-1611. doi: 10.1093/brain/awq085 [DOI] [PubMed] [Google Scholar]
- 8.Ingram G, Hakobyan S, Robertson NP, Morgan BP. Elevated plasma C4a levels in multiple sclerosis correlate with disease activity. J Neuroimmunol. 2010;223(1-2):124-127. doi: 10.1016/j.jneuroim.2010.03.014 [DOI] [PubMed] [Google Scholar]
- 9.Keller CW, Oechtering J, Wiendl H, Kappos L, Kuhle J, Lunemann JD. Impact of complement activation on clinical outcomes in multiple sclerosis. Ann Clin Transl Neurol. 2021;8(4):944-950. doi: 10.1002/acn3.51334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oechtering J, Stein K, Schaedelin SA, et al. Complement activation is associated with disease severity in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2024;11(2):e200212. doi: 10.1212/NXI.0000000000200212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coulthard LG, Hawksworth OA, Woodruff TM. Complement: the emerging architect of the developing brain. Trends Neurosci. 2018;41(6):373-384. doi: 10.1016/j.tins.2018.03.009 [DOI] [PubMed] [Google Scholar]
- 12.Saez-Calveras N, Stuve O. The role of the complement system in multiple sclerosis: a review. Front Immunol. 2022;13:970486. doi: 10.3389/fimmu.2022.970486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Absinta M, Sati P, Masuzzo F, et al. Association of chronic active multiple sclerosis lesions with disability in vivo. JAMA Neurol. 2019;76(12):1474-1483. doi: 10.1001/jamaneurol.2019.2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cagol A, Schaedelin S, Barakovic M, et al. Association of brain atrophy with disease progression independent of relapse activity in patients with relapsing multiple sclerosis. JAMA Neurol. 2022;79(7):682-692. doi: 10.1001/jamaneurol.2022.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sastre-Garriga J, Pareto D, Battaglini M, et al. MAGNIMS consensus recommendations on the use of brain and spinal cord atrophy measures in clinical practice. Nat Rev Neurol. 2020;16(3):171-182. doi: 10.1038/s41582-020-0314-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Disanto G, Benkert P, Lorscheider J, et al. The Swiss multiple sclerosis cohort-study (SMSC): a prospective Swiss wide investigation of key phases in disease evolution and new treatment options. PLoS One. 2016;11(3):e0152347. doi: 10.1371/journal.pone.0152347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227-231. doi: 10.1002/ana.410130302 [DOI] [PubMed] [Google Scholar]
- 18.Lorscheider J, Buzzard K, Jokubaitis V, et al. Defining secondary progressive multiple sclerosis. Brain. 2016;139(pt 9):2395-2405. doi: 10.1093/brain/aww173 [DOI] [PubMed] [Google Scholar]
- 19.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi: 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 20.Andermatt S, Pezold S, Cattin PC. Automated segmentation of multiple sclerosis lesions using multi-dimensional gated recurrent units. Lecture Notes Comp Sci. 2018;10670:31-42. doi: 10.1007/978-3-319-75238-9_3 [DOI] [Google Scholar]
- 21.Palumbo L, Bosco P, Fantacci ME, et al. Evaluation of the intra- and inter-method agreement of brain MRI segmentation software packages: a comparison between SPM12 and FreeSurfer v6.0. Phys Med. 2019;64:261-272. doi: 10.1016/j.ejmp.2019.07.016 [DOI] [PubMed] [Google Scholar]
- 22.Andersson M, Alvarez-Cermeno J, Bernardi G, et al. Cerebrospinal fluid in the diagnosis of multiple sclerosis: a consensus report. J Neurol Neurosurg Psychiatry. 1994;57(8):897-902. doi: 10.1136/jnnp.57.8.897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qureshi F, Hu W, Loh L, et al. Analytical validation of a multi-protein, serum-based assay for disease activity assessments in multiple sclerosis. Proteomics Clin Appl. 2023;17(3):e2200018. doi: 10.1002/prca.202200018 [DOI] [PubMed] [Google Scholar]
- 24.Meier S, Willemse E, Schaedelin S, et al. Serum glial fibrillary acidic protein compared with neurofilament light chain as biomarker for multiple sclerosis disease progression. JAMA Neurol. 2023;80(3):287-297. doi: 10.1001/jamaneurol.2022.5250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang J, Khademi M, Fugger L, et al. Inflammation-related plasma and CSF biomarkers for multiple sclerosis. Proc Natl Acad Sci U S A. 2020;117(23):12952-12960. doi: 10.1073/pnas.1912839117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magliozzi R, Howell OW, Nicholas R, et al. Inflammatory intrathecal profiles and cortical damage in multiple sclerosis. Ann Neurol. 2018;83(4):739-755. doi: 10.1002/ana.25197 [DOI] [PubMed] [Google Scholar]
- 27.Henry CJ, Ornelles DA, Mitchell LM, Brzoza-Lewis KL, Hiltbold EM. IL-12 produced by dendritic cells augments CD8+ T cell activation through the production of the chemokines CCL1 and CCL17. J Immunol. 2008;181(12):8576-8584. doi: 10.4049/jimmunol.181.12.8576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan Z, Zhu T, Liu Y, Zhang N. Role of the CXCL13/CXCR5 Axis in autoimmune diseases. Front Immunol. 2022;13:850998. doi: 10.3389/fimmu.2022.850998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tokunaga R, Zhang W, Naseem M, et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation - a target for novel cancer therapy. Cancer Treat Rev. 2018;63:40-47. doi: 10.1016/j.ctrv.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vandendriessche S, Cambier S, Proost P, Marques PE. Complement receptors and their role in leukocyte recruitment and phagocytosis. Front Cel Dev Biol. 2021;9:624025. doi: 10.3389/fcell.2021.624025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47(6):707-717. doi: [DOI] [PubMed] [Google Scholar]
