Abstract
Lipoprotein(a) [Lp(a)] is a well-established cardiovascular disease (CVD) risk factor with elevated Lp(a) levels contributing to a higher incidence of atherosclerotic CVD (ASCVD). However, no Lp(a)-specific interventions are currently available in the primary CVD prevention in individuals with elevated Lp(a) levels. RNA-based therapies targeting Lp(a) are under investigation in phase III clinical trials. However, these trials are mostly focused on the secondary prevention of patients with established ASCVD. Aspirin (ASA) has demonstrated efficacy in reducing major adverse cardiovascular events in secondary prevention; however, its role in primary prevention is limited due to an increased risk of haemorrhagic events. Consequently, current guidelines recommend ASA for primary prevention only in patients with a high risk for CVD and low haemorrhagic risk. Identifying sub-populations that may benefit from ASA in primary prevention is an ongoing research focus. To that effect, some evidence suggests that individuals without established ASCVD but with elevated Lp(a) levels may represent such a sub-group, where the potential cardiovascular benefits of ASA may outweigh the risk of bleeding. However, though promising, these data are limited, largely retrospective and require further validation. The mechanisms through which ASA may confer benefit in this population have not been fully elucidated but potential pathways include a modest reduction in Lp(a) levels and the inhibition of Lp(a)-mediated platelet activation and aggregation. Whilst preliminary findings are promising, there are still significant gaps in the literature, underscoring the need for more rigorous, prospective studies to elucidate the role of ASA in this specific context.
Keywords: aspirin (ASA), cardiovascular disease, hyperlipoproteinaemia(a), lipoprotein(a), primary prevention
Introduction
Lipoprotein(a) [Lp(a)] constitutes a novel and potentially modifiable cardiovascular disease (CVD) risk factor. Even though it has been nearly 60 years since its discovery by Berg,1 various aspects regarding its metabolism and function are still under investigation. The association of Lp(a) with the development and progression of CVD has been revealed through Mendelian randomization analysis and genome-based studies, which clearly indicate a causal link between Lp(a) levels and multiple aspects of atherosclerosis such as coronary artery disease (CAD), ischaemic stroke and aortic aneurysm.2,3 The increased risk of CVD in patients with elevated Lp(a) levels highlights the need for easily applicable methods for both primary and secondary prevention in this population.
The role of aspirin (ASA) in secondary CVD prevention is well established. Specifically, ASA is considered a first-line treatment in all cases with at least one cardiovascular event of atherosclerotic origin such as myocardial infarction or ischaemic stroke.4 However its benefit in primary prevention is controversial. Current data support that despite the mitigation of major acute cardiovascular events (MACE), ASA is unable to decrease mortality in the general population due to a significant increase in bleeding risk.5
However, there may be particular population sub-groups with increased CVD prevalence where the benefit of ASA could be more prominent. Individuals with elevated Lp(a) levels may be a suitable population for investigating this hypothesis mainly due to the anti-inflammatory and antithrombotic properties of ASA, which could potentially compete with and inhibit the atherothrombotic activity of Lp(a). Therefore, a potential favourable effect of ASA in individuals with high Lp(a) levels is under investigation.
This comprehensive review aims to provide readers with the current data on the use of ASA as a primary prevention tool for atherosclerotic CVD (ASCVD) in patients with elevated Lp(a) levels.
Review
Lp(a): structure, pathophysiology and role in CVD
Elevated Lp(a) levels are an independent risk factor for the development of ASCVD and an important determinant of the residual CVD risk in patients in whom other established risk factors, such as elevated low-density lipoprotein (LDL) cholesterol (LDL-C) levels, hypertension and diabetes mellitus (DM), are well controlled.6,7
Recently, a Mendelian randomization analysis in patients of European descent showcased a causal association of Lp(a) levels with CAD (OR 1.003, 95% CI 1.001–1.004; p=0.010), aortic aneurysm (OR 1.005, 95% CI 1.001–1.010; p=0.009) and large artery atherosclerosis stroke (OR 1.003, 95% CI 1.002–1.004; p=9.50×10−11) in the general population.3
Though the association of Lp(a) with CVD risk is linear, previously young healthy individuals face an apparently increased risk for myocardial infarction and calcific aortic valve stenosis when Lp(a) exceeds the 75th percentile (>30 mg/dL in the general population).8,9 An Lp(a) concentration over the 90th percentile (>70 mg/dL) potentiates the hazard for heart failure in these individuals, whilst higher values, over the 95th percentile (>100 mg/dL) are associated with fatal CVD events and ischaemic strokes.9 Globally, almost 20% of the population has Lp(a) values ≥100–125 nmol/L (∼50 mg/dL).10
Lp(a) is synthesized almost exclusively in the liver but its catabolism and secretion are not fully understood, with LDL receptors being partly responsible for its clearance from the circulation.11–13
The distinctive properties of Lp(a) in regard to weight, density and electrophoretic mobility are mainly attributed to its unique structure.14 Lp(a) consists of a lipid-rich core containing cholesterol esters and triglycerides, surrounded by a hydrophilic outer layer of phospholipids and non-esterified cholesterol.15 Similar to LDL, Lp(a) contains apolipoprotein B-100 (apoB100) covalently attached to apolipoprotein(a) [apo(a)] through a single disulfide linkage between two cysteine residues.8 Apo(a) consists of repeating triple-loop protein units known as kringles (KIV1–KIV10), with all but KIV2 found in single copies. KIV2 is found in multiple copies (1 to more than 40), giving rise to apo(a) isoforms of unequal size and noteworthy heterogeneity between individuals.14,16 The number of copies is directly and inversely associated with Lp(a) plasma concentration due to an innate increased ability of hepatocytes to produce proteins of low molecular weight.17 Lp(a) plasma concentration ranges from <1 mg/dL to >1,000 mg/dL between individuals with almost 90% of Lp(a) levels being genetically predisposed.18,19
The pathophysiological activity of Lp(a) has yet to be fully elucidated; however, current data support its pro-atherogenic, pro-inflammatory and potentially prothrombotic properties.20
Pro-atherogenic properties
Lp(a) interacts directly with numerous receptors and molecules which accounts for most of its properties.21 Due to its similarity with LDL-C, Lp(a) is attached through its hydrophilic outer layer to the endothelial surface, thus permitting its entrance and accumulation in the arterial sub-intimal space.22 It can then be integrated into macrophages leading to foam cell formation.23 Moreover, there is evidence that Lp(a) may inhibit TGFβ, thus enhancing human muscle cell proliferation and migration and vascular remodelling.11,24 Although the pro-atherogenic action of Lp(a) resembles that of LDL, it is enhanced by its ability to remain attached to the extracellular matrix for a longer time.25
