Abstract
Alzheimer’s disease (AD) is a progressive neurological condition characterized by a loss in cognitive functions, with no disease-modifying medication now available. It is crucial for early detection and treatment of Alzheimer’s disease before clinical manifestation. The stage between cognitively healthy older persons and AD is known as mild cognitive impairment (MCI). To predict the transition from one-stage MCI to probable AD, five ensemble learning approach was used (Stacking, Gradient boost Bagging, Adaptive boost and Voting), an integrated model that combines not only cross-sectional neuroimaging biomarkers at baseline but also longitudinal cerebrospinal fluid (CSF) and cognitive performance biomarkers from the Alzheimer’s Disease Neuroimaging Initiative cohort (ADNI). The adaptive boost, stacking and bagging ensemble approach has shown potential to identify those at risk of developing Alzheimer’s disease, this would benefit them the most from a clinical trial or to use as a stratification approach inside clinical trials.
Keywords: Machine learning, Ensemble, Adaptive Boost, Stacking, Gradient Boost, Bagging, Voting, Disease progression, Alzheimer’s disease
1. Introduction
The most widely recognized explanation of Alzheimer’s disease (AD) states that it begins as an abnormal amyloid buildup in the brain and progresses to dementia over a number of decades (Rudimar et al., 2018; Nicolai et al., 2020; Alvin et al.,2020; Sarah et al., 2020; Laurent et al., 2019). According to estimates from the Alzheimer Association as of March 2012, 5.4 million Americans have been diagnosed with AD, and more than 95% of them are 65 or older. Additionally, AD affects almost half of the population over the age of 85 (Modupe et al. 2022; Qi et al., 2014; Gesine and Peter, 2009; Jorge et al., 2018). The condition produces little strokes in the brain, which results in the slow cell death and nerve dysfunction in the brain. A person suffering from the condition may be unaware of the strokes because they occur without any perception (Malavika et al., 2020). The rate of advancement of Alzheimer’s disease (AD) differs amongst persons, thus rendering it impossible to gather precise projections of disease progression or time until specific medical outcomes for individual patients (Eric et al., 2017). This implies that effective prevention will necessitate predicting who will acquire AD decades before symptoms appear. As a result, there is a rising interest in establishing precise methods of identifying persons who are predisposed to developing symptomatic AD, in order to ensure they could be targeted for preventive interventions such as risk factor reduction, behavioural modification, or pharmacologic treatment. (Deborah and Sei, 2011; Lei et al., 2012).
Considering the disease cannot be cured, the acceptable treatment is limited to slow down the progression of the ailment. Early disease detection will be beneficial for the doctor, the patient’s family, other close friends, etc. As a result, machine learning approaches are utilized to detect the disease early. In order to achieve the finest degree of accurateness, five strategies of ensemble methods are applied. Gradient boost, Stacking, Bagging, Voting and Adaptive boost Classifier are the approaches used. Using Python script for implementation, the most suitable and accurate model can possibly be recognized.
Prior models for Alzheimer’s disease risk prediction are often based on preset health profile variables such as sociodemographic (age, gender, education), lifestyle (physical activity), midlife health risk factors (systolic blood pressure, BMI, and total cholesterol level), and cognitive profiles (Alexander et al.,2019; Ji et al., 2020). Aside from identifying Alzheimer’s disease, forecasting the severity of cognitive impairment is a clinically significant challenge. Previous research has demonstrated that markers of primary AD pathology, neurodegeneration (structural MRI, FDG-PET), or biomarker combinations can predict whether a person would progress from moderate cognitive impairment (MCI) to AD dementia (Nicolai et al., 2019; Longfei et al., 2020; Xiaoying et al., 2017; Ting et al., 2019; Gang et al., 2011; Sarah et al., 2020). The moment these alterations start to take place and the point at which they may be distinguished from normal aging are, however, not well understood. This question is critical for establishing measurements that may be more sensitive to recognizing persons in the preclinical stage of the disease, as well as for therapeutic implications. As more effective pharmacological therapies for Alzheimer’s disease become available, it will become increasingly vital to develop and deploy preventive techniques to identify persons with preclinical dementia earlier in the illness’s natural course. Artificial intelligence-based 18F-FDG-PET analysis, including machine learning and deep learning, has gradually entered mainstream computing (Charles et al., 2000; Janani et al.,2021; Mohamed et al., 2018; Aliaa et al., 2022).
