Abstract
Background
The prognostic value of C-reactive protein (CRP) in patients with bladder cancer (BCa) has been widely analysed; however, the results remain conflicting. Therefore, we performed this meta-analysis to identify the precise role of CRP level in predicting BCa prognosis.
Methods
PubMed, Web of Science, Embase and Cochrane Library databases were comprehensively searched until 19 April 2024. The impact of CRP level on predicting the prognosis of patients with BCa was examined using combined hazard ratios (HRs) and 95% confidence intervals (CIs). The relationship between CRP level and BCa clinicopathological characteristics was investigated by combining the odds ratios (ORs) with 95%CIs.
Results
Twenty studies with 7276 patients were enrolled in this study. As revealed by pooled data, elevated CRP levels were markedly related to poor overall survival (OS) (HR = 2.02, 95%CI = 1.41–2.90, p < .001), inferior cancer-specific survival (CSS) (HR = 1.46, 95%CI = 1.29–1.66, p < .001), shortened recurrence-free survival (RFS) (HR = 1.25, 95%CI = 1.17–1.33, p < .001) and dismal progression-free survival (PFS) (HR = 2.28, 95%CI = 1.80–2.90, p < .001) in BCa patients. Nevertheless, there was no significant relationship between CRP level and sex, tumour size, tumour grade or lymph node metastasis (LNM) in BCa.
Conclusions
Elevated CRP levels were significantly related to poor OS, CSS, RFS and PFS of BCa patients with BCa. CRP could act as a reliable biomarker for predicting the short- and long-term survival of patients with BCa in clinical practice.
Keywords: C-reactive protein, bladder cancer, meta-analysis, prognosis, biomarker
Key Messages
As far as we know, this work is the first to investigate the effect of C-reactive protein (CRP) on predicting bladder cancer (BCa) prognosis.
The combined data demonstrated the elevated CRP level was notably related to poor overall survival (OS), cancer-specific survival (CSS), recurrence-free survival (RFS) and progression-free survival (PFS) of BCa patients.
CRP could act as a reliable biomarker for predicting short- and long-time survival of BCa patients in clinical practice.
Introduction
Bladder cancer (BCa) has the highest prevalence among all urinary tract cancers. BCa ranks 10th among malignant tumours worldwide in 2020 [1]. It was estimated that 573,278 new cases of BCa were diagnosed and 212,536 deaths due to BCa occurred in 2020 globally [1]. There is an estimated 75% rate of non-muscle invasive BCa (NMIBC) (Ta/T1) in the general population, and it represents a heterogeneous group with varying risks of recurrence and progression to muscle invasive BCa (MIBC) (T2–T4) [2]. NMIBC has favourable prognostic outcomes, with a 5-year overall survival (OS) rate of >90% [3]. The gold standard treatment for localized MIBC is radical cystectomy and pelvic lymph node dissection, with an OS rate of approximately 60% over 5 years [4]. BCa is multicentric in nature, with a high rate of recurrence after surgery, requiring frequent review and long-term follow-up, which is a great burden on healthcare systems. Thus, identifying prognostic indicators for patients with BCa would be helpful for predicting survival and recurrence.
C-reactive protein (CRP), first detected in the serum of patients with pneumococcal pneumonia in 1930 [5], is a typical acute-phase protein that is primarily generated in the liver. CRP is an indicator of inflammation and a frequently used systemic inflammation marker [6]. As a ‘robust biomarker’, CRP measurement is one of the most requested laboratory tests because it may provide valuable information in a wide range of clinical conditions [7]. The significance of CRP in various cancers, including breast cancer [8], oesophageal squamous cell carcinoma [9], hepatocellular carcinoma [10], head and neck cancer [11] and pancreatic ductal adenocarcinoma [12], has been explored previously. The prognostic impact of CRP on BCa has been extensively analysed, but the results remain conflicting [13–32]. In certain studies, high CRP served as a significant marker to predict the prognosis of BCa patients [13,17,22,23,27,29], while in others, CRP was not significantly related to the prognosis of BCa [21,28,30,31]. Therefore, we performed the present meta-analysis to identify the precise significance of CRP level in predicting BCa prognosis. Moreover, the relationship between CRP level and BCa clinicopathological features was examined in this meta-analysis.
Materials and methods
Study guideline
This meta-analysis was performed following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [33].
Ethics statement
This meta-analysis relied on previously published data, which exempted the requirement for informed consent and ethical approval.
Literature retrieval
PubMed, Web of Science, Embase and Cochrane Library databases were comprehensively searched until 19 April 2024, using the following strategies: (C-reactive protein or CRP) and (bladder cancer or bladder carcinoma or bladder tumour or urinary bladder cancer or urinary bladder neoplasm or urothelial carcinoma). Only publications published in English were included. In addition, reference lists of the retrieved studies were manually searched to identify other relevant studies.
Eligibility criteria
Studies satisfying the following criteria were included: (1) BCa was confirmed through pathology; (2) studies exploring the relationship between CRP levels and BCa prognosis; (3) studies with available or calculable (by Tierney’s method) hazard ratios (HRs) with 95% confidence intervals (CIs) of survival outcomes [34]; (4) studies with identified CRP thresholds; and (5) English studies. The following studies were excluded: (1) case reports, meeting abstracts, reviews, comments and letters, (2) duplicate patients and (3) animal studies.
Data collection and quality evaluation
Data were collected in qualified studies by two researchers (X.F. and Z.Z.) independently, and any discrepancy was settled through negotiation with a third investigator (S.M.) until consensus was reached. The following data were collected: first author, publication year, country, sample size, age, sex, study design, study period, study centre, cT stage, treatment, threshold, threshold determination approach, survival endpoints, survival analysis types, follow-up and HRs with 95%CIs. The survival endpoints included OS, cancer-specific survival (CSS), recurrence-free survival (RFS) and progression-free survival (PFS). As the metric of discernment, the Newcastle-Ottawa Scale (NOS) [35] was employed to evaluate the incorporated literature. NOS evaluates study quality from three domains: selection, comparability and outcomes. In addition, the total score is 0–9, with NOS scores ≥6 indicating high-quality studies.
Statistical analysis
The effect of CRP level on predicting BCa prognosis was analysed based on the combined HRs and 95%CIs. The Cochrane Q test and I2 statistics were used to assess among-study heterogeneity. p < .10 or I2 >50% was identified as indicators of significant heterogeneity, so a random-effects model should be adopted; otherwise, a fixed-effects model should be applied. We also conducted subgroup analyses based on various clinical factors for further investigation. The relationship between CRP and BCa clinicopathological factors was investigated by combining the odds ratios (ORs) with 95%CIs. Publication bias was assessed using Begg’s and Egger’s tests. Stata version 12.0 software (Stata Corp., College Station, TX) was used for the statistical analysis. Statistical significance was set at p < .05.