Pro-inflammatory properties
A core mediator of the pro-inflammatory properties of Lp(a) seems to be its oxidized phospholipid (OxPL) content, primarily attached to the KIV10 domain of apo(a).11 Lp(a) is the main carrier of OxPL in circulation.26 OxPL carry a highly oxidized state and provokes potent inflammation in the sub-intimal space, promoting the formation of atheromatous plaques.20 The inflammatory state also induces the production of matrix-degradative enzymes, which contribute to plaque vulnerability and rupture.11 Additionally, in vitro studies have proposed the Lp(a)-mediated expression of multiple adhering molecules with pro-inflammatory properties such as selectin E and vascular cell adhesion molecule 1 (VCAM1), as one of the main pro-inflammatory actions of Lp(a).27 Finally, Klezovitch et al. suggested that Lp(a) acts as a chemotactic factor for macrophages and monocytes and induces the expression of cytokines such as IL-8.28
Prothrombotic properties
Due to its morphological similarity to plasminogen, Lp(a) may inhibit plasminogen activation pathways that are regulated by urokinase, streptokinase and tissue plasminogen activator.26,29 Further, Lp(a) may compete with the binding of plasminogen to fibrin, fibrinogen and cellular receptors of endothelium, macrophages and monocytes, possibly through its KIV10 domain.26,30 Both the potential inhibition of plasminogen activation and the potential antagonistic effect of Lp(a) on plasminogen function may contribute to an Lp(a)-mediated impairment of plasmin formation and thrombus lysis.20 However, despite in vitro observation supporting this hypothesis, in vivo studies have not definitely confirmed the antithrombolytic role of Lp(a) in patients with elevated Lp(a) concentrations, except in those with extreme values.31,32 In a Mendelian randomization study on 41,231 participants of Danish origin, levels of Lp(a) did not correlate with the risk for deep venous thrombosis and pulmonary embolism. However, the study did show a positive relationship between Lp(a) levels with atherosclerotic stenosis and atherothrombosis.33 Finally, an observational study in patients undergoing percutaneous coronary intervention suggested that Lp(a) may contribute to platelet activation and aggregation and the subsequent development of ischaemic events by accelerating fibrin generation and increasing adenosine diphosphate-induced platelet aggregation. However, the authors noted that more data are needed to support this hypothesis in the general population.34
Other properties
The similarity of apo(a), because of its kringle formation, with multiple proteins involved in angiogenesis, such as plasminogen and angiostatin, has raised multiple questions about its role in this process. In vitro studies using recombinant apo(a) particles reported on the possible antiangiogenic properties of apo(a) that could potentially inhibit vascular-rich pathological conditions such as cancer and retinopathy.21 However, there is a lack of in vivo studies using an intact Lp(a) molecule and it is uncertain whether apo(a) retains the aforementioned properties when attached to LDL-C.
To date, no available, approved lipid-lowering treatment has been shown to significantly reduce Lp(a) levels. Currently, RNA-based gene-silencing agents targeting Lp(a) production and leading to a robust decrease in Lp(a) plasma concentration are under investigation in phase III clinical trials with the first results being eagerly awaited within the next 2 years.35,36
ASA: mechanism of action and its role in the prevention of CVD
ASA was first introduced in the market in powder form in 1900 and in tablet form in 1904. It was created in 1897 from salicin, a substance isolated from willow tree bark that is known since ancestry for its anti-inflammatory, antipyretic and antianalgesic properties.37
ASA constitutes a cornerstone of CVD prevention. The principal mechanisms of action of ASA are the following:
ASA irreversibly inhibits the enzyme cyclooxygenase-1 (COX1), which is widely expressed in human tissues and to a high degree in platelets and vascular endothelial cells.36,38 To a lesser degree, ASA inhibits COX2, which is mainly induced during inflammation.37,39 Both enzymes catalyze the formation of prostanoids, amongst them thromboxane A2 (TXA2), a platelet-aggregating agent with vasoconstrictive and smooth muscle cell mitogenic activity.38,40 This imparts ASA with its antiplatelet properties, which benefit individuals in states of increased atherothrombotic risk.41,42
ASA can also act at the gene level, suppressing the expression of multiple transcription factors such as NF-κB and Runt-related transcription factor 1 (RUNX1).43,44 NF-κB participates in inflammatory processes, including atherogenesis, and its suppression can alter the composition of the atheromatous plaque and interrupt the expansion of atheromatosis in animal models.45,46 On the other hand, RUNX1 regulates numerous pathways in megakaryocytes.47 An in vitro human study suggested that RUNX1 inhibition by ASA could decrease the production of proteins that modulate platelet activation, thus supporting its antiplatelet action and antiatherogenic properties.44
In vivo studies have showcased the prevention of clot formation as an additional antithrombotic mechanism of ASA. Specifically, ASA seems to lead to unstable fibrin formation and reduced thrombin production.48,49
Role of ASA in the secondary prevention of CVD
The antiplatelet properties of ASA and the survival benefit following its use in patients with ASCVD have established its importance in secondary prevention. The first observations for this implication were made in randomized controlled trials (RCTs) conducted in the 1970s and 1980s.50 Subsequent meta-analyses reported a significant decrease in all-cause mortality as well as non-fatal strokes and non-fatal coronary episodes, which outweigh the risk of major bleeding events, thus establishing the use of ASA as the cornerstone of secondary prevention in patients with ASCVD.51–55
In accordance with previous evidence, the current guidelines suggest lifelong ASA administration for the secondary prevention of events of atherosclerotic origin in patients at high risk, including patients with acute coronary syndrome, acute ischaemic stroke, history of CAD, or peripheral artery disease and in cases of coronary revascularization procedures (percutaneous coronary intervention and coronary artery bypass surgery). Data have supported the equality of ASA doses (range of 75–325 mg daily) to prevent CVD events, and because the bleeding risk is dose-dependent, lower applicable doses were recommended (75–100 mg daily).56
Role of ASA in the primary prevention of CVD
Considering its recognized efficacy in reducing CVD risk in individuals with established ASCVD, it is reasonable to question the potential role of ASA in primary prevention.
