ABSTRACT
Japanese encephalitis virus (JEV) genotype IV (GIV) is one of the least common and most neglected genotypes worldwide, having been identified only on a few Indonesian islands until it was recently found to be the cause of outbreaks that occurred in several Australian states in early 2022. Given the limited availability of information, the vector range for JEV GIV remains unknown; thus, understanding this range could prove invaluable for future prevention efforts in new areas. Herein, we experimentally exposed four mosquito colonies originated from various countries with no previous reports of GIV to JEV GIV strain 19CxBa-83-Cv, which was isolated from Culex vishnui Theobald collected in Bali in 2019. At 7 and 14 days post-JEV GIV exposure through a membrane feeding method, mosquito bodies, head–wings–legs, and saliva were harvested for infection, dissemination, and transmission efficiency analyses. The results showed robust transmission efficiencies of the virus by Culex tritaeniorhynchus Giles (∼74%) and Aedes albopictus Skuse (∼52%) from Japan, followed by Culex quinquefasciatus Say from Vietnam (∼35%) and Culex pipiens form molestus from Turkey (∼18%). Although significant differences were observed, we found that the four mosquito species could transmit JEV GIV. The efficiency of biological transmission of this restricted genotype by mosquitoes from various origins suggests that these mosquito species could support localized transmission if the genotype were introduced to their respective areas. This study emphasizes the importance of remaining vigilant and continuing arbovirus surveillance in all locations.
KEYWORDS: Japanese encephalitis virus, genotype IV, Ae. albopictus, Cx. pipiens, Cx. tritaeniorhynchus, Cx. quinquefasciatus, vector competence
Introduction
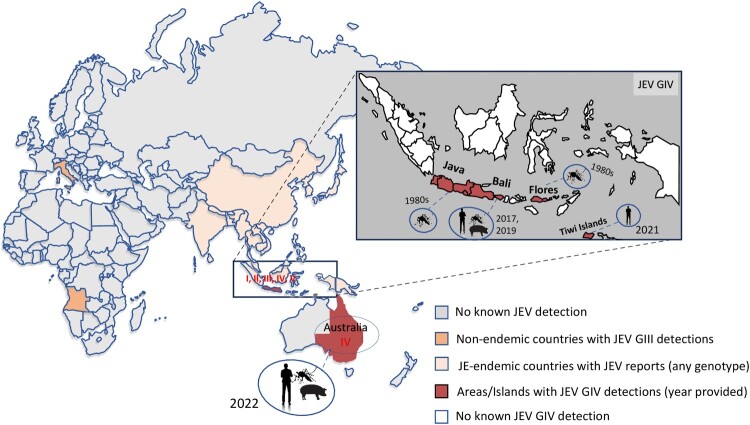
Japanese encephalitis (JE) is an acute neurological infectious disease caused by the JE virus (JEV; family Flaviviridae, genus Orthoflavivirus, virus species Orthoflavivirus japonicum) [1,2]. Although most human infections are asymptomatic, approximately one in 250 individuals who progresses to the encephalitic stage faces a perilous outcome, with a significant portion of the population succumbing to devastating effects of this infection [1,3]. Over 30% of survivors experience varying degrees of central nervous system sequelae, making JE a threat to public health [3,4]. JEV transmission is facilitated by arthropod vectors, and the infection claims the lives of 13,600–20,400 individuals annually, according to a report from the World Health Organization [5]. While the virus is considered to have first emerged in Southeast Asia, or the Malay Archipelago to be precise [6,7], its current distribution extends across Asia, even reaching as far as the South Pacific region of Australia (Figure 1) [8,9].
Figure 1.
Areas where Japanese encephalitis virus detections had been reported, with particular emphasis on JEV GIV distribution. Outside JE-endemic areas (Asian countries), there were JEV GIII detections in Italy and Angola, and JEV GIV detections in Australia. Source of virus and year of JEV GIV detection/isolation were also illustrated.