Advances in artificial intelligence, particularly in the field of machine learning, pose novel challenges as a result of the merging of computer science and biomedical sciences (Pushkar, 2019). The topic of big data with high data dimensions is being researched in the field of medicine, particularly with regard to magnetic resonance imaging (MRI) images. As the Internet and databases advance, big data continues to grow and advance tremendously. This is especially true in the case of medical large data and imagery. As a result, the issue of increasing data demonstrates the concept and potential of big data (Firouzeh et al., 2019).
2. Related Study
Nria et al. (2020) described the variations in the short-term temporal network dynamics of undirected and weighted whole-brain functional connectivity between healthy aging persons and people with mild cognitive impairment (MCI). The Network Change Point Detection technique was used to identify major change points in the resting-state fMRI register, and the fluctuations in the topological features of the sub-networks between significant change points were investigated. Ji et al. (2020) used large-scale administrative health data from the Korean National Health Insurance Service database between 2002 and 2010 to test the feasibility of using trained and validated random forest, support vector machine, and logistic regression machine learning to predict AD incidents in 1, 2, 3, and 4 subsequent years.
Shweta et al. (2020) used machine learning methods to identify dementia in its early stages, including Random Forest Classifier, (SVM), Decision Tree Classifier, Extra Tree Classifier, Neighbours Classifier, and Logistic Regression. Gender, age, education, MMSE, CDR, ASF, Handedness, and the number of hospital visits of patients classified as demented or non-demented make up the data for inquiry. Gopi et al. (2020) designed a decision tree model to forecast Alzheimer’s disease (AD) in the future. In this study, demographic variables from 150 participants and 373 MRI sessions were evaluated. To perform predictive analysis on Alzheimer’s disease patients, pruned decision trees (J48) were used.
Weiming et al. (2020) created an ELM-based grading approach to efficiently fuse multimodal data and predict MCI-to-AD conversion. First, features from magnetic resonance (MR) images were retrieved, and then valuable features were chosen using a feature selection method. subsequently these grading scores from several modalities were entered into a classifier to distinguish participants with progressing MCI from those with stable MCI. The research by Matoug et al. (2019) described a pseudo-automatic method that reads volumetric MRI, extracts the middle slices of the brain region, performs segmentation to find the region of the brain’s ventricle, generates a feature vector that describes this region, creates a SQL database that contains the generated data, and then categorizes the images using the extracted features.
Xiaojing et al. (2016) developed a machine learning method to distinguish patients with AD or moderate cognitive impairment (MCI) from healthy elderly and to predict AD conversion in MCI patients by computing and evaluating localized morphological variations in the brain between groups. Asymmetric diffeomorphic registration was used to calculate the distance between each pair of subjects, which was then followed by an embedding algorithm and a learning approach for classification.
3. Proposed Methodology
An all-encompassing meta-approach to machine learning called ensemble learning aims to improve predictive performance by pooling predictions from many models called base leaners. Whilst one can create an apparently infinite number of ensembles to tackle model prediction, three strategies dominate the field of ensemble learning which are Boosting, Stacking and Bagging. Sequential and parallel ensemble techniques are the two primary kinds of ensemble methods.
Sequential ensemble. approaches produce base learners in a sequential order. The sequential production of basic learners fosters dependability among the base learners. The model’s performance is then improved by giving bigger weights to previously misrepresented learners.
Parallel ensemble approaches generate base learners in a parallel fashion, such as random forest. Parallel techniques make use of the parallel generation of base learners to develop independence among the base learners. The independence of base learners considerably lowers the inaccuracy caused by the use of averages.
In base learning, the majority of ensemble strategies use a single algorithm, resulting in homogeneity across all base learners. Homogenous base learners are base learners of the same type with similar characteristics. Heterogeneous base learners, resulting in heterogeneous ensembles.