Use of ASA in the general population
Multiple RCTs and subsequent meta-analyses have been conducted comparing the use of ASA versus placebo in the primary prevention of the general population, indicating a protective role of ASA against the first occurrence of MACE. However, this was not associated with a significant reduction in CVD mortality or all-cause deaths and, at the same time, treatment with ASA led to a significantly increased incidence of major bleeding events, even at low doses, leading to a slightly negative net benefit.57–59
The risk for complications, accompanied by its inability to reduce CVD mortality, and its controversial efficacy to prevent atherosclerotic episodes have so far discouraged ASA administration in the general population for primary prevention purposes.60
Use of ASA in specific populations
The use of ASA as a primary prevention tool has also been investigated in individuals at high CVD risk, where its benefits might outweigh the bleeding risk. One such state is DM, with people with DM facing a 2–3-fold increased CVD risk.61 RCTs and subsequent meta-analyses comparing low-dose ASA versus control or placebo in people with DM for primary prevention reported a reduction in total ASCVD events, which, however, was accompanied by a significant increase in total haemorrhagic risk.62,63 Studies on other populations at high risk, such as the elderly and those with chronic kidney disease, also failed to report a favourable effect of ASA.64–66
Consequently, current guidelines propose a limited role of ASA in primary CVD prevention, mainly in populations at high risk.67 Specifically, the 2019 American College of Cardiology/American Heart Association guidelines support daily use of low-dose ASA, 75–100 mg, for primary prevention purposes in individuals aged 40–70 years who belong to high CVD risk groups (≥10% risk for ASCVD events for the following 10 years) and have a low bleeding risk.68 The 2020 American Diabetic Association recommendations support 75–162 mg ASA administration in people with DM, in whom the CVD risk outmatches the bleeding hazard.69 The 2021 European guidelines indicate that low-dose ASA may be considered in individuals with DM of at least high CVD risk or in individuals without DM but with exceptionally high CVD risk who are <70 years of age and with a low bleeding risk.70
Currently, the use of ASA in primary CVD prevention in individuals with elevated Lp(a) levels is under investigation.
ASA in patients with high Lp(a): current data and perspectives
To date, none of the existing medications has been shown to strongly interrupt the Lp(a)-mediated atherothrombotic process; therefore, a large population sub-group remains at high risk of ASCVD events even after regulating the other risk factors.7 Due to its antithrombotic properties, ASA may potentially have an essential role in the prevention of CVD in those patients.
The first report for a putative benefit of ASA use in individuals with elevated Lp(a) levels was published in 2009 from a secondary analysis of the Women’s Health Study. In this RCT, 39,879 middle-aged or older women without a history of CVD at baseline received 100 mg ASA or placebo every other day and were followed for a total of 10 years for the development of MACE.71 A sub-population of 25,131 white women was subsequently examined for the presence of a specific single nucleotide polymorphism (SNP) in the LPA gene, the rs3798220-C (present in 3–4% of the population), which leads to high Lp(a) plasma concentrations.72 In the sub-study, the initial Lp(a) levels were found to be greater in SNP carriers, especially the homozygotes (153.9 mg/dL versus 79.5 mg/dL in heterozygotes and 10.0 mg/dL in non-carriers). The presence of the polymorphism more than doubled the risk for MACE (age-adjusted HR 2.22, 95% CI 1.39–3.53). On the other hand, ASA achieved a more than 50% reduction in CVD events in these individuals as compared to carriers who received placebo (age-adjusted HR 0.44, 95% CI 0.20–0.94), whereas it had no significant effect in the arm of non-carriers (age-adjusted HR 0.91, 95% CI 0.77–1.08).
Similarly, an analysis of data from 12,815 participants (≥70 years and of European origin) of the ASPREE trial (Aspirin in Reducing Events in the Elderly),73 who received 100 mg of ASA daily, showed that during a 4.7-year observation period the presence of the rs3798220-C genotype significantly increased CVD events only in the placebo group but not in the ASA group (HR 1.90; 95% CI 1.11–3.24 versus HR 0.54; 95% CI 0.17–1.70, respectively). In the same study, a high Lp(a) genomic risk score, based on the presence or absence of 43 specific SNPs, was associated with an increased risk of MACE in the placebo group, which was somewhat attenuated in the ASA group (HR 1.70; 95% CI 1.14–2.55 versus HR 1.41; 95% CI 0.90–2.23, respectively). Overall, ASA achieved a reduction of MACE in all participants by 1.7 events per 1,000 person-years but with an equivalent increase in bleeding episodes. The net benefit was positive only in carriers of the rs3798220-C SNP and in the cases with high Lp(a) genomic risk score. In these sub-groups, ASA mitigated MACE more efficiently, by 11.4 and 3.3 events per 1,000 person-years, respectively.74
Both studies mentioned above were conducted exclusively on populations of European origin. It remains unclear whether these findings can be generalized to individuals of other ethnic origins. The rs3798220-C polymorphism is rare in Black people and has been found only in heterozygotes. Of particular interest would be a similar genetic-based study on Black participants, using genotypes prevalent in this population that lead to increased Lp(a), for example, the rs9457951.75 An additional limitation in both analyses is the lack of information regarding the effect of ASA on mortality. Furthermore, those studies are solely genome based and could not provide data for the benefit of ASA in relation to Lp(a) concentrations.