- 32.Sadaba MC, Tzartos J, Paino C, et al. Axonal and oligodendrocyte-localized IgM and IgG deposits in MS lesions. J Neuroimmunol. 2012;247(1-2):86-94. doi: 10.1016/j.jneuroim.2012.03.020 [DOI] [PubMed] [Google Scholar]
- 33.Loveless S, Neal JW, Howell OW, et al. Tissue microarray methodology identifies complement pathway activation and dysregulation in progressive multiple sclerosis. Brain Pathol. 2018;28(4):507-520. doi: 10.1111/bpa.12546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooze BJ, Dickerson M, Loganathan R, et al. The association between neurodegeneration and local complement activation in the thalamus to progressive multiple sclerosis outcome. Brain Pathol. 2022;32(5):e13054. doi: 10.1111/bpa.13054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arvieux J, Yssel H, Colomb MG. Antigen-bound C3b and C4b enhance antigen-presenting cell function in activation of human T-cell clones. Immunology. 1988;65(2):229-235. [PMC free article] [PubMed] [Google Scholar]
- 36.Dempsey PW, Allison MED, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271(5247):348-350. doi: 10.1126/science.271.5247.348 [DOI] [PubMed] [Google Scholar]
- 37.Kemper C, Atkinson JP. T-cell regulation: with complements from innate immunity. Nat Rev Immunol. 2007;7(1):9-18. doi: 10.1038/nri1994 [DOI] [PubMed] [Google Scholar]
- 38.Kemper C, Mitchell LM, Zhang L, Hourcade DE. The complement protein properdin binds apoptotic T cells and promotes complement activation and phagocytosis. Proc Natl Acad Sci U S A. 2008;105(26):9023-9028. doi: 10.1073/pnas.0801015105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cinti I, Denton AE. Lymphoid stromal cells-more than just a highway to humoral immunity. Oxf Open Immunol. 2021;2(1):iqab011. doi: 10.1093/oxfimm/iqab011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elliott C, Belachew S, Wolinsky JS, et al. Chronic white matter lesion activity predicts clinical progression in primary progressive multiple sclerosis. Brain. 2019;142(9):2787-2799. doi: 10.1093/brain/awz212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Magliozzi R, Howell O, Vora A, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130(Pt 4):1089-1104. doi: 10.1093/brain/awm038 [DOI] [PubMed] [Google Scholar]
- 42.Cumpelik A, Heja D, Hu Y, et al. Dynamic regulation of B cell complement signaling is integral to germinal center responses. Nat Immunol. 2021;22(6):757-768. doi: 10.1038/s41590-021-00926-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson MJ, Quast I, Tarlinton DM. Complement-in' the germinal center response. Nat Immunol. 2021;22(6):673-674. doi: 10.1038/s41590-021-00946-w [DOI] [PubMed] [Google Scholar]
- 44.Absinta M, Maric D, Gharagozloo M, et al. A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature. 2021;597(7878):709-714. doi: 10.1038/s41586-021-03892-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michailidou I, Willems JG, Kooi EJ, et al. Complement C1q-C3-associated synaptic changes in multiple sclerosis hippocampus. Ann Neurol. 2015;77(6):1007-1026. doi: 10.1002/ana.24398 [DOI] [PubMed] [Google Scholar]
- 46.Werneburg S, Jung J, Kunjamma RB, et al. Targeted complement inhibition at synapses prevents microglial synaptic engulfment and synapse loss in demyelinating disease. Immunity. 2020;52(1):167-182.e7. doi: 10.1016/j.immuni.2019.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piddlesden SJ, Storch MK, Hibbs M, Freeman AM, Lassmann H, Morgan BP. Soluble recombinant complement receptor 1 inhibits inflammation and demyelination in antibody-mediated demyelinating experimental allergic encephalomyelitis. J Immunol. 1994;152(11):5477-5484. doi: 10.4049/jimmunol.152.11.5477 [DOI] [PubMed] [Google Scholar]
- 48.Mead RJ, Singhrao SK, Neal JW, Lassmann H, Morgan BP. The membrane attack complex of complement causes severe demyelination associated with acute axonal injury. J Immunol. 2002;168(1):458-465. doi: 10.4049/jimmunol.168.1.458 [DOI] [PubMed] [Google Scholar]
- 49.Tran GT, Hodgkinson SJ, Carter N, Killingsworth M, Spicer ST, Hall BM. Attenuation of experimental allergic encephalomyelitis in complement component 6-deficient rats is associated with reduced complement C9 deposition, P-selectin expression, and cellular infiltrate in spinal cords. J Immunol. 2002;168(9):4293-4300. doi: 10.4049/jimmunol.168.9.4293 [DOI] [PubMed] [Google Scholar]
- 50.Mead RJ, Neal JW, Griffiths MR, et al. Deficiency of the complement regulator CD59a enhances disease severity, demyelination and axonal injury in murine acute experimental allergic encephalomyelitis. Lab Invest. 2004;84(1):21-28. doi: 10.1038/labinvest.3700015 [DOI] [PubMed] [Google Scholar]
- 51.Weerth SH, Rus H, Shin ML, Raine CS. Complement C5 in experimental autoimmune encephalomyelitis (EAE) facilitates remyelination and prevents gliosis. Am J Pathol. 2003;163(3):1069-1080. doi: 10.1016/S0002-9440(10)63466-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request.