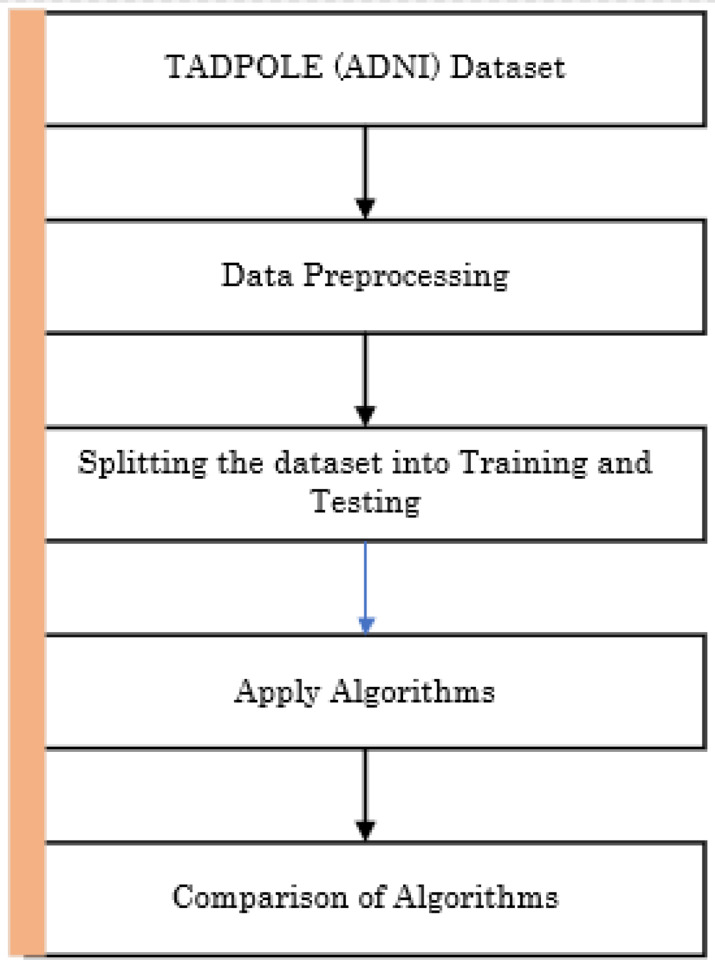
In the healthcare sector, machine learning has an enormous impact. The healthcare area has an immense quantity of datasets to design an advanced and scientific way to diagnose the disease at an early stage. As a result, some machine learning algorithms are employed for predicting symptoms and choosing the top precision supplier among all of these approaches. The proposed strategy as in Fig-1 employs the ensemble technique for forecasting the five phases of Alzheimer’s disease in advance. Adaptive Boost, Gradient Boost, Stacking, Voting and Bagging are the algorithms used and the implementation is done in Python programming language.
Figure 1.
Proposed process framework
3.1. Data Collection
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer’s disease (AD). Table 1 shows the specifics of the dataset used in this investigation. The baseline combined dataset included 12741 occurrences, 3821 of which were Mild Cognitive Impairment - CN, 2319 Early Mild Cognitive Impairment - EMCI, 389 Significant Memory Concern - SMC, 4644 Late Mild Cognitive Impairment - LMCI and 1568 participants recorded to have developed Alzheimer Disease - AD. The dataset includes people ranging in age from 55 to 96, both male and female, as well as many other characteristics that can be utilized to train and execute algorithms to detect the impact of Alzheimer’s disease.
Table 1.