Two recently published observational studies aim to shed light on previously unresolved issues. Both were published in 2024 and were based on available data from previously published large studies. They investigated the role of ASA in primary prevention of populations with various Lp(a) concentrations regardless of their ethnic origin. The first study was conducted using data from the Multi-Ethnic Study of Atherosclerosis (MESA), a prospective cohort study on 6,814 participants without a history of CVD at baseline who were followed for identification of MACE.76 Cases with available information regarding Lp(a) levels and regular ASA use were categorized into two cohorts, one of 1,760 individuals with Lp(a) ≤50 mg/dL and one of 423 individuals with Lp(a) >50 mg/dL. ASA administration led to a significant reduction of coronary heart disease events in the group with elevated Lp(a) by almost 50% (HR 0.54, 95% CI 0.31–0.93) but not in the group with Lp(a) ≤50 mg/dL (HR 0.80, 95% CI 0.58–1.10). Notably, those with Lp(a) >50 mg/dL who used ASA had similar coronary heart disease risk as those with Lp(a) ≤50 mg/dL regardless of ASA use. Overall, ASA increased the rate of major bleeding events independently of Lp(a) concentrations (17.5% versus 12.5% in ASA users versus non-ASA users, respectively; p<0.001).77
The second study used data from the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994)78 to examine the relationship between ASA use, Lp(a) levels and CVD mortality in individuals without a history of MACE at baseline. Only participants on regular ASA use with available baseline Lp(a) measurements were included in this retrospective study, reaching a number of 2,990 eligible cases. The study reported a protective effect of ASA in cases with Lp(a) >50 mg/dL (1.2 fatal events (95% CI 0.1–2.3) versus 3.9 fatal events (95% CI 2.8–4.9) per 1,000 person-years in cases with versus without regular ASA use, respectively). Furthermore, in multivariable modelling, regular ASA use reduced the risk of fatal CVD episodes by more than 50% in individuals with elevated Lp(a) (HR 0.48, 95% CI 0.28–0.83) but it had no effect when Lp(a) was within normal range (HR 1.01, 95% CI 0.81–1.25).79 Both aforementioned studies show a favourable effect of aspirin when Lp(a) levels are elevated; however, there are considerable limitations due to their observational and retrospective nature. Furthermore, no information is provided regarding the ASA dose and the exact frequency of its administration.
A summary of the main studies investigating the effect of ASA in individuals with elevated Lp(a) levels is shown in Table 1.
Table 1.
Summary of the main studies investigating the effect of ASA in individuals with elevated Lp(a) levels.
| Author (Year) | Population database | Participants | Median follow-up | ASA dosage | Outcomes | Lp(a)-related parameters | Results | Limitations |
|---|---|---|---|---|---|---|---|---|
| Chasman et al. (2009)72 | WHS | 25,131 white women ≥45 years of age | 9.9 years | 100 mg alternate days | MACE | Carriers of SNP rs3798220-C | Significantly reduced CVD risk with ASA only amongst SNP carriers (HR 0.44; 95% CI 0.20–0.94) |
|
| Lacaze et al. (2022)74 | ASPREE | 12,815 individuals of European origins ≥70 years of age | 4.7 years | 100 mg once daily |
|
|
|
|
| Bhatia et al. (2024)77 | MESA | 2,183 individuals from the USA, ≥45 years old | 15.7 years | Self-reported ASA use ≥3 days/week |
|
Participants with Lp(a) >50 mg/dL versus ≤50 mg/dL |
|
|
| Razavi et al. (2024)79 | NHANES III | 2,990 individuals from the USA, 40–70 years old | 26 years | Self-reported ASA ≥30 times in the previous month |
|
Participants with Lp(a) ≥50 mg/dL versus <50 mg/dL | ASA reduced the risk of fatal CVD episodes by more than 50% in individuals with elevated Lp(a) (HR 0.48; 95% CI 0.28–0.83) but it had no effect when Lp(a) was within normal range (HR 1.01; 95% CI 0.81–1.25) |
|
ASA, aspirin; ASCVD, atherosclerotic cardiovascular disease; ASPREE, Aspirin in Reducing Events in the Elderly; CAD, coronary artery disease; CHD, coronary heart disease; CVD, cardiovascular disease; Lp(a), lipoprotein(a); MACE, major adverse cardiovascular events; MESA, Multi-Ethnic Study of Atherosclerosis; NHANES III, Third National Health and Nutrition Examination Survey; SNP, single nucleotide polymorphism; WHS, Women’s Health Study.
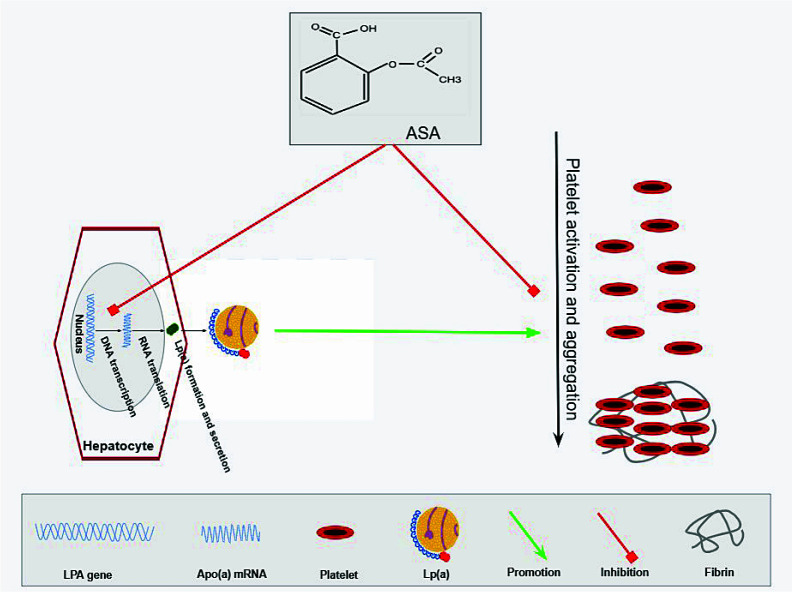
The mechanisms through which ASA may reduce CVD risk in the primary prevention of individuals with elevated Lp(a) levels have not been fully elucidated. The two main mechanisms proposed as being, at least partly, responsible for the beneficial effect of ASA are the following (Figure 1):
Figure 1. Potential mechanisms through which ASA reduces CVD risk in the primary prevention of individuals with elevated Lp(a) levels.