Results
Search results
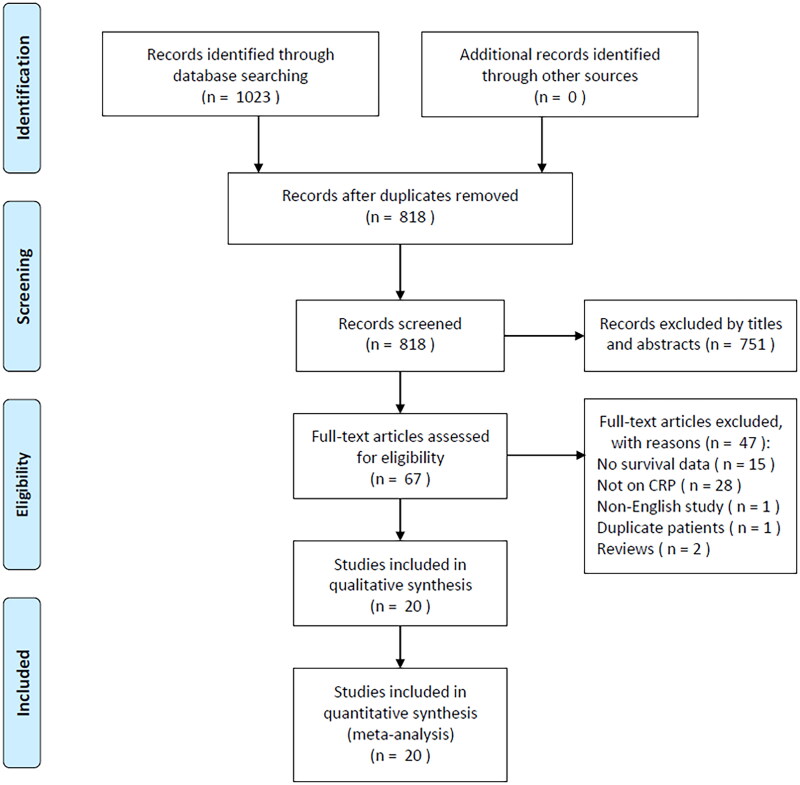
Altogether 1023 studies were obtained through primary retrieval, among which duplicates were removed, yielding 818 articles (Figure 1). After reading the title and abstract, we eliminated 751 articles due to irrelevance and animal studies. The full texts of the remaining 67 studies were read, and 47 were discarded due to unavailable survival information (n = 15), irrelevance to CRP (n = 28), non-English study (n = 1), duplicate patients involved (n = 1) and reviews (n = 2). Finally, 20 studies with 7276 patients were included in the present work [13–32] (Figure 1; Table 1).
Figure 1.
PRISMA flow diagram of study selection.
Table 1.
Baseline characteristics of included studies in this meta-analysis.
| Author | Country | Sample size | Age (years) Median (range) |
Gender (M/F) | Study design | Study period | Study center | cT stage | Treatment | Cut-off value (mg/dL) | Cut-off determination | Survival endpoints | Survival analysis | Follow-up (months) Median (range) |
NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hilmy et al. [13] | UK | 105 | ≤65 y: 37 >65 y: 68 |
75/30 | Retrospective | 1992–1999 | Single center | T1–T4 | Mixed | 1 | Literature | OS, CSS | Multivariate | To Sep 2004 | 7 |
| Yoshida et al. [14] | Japan | 88 | 70 (63–75) | 63/25 | Retrospective | 1997–2006 | Single center | T2–T4 | Mixed | 0.5 | Literature | CSS | Multivariate | 33 (3–117) | 8 |
| Gakis et al. [15] | Germany | 246 | 65 (43–84) | 191/55 | Retrospective | 1999–2009 | Single center | T1–T4 | Mixed | 0.5 | Literature | CSS | Multivariate | 30 (6–116) | 8 |
| Gondo et al. [16] | Japan | 189 | 68 (38–85) | 158/31 | Retrospective | 2000–2009 | Single center | T1–T4 | Surgery | 0.5 | Literature | CSS | Univariate | 25.1 (2.1–127.9) | 8 |
| Nakagawa et al. [17] | Japan | 114 | 67 (32–84) | 92/22 | Retrospective | 1990–2010 | Single center | Recurrent/metastatic | Surgery | 0.5 | Literature | OS | Multivariate | 11.0 (0.2–206.7) | 8 |
| Sejima et al. [18] | Japan | 249 | 72 (47–91) | 214/35 | Retrospective | 2003–2011 | Multicentre | T1–T4 | Surgery | 0.5 | Literature | CSS | Multivariate | 24.8 (0.7–110.1) | 9 |
| Grimm et al. [19] | Germany | 664 | 70 (35–97) | 511/153 | Retrospective | 2004–2013 | Single center | T1–T4 | Surgery | 0.5 | Median value | CSS | Multivariate | 24 (3–108) | 7 |
| Mbeutcha et al. [20] | Austria | 1117 | 67 | 855/262 | Retrospective | 1996–2007 | Multicentre | Ta–T1 | Surgery | 0.5 | Literature | RFS, PFS | Univariate | 64 | 9 |
| Mao et al. [21] | China | 207 | 66 | 169/38 | Retrospective | 2010–2012 | Single center | Ta–T1 | Surgery | 0.34 | ROC curve | RFS, PFS | Multivariate | 1–48 | 8 |
| Albisinni et al. [22] | Belgium | 134 | 72 | 110/24 | Prospective | 2013–2018 | Multicentre | T1–T4 | Surgery | 0.91 | ROC curve | CSS, RFS | Univariate | 21.1 (5–37) | 9 |
| Ma et al. [23] | China | 169 | 66.6 (32–87) | 245/24 | Retrospective | 2009–2018 | Single center | T1–T4 | Surgery | 0.5 | X-tile | OS, CSS, PFS | Multivariate | 32.3 (2–108) | 8 |
| Mari et al. [24] | Italy | 694 | 79 | 212/43 | Retrospective | 2008–2015 | Single center | T1–T4 | Surgery | 0.3 | ROC curve | OS, CSS, RFS | Univariate | 1–60 | 7 |
| Ogawa et al. [25] | Japan | 26 | 91 (77–101) | 11/15 | Retrospective | 2012–2019 | Single center | T2–T4 | No anti-cancer treatment | 2.0 | ROC curve | OS | Univariate | 9.5 (1–25) | 8 |
| Tamalunas et al. [26] | Germany | 1049 | 70 | 793/250 | Prospective | 2004–2018 | Single center | T1–T4 | Surgery | 0.5 | Median value | OS, CSS | Multivariate | 22 (1–170) | 9 |
| Gui et al. [27] | China | 175 | 59.5 | 124/51 | Retrospective | 2005–2016 | Single center | T2–T4 | Surgery | 0.5 | ROC curve | OS | Univariate | 1–100 | 8 |
| Tomioka et al. [28] | Japan | 27 | 71 | 25/2 | Retrospective | 2015–2019 | Multicentre | T2–T4 | Chemotherapy | 0.475 | ROC curve | PFS | Multivariate | 1–60 | 9 |
| Wang et al. [29] | China | 199 | 66 | 174/25 | Retrospective | 2010–2019 | Single center | T1–T4 | Surgery | 0.68 | ROC curve | OS, PFS | Multivariate | 20 | 8 |
| Caglayan and Horsanali [30] | Turkey | 74 | 67.4 | 64/10 | Retrospective | 2016–2020 | Single center | Ta–T1 | Surgery | 0.5 | Literature | OS, CSS, RFS | Univariate | 38.7 | 8 |
| Iemura et al. [31] | Japan | 41 | 72 (52–88) | 35/5 | Retrospective | 2007–2017 | Single center | T2–T4 | Surgery | 0.5 | Literature | CSS | Multivariate | 14 | 7 |
| Nishikawa et al. [32] | Japan | 1709 | 72 (65–78) | 1402/307 | Retrospective | 2000–2019 | Multicentre | Ta–T1 | Surgery | 0.5 | Literature | CSS, RFS, PFS | Multivariate | 1–200 | 9 |
OS: overall survival; CSS: cancer-specific survival; RFS: recurrence-free survival; PFS: progression-free survival; NOS: Newcastle-Ottawa Scale; ROC: receiver operating characteristics.