Dataset statistics
| Alzheimer Stages | No. of Records |
|---|---|
| Mild Cognitive Impairment - CN | 3821 |
| Significant Memory Concern - SMC | 389 |
| Early Mild Cognitive Impairment - EMCI | 2319 |
| Late Mild Cognitive Impairment - LMCI | 4644 |
| Alzheimer Disease - AD | 1568 |
| Total | 12741 |
3.2. Data Preprocessing
All modifications to the raw data before they are delivered to the machine learning algorithm is referred to as data preprocessing. Poor classification performance is likely to result from training a model on an unprocessed dataset. Preprocessing is essential for accelerating training methods like clustering and scaling. Real-world data is frequently inaccurate and lacking in specific behaviours or trends. It is also frequently inconsistent and incomplete. A tried-and-true technique for tackling such problems is data preprocessing. The dataset was first examined to see if there were any categorical values, and it did contain a few of them. The gender and marital status attribute columns are among them and are changed into the numbers 0–1 and 1–4. In order to better comprehend them, we have examined the correlation between qualities using the “correlation matrix” function based on group attributes and plotted them. The dataset is then examined for any null or missing values.
3.3. Splitting the dataset into Training and Testing
Based on the holdout approach, the dataset is split into a training set and a testing set (Bookheimer et al., 2000). According to several researchers in the literature, 80% of the dataset (used as a testing set) is sufficient to produce accurate results (Huang et al., 2017; Seijo-Pardo et al., 2017). As a result, the prediction model was created using 80% and 20% size of testing and training set required to achieve the best results.
3.4. Apply Algorithm
In the present investigation, a comparative analysis with well-known methods has been done using the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). Bagging, Stacking, Voting, Adaptive Boost, and Gradient Boost are the ensemble methods used for early AD detection. The rest of this subsection presents a summary of the selected techniques.
Boosting
The term “boosting” refers to a class of algorithms that can convert weak models into strong models. Boosting works by placing weak learners in succession such that weak learners can learn from the next learner in the sequence, resulting in improved prediction models. Gradient boosting, Adaptive Boosting (AdaBoost), and XGBoost (Extreme Gradient Boosting) are all examples of boosting, however AdaBoost and Gradient Boost has been chosen for the study.
Adaptive Boost
AdaBoost employs weak learners in the form of decision trees, which typically feature one split, also known as decision stumps. AdaBoost’s fundamental deciding stump is made up of inputs with equal weights.
Gradient Boost
Gradient boosting increases the ensemble’s predictor count incrementally, with earlier predictors correcting subsequent ones in order to improve the precision of the model. In order to mitigate the consequences of previous prediction failures, new predictors are fitted. The gradient booster uses the gradient of descent to find and fix predicted errors made by students.
Bagging
This strategy, also known as “Bootstrap Aggregating,” summarizes the key elements of this approach. Bootstrapping and aggregation are the two methods of bagging.
Bootstrapping is a sampling strategy where samples are taken utilizing the method of substitution from the entire sample (set). The sampling with the substitution method aids in the randomization of the selection process. The process is finished by applying the base learning algorithm to the samples.
Aggregation is used to include all potential outcomes of the prediction and randomize the result. Predictions made without aggregation won’t be accurate because all possible outcomes won’t be taken into account. As a result, the aggregate is based either on all of the results from the predictive models or on the probability bootstrapping techniques.
Voting
Voting ensembles are a subset of ensemble techniques. They use several models to train on the dataset and provide predictions because they are one of the ensemble methods. The entire dataset is fed to several models of various machine learning algorithms in Voting Classifiers, and each algorithm makes predictions after being trained on the data. The most common approach is used to obtain the final prediction from the model after all of the models have predicted the sample data. The category that the various algorithms predicted most accurately in this case will be treated as the model’s final prediction.
Stacking
Also known as stacked generalization, is an ensemble method that combines models using another machine learning algorithm. The basic idea is to train machine learning algorithms on training datasets and then use these models to generate new datasets. The new dataset is then fed into the combiner machine learning algorithm.
3.5. Model Evaluation
Fig-5 is confusion matrix representing adaptive boost method with the various labels encoded (CN-0, SMC-1, EMCI-2, LMCI-3, and AD-4). Table 2 is a statistical breakdown of the data in Fig-5, Table 3 displays the key performance parameters Precision, Recall, and F1 scores for five phases of Alzheimer disease and Table 4 shows the performance of the categorization models on the test data.
Figure 5.