The aspirin (ASA)-mediated reduction of lipoprotein(a) [Lp(a)]-associated cardiovascular disease (CVD) risk could be summarized in a mechanism triangle: on the one side, Lp(a) seems to promote platelet activation and aggregation, especially through the adenosine diphosphate-induced platelet activation mechanism. On the other side, ASA seems to slightly reduce the potential of hepatocytes to produce Lp(a) molecules acting in the pre-transcription stage, which in conjunction with its main effect, the inhibition of platelet activation and aggregation mainly through the inhibition of TXA2 production, may result in a reduced thrombotic status when administered in individuals with elevated Lp(a) levels. Apo(a), apolipoprotein(a).
ASA seems to slightly reduce Lp(a) levels. In 1999, Kagawa et al.80 reported a reduction of up to 73% in apo(a) levels in human hepatocyte cultures exposed to a solution containing 5 mmol/L of ASA as compared to controls. ASA seems to reduce apo(a) production by inhibiting LPA gene transcription and apo(a) mRNA expression.80–82 Subsequent studies concluded that low ASA doses may reduce Lp(a) levels by 18–56% or even >80% in case of extreme initial Lp(a) concentrations; these studies included patients with known ASCVD, and ASA-mediated Lp(a) reduction was principally observed in individuals with Lp(a) >30 mg/dL.81,82 There is a lack of related studies examining the effect of ASA on Lp(a) levels in the era of primary prevention and thus it remains unclear whether ASA retains such a potent beneficial effect.
Lp(a) may promote platelet activation and aggregation. Due to its strong antiplatelet and antithrombotic potential, ASA may reduce CVD risk in individuals with high Lp(a) levels by antagonizing the effect of Lp(a) on thrombus formation.34,83
Conclusions
High Lp(a) levels constitute a significant contributor to the residual CVD risk in primary prevention. To date, no lipid-lowering treatment specifically targeting Lp(a) is available. Two novel RNA-related agents (pelacarsen and olpasiran) are currently under investigation in phase III clinical trials, with the results being expected within 2025 and 2026, respectively.35,36 However, both trials are focused on secondary prevention in patients with established ASCVD and Lp(a) >70 mg/dL. Thus, it needs to be emphasized that even a positive result of any of these trials would have no bearing on the effect of Lp(a) lowering in primary prevention, and therefore no agent would still be available for the reduction of cardiovascular risk in individuals with elevated Lp(a) levels but without a history of ASCVD.
The use of ASA in the primary prevention of CVD in individuals with high Lp(a) levels may be an essential tool for minimizing CVD risk in those individuals. Current data, though limited, are encouraging. However, there are important gaps that continue to exist in the literature and more meticulous research is needed. Evidence from RCTs supports ASA administration after genome analysis in Europeans. However, such procedures are not easily applicable. Given the proven pro-atherogenic properties of Lp(a), there is a need for well-designed studies that aim to elucidate any relationship between Lp(a) concentration, CVD risk and net benefit of ASA amongst different ethnicities.
Moreover, no data are available on the effect of ASA in the primary prevention of patients with elevated Lp(a) levels in conjunction with any of the other known CVD risk factors. In such cases, an even more profound favourable effect of ASA use may be apparent. There is evidence supporting the notion that the coexistence of DM and elevated Lp(a) levels synergistically amplify overall CVD risk.84 Patients with DM and elevated Lp(a) would thus be a suitable population in which to investigate this hypothesis.
Acknowledgements
None.
Footnotes
Contributions: Conceived the concepts: CEK; analysed the data: CEK; wrote the first draft of the manuscript: EG, DT, CEK; contributed to the writing of the manuscript: EG, LSR, DT, MG; agreed with manuscript results and conclusions: EG, LSR, DT, MG, CEK; jointly developed the structure and arguments for the paper: CEK, EG, DT; made critical revisions and approved final version: CEK, LSR; all authors reviewed and approved the final manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: LSR received honoraria for lectures from Amgen, Sanofi Aventis, Viatris, Novartis and Vianex, outside the submitted work. The other authors declare that they have no conflicts of interest relevant to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2024/12/dic.2024-10-2-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2024 Giannopoulou E, Rallidis LS, Tsamoulis D, Gianniou M, Kosmas CE. https://doi.org/10.7573/dic.2024-10-2. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: 6 Green Lane Business Park, 238 Green Lane, New Eltham, London, SE9 3TL, UK.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights, and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Berg K. A new serum type system in man-the LP system. Acta Pathol Microbiol Scand. 1963;59:369–382. doi: 10.1111/j.1699-0463.1963.tb01808.x. [DOI] [PubMed] [Google Scholar]
- 2.Duarte Lau F, Giugliano RP. Lipoprotein(a) and its significance in cardiovascular disease: a review. JAMA Cardiol. 2022;7(7):760–769. doi: 10.1001/jamacardio.2022.0987. [DOI] [PubMed] [Google Scholar]
- 3.Wang S, Zha L, Chen J, et al. The relationship between lipoprotein(a) and risk of cardiovascular disease: a Mendelian randomization analysis. Eur J Med Res. 2022;27(1):211. doi: 10.1186/s40001-022-00825-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy E, Curneen JMG, McEvoy JW. Aspirin in the modern era of cardiovascular disease prevention. Methodist Debakey Cardiovasc J. 2021;17(4):36–47. doi: 10.14797/mdcvj.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimitriadis K, Lazarou E, Tsioufis P, Soulaidopoulos S, Tsioufis K. Aspirin for primary prevention of cardiovascular diseases: “WALTZ” with the evidence. Curr Cardiol Rep. 2022;24(9):1139–1147. doi: 10.1007/s11886-022-01740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoogeveen RC, Ballantyne CM. Residual cardiovascular risk at low LDL: remnants, Lipoprotein(a), and inflammation. Clin Chem. 2021;67(1):143–153. doi: 10.1093/clinchem/hvaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinci P, Di Girolamo FG, Panizon E, et al. Lipoprotein(a) as a risk factor for cardiovascular diseases: pathophysiology and treatment perspectives. Int J Environ Res Public Health. 2023;20(18):6721. doi: 10.3390/ijerph20186721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tasdighi E, Adhikari R, Almaadawy O, Leucker TM, Blaha MJ. LP(a): Structure, genetics, associated cardiovascular risk, and emerging therapeutics. Annu Rev Pharmacol Toxicol. 2024;64:135–157. doi: 10.1146/annurev-pharmtox-031023-100609. [DOI] [PubMed] [Google Scholar]