Enrolled study features
Table 1 presents the fundamental characteristics of all the enrolled studies. These articles were published between 2005 and 2023 and were all English publications. Eight studies were performed in Japan [14,16–18,25,28,31,32], four in China [21,23,27,29], three in Germany [15,19,26] and one each in UK [13], Austria [20], Belgium [22], Italy [24] and Turkey [30], respectively. The sample sizes were 26–1709 (median, 182). Two were prospective trials [22,26], 18 with a retrospective design [13–21,23–25,27–32]. Thirteen studies used 0.5 mg/dL as CRP cut-off value [14–20,23,26,27,30–32] and seven studies adopted cutoff values other than 0.5 mg/dL [13,21,22,24,25,28,29]. Nine articles mentioned the significance of CRP for predicting OS [13,17,23–27,29,30], 13 studies analysed the relationship of CRP with CSS [13–16,18,19,22–24,26,30–32], six studies presented the relationship between CRP and RFS [20–22,24,30,32] and six studies showed the prognostic role of CRP for PFS [20,21,23,28,29,32]. Seven articles provided HRs and 95%CIs from univariate regression [16,20,22,24,25,27,30] and 13 studies applied multivariate analysis [13–15,17–19,21,23,26,28,29,31,32]. The NOS scores were 7–9, revealing high-quality studies (Table 1).
CRP and OS
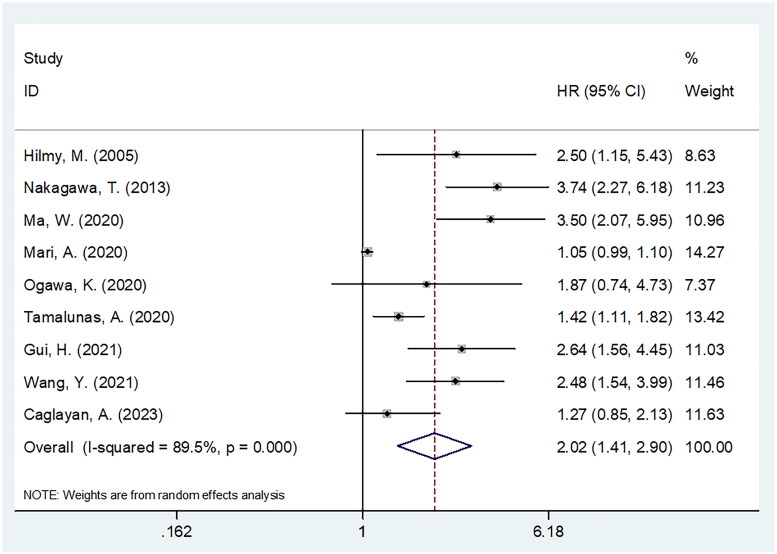
Altogether, nine articles involving 2605 cases [13,17,23–27,29,30] reported the role of CRP in predicting OS of BCa patients. Owing to the obvious heterogeneity (I2 = 89.5%, p < .001), we selected the random-effects model. As shown in Figure 2 and Table 2, the pooled results indicated that CRP significantly predicted the prognosis of dismal OS of BCa (HR = 2.02, 95%CI = 1.41–2.90, p < .001). Subgroup analysis revealed that CRP level still significantly predicted OS, regardless of the study design (Table 2). Moreover, elevated CRP significantly predicted dismal OS in Asian countries, sample size <200, in cT stage T1–T4, T2–T4, and recurrent/metastatic, treatment with surgery and systemic treatments, threshold of 0.5 mg/dL, and multivariate survival analysis subgroups (p < .05; Table 2).
Figure 2.
Meta-analysis of the association between elevated CRP and OS in patients with BCa.
Table 2.
Subgroup analysis of prognostic value of CRP for OS in patients with BCa.
| Subgroups | No. of studies | No. of patients | Effects model | HR (95%CI) | p | Heterogeneity |
|
|---|---|---|---|---|---|---|---|
| I2 (%) | Ph | ||||||
| Total | 9 | 2605 | Random | 2.02 (1.41–2.90) | <.001 | 89.5 | <0.001 |
| Geographical region | |||||||
| Asian countries | 6 | 757 | Random | 2.45 (1.72–3.49) | <.001 | 60.8 | 0.026 |
| Non-Asian countries | 3 | 1848 | Random | 1.33 (0.95–1.86) | .100 | 80.2 | 0.006 |
| Sample size | |||||||
| <200 | 7 | 862 | Random | 2.46 (1.80–3.36) | <.001 | 53.0 | 0.047 |
| ≥200 | 2 | 1743 | Random | 1.19 (0.89–1.59) | .241 | 81.7 | 0.019 |
| Study design | |||||||
| Prospective | 1 | 1049 | – | 1.42 (1.11–1.82) | .005 | – | – |
| Retrospective | 8 | 1556 | Random | 2.16 (1.35–3.45) | .001 | 90.3 | <0.001 |
| cT stage | |||||||
| Ta–T1 | 1 | 74 | – | 1.27 (0.80–2.01) | .308 | – | – |
| T1–T4 | 5 | 2216 | Random | 1.87 (1.21–2.89) | .005 | 90.2 | <0.001 |
| Recurrent/metastatic | 1 | 114 | – | 3.74 (2.27–6.17) | <.001 | – | – |
| T2–T4 | 2 | 201 | Fixed | 2.43 (1.54–3.83) | <.001 | 0 | 0.526 |
| Treatment | |||||||
| Surgery | 7 | 2474 | Random | 1.99 (1.34–2.97) | .001 | 91.5 | <0.001 |
| Mixed | 1 | 105 | – | 2.50 (1.15–5.43) | .021 | – | – |
| No anti-cancer treatment | 1 | 26 | – | 1.87 (0.74–4.73) | .186 | – | – |
| Cut-off value (mg/dL) | |||||||
| 0.5 | 5 | 1581 | Random | 2.22 (1.42–3.50) | .001 | 81.8 | <0.001 |
| ≠0.5 | 4 | 1024 | Random | 1.78 (0.98–3.23) | .060 | 83.7 | <0.001 |
| Cut-off value determination | |||||||
| Literature | 3 | 293 | Random | 2.25 (1.10–4.64) | .027 | 79.7 | <0.001 |
| ROC curve | 4 | 1094 | Random | 1.84 (0.99–3.40) | .052 | 88.1 | <0.001 |
| Median value | 1 | 1049 | – | 1.42 (1.11–1.82) | .005 | – | – |
| X-tile | 1 | 169 | – | 3.50 (2.07–5.95) | <.001 | – | – |
| Survival analysis | |||||||
| Univariate | 4 | 969 | Random | 1.50 (0.95–2.36) | .081 | 78.3 | 0.003 |
| Multivariate | 5 | 1636 | Random | 2.51 (1.60–3.95) | <.001 | 78.7 | <0.001 |
OS: overall survival; BCa: bladder cancer; ROC: receiver operating characteristics.