Confusion matrix of Adaptive boost
Table 2:
Statistics of Adaboost confusion matrix
| CN | SMC | EMCI | LMCI | AD | TOTAL | |
|---|---|---|---|---|---|---|
| CN | 779 | 17 | 46 | 64 | 29 | 935 |
| SMC | 4 | 900 | 0 | 0 | 4 | 908 |
| EMCI | 71 | 6 | 731 | 78 | 40 | 926 |
| LMCI | 88 | 9 | 86 | 642 | 125 | 950 |
| AD | 36 | 1 | 25 | 44 | 819 | 925 |
| TOTAL | 978 | 933 | 888 | 828 | 1017 | 4644 |
Table 3.
Performance evaluation using Recall, F1 Score and Precision.
| Methodology | PRECISION | RECALL | F1 SCORE | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CN | SMC | EMCI | LMCI | AD | CN | SMC | EMCI | LMCI | AD | CN | SMC | EMCI | LMCI | AD | |
| Bagging | 80 | 96 | 81 | 77 | 78 | 82 | 99 | 81 | 63 | 88 | 81 | 98 | 81 | 69 | 83 |
| Gboost | 75 | 92 | 82 | 75 | 75 | 79 | 98 | 79 | 60 | 85 | 77 | 95 | 80 | 66 | 80 |
| Adaboost | 80 | 96 | 82 | 78 | 81 | 83 | 99 | 79 | 68 | 89 | 81 | 98 | 81 | 72 | 84 |
| Voting | 75 | 94 | 81 | 80 | 77 | 84 | 99 | 80 | 55 | 89 | 79 | 97 | 81 | 65 | 83 |
| Stacking | 79 | 99 | 85 | 74 | 86 | 84 | 99 | 80 | 74 | 86 | 81 | 99 | 82 | 74 | 86 |
Table 4.
Classification Performance
| General Accuracy (%) on Train and Test Set Data | ||
|---|---|---|
| Methodology | Train Set | Test Set |
| GBoost | 88 | 95 |
| Voting | 92 | 81 |
| Stacking | 93 | 97 |
| Bagging | 93 | 97 |
| AdaBoost | 93 | 97 |
4. Result and Discussion
In this study, every one of the classification models is trained using 10 folds cross-validation on the training dataset. In order to prevent model overfitting, cross-validation has been carried out with the training dataset. The learners’ effectiveness has been assessed using an unobserved test dataset. Table 4 shows the categorization performance of the models across both the training and testing periods.
According to the results analysis in Fig-4, three ensemble approaches, Stacking, Adaboost, and Bagging beat the other two classifiers and provided an accuracy of 97% over the test time. Voting and GBoost have reasonable accuracies of 81% and 95%, respectively. It is also worth noting that the results obtained on test data are very similar to the findings obtained throughout the cross-validation period. This demonstrates that the created models were not overfitted during the training phase. AdaBoost ensemble model accurately categorized the majority of the unseen instances of the CN, SMC, EMCI, LMCI and AD classes, with class precisions [80%, 96%, 82%, 78%, 81%], class recall [83%, 99%, 79%, 68%, 89%], F1 Score [81%, 98%, 81%, 72%, 84%] respectively. The SMC class findings suggest that testing instances have been misclassified. Whilst in Fig-5, 17 out of 933 instances of the SMC class were misclassified as the CN class. This is due to the fact that patients in the SMC class have clinical assessment characteristics that are extremely comparable to those found in the CN class. Furthermore, 78 of the 828 LMCI cases have been incorrectly classified as EMCI. This is due to the characteristic values of the EMCI and LMCI classes intersect.
Figure 4.
Gender and diagnosis statistics
5. Conclusion
This research compares and evaluates recent work in the prognosis and prediction of Alzheimer’s disease using ensemble learning approaches. Obviously, significant machine learning advances have been reported that cover the categories of data employed and the effectiveness of algorithms based on machine learning for identifying the earliest stages of Alzheimer’s. It has become evident that machine learning improves the precision of forecasts, particularly when compared to traditional statistical techniques. There are a number of improvements to our dataset and methodology that are important steps for future research. Here, we limited ourselves to modelling 14 biomarkers that are commonly measured in AD clinical trials. We had excluded some interesting ones due to high number of null values; whilst over sampling is good to balance up data this can equally lead to model overfitting, in future other methods would be employed to handle the situation of data imbalance.