JEV is a single-stranded, positive-sense RNA virus containing a genome spanning 10,965 nucleotides, with a single open reading frame encoding a polyprotein that is converted into three structural (core (C), precursor membrane (M), and envelope (E)) and seven nonstructural (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) proteins [10,11]. Based on sequence of the E gene, JEV could be phylogenetically categorized into five different genotypes, GI to GV [12]. The most dominant genotype circulating in Asia was GIII, before it was replaced by GI in the 1990s [13]. The other three genotypes are rarely detected and have a limited distribution. GII has been considered endemic in Indonesia and Malaysia, with sporadic detections in Australia in 1995 and 1998 and in South Korea before 1951 [14]. GV was once restricted to Malaysia but has emerged in China and South Korea since 2009 [15–18]. GIV was first detected solely on three Indonesian islands – Bali, Java, and Flores – in the 1980s [19]. In 2021, its presence was noted on the Tiwi Islands, followed by an emergence in southeastern Australia in early 2022 [20] (Figure 1). The first documented human case of JEV GIV was reported in 2019, involving an Australian tourist who contracted the infection on Bali Island, resulting in fatality, with the virus isolated from cerebrospinal fluid sampled in a hospital in Queensland [21]. The emergence of cases in early 2022 across Australian states suggests potential divergent pathways, with initial cases on the Tiwi Islands followed by subsequent occurrences and positive identifications in sentinel pigs and mosquitoes of mainland Australia [20]. This marked the first instance of human cases and mortality attributed to JEV GIV beyond endemic regions, unrelated to travel.
The primary arthropod vector for JEV transmission in most Asian countries is the rice paddy-breeding Culex tritaeniorhynchus [22,23], whereas in Australia, it is Culex annulirostris [24]. In addition, at least 12 other species, belonging to the genera Aedes, Culex, and Armigeres, have been confirmed to be JEV vectors, with established experimental vector competence and evidence of the presence of JEV in wild-caught mosquitoes [25]. Most of these extensive studies assessed GIII, GI, GII, and GV (in the order of the number of studies) [25–28] and none has evaluated JEV GIV in any mosquito species. While the impact of genotypes on the vector competence of the same species remains unknown, it has been proposed that the diversity of host varieties and the high replication efficiency of mosquito vectors may have contributed to the displacement of GIII by GI [13,29].
To comprehend the potential risk of disease endemicity in an area, it is necessary to understand the probable vertebrate hosts, the vector status of local mosquito species, and the pathogenic potential of the virus [30]. Revealing the range of competent vectors for transmitting JEV GIV will enhance our understanding of this virus. Notably, the mosquito species responsible for numerous JEV outbreaks in Asia, Cx. tritaeniorhynchus [31–33], should be investigated as potential vectors for JEV GIV. Additionally, it is important to examine the competency of other significant secondary vectors, such as Culex quinquefasciatus and Culex pipiens form molestus, which may also serve as primary vectors in certain regions [34,35]. Furthermore, considering the widespread distribution of Aedes albopictus, a highly invasive species [36,37], it is imperative to elucidate its competency as well. Not only are these four species abundant in nonendemic areas surrounding disease-endemic areas, but they also have the ability to transmit JEV and a high likelihood to serve as vectors for JEV GIV.
Demonstrated as the newest genotype undergoing rapid evolutionary changes, JEV GIV exhibits favourable adaptability to hosts or vectors, facilitating its introduction into nonendemic regions [11]. In this study, we evaluated the infection, dissemination, and transmission efficiency (TE) rates of JEV GIV by four mosquito species that originated from various countries and analysed their overall vector competence.
Materials and methods
Mosquito species
The laboratory colonies used in this study included Cx. tritaeniorhynchus, Ae. albopictus, Cx. pipiens form molestus, and Cx. quinquefasciatus (Table 1). The Cx. tritaeniorhynchus colony was maintained at 28°C and 55–60% relative humidity, with an 11/13-h light/dark cycle. Ae. albopictus, Cx. pipiens form molestus, and Cx. quinquefasciatus were maintained at 25°C and 55–60% relative humidity, with a 16/8-h light/dark cycle. All colonies were provided ad libitum access to a 3% sucrose solution in cotton pledgets.
Table 1.