CRP and CSS
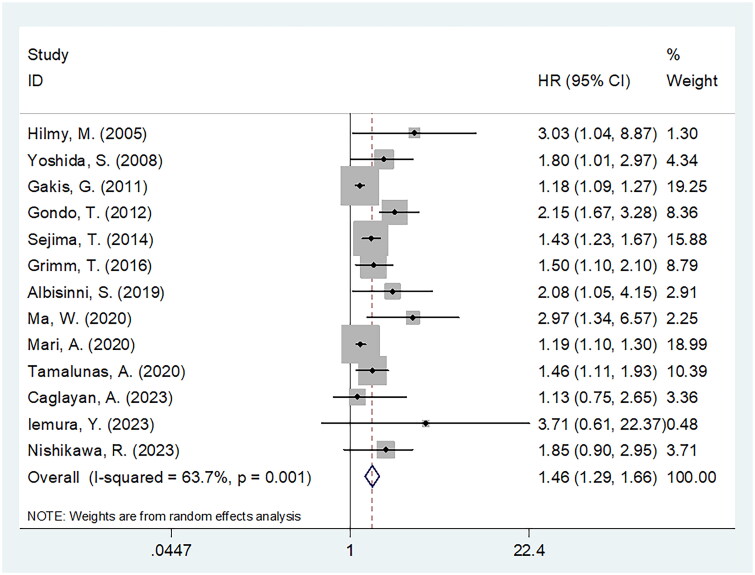
Thirteen articles enrolling 5411 cases [13–16,18,19,22–24,26,30–32] provided the data on association between CRP and CSS in BCa. We applied the random-effects model and obtained HR = 1.46, 95%CI = 1.29–1.66, p < .001, which indicated a significant relation between a high CRP level and dismal CSS of BCa patients with BCa (Figure 3; Table 3). As demonstrated by the subgroup analysis, CRP still remarkably predicted CSS regardless of geographical region, sample size, study design, study centre or survival analysis type (Table 3). Furthermore, CSS significantly predicted BCa in the cT stage of T1–T4 and T2–T4, treatment of surgery, threshold of 0.5 mg/dL, threshold determination methods of literature, median value and X-tile software subgroups (p < .05; Table 3).
Figure 3.
Meta-analysis of the association between elevated CRP and CSS in patients with BCa.
Table 3.
Subgroup analysis of prognostic value of CRP for CSS in patients with BCa.
| Subgroups | No. of studies | No. of patients | Effects model | HR (95%CI) | p | Heterogeneity |
|
|---|---|---|---|---|---|---|---|
| I2 (%) | Ph | ||||||
| Total | 13 | 5411 | Random | 1.46 (1.29–1.66) | <.001 | 63.7 | 0.001 |
| Geographical region | |||||||
| Asian countries | 7 | 2519 | Fixed | 1.58 (1.39–1.79) | <.001 | 37.4 | 0.143 |
| Non-Asian countries | 6 | 2892 | Fixed | 1.21 (1.15–1.28) | <.001 | 45.9 | 0.100 |
| Sample size | |||||||
| <200 | 7 | 4611 | Fixed | 2.01 (1.60–2.52) | <.001 | 0 | 0.488 |
| ≥200 | 6 | 800 | Random | 1.29 (1.17–1.41) | <.001 | 51.5 | 0.067 |
| Study design | |||||||
| Prospective | 2 | 1183 | Fixed | 1.53 (1.19–1.98) | .001 | 0 | 0.349 |
| Retrospective | 11 | 4228 | Random | 1.45 (1.26–1.66) | <.001 | 66.5 | 0.001 |
| Study center | |||||||
| Single center | 10 | 3319 | Random | 1.44 (1.24–1.66) | <.001 | 65.4 | 0.002 |
| Multicentre | 3 | 2092 | Fixed | 1.48 (1.28–1.71) | <.001 | 0 | 0.441 |
| cT stage | |||||||
| Ta–T1 | 2 | 1783 | Fixed | 1.47 (0.95–2.26) | .083 | 18.9 | 0.267 |
| T1–T4 | 9 | 3499 | Random | 1.44 (1.26–1.65) | <.001 | 71.6 | <0.001 |
| T2–T4 | 2 | 129 | Fixed | 1.91 (1.14–3.20) | .014 | 0 | 0.451 |
| Treatment | |||||||
| Surgery | 10 | 4972 | Random | 1.54 (1.30–1.82) | <.001 | 63.3 | 0.004 |
| Mixed | 3 | 439 | Random | 1.54 (0.97–2.43) | .068 | 61.7 | 0.074 |
| Cut-off value (mg/dL) | |||||||
| 0.5 | 10 | 4478 | Random | 1.53 (1.30–1.81) | <.001 | 64.5 | 0.002 |
| ≠0.5 | 3 | 933 | Random | 1.65 (0.95–2.88) | .074 | 62.6 | 0.069 |
| Cut-off value determination | |||||||
| Literature | 8 | 2701 | Random | 1.55 (1.25–1.91) | <.001 | 68.8 | 0.002 |
| ROC curve | 2 | 828 | Random | 1.41 (0.85–2.34) | .180 | 60.0 | 0.114 |
| Median value | 2 | 1713 | Fixed | 1.48 (1.20–1.82) | <.001 | 0 | 0.901 |
| X-tile | 1 | 169 | – | 2.97 (1.34–6.57) | .007 | – | – |
| Survival analysis | |||||||
| Univariate | 4 | 1091 | Random | 1.53 (1.04–2.26) | .031 | 77.6 | 0.004 |
| Multivariate | 9 | 4320 | Random | 1.49 (1.26–1.76) | <.001 | 58.6 | 0.031 |
CSS: cancer-specific survival; BCa: bladder cancer; ROC: receiver operating characteristics.
CRP and RFS
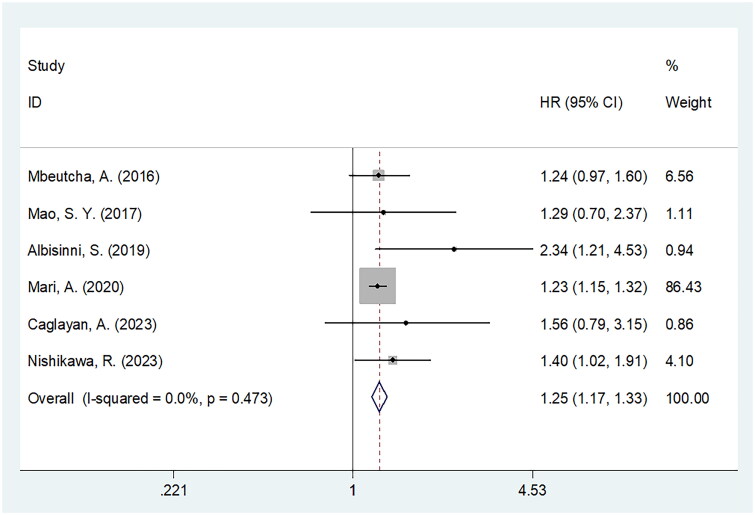
Six articles comprising 3935 patients showing the prognostic efficiency of CRP for RFS [20–22,24,30,32] in BCa. The heterogeneity was non-significant (I2 = 0, p = .473); thus, the fixed-effects model was adopted to obtain HR = 1.25, 95%CI = 1.17–1.33, p < .001), which suggested that a high CRP level was markedly related to inferior RFS of BCa (Figure 4; Table 4). As revealed by the subgroup analysis, CRP still significantly predicted RFS regardless of geographical region, sample size, study centre, study design, threshold, threshold determination approach or survival analysis type (Table 4).
Figure 4.
Meta-analysis of the association between elevated CRP and RFS in patients with BCa.
Table 4.