Figure 2.
Age statistics
Figure 3.
Marital statistics
Acknowledgement
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12–2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California.
Funding
There was no third-party funding for this study.
Footnotes
Declarations
Ethics approval and consent to participate
Neither ethics approval nor consent for publication were applicable given the study design.
Consent for publication
The authors consent to publication of this paper.
Competing interests
Authors have no financial or non-financial conflicts of interest to declare.
Additional Declarations:
No competing interests reported.
Contributor Information
Placida Orochi Orlunwo, Ignatius Ajuru University of Education.
Friday Eleonu Onuodu, Ignatius Ajuru University of Education.
References
- Kulminski AlexanderM., Shu Leonardo, Loika Yury, He Liang, Nazarian Alireza, Arbeev Konstantin, Ukraintseva Svetlana, Yashin Anatoliy and Culminskaya Irina. (2019). Genetic and regulatory architecture of Alzheimer’s disease in the APOE region. DOI: 10.1002/dad2.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gawady Aliaa, Makhlouf Mohamed A., Tawfik BenBella S. and Nassar Hamed. (2022). Machine Learning Framework for the Prediction of Alzheimer’s Disease Using Gene Expression Data Based on Efficient Gene Selection. doi. 10.3390/sym14030491. [DOI] [Google Scholar]
- Bachman Alvin H. and Babak A. Ardekani for the Alzheimer’s Disease Neuroimaging Initiative. (2020). Change point analyses in prodromal Alzheimer’s disease. doi. 10.1016/j.bionps.2020.100028 [DOI] [Google Scholar]
- Alzheimer Disease Neurological Imaging (ADNI). (2021). TAPOLE challenge data. https://ida.loni.usc.edu/pages/access/studyData.jsp?categoryId=43&subCategoryId=94 [Google Scholar]
- Bookheimer S.Y., Strojwas M.H., Cohen M.S., Saunders A.M., Pericak-Vance M.A., Mazziotta J.C., Small G. W., 2000, “Patterns of brain activation in people at risk of Alzheimer’s disease”, New England journal of medicine, Vol. 343, No. 7, pp.450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall1 Charles B. , Lipton Richard B., Sliwinski Martin and Stewart Walter F.. (2000). A change point model for estimating the onset of cognitive decline in preclinical Alzheimer’s disease. Statist. Med. 2000; 19:1555{1566. [DOI] [PubMed] [Google Scholar]
- Barnes Deborah E and Lee Sei J. (2011). Predicting Alzheimer’s risk: Why and how?. Barnes and Lee Alzheimer’s Research & Therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razavi Firouzeh, Tarokh Mohammad Jafar and Alborzi1 Mahmood. (2019). An intelligent Alzheimer’s disease diagnosis method using unsupervised feature learning. doi. 10.1186/s40537-019-0190-7. [DOI] [Google Scholar]
- Gang Chen B. Douglas Ward, Chunming Xie, Wenjun Li, Zhilin Wu, Jones Jennifer L., Franczak Malgorzata, Antuono Piero and Li Shi-Jiang. (2011). Classification of Alzheimer Disease, Mild Cognitive Impairment, and Normal Cognitive Status with Large-Scale Network Analysis Based on Resting-State Functional MR Imaging. doi: 10.1148/radiol.10100734/-/DC1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battineni Gopi, Chintalapudi Nalini and Amenta Francesco. (2020). Late-Life Alzheimer’s Disease (AD) Detection Using Pruned Decision Trees. DOI: 10.23937/2469-5866/1410033 [DOI] [Google Scholar]