Laboratory-established mosquito colonies used in the current study.
| Mosquito species | Colony name | Year of colonization | Source of colony | Generation | Initial JEV titre (in log10 FFU/mL) |
|---|---|---|---|---|---|
| Cx. tritaeniorhynchus | Yasugi | 2018 | Shimane Prefecture, Japan | F30 | 7.32 |
| Ae. albopictus | NMT | 2019 | Gunma Prefecture, Japan | F14 | 6.57 |
| Cx. pipiens form molestus | – | 2023 | Turkey | F6 | 5.66 |
| Cx. quinquefasciatus | N4-11 | 2023 | Hanoi, Vietnam | F4 | 6.78 |
Virus strain used and its preparation
JEV GIV strain 19CxBa-83-Cv (GenBank accession no. LC579814), isolated from Culex vishnui which were collected in Bali Province [38], was used in the study. The virus was propagated in Ae. albopictus-derived C6/36 cells (American Type Culture Collection (ATCC)) three times, then passed once on Vero cells (ATCC) to exclude insect-specific viruses, and finally propagated in C6/36 cells prior to infection experiments. The virus was grown in C6/36 cells in 5 mL of a culture medium consisting of Eagle’s Minimum Essential Medium (MEM) (Sigma-Aldrich, St. Louis, MO, USA), with 2% fetal bovine serum (FBS) (Sigma-Aldrich) at 28°C and 5% CO2 for 3–4 days in T25 flasks. Light microscopy was used to determine the infection in the cell monolayer and evaluate the cytopathic effect for harvesting. Cell suspensions were centrifuged at 10,000g for 10 min at 5°C, and only supernatants were collected to exclude remaining dead cells. The supernatants were aliquoted for virus quantification by either a plaque assay or a focus-forming assay and finally used for in vivo infection experiments.
Vector competence analysis
Adult females from the four colonies were tested for vector competence by exposing them to 3 mL of an infectious blood meal consisting of a JEV GIV supernatant and defibrinated rabbit blood (Nippon Bio-Supp. Center, Tokyo, Japan), supplemented with adenosine triphosphate (final concentration 3 mM), following previous studies [26,39]. Mosquitoes were exposed to the infectious blood meal through a Hemotek membrane feeding system (Hemotek Ltd Accrington, Lancashire, UK). Seven- to 12-day-old adult female mosquitoes were first starved of sucrose overnight (12 ± 2 h) and then allowed to feed on the next day in a lighted (Ae. albopictus) or dark (Cx. tritaeniorhynchus, Cx. pipiens form molestus, and Cx. quinquefasciatus) room for 1 h at room temperature (RT). The virus titres in the blood meal (log10 PFU/mL) are provided in Table 1. Only fully fed individuals were anaesthetized using CO2 and randomly divided into two groups, which were incubated at 28°C and processed either 7 or 14 days postexposure (dpe). At the harvesting time points (7 or 14 dpe), mosquitoes were immobilized again with CO2, and their legs and wings were removed, saliva samples collected, and the head dissected to be combined with the leg and wing samples. Saliva was collected by inserting the proboscis into a micropipette tip containing 10 μL of FBS for 50 ± 5 min and later diluted with 100 μL of MEM containing 2% FBS, 2% penicillin–streptomycin, and 2% amphotericin B for storage. All harvested body parts and saliva were stored at −80°C for downstream analysis. Infection experiments of these Culex and Aedes mosquitoes were conducted in a biosafety level 2 laboratory within insectaries at the National Institute of Infectious Diseases, Japan.
Molecular detection of JEV GIV RNA and virus quantification
RNA was extracted from samples of body parts using the Nucleospin RNA kit (Takara Bio, Shiga, Japan) following the manufacturer’s protocol, with a slight modification. Body parts were initially homogenized in RA1 buffer containing 1% mercaptoethanol using a TissueLyser II (QIAGEN, Venlo, Netherlands) at a frequency of 25 Hz/s for 30 s; the homogenate was centrifuged at 12,000 rpm for 3 min, filtered, and treated with DNase. The final RNA was eluted with 60 μL of RNase-free dH2O.
Real-time quantitative reverse transcription–PCR (RT–qPCR) was employed to detect JEV GIV RNA in samples. Standards for RT–qPCR were constructed following a previous study [40]. In brief, a viral RNA standard for JEV GIV was prepared by reverse transcription PCR (RT–PCR) amplification of a partial sequence from NS2A region using specific primers. Subsequent PCRs added a T7 promoter sequence for RNA synthesis. The resulting PCR products were transcribed in vitro to generate RNA transcripts, which were then purified and quantified to determine the copy number of the RNA standard. JEV GIV strain 19CxBa-83-Cv was used as the template for designing outer primers, while inner primers and probe were based on a previous study [41]. Primers and probe for RT–qPCR were provided in Table S1.