- 9.Kronenberg F, Mora S, Stroes ESG, et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. Eur Heart J. 2022;43(39):3925–3946. doi: 10.1093/eurheartj/ehac361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsimikas S, Marcovina SM. Ancestry, Lipoprotein(a), and cardiovascular risk thresholds: JACC review topic of the week. J Am Coll Cardiol. 2022;80(9):934–946. doi: 10.1016/j.jacc.2022.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Lampsas S, Xenou M, Oikonomou E, et al. Lipoprotein(a) in atherosclerotic diseases: from pathophysiology to diagnosis and treatment. Molecules. 2023;28(3):969. doi: 10.3390/molecules28030969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasławska A, Tomasik PJ. Lipoprotein(a)-60 years later-what do we know? Cells. 2023;12(20):2472. doi: 10.3390/cells12202472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCormick SPA, Schneider WJ. Lipoprotein(a) catabolism: a case of multiple receptors. Pathology. 2019;51(2):155–164. doi: 10.1016/j.pathol.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Tsioulos G, Kounatidis D, Vallianou NG, et al. Lipoprotein(a) and atherosclerotic cardiovascular disease: where do we stand? Int J Mol Sci. 2024;25(6):3537. doi: 10.3390/ijms25063537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jawi MM, Frohlich J, Chan SY. Lipoprotein(a) the insurgent: a new insight into the structure, function, metabolism, pathogenicity, and medications affecting Lipoprotein(a) molecule. J Lipids. 2020;2020:3491764. doi: 10.1155/2020/3491764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scharnagl H, Stojakovic T, Dieplinger B, et al. Comparison of lipoprotein (a) serum concentrations measured by six commercially available immunoassays. Atherosclerosis. 2019;289:206–213. doi: 10.1016/j.atherosclerosis.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Khovidhunkit W. Lipoprotein(a) In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext. South Dartmouth, MA: [Accessed December 23, 2024]. https://www.endotext.org/ [Google Scholar]
- 18.Kosmas CE, Bousvarou MD, Papakonstantinou EJ, et al. Novel pharmacological therapies for the management of Hyperlipoproteinemia(a) Int J Mol Sci. 2023;24(17):13622. doi: 10.3390/ijms241713622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart SL, Savenkov O, Hurd MA, et al. Genetic and metabolic determinants of Lipoprotein (a) J Clin Lipidol. 2023;17(4):e5–e6. doi: 10.1016/j.jacl.2023.05.009. [DOI] [Google Scholar]
- 20.Rehberger Likozar A, Zavrtanik M, Šebeštjen M. Lipoprotein(a) in atherosclerosis: from pathophysiology to clinical relevance and treatment options. Ann Med. 2020;52(5):162–177. doi: 10.1080/07853890.2020.1775287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalaivani V, Jaleel A. Apolipoprotein(a), an enigmatic anti-angiogenic glycoprotein in human plasma: a curse or cure? Pharmacol Res. 2020;158:104858. doi: 10.1016/j.phrs.2020.104858. [DOI] [PubMed] [Google Scholar]
- 22.Sukkari MH, Al-Bast B, Al Tamimi R, Giesing W, Siddique M. Is there a benefit of aspirin therapy for primary prevention to reduce the risk of atherosclerotic cardiovascular disease in patients with elevated Lipoprotein (a) – a review of the evidence. Am J Prev Cardiol. 2023;15:100579. doi: 10.1016/j.ajpc.2023.100579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ugovšek S, Šebeštjen M. Lipoprotein(a) – the crossroads of atherosclerosis, atherothrombosis and inflammation. Biomolecules. 2021;12(1):26. doi: 10.3390/biom12010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grainger DJ, Kirschenlohr HL, Metcalfe JC, Weissberg PL, Wade DP, Lawn RM. Proliferation of human smooth muscle cells promoted by lipoprotein(a) Science. 1993;260(5114):1655–1658. doi: 10.1126/science.8503012. [DOI] [PubMed] [Google Scholar]
- 25.Kiechl S, Willeit J. The mysteries of lipoprotein(a) and cardiovascular disease revisited. J Am Coll Cardiol. 2010;55(19):2168–2170. doi: 10.1016/j.jacc.2009.12.048. [DOI] [PubMed] [Google Scholar]
- 26.Kronenberg F. Lipoprotein(a) Handb Exp Pharmacol. 2022;270:201–232. doi: 10.1007/164_2021_504. [DOI] [PubMed] [Google Scholar]
- 27.Allen S, Khan S, Tam Sp, Koschinsky M, Taylor P, Yacoub M. Expression of adhesion molecules by lp(a): a potential novel mechanism for its atherogenicity. FASEB J. 1998;12(15):1765–1776. doi: 10.1096/fasebj.12.15.1765. [DOI] [PubMed] [Google Scholar]
- 28.Klezovitch O, Edelstein C, Scanu AM. Stimulation of interleukin-8 production in human THP-1 macrophages by apolipoprotein(a). Evidence for a critical involvement of elements in its C-terminal domain. J Biol Chem. 2001;276(50):46864–46869. doi: 10.1074/jbc.M107943200. [DOI] [PubMed] [Google Scholar]
- 29.Durlach V, Bonnefont-Rousselot D, Boccara F, et al. Lipoprotein(a): pathophysiology, measurement, indication and treatment in cardiovascular disease. A consensus statement from the Nouvelle Société Francophone d’Athérosclérose (NSFA) Arch Cardiovasc Dis. 2021;114(12):828–847. doi: 10.1016/j.acvd.2021.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Koschinsky ML, Marcovina SM. Structure-function relationships in apolipoprotein(a): insights into lipoprotein(a) assembly and pathogenicity. Curr Opin Lipidol. 2004;15(2):167–174. doi: 10.1097/00041433-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Boffa MB. Beyond fibrinolysis: the confounding role of Lp(a) in thrombosis. Atherosclerosis. 2022;349:72–81. doi: 10.1016/j.atherosclerosis.2022.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Nordestgaard BG, Langsted A. Lipoprotein (a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res. 2016;57(11):1953–1975. doi: 10.1194/jlr.R071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG. Genetic evidence that lipoprotein(a) associates with atherosclerotic stenosis rather than venous thrombosis. Arterioscler Thromb Vasc Biol. 2012;32(7):1732–1741. doi: 10.1161/ATVBAHA.112.248765. [DOI] [PubMed] [Google Scholar]
- 34.Zhu P, Tang XF, Song Y, et al. Association of lipoprotein(a) with platelet aggregation and thrombogenicity in patients undergoing percutaneous coronary intervention. Platelets. 2021;32(5):684–689. doi: 10.1080/09537104.2020.1802412. [DOI] [PubMed] [Google Scholar]
- 35.Assessing the impact of lipoprotein (a) lowering with pelacarsen (TQJ230) on major cardiovascular events in patients with CVD (Lp(a)HORIZON) ClinicalTrials.gov Identifier: NCT04023552. [Accessed October 4, 2024]. https://clinicaltrials.gov/study/NCT04023552 .