Subgroup analysis of prognostic value of CRP for RFS in patients with BCa.
| Subgroups | No. of studies | No. of patients | Effects model | HR (95%CI) | p | Heterogeneity |
|
|---|---|---|---|---|---|---|---|
| I2 (%) | Ph | ||||||
| Total | 6 | 3935 | Fixed | 1.25 (1.17–1.33) | <.001 | 0 | 0.473 |
| Geographical region | |||||||
| Asian countries | 3 | 1990 | Fixed | 1.40 (1.08–1.81) | .012 | 0 | 0.923 |
| Non-Asian countries | 3 | 1945 | Fixed | 1.24 (1.16–1.32) | <.001 | 44.6 | 0.165 |
| Sample size | |||||||
| <200 | 2 | 208 | Fixed | 1.93 (1.20–3.11) | .007 | 0 | 0.406 |
| ≥200 | 4 | 3727 | Fixed | 1.24 (1.16–1.32) | <.001 | 0 | 0.895 |
| Study design | |||||||
| Prospective | 1 | 134 | – | 2.34 (1.21–4.53) | .012 | – | – |
| Retrospective | 5 | 3801 | Fixed | 1.24 (1.16–1.32) | <.001 | 0 | 0.905 |
| Study center | |||||||
| Single center | 3 | 2960 | Fixed | 1.23 (1.15–1.32) | <.001 | 0 | 0.790 |
| Multicentre | 3 | 975 | Fixed | 1.36 (1.13–1.64) | .001 | 36.4 | 0.207 |
| cT stage | |||||||
| Ta–T1 | 4 | 3107 | Fixed | 1.31 (1.10–1.57) | .003 | 0 | 0.900 |
| T1–T4 | 2 | 828 | Random | 1.55 (0.85–2.85) | .154 | 72.3 | 0.058 |
| Cut-off value (mg/dL) | |||||||
| 0.5 | 3 | 2900 | Fixed | 1.32 (1.09–1.59) | .004 | 0 | 0.747 |
| ≠0.5 | 3 | 1035 | Fixed | 1.24 (1.16–1.33) | <.001 | 44.8 | 0.163 |
| Cut-off value determination | |||||||
| Literature | 3 | 2900 | Fixed | 1.32 (1.09–1.59) | .004 | 0 | 0.747 |
| ROC curve | 3 | 1035 | Fixed | 1.24 (1.16–1.33) | <.001 | 44.8 | 0.163 |
| Survival analysis | |||||||
| Univariate | 4 | 2019 | Fixed | 1.24 (1.16–1.33) | <.001 | 25.6 | 0.258 |
| Multivariate | 2 | 1916 | Fixed | 1.37 (1.04–1.82) | .027 | 0 | 0.825 |
RFS: recurrence-free survival; BCa: bladder cancer; ROC: receiver operating characteristics.
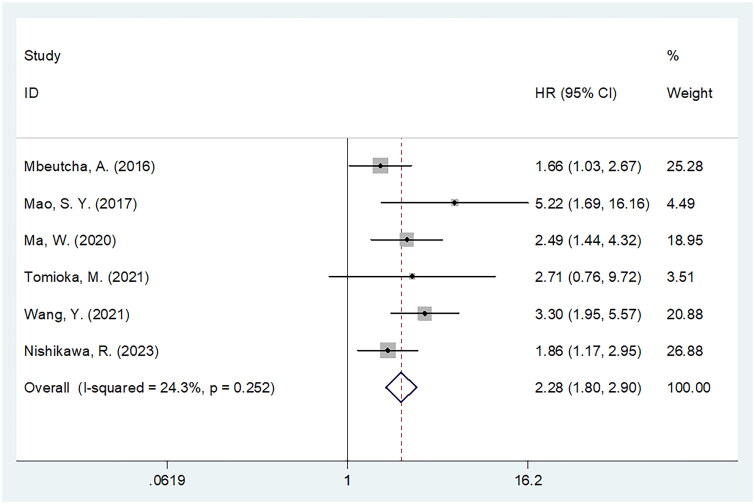
CRP and PFS
A total of six studies involving 3428 cases [20,21,23,28,29,32] showed the impact of CRP on the PFS of BCa. As indicated by the pooled data, a high CRP level was significantly associated with dismal PFS of BCa (HR = 2.28, 95%CI = 1.80–2.90, p < .001; Figure 5, Table 5). Subgroup analysis showed that the significance of CRP level in predicting PFS was not influenced by geographical region, sample size, study centre, threshold, threshold determination method or survival analysis type (Table 5).
Figure 5.
Meta-analysis of the association between elevated CRP and PFS in patients with BCa.
Table 5.
Subgroup analysis of prognostic value of CRP for PFS in patients with BCa.
| Subgroups | No. of studies | No. of patients | Effects model | HR (95%CI) | p | Heterogeneity |
|
|---|---|---|---|---|---|---|---|
| I2 (%) | Ph | ||||||
| Total | 6 | 3428 | Fixed | 2.28 (1.80–2.90) | <.001 | 24.3 | 0.252 |
| Geographical region | |||||||
| Asian countries | 5 | 2311 | Fixed | 2.54 (1.93–3.36) | <.001 | 6.9 | 0.367 |
| Non-Asian countries | 1 | 1117 | – | 1.66 (1.03–2.67) | .037 | – | – |
| Sample size | |||||||
| <200 | 3 | 395 | Fixed | 2.87 (2.00–4.13) | <.001 | 0 | 0.768 |
| ≥200 | 3 | 3033 | Fixed | 1.92 (1.39–2.64) | <.001 | 41.0 | 0.183 |
| Study center | |||||||
| Single center | 3 | 575 | Fixed | 3.07 (2.14–4.39) | <.001 | 0 | 0.479 |
| Multicentre | 3 | 2853 | Fixed | 1.81 (1.31–2.49) | <.001 | 0 | 0.771 |
| cT stage | |||||||
| Ta–T1 | 3 | 3033 | Fixed | 1.92 (1.39–2.64) | <.001 | 41.0 | 0.183 |
| T1–T4 | 2 | 368 | Random | 2.89 (1.98–4.22) | <.001 | 0 | 0.471 |
| T2–T4 | 1 | 27 | – | 2.71 (0.75–9.72) | .126 | – | – |
| Treatment | |||||||
| Surgery | 5 | 3401 | Fixed | 2.27 (1.78–2.90) | <.001 | 38.8 | 0.163 |
| Chemotherapy | 1 | 27 | – | 2.71 (0.75–9.72) | .126 | – | – |
| Cut-off value (mg/dL) | |||||||
| 0.5 | 2 | 1286 | Fixed | 1.76 (1.26–2.45) | .001 | 0 | 0.740 |
| ≠0.5 | 4 | 2142 | Fixed | 3.04 (2.15–4.29) | <.001 | 0 | 0.681 |
| Cut-off value determination | |||||||
| Literature | 2 | 2826 | Fixed | 1.76 (1.26–2.45) | .001 | 0 | 0.740 |
| ROC curve | 3 | 433 | Fixed | 3.46 (2.21–5.40) | <.001 | 0 | 0.711 |
| X-tile | 1 | 169 | – | 2.49 (1.44–4.32) | .001 | – | – |
| Survival analysis | |||||||
| Univariate | 1 | 1117 | – | 1.66 (1.03–2.67) | .037 | – | – |
| Multivariate | 5 | 2311 | Fixed | 2.54 (1.93–3.36) | <.001 | 6.9 | 0.367 |
PFS: progression-free survival; BCa: bladder cancer; ROC: receiver operating characteristics.
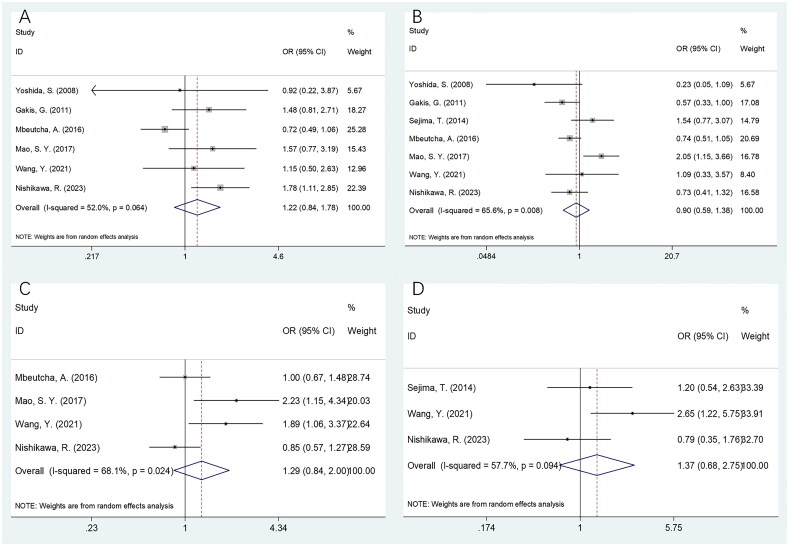
The relation of CRP with clinicopathological features of BCa
Seven studies involving 3815 patients [14,15,18,20,21,29,32] reported the relationship between CRP and BCa clinicopathological features. Based on the combined data, there was a non-significant relationship between CRP and sex (OR = 1.22, 95%CI = 0.84–1.78, p = .297), tumour grade (OR = 0.90, 95%CI = 0.59–1.38, p = .637), tumour size (OR = 1.29, 95%CI = 0.84–2.00, p = .244) or lymph node metastasis (LNM) (OR = 1.37, 95%CI = 0.68–2.75, p = .384) in patients with BCa (Figure 6; Table 6).