- Huang M., Yang W., Feng Q., & Chen W., 2017, “Longitudinal measurement and hierarchical classification framework for the prediction of Alzheimer’s disease”. Scientific reports, Vol. 7, No. 1, pp. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopalan Janani, Tong Li, Hassanzadeh Hamid Reza and Wang May D.. (2021). Multimodal deep learning models for early detection of Alzheimer’s disease stage. doi. 10.1038/s41598-020-74399-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Ji Hwan, Cho Han Eol, Kim Jong Hun, Wall Melanie M., Stern Yaakov, Lim Hyunsun, Yoo Shinjae, Kim Hyoung Seopand Cha Jiook. (2020). Machine learning prediction of incidence of Alzheimer’s disease using large-scale administrative health data. Doi. 10.1038/s41746-020-0256-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del-Aguila Jorge L. , Fernández Maria Victoria, Schindler Suzanne, Ibanez Laura, Deming Yuetiva, Ma Shengmei, Saef Ben, Black Kathleen, Budde John, Norton Joanne, Chasse Rachel, Alzheimer’s Disease Neuroimaging Initiative (ADNI), Harari Oscar, Goate Alison, Xiong Chengjie, Morris John C., and Carlos Cruchaga. (2018). Assessment of the genetic architecture of Alzheimer’s Disease risk in rate of memory decline. doi: 10.3233/JAD-170834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes Laurent, Albert Marilyn, Moghekar Abhay, Soldan Anja, Pettigrew Corinne and Miller Michael I.. (2019). Identifying Changepoints in Biomarkers During the Preclinical Phase of Alzheimer’s Disease. Doi: 10.3389/fnagi.2019.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Lei, Boyle Patricia, Wilson Robert S., Segawa Eisuke, Leurgans Sue, De Jager Philip L., and Bennett David A.. (2012). A Random Change Point Model for Cognitive Decline in Alzheimer’s Disease and Mild Cognitive Impairment. DOI: 10.1159/000339365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Longfei, Li Fangyu, Wei Cuibai, Zhu Min, Qu Qiumin, Qin Wei, Tang Yi, Shen Luxi, Wang Yanjiang, Shen Lu, Li Honglei, Peng Dantao, Tan Lan, Luo Benyan, Guo Qihao, Tang Muni, Du Yifeng, Zhang Jiewen, Zhang Junjian, Lyu Jihui, Li Ying, Zhou Aihong, Wang Fen, Chu Changbiao, Song Haiqing, Wu Liyong, Zuo Xiumei, Han Yue, Liang Junhua, Wang Qi, Jin Hongmei, Wang Wei, Lu Yang, Li Fang, Zhou Yuying, Zhang Wei, Liao Zhengluan, Qiu Qiongqiong, Li Yan, Kong Chaojun, Li Yan, Jiao Haishan, Lu Jie and Jia Jianping. (2020). Prediction of Alzheimer’s disease using multi-variants from a Chinese genome-wideassociation study. doi: 10.1093/brain/awaa364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavika G., Rajathi N., Vanitha V. and Parameswari P. (2020). Alzheimer Disease Forecasting using Machine Learning Algorithm. doi. 10.21786/bbrc/13.11/4 [DOI] [Google Scholar]
- Matoug S., Abdel-Dayem A., Passi K, Gross W. and Alqarni M. (2019). Predicting Alzheimer’s disease by classifying 3D-Brain MRI images using SVM and other well-defined classifiers. Doi: 10.1088/1742-6596/341/1/012019. [DOI] [Google Scholar]
- Odusami Modupe, Maskeliunas Rytis and Damaševičius Robertas. (2022). An Intelligent System for Early Recognition of Alzheimer’s Disease Using Neuroimaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahyoub Mohamed, Randles Martin, Baker Thar, Yang Po, ADNI. (2018). Effective Use of Data Science Toward Early Prediction of Alzheimer’s Disease. DOI 10.1109/HPCC/SmartCity/DSS.2018.00240. [DOI] [Google Scholar]