The copy number of viral genomic RNA in JEV GIV-infected individuals was determined through RNA extraction and real-time qPCR targeting a specific region in NS2A. The reaction mixture consisted of 0.9 μL of JEV GIV inner primers (forward and reverse, 10 μM each), 0.25 μL of a probe (10 μM), 2.5 μL of the TaqMan Fast Virus 1-Step master mix (Thermo Fisher Scientific), 1 μL of an RNA sample, and 4.45 μL of RNase-free water to a total volume of 10 μL. The cycling conditions were as follows: the RT step at 50°C for 5 min, followed by denaturation at 95°C for 20 s and 35 cycles at 95°C for 3 s and 60°C for 30 s. The results were analysed in duplicate using the PikoReal software version 2.2 (Thermo Fisher Scientific), and samples were considered positive for JEV GIV RNA at a cycle threshold (Ct) value of 32 or lower. The cut-off value was chosen based on serial dilution results, which showed inconsistent detection of the target RNA above this threshold, ensuring reliable detection while minimizing false positives. JEV GIV RNA detection in the mosquito abdomen and thorax indicated infection, whereas the presence of the virus in the mosquito head, wings, and legs of infected individuals indicated disseminated infection.
An aliquot of a diluted saliva sample (20 μL) was inoculated to Vero cell cultures in 24-well plates containing 300 μL/well of MEM supplemented with 2% FBS, 2% penicillin–streptomycin, and 2% amphotericin B. After 1-h incubation at 37°C, 500 μL of the same medium was added to each well. Cells that showed cytopathic effects within 7 days postinoculation were considered infectious or able to transmit the virus. Selected samples were further analysed by a plaque assay or RT–PCR following a previous method [26,42]. A JEV universal primer set (forward: 5′-GCYGARCAGAAYCAATGGAGC-3′ and reverse: 5′-GCTCCWAGCCACATGAACCA-3′) and the following PCR conditions were used: 50°C for 30 s, followed by 94°C for 2 min and 35 cycles at 94°C for 15 s, 53°C for 15 s, and 72°C for 30 s.
Vero cells were used for viral quantification by plaque and focus-forming assays. The cells were seeded in 12-well plates at a concentration of 2 × 105 cells/well. An aliquot of a diluted saliva sample (50 μL) was added with 200 μL of MEM and further serially diluted to 10−1 and 10−2, with 200 μL of each dilution added per well. After incubation for 1 h at 37°C, the inoculum was removed; 1 mL of MEM supplemented with 1% methylcellulose was added to each well, and incubation continued at 37°C for 3 days. Subsequent fixing and staining steps were different for the plaque and focus-forming assays.
In the plaque assay, cells were fixed by adding a 10% neutral buffered formalin solution (Fujifilm Wako Pure Chemical Industries, Ltd., Tokyo, Japan), followed by incubation at RT for 2 h. After the fixation step, the cells were washed with running water, and 500 μL of 1× methylene blue (Fujifilm Wako Pure Chemical Industries, Ltd.) was added. The stain was removed after 2 h of incubation at RT, and the resulting viral titre was determined as log10 PFU/mL.
In the focus-forming assay, cells were fixed with 2.6% paraformaldehyde in each well, followed by incubation at RT for 20 min, a subsequent PBS wash, and additional 20-min incubation with 0.1% Triton X-100 at RT. Following the fixation step, the cells were washed with PBS and then incubated for 1 h at 37°C with a mouse monoclonal anti-flavivirus group antigen antibody derived from hybridoma cells (D1-4G2-4-15, ATCC HB-112), followed by a 30-min incubation at RT with a secondary antibody (Dako Envision+ System HRP-labeled polymer; Agilent Technologies, Santa Clara, CA) on a slow-speed laboratory shaker. Lastly, a substrate (Dab+Chromosome substrate kit; Agilent Technologies) was introduced, and the resulting viral titre was determined as log10 FFU/mL.