Figure 6.
Meta-analyses of the correlation between CRP and clinicopathological parameters in patients with BCa. (A) Gender (male vs. female); (B) tumor grade (G3 vs. G1–G2); (C) tumor size (cm) (≥3 vs. <3); and (D) LNM (yes vs. no).
Table 6.
The correlation between CRP and clinicopathological features in patients with BCa.
| Variables | No. of studies | No. of patients | Effects model | OR (95%CI) | p | Heterogeneity |
|
|---|---|---|---|---|---|---|---|
| I2 (%) | Ph | ||||||
| Gender (male vs. female) | 6 | 3566 | Random | 1.22 (0.84–1.78) | .297 | 52.0 | 0.064 |
| Tumor grade (G3 vs. G1–G2) | 7 | 3815 | Random | 0.90 (0.59–1.38) | .637 | 65.6 | 0.008 |
| Tumor size (cm) (≥3 vs. <3) | 4 | 3232 | Random | 1.29 (0.84–2.00) | .244 | 68.1 | 0.024 |
| LNM (yes vs. no) | 3 | 2157 | Random | 1.37 (0.68–2.75) | .384 | 57.7 | 0.094 |
CRP: C-reactive protein; LNM: lymph node metastasis; BCa: bladder cancer.
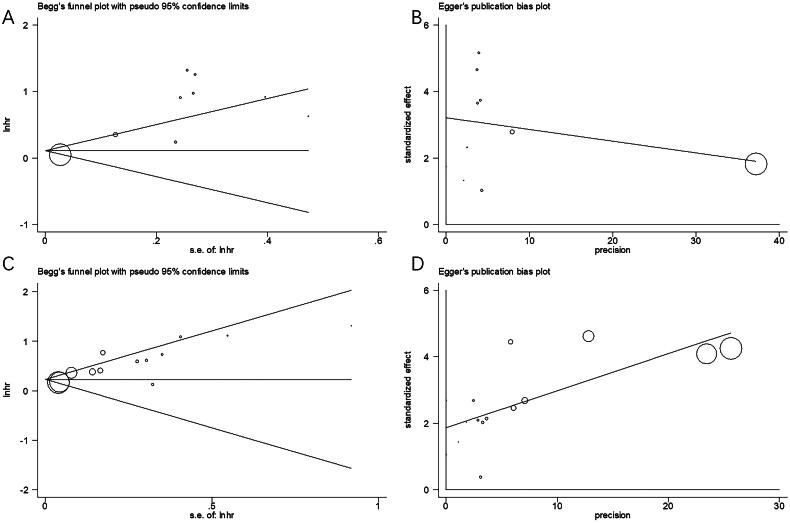
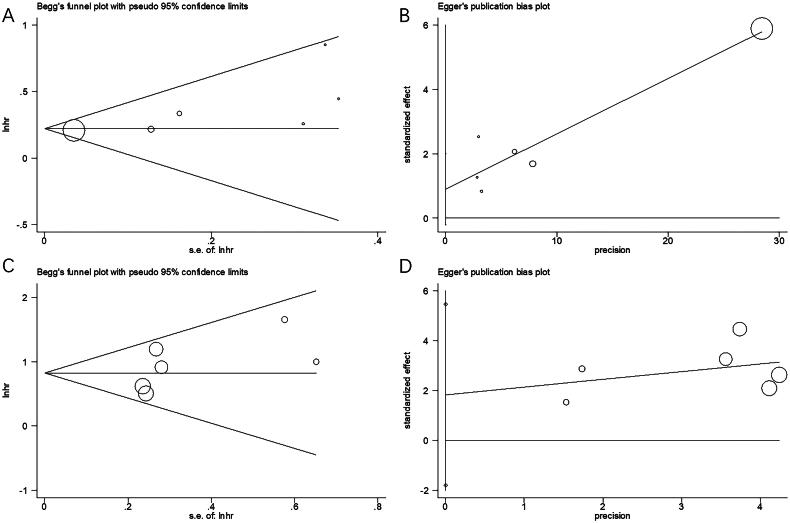
Publication bias
Publication bias was analysed using Begg’s and Egger’s tests in the current study (Figures 7 and 8). Publication bias was not detected for OS, CSS, RFS and PFS (Figures 7 and 8).
Figure 7.
Publication bias of the studies. (A) Begg’s test for OS, p = .466; (B) Egger’s test for OS, p = .182; (C) Begg’s test for CSS, p = .127; and (D) Egger’s test for CSS, p = .907.
Figure 8.
Publication bias of the studies. (A) Begg’s test for RFS, p = .133; (B) Egger’s test for CSS, p = .086; (C) Begg’s test for PFS, p = .707; and (D) Egger’s test for PFS, p = .234.
Discussion
The efficiency of CRP in predicting BCa prognosis has been previously analysed, but there are no consistent findings. In this study, we synthesized data from 20 articles involving 7276 cases. Our pooled data showed that CRP level significantly predicted the dismal OS, CSS, RFS and PFS of patients with BCa. Moreover, the prognostic power of CRP was stable in the subgroup analysis. Nonetheless, CRP levels were not significantly related to sex, tumour grade, tumour size or LNM of BCa. Collectively, CRP levels significantly and reliably predicted the short- and long-term prognosis of patients with BCa. To the best of our knowledge, this is the first study to investigate the effect of CRP level on predicting BCa prognosis.
The precise mechanisms underlying the significance of CRP in predicting BCa prognosis have not been comprehensively analysed. These results are interpreted as follows: first, acute-phase proteins such as CRP are produced by the liver when stimulated by cytokines, such as interleukin-6 (IL-6) [36]. The presence of chronic inflammation may contribute to carcinogenesis and cancer occurrence or progression [37]. The circulating concentration of vesicular endothelial growth factor (VEGF) and CRP is directly related, so an elevated circulating CRP level may indicate a tumour’s aggressiveness or phenotype [38]. Second, high serum levels of IL-6 are associated with elevated CRP levels in the blood. Because of its role in regulating the JAK–STAT signalling pathway, IL-6 regulates the expression of genes that are related to proliferation [39]. Third, patients with elevated CRP concentrations may have impaired T-lymphocytic responses to tumours, which may lead to the development of BCa 40. Tumor antigens may trigger an increase in CRP levels because of innate and adaptive immune responses.
This meta-analysis indicated that elevated CRP was significantly associated with diverse survival outcomes of BCa. Notably, integrating additional biomarkers with CRP or providing a weighted nomogram for CRP usage has been crucial for enhancing clinical decision-making and care delivery. However, due to the inherent nature of meta-analysis study, a weighted nomogram cannot be performed in the current study. A panel incorporating CRP with other biomarkers is also important for clinical decision-making of BCa. Because of limited data in this meta-analysis, prognostic panels are not performed in this study. Current evidence showed that panels with acute phase proteins were novel and reliable tools for prognostication and response evaluation in various cancers [40] including non-small cell lung cancer [41], pancreatic ductal adenocarcinoma [42] and diffuse large B‑cell lymphoma [43]. Therefore, prognostic nomograms and panels incorporating CRP should be investigated and developed in future studies.