- 36.Olpasiran trials of cardiovascular events and lipoprotein(a) reduction (OCEAN(a)) – outcomes trial. ClinicalTrials.gov Identifier: NCT05581303. [Accessed October 4, 2024]. https://clinicaltrials.gov/study/NCT05581303 .
- 37.Hybiak J, Broniarek I, Kiryczyński G, et al. Aspirin and its pleiotropic application. Eur J Pharmacol. 2020;866:172762. doi: 10.1016/j.ejphar.2019.172762. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell JA, Kirkby NS, Ahmetaj-Shala B, et al. Cyclooxygenases and the cardiovascular system. Pharmacol Ther. 2021;217:107624. doi: 10.1016/j.pharmthera.2020.107624. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell JA, Kirkby NS. Eicosanoids, prostacyclin and cyclooxygenase in the cardiovascular system. Br J Pharmacol. 2019;176(8):1038–1050. doi: 10.1111/bph.14167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santos-Gallego CG, Badimon J. Overview of aspirin and platelet biology. Am J Cardiol. 2021;144(Suppl 1):S2–S9. doi: 10.1016/j.amjcard.2020.12.018. [DOI] [PubMed] [Google Scholar]
- 41.Warner TD, Nylander S, Whatling C. Anti-platelet therapy: cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br J Clin Pharmacol. 2011;72(4):619–633. doi: 10.1111/j.1365-2125.2011.03943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patrono C. Low-dose aspirin for the prevention of atherosclerotic cardiovascular disease. Eur Heart J. 2024;45(27):2362–2376. doi: 10.1093/eurheartj/ehae324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Block RC, Abdolahi A, Smith B, et al. Effects of low-dose aspirin and fish oil on platelet function and NF-kappaB in adults with diabetes mellitus. Prostaglandins Leukot Essent Fatty Acids. 2013;89(1):9–18. doi: 10.1016/j.plefa.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voora D, Rao AK, Jalagadugula GS, et al. Systems pharmacogenomics finds RUNX1 Is an aspirin-responsive transcription factor linked to cardiovascular disease and colon cancer. EBioMedicine. 2016;11:157–164. doi: 10.1016/j.ebiom.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cyrus T, Sung S, Zhao L, Funk CD, Tang S, Praticò D. Effect of low-dose aspirin on vascular inflammation, plaque stability, and atherogenesis in low-density lipoprotein receptor-deficient mice. Circulation. 2002;106(10):1282–1287. doi: 10.1161/01.cir.0000027816.54430.96. [DOI] [PubMed] [Google Scholar]
- 47.Elagib KE, Racke FK, Mogass M, Khetawat R, Delehanty LL, Goldfarb AN. RUNX1 and GATA-1 coexpression and cooperation in megakaryocytic differentiation. Blood. 2003;101(11):4333–4341. doi: 10.1182/blood-2002-09-2708. [DOI] [PubMed] [Google Scholar]
- 48.Undas A, Brummel-Ziedins KE, Mann KG. Antithrombotic properties of aspirin and resistance to aspirin: beyond strictly antiplatelet actions. Blood. 2007;109(6):2285–2292. doi: 10.1182/blood-2006-01-010645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Undas A, Brummel-Ziedins K, Mann KG. Why does aspirin decrease the risk of venous thromboembolism? On old and novel antithrombotic effects of acetyl salicylic acid. J Thromb Haemost. 2014;12(11):1776–1787. doi: 10.1111/jth.12728. [DOI] [PubMed] [Google Scholar]
- 50.Jacobsen AP, Raber I, McCarthy CP, et al. Lifelong aspirin for all in the secondary prevention of chronic coronary syndrome: still sacrosanct or is reappraisal warranted? Circulation. 2020;142(16):1579–1590. doi: 10.1161/CIRCULATIONAHA.120.045695. [DOI] [PubMed] [Google Scholar]
- 51.Antiplatelet Trialists’ Collaboration. Secondary prevention of vascular disease by prolonged antiplatelet treatment. Antiplatelet Trialists’ Collaboration. Br Med J (Clin Res Ed) 1988;296(6618):320–331. [PMC free article] [PubMed] [Google Scholar]
- 52.Collaborative overview of randomised trials of antiplatelet therapy--I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308(6921):81–106. [PMC free article] [PubMed] [Google Scholar]
- 53.Collaborative overview of randomised trials of antiplatelet therapy--II: Maintenance of vascular graft or arterial patency by antiplatelet therapy. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308(6922):159–168. [PMC free article] [PubMed] [Google Scholar]
- 54.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lièvre M, Cucherat M. Aspirin in the secondary prevention of cardiovascular disease: an update of the APTC meta-analysis. Fundam Clin Pharmacol. 2010;24(3):385–391. doi: 10.1111/j.1472-8206.2009.00769.x. [DOI] [PubMed] [Google Scholar]
- 56.Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–3337. doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
- 57.Abdelaziz HK, Saad M, Pothineni NVK, et al. Aspirin for primary prevention of cardiovascular events. J Am Coll Cardiol. 2019;73(23):2915–2929. doi: 10.1016/j.jacc.2019.03.501. [DOI] [PubMed] [Google Scholar]
- 58.Wang M, Yu H, Li Z, Gong D, Liu X. Benefits and risks associated with low-dose aspirin use for the primary prevention of cardiovascular disease: a systematic review and meta-analysis of randomized control trials and trial sequential analysis. Am J Cardiovasc Drugs. 2022;22(6):657–675. doi: 10.1007/s40256-022-00537-6. [DOI] [PubMed] [Google Scholar]
- 59.Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036–1046. doi: 10.1016/S0140-6736(18)31924-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fegers-Wustrow I, Gianos E, Halle M, Yang E. Comparison of American and European guidelines for primary prevention of cardiovascular disease: JACC guideline comparison. J Am Coll Cardiol. 2022;79(13):1304–1313. doi: 10.1016/j.jacc.2022.02.001. [DOI] [PubMed] [Google Scholar]