- Franzmeier Nicolai, Neitzel Julia, Rubinski Anna, Smith Ruben, Strandberg Olof, Ossenkoppele Rik, Hansson Oskar, Ewers Michael and Alzheimer’s Disease Neuroimaging Initiative (ADNI). (2020). Functional brain architecture is associated with the rate of tau accumulation in Alzheimer’s disease. doi. 10.1038/s41467-019-14159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzmeier Nicolai, Koutsouleris Nikolaos, Benzinger Tammie, Goate Alison, Karch Celeste M., Fagan Anne M., McDade Eric, Duering Marco, Dichgans Martin, Levin Johannes, Gordon Brian A., Lim Yen Ying, Masters Colin L., Rossor Martin, Fox Nick C., O’Connor Antoinette, Chhatwal Jasmeer, Salloway Stephen, Danek Adrian, Hassenstab Jason, Schofield Peter R., Morris John C., Bateman Randall J., the Alzheimer’s disease neuroimaging initiative (ADNI), the Dominantly Inherited Alzheimer Network (DIAN) and Ewers Michael. (2019). Predicting sporadic Alzheimer’s disease progression via inherited Alzheimer’s disease-informed machine-learning. DOI: 10.1002/alz.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatkoti Pushkar. (2019). Early Diagnosis of Alzheimer’s Disease: A Multiclass Deep Learning Framework with Modified k-Sparse Autoencoder Classification. School of Computing and Mathematics Charles Sturt University, Australia. [Google Scholar]
- Zhou Qi, Goryawala Mohammed, Cabrerizo Mercedes, Barker Warren, Duara Ranjan, and Adjouadi Malek. (2014). Significance of Normalization on Anatomical MRI Measures in Predicting Alzheimer’s Disease. dx.doi. 10.1155/2014/541802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frozza Rudimar L., Lourenco Mychael V. and De Felice Fernanda G.. (2018). Challenges for Alzheimer’s Disease Therapy: Insights from Novel Mechanisms Beyond Memory Defects. Doi: 10.3389/fnins.2018.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruningk Sarah C., Hensel Felix, Jutzeler Catherine R., Rieck Bastian. (2020). Image analysis for Alzheimer’s disease prediction: Embracing pathological hallmarks for model architecture design. ML4H Extended Abstract arXiv Index:1–8. [Google Scholar]
- Neunera Sarah M., TCW Julia., Goate Alison M.. (2020). Genetic architecture of Alzheimer’s disease. doi. 10.1016/j.nbd.2020.104976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seijo-Pardo B., Porto-Díaz I., Bolón-Canedo V., Alonso-Betanzos A, 2017, “Ensemble feature selection: homogeneous and heterogeneous approaches”, Knowledge-Based Systems, Vol. 118, pp. 124–139. [Google Scholar]
- Madiwalar Shweta, Patil Sujata, Shashidhar H., and Parameshachari B. D. (2020). Classification and Investigation of Alzheimer Disease Using Machine Learning Algorithms. doi. 10.21786/bbrc/13.13/. [DOI] [Google Scholar]
- Shen Ting, Jiang Jiehui, Lu Jiaying, Wang Min, Zuo Chuantao, Yu Zhihua, and Yan Zhuangzhi. (2019). Predicting Alzheimer Disease from Mild Cognitive Impairment with a Deep Belief Network Based on 18F-FDG-PET Images. DOI: 10.1177/1536012119877285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Weiming, Gao Qinquan, Yuan Jiangnan, Chen Zhiying, Feng Chenwei, Chen Weisheng, Du Min and Tong Tong for the Alzheimer’s Disease Neuroimaging Initiative. (2020). Predicting Alzheimer’s Disease Conversion from Mild Cognitive Impairment Using an Extreme Learning Machine-Based Grading Method with Multimodal Data. doi: 10.3389/fnagi.2020.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Xiaojing, Chen Lifang, Jiang Chunxiang, Zhang Lijuan and Alzheimer’s Disease Neuroimaging Initiative. (2017). Prediction and classification of Alzheimer disease based on quantification of MRI deformation. DOI: 10.1371/journal.pone.0173372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Xiaoying, Miller Michael I. and Younes Laurent. (2017). Biomarker Change-Point Estimation with Right Censoring in Longitudinal Studies. DOI: 10.1214/17-AOAS1056. [DOI] [PMC free article] [PubMed] [Google Scholar]