Moreover, identifying CRP status at the moment of transurethral resection of bladder tumour (TURBT) would be novel. In this meta-analysis, the majority of BCa patients were treated with surgery. Our results showed that CRP remained a significant prognostic marker for BCa patients receiving surgery.
The cut-off values of CRP were not consistent in included studies. Interestingly, when we searched the literatures including urothelial carcinoma, a total of 47 studies were finally eligible (Supplementary Table S1). The cut-off values (mg/dL) ranged from 0.13 to 4.2, with a median value of 0.5. Moreover, 0.5 (mg/dL) was still the most prevalently selected cut-off value, which was used in 26 studies out of the 47 studies. The above-mentioned results on cut-off value were in consistent with the data in the current meta-analysis.
Recently, many studies have also reported the significance of CRP in predicting the prognosis of different cancers by conducting meta-analysis [44–47]. As shown by Zhang et al. in their meta-analysis comprising 3202 cases, a high serum CRP level was closely related to poor OS and PFS in ovarian cancer [44]. According to Yu and Zhang in their meta-analysis incorporating 2149 cases, higher CRP levels were significantly related to dismal OS in Asian patients with endometrial cancer [45]. Zhou et al. conducted a meta-analysis of 16 articles and based on their results, a high CRP level was associated with poor OS, CSS and PFS in prostate cancer cases [46]. In a recent meta-analysis encompassing 2204 cases, Yang et al. discovered a significant association between high CRP levels and poor OS and shortened PFS in cervical cancer [47]. Another recent meta-analysis of 2314 patients indicated that CRP level significantly predicted the dismal OS of patients with diffuse large B-cell lymphoma patients [48].
The present study had some limitations. First, many enrolled articles had a retrospective design. Therefore, heterogeneity may exist because of the retrospective nature of the studies. Second, the CRP threshold was nonuniform in the included articles, although the majority of these studies used 0.5 mg/dL. The CRP cut-off is the most compelling to us, even though there are multiple CRP cut-off values. Third, some studies only reported univariate HRs during raw data extraction, which may have led to an overestimation of effect sizes in the pooled results. Therefore, large-scale trials applying a standard CRP threshold should be conducted for further validation.
Conclusions
In summary, elevated CRP levels were notably related to poor OS, CSS, RFS and PFS of BCa patients with BCa. CRP could serve as a reliable biomarker for predicting short- and long-term BCa prognoses in clinical practice.
Supplementary Material
Funding Statement
This work was supported by Huzhou Science and Technology Plan Public Welfare Applied Research Project (No. 2022GYB07).
Author contributions
XF and ZZ performed the article search and selection. XF, ZZ and SM collected relevant data. XF and ZZ performed the analyses, and ZZ and SM interpreted the results and drafted this paper. XF, ZZ and SM revised the manuscript and provided some critical suggestions. All authors have approved the final version of the manuscript.
Ethical approval
Not applicable.
Consent form
Not applicable.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Lobo N, Afferi L, Moschini M, et al. Epidemiology, screening, and prevention of bladder cancer. Eur Urol Oncol. 2022;5(6):628–639. doi: 10.1016/j.euo.2022.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Catto JWF, Downing A, Mason S, et al. Quality of life after bladder cancer: a cross-sectional survey of patient-reported outcomes. Eur Urol. 2021;79(5):621–632. doi: 10.1016/j.eururo.2021.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitra AP, Cai J, Miranda G, et al. Management trends and outcomes of patients undergoing radical cystectomy for urothelial carcinoma of the bladder: evolution of the University of Southern California experience over 3,347 cases. J Urol. 2022;207(2):302–313. doi: 10.1097/ju.0000000000002242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kushner I. C-reactive protein – my perspective on its first half century, 1930–1982. Front Immunol. 2023;14:1150103. doi: 10.3389/fimmu.2023.1150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharya S, Munshi C.. Biological significance of C-reactive protein, the ancient acute phase functionary. Front Immunol. 2023;14:1238411. doi: 10.3389/fimmu.2023.1238411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plebani M. Why C-reactive protein is one of the most requested tests in clinical laboratories? Clin Chem Lab Med. 2023;61(9):1540–1545. doi: 10.1515/cclm-2023-0086. [DOI] [PubMed] [Google Scholar]
- 8.Andersen HH, Bojesen SE, Johansen JS, et al. Prognostic value of pretreatment plasma C-reactive protein in patients with early-stage breast cancer. Cancer Epidemiol Biomarkers Prev. 2024;33(5):662–670. doi: 10.1158/1055-9965.Epi-23-1299. [DOI] [PubMed] [Google Scholar]
- 9.Harada K, Matsumoto C, Toihata T, et al. C-reactive protein levels after esophagectomy are associated with increased surgical complications and poor prognosis in esophageal squamous cell carcinoma patients. Ann Surg Oncol. 2023;30(3):1554–1563. doi: 10.1245/s10434-022-12831-3. [DOI] [PubMed] [Google Scholar]
- 10.Kaneko S, Asahina Y, Murakawa M, et al. Prognostic significance of C-reactive protein in unresectable hepatocellular carcinoma treated with atezolizumab and bevacizumab. Hepatol Res. 2024;54(6):562–574. doi: 10.1111/hepr.14001. [DOI] [PubMed] [Google Scholar]
- 11.Adachi M, Nakayama M, Matsumoto S, et al. Elevation of C-reactive protein during concurrent chemoradiotherapy is a poor predictive factor for head and neck cancer. Auris Nasus Larynx. 2023;50(4):601–606. doi: 10.1016/j.anl.2022.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Bonazzi VF, Aoude LG, Brosda S, et al. C-reactive protein is a prognostic biomarker in pancreatic ductal adenocarcinoma patients. Asia Pac J Clin Oncol. 2023. doi: 10.1111/ajco.13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilmy M, Bartlett JM, Underwood MA, et al. The relationship between the systemic inflammatory response and survival in patients with transitional cell carcinoma of the urinary bladder. Br J Cancer. 2005;92(4):625–627. doi: 10.1038/sj.bjc.6602406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida S, Saito K, Koga F, et al. C-reactive protein level predicts prognosis in patients with muscle-invasive bladder cancer treated with chemoradiotherapy. BJU Int. 2008;101(8):978–981. doi: 10.1111/j.1464-410X.2007.07408.x. [DOI] [PubMed] [Google Scholar]
- 15.Gakis G, Todenhöfer T, Renninger M, et al. Development of a new outcome prediction model in carcinoma invading the bladder based on preoperative serum C-reactive protein and standard pathological risk factors: the TNR-C score. BJU Int. 2011;108(11):1800–1805. doi: 10.1111/j.1464-410X.2011.10234.x. [DOI] [PubMed] [Google Scholar]
- 16.Gondo T, Nakashima J, Ohno Y, et al. Prognostic value of neutrophil-to-lymphocyte ratio and establishment of novel preoperative risk stratification model in bladder cancer patients treated with radical cystectomy. Urology. 2012;79(5):1085–1091. doi: 10.1016/j.urology.2011.11.070. [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa T, Hara T, Kawahara T, et al. Prognostic risk stratification of patients with urothelial carcinoma of the bladder with recurrence after radical cystectomy. J Urol. 2013;189(4):1275–1281. doi: 10.1016/j.juro.2012.10.065. [DOI] [PubMed] [Google Scholar]