- 61.Emerging Risk Factors Collaboration. Sarwar N, Gao P, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.ASCEND Study Collaborative Group. Bowman L, Mafham M, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529–1539. doi: 10.1056/NEJMoa1804988. [DOI] [PubMed] [Google Scholar]
- 63.Masson W, Barbagelata L, Lavalle-Cobo A, et al. Low-doses aspirin in the primary prevention of cardiovascular disease in patients with diabetes: meta-analysis stratified by baseline cardiovascular risk. Diabetes Metab Syndr. 2022;16(1):102391. doi: 10.1016/j.dsx.2022.102391. [DOI] [PubMed] [Google Scholar]
- 64.McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379(16):1519–1528. doi: 10.1056/NEJMoa1803955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bellos I, Marinaki S, Lagiou P, Benetou V. Aspirin for the primary prevention of cardiovascular diseases in patients with chronic kidney disease: an updated meta-analysis. Am J Cardiovasc Drugs. 2024;24(2):241–253. doi: 10.1007/s40256-024-00630-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pallikadavath S, Ashton L, Brunskill NJ, Burton JO, Gray LJ, Major RW. Aspirin for the primary prevention of cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. Eur J Prev Cardiol. 2022;28(17):1953–1960. doi: 10.1093/eurjpc/zwab132. [DOI] [PubMed] [Google Scholar]
- 67.Li XY, Li L, Na SH, Santilli F, Shi Z, Blaha M. Implications of the heterogeneity between guideline recommendations for the use of low dose aspirin in primary prevention of cardiovascular disease. Am J Prev Cardiol. 2022;11:100363. doi: 10.1016/j.ajpc.2022.100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74(10):e177–e232. doi: 10.1016/j.jacc.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.American Diabetes Association. Standards of Medical Care in Diabetes-2020 Abridged for Primary Care Providers. Clin Diabetes. 2020;38(1):10–38. doi: 10.2337/cd20-as01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Visseren FLJ, Mach F, Smulders YM, et al. ESC National Cardiac Societies; ESC Scientific Document Group. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–3337. doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
- 71.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352(13):1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 72.Chasman DI, Shiffman D, Zee RY, et al. Polymorphism in the apolipoprotein(a) gene, plasma lipoprotein(a), cardiovascular disease, and low-dose aspirin therapy. Atherosclerosis. 2009;203(2):371–376. doi: 10.1016/j.atherosclerosis.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McNeil JJ, Wolfe R, Woods RL, et al. ASPREE Investigator Group. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509–1518. doi: 10.1056/NEJMoa1805819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lacaze P, Bakshi A, Riaz M, et al. Aspirin for primary prevention of cardiovascular events in relation to Lipoprotein(a) genotypes. J Am Coll Cardiol. 2022;80(14):1287–1298. doi: 10.1016/j.jacc.2022.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee SR, Prasad A, Choi YS, et al. LPA gene, ethnicity, and cardiovascular events. Circulation. 2017;135(3):251–263. doi: 10.1161/CIRCULATIONAHA.116.024611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.MESA. Overview and Protocol. [Accessed October 4, 2024]. https://www.mesa-nhlbi.org/aboutMESAOverviewProtocol.aspx .
- 77.Bhatia HS, Trainor P, Carlisle S, et al. Aspirin and cardiovascular risk in individuals with elevated Lipoprotein(a): the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2024;13(3):e033562. doi: 10.1161/JAHA.123.033562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burt VL, Harris T. The third National Health and Nutrition Examination Survey: contributing data on aging and health. Gerontologist. 1994;34(4):486–490. doi: 10.1093/geront/34.4.486. [DOI] [PubMed] [Google Scholar]
- 79.Razavi AC, Richardson LC, Coronado F, et al. Aspirin use for primary prevention among US adults with and without elevated Lipoprotein(a) Am J Prev Cardiol. 2024;18:100674. doi: 10.1016/j.ajpc.2024.100674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kagawa A, Azuma H, Akaike M, Kanagawa Y, Matsumoto T. Aspirin reduces apolipoprotein(a) (apo(a)) production in human hepatocytes by suppression of apo(a) gene transcription. J Biol Chem. 1999;274(48):34111–34115. doi: 10.1074/jbc.274.48.34111. [DOI] [PubMed] [Google Scholar]
- 81.Akaike M, Azuma H, Kagawa A, et al. Effect of aspirin treatment on serum concentrations of lipoprotein(a) in patients with atherosclerotic diseases. Clin Chem. 2002;48(9):1454–1459. [PubMed] [Google Scholar]
- 82.Ranga GS, Kalra OP, Tandon H, Gambhir JK, Mehrotra G. Effect of aspirin on lipoprotein(a) in patients with ischemic stroke. J Stroke Cerebrovasc Dis. 2007;16(5):220–224. doi: 10.1016/j.jstrokecerebrovasdis.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 83.Liu H, Fu D, Luo Y, Peng D. Independent association of Lp(a) with platelet reactivity in subjects without statins or antiplatelet agents. Sci Rep. 2022;12(1):16609. doi: 10.1038/s41598-022-21121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsamoulis D, Kosmas CE, Rallidis LS. Is inverse association between lipoprotein(a) and diabetes mellitus another paradox in cardiometabolic medicine? Expert Rev Endocrinol Metab. 2024;19(1):63–70. doi: 10.1080/17446651.2023.2293108. [DOI] [PubMed] [Google Scholar]