- 18.Sejima T, Morizane S, Yao A, et al. Prognostic impact of preoperative hematological disorders and a risk stratification model in bladder cancer patients treated with radical cystectomy. Int J Urol. 2014;21(1):52–57. doi: 10.1111/iju.12161. [DOI] [PubMed] [Google Scholar]
- 19.Grimm T, Buchner A, Schneevoigt B, et al. Impact of preoperative hemoglobin and CRP levels on cancer-specific survival in patients undergoing radical cystectomy for transitional cell carcinoma of the bladder: results of a single-center study. World J Urol. 2016;34(5):703–708. doi: 10.1007/s00345-015-1680-7. [DOI] [PubMed] [Google Scholar]
- 20.Mbeutcha A, Shariat SF, Rieken M, et al. Prognostic significance of markers of systemic inflammatory response in patients with non-muscle-invasive bladder cancer. Urol Oncol. 2016;34(11):483.e17–483.e24. doi: 10.1016/j.urolonc.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Mao SY, Huang TB, Xiong DD, et al. Prognostic value of preoperative systemic inflammatory responses in patients with non-muscle invasive bladder cancer undergoing transurethral resection of bladder tumor. Int J Clin Exp Path. 2017;10:5799–5810. [Google Scholar]
- 22.Albisinni S, Moussa I, Aoun F, et al. The impact of postoperative inflammatory biomarkers on oncologic outcomes of bladder cancer. Prog Urol. 2019;29(5):270–281. doi: 10.1016/j.purol.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Ma W, Mao S, Bao M, et al. Prognostic significance of red cell distribution width in bladder cancer. Transl Androl Urol. 2020;9(2):295–302. doi: 10.21037/tau.2020.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mari A, Muto G, Di Maida F, et al. Oncological impact of inflammatory biomarkers in elderly patients treated with radical cystectomy for urothelial bladder cancer. Arab J Urol. 2020;19(1):2–8. doi: 10.1080/2090598x.2020.1814974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogawa K, Shimizu Y, Uketa S, et al. Prognosis of patients with muscle invasive bladder cancer who are intolerable to receive any anti-cancer treatment. Cancer Treat Res Commun. 2020;24:100195. doi: 10.1016/j.ctarc.2020.100195. [DOI] [PubMed] [Google Scholar]
- 26.Tamalunas A, Buchner A, Kretschmer A, et al. Impact of routine laboratory parameters in patients undergoing radical cystectomy for urothelial carcinoma of the bladder: a long-term follow-up. Urol Int. 2020;104(7–8):551–558. doi: 10.1159/000506263. [DOI] [PubMed] [Google Scholar]
- 27.Gui H, Song Y, Yin Y, et al. Prognostic value of preoperative inflammation-based predictors in patients with bladder carcinoma after radical cystectomy. Open Med. 2021;16(1):816–825. doi: 10.1515/med-2021-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomioka M, Yoneyama T, Tobisawa Y, et al. Ghrelin after chemotherapy as a prognostic predictor of progression-free survival in patients with muscle-invasive bladder cancer. Transl Androl Urol. 2021;10(3):1192–1201. doi: 10.21037/tau-20-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Wang K, Ni J, et al. Combination of C-reactive protein and neutrophil-to-lymphocyte ratio as a novel prognostic index in patients with bladder cancer after radical cystectomy. Front Oncol. 2021;11:762470. doi: 10.3389/fonc.2021.762470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caglayan A, Horsanali MO.. Can peripheral blood systemic immune response parameters predict oncological outcomes in patients with non-muscle-invasive bladder cancer? Niger J Clin Pract. 2023;26(5):591–598. doi: 10.4103/njcp.njcp_399_22. [DOI] [PubMed] [Google Scholar]
- 31.Iemura Y, Miyake M, Fukui S, et al. Depth of invasion to the bladder wall as a prognostic factor and its association with circulating cell-free DNA levels in patients with muscle-invasive bladder cancer. Curr Urol. 2023;17(4):229–235. doi: 10.1097/CU9.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishikawa R, Miyake M, Morizane S, et al. C-reactive protein as a prognostic predictor for non-muscle invasive bladder cancer after intravesical bacillus Calmette-Guérin therapy: a Japan Urological Oncology Group study analysis. Int J Urol. 2023;30(3):299–307. doi: 10.1111/iju.15106. [DOI] [PubMed] [Google Scholar]
- 33.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8(1):16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stang A. Critical evaluation of the Newcastle-Ottawa Scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 36.Gyawali P, Hinwood M, Chow WZ, et al. Exploring the relationship between fatigue and circulating levels of the pro-inflammatory biomarkers interleukin-6 and C-reactive protein in the chronic stage of stroke recovery: a cross-sectional study. Brain Behav Immun Health. 2020;9:100157. doi: 10.1016/j.bbih.2020.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McFarland DC, Doherty M, Atkinson TM, et al. Cancer-related inflammation and depressive symptoms: systematic review and meta-analysis. Cancer. 2022;128(13):2504–2519. doi: 10.1002/cncr.34193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olszewska E, Pietrewicz TM, Świderska M, et al. A case-control study on the changes in high-sensitivity C-reactive protein and tumor necrosis factor-alpha levels with surgical treatment of OSAS. Int J Mol Sci. 2022;23(22):14116. doi: 10.3390/ijms232214116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu M, Zhu M, Du Y, et al. Serum C-reactive protein and risk of lung cancer: a case-control study. Med Oncol. 2013;30(1):319. doi: 10.1007/s12032-012-0319-4. [DOI] [PubMed] [Google Scholar]
- 40.Li S, Amakye WK, Zhao Z, et al. Prognostic value of anthropometric- and biochemistry-based nutrition status indices on blood chemistry panel levels during cancer treatment. Nutrition. 2024;126:112520. doi: 10.1016/j.nut.2024.112520. [DOI] [PubMed] [Google Scholar]
- 41.Schneider MA, Rozy A, Wrenger S, et al. Acute phase proteins as early predictors for immunotherapy response in advanced NSCLC: an explorative study. Front Oncol. 2022;12:772076. doi: 10.3389/fonc.2022.772076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ali N, Debernardi S, Kurotova E, et al. Evaluation of urinary C-reactive protein as an early detection biomarker for pancreatic ductal adenocarcinoma. Front Oncol. 2024;14:1450326. doi: 10.3389/fonc.2024.1450326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie S, Zhu L, Wang L, et al. Assessment and prognostic significance of a serum cytokine panel in diffuse large B‑cell lymphoma. Oncol Lett. 2024;27(5):237. doi: 10.3892/ol.2024.14370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang W, Zhang Z, Qian L.. Prognostic and clinicopathological significance of C-reactive protein in patients with ovarian cancer: a meta-analysis. World J Surg Oncol. 2024;22(1):8. doi: 10.1186/s12957-023-03290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu X, Zhang X.. Prognostic role of C-reactive protein in patients with endometrial cancer: a meta-analysis. Biomark Med. 2024;18(6):279–289. doi: 10.2217/bmm-2023-0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou K, Li C, Chen T, et al. C-reactive protein levels could be a prognosis predictor of prostate cancer: a meta-analysis. Front Endocrinol. 2023;14:1176102. doi: 10.3389/fendo.2023.1111277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang S, Zhang Z, Shen L.. Prognostic significance of C-reactive protein in patients with cervical cancer: a meta-analysis. Front Oncol. 2023;13:1232409. doi: 10.3389/fonc.2023.1232409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang Z, Wang K, Huang S, et al. Prognostic value of baseline C-reactive protein in diffuse large B-cell lymphoma: a systematic review and meta-analysis. Transl Cancer Res. 2023;12(8):2169–2180. doi: 10.21037/tcr-23-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.