Statistical analysis
Graphs were created using GraphPad version 9.5.1 (GraphPad Software, San Diego, CA, USA). Statistical analysis was performed using the GraphPad Software or R software (ver. 4.4.0). One-way ANOVA and the nonparametric Kruskal–Wallis test for multiple comparison, corrected with the Dunn test, were employed to compare the viral loads between colonies at each collection time point and between two collection time points for each colony. Two-tailed Fisher’s exact test on the GraphPad website (https://www.graphpad.com/quickcalcs/contingency1/) was applied to determine the significance of differences between colonies in their infection rates (IRs; the number of JEV-positive thorax/abdomen samples per total number of tested mosquitoes), dissemination rates (DRs; the number of JEV-positive head/wing/leg samples per total number of infected mosquitoes), and transmission efficiency rates (TEs; the number of JEV-positive saliva samples per total number of tested samples).
Results
Cx. tritaeniorhynchus
The Cx. tritaeniorhynchus colony presented 98% (46/47) and 95% (62/65) IRs at 7 and 14 dpe, respectively, with DRs of 98% (45/46) and 100% (62/62) at 7 and 14 dpe, respectively (Table 2). The TE was 40% (19/47) at the earlier time point, increasing to 74% (48/65) at the later time point.
Table 2.
Results of the infection (IR), dissemination (DR), and transmission rates (TR) in JEV GIV-exposed mosquito colonies.
| Colony name | Total number processed | Collection time pointsa | |||||
|---|---|---|---|---|---|---|---|
| 7 dpe | 14 dpe | ||||||
| IR | DR | TE | IR | DR | TE | ||
| Cx. tritaeniorhynchus | 112 | 46/47 (98, 89–99)a | 45/46 (98, 88–99)a | 19/47 (40, 26–55)a | 62/65 (95, 87–99)a | 62/62 (100, 94–100)a | 48/65 (74, 61–83)a |
| Ae. albopictus | 49 | 13/24 (54, 33–74)bc | 12/13 (92, 63–100)ab | 5/24 (21, 7–42)ab | 20/25 (80, 59–93)b | 20/20 (100, 83–100)a | 13/25 (52, 31–72)ac |
| Cx. pipiens form molestus | 42 | 5/20 (25, 9–49)b | 3/5 (60, 15–95)b | 2/20 (10, 1–31)b | 10/22 (45, 24–68)c | 6/10 (60, 26–88)b | 4/22 (18, 5–40)b |
| Cx. quinquefasciatus | 64 | 22/30 (73, 54–88)c | 14/22 (63, 41–83)b | 8/30 (27, 12–45)ab | 16/34 (47, 30–65)c | 12/16 (75, 48–93)b | 12/34 (35, 19–53)bc |
IR/Infection rate, DR/Dissemination rate, TE/Transmission efficiency. Rate (IR, DR, or TE) and 95% confidence interval (value, LCI-UCI) are provided. Two-tailed Fisher’s exact test was applied to compare colonies in each evaluation point (IR, DR or TE) in respective time points. Statistical significance between colonies is represented by letters (a, b, or c). Colonies that do not share the same letter in a column demonstrate significant differences (p-value < .05), which were provided in Table S2.
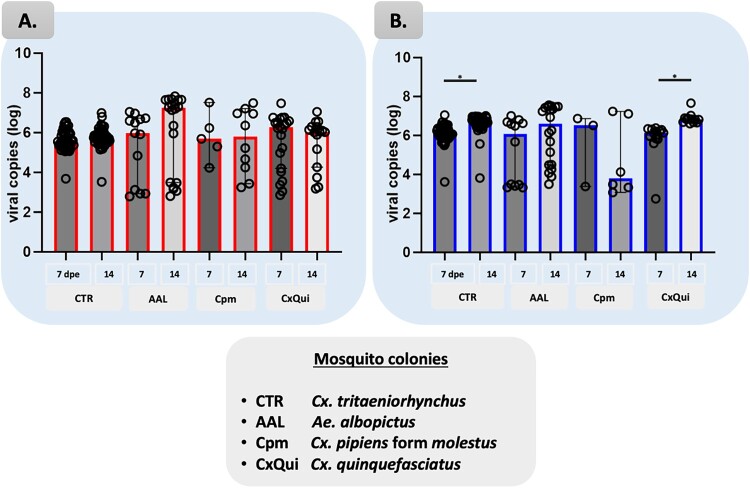
The viral load in the abdomen/thorax samples of Cx. tritaeniorhynchus clustered between 5.00 to 7.00 log10 RNA viral copies/μL with a few samples showing levels as low as 3.52 log10 RNA viral copies/μL (Figure 2, Table S5). The median RNA copies of JEV GIV in the abdomen–thorax were 5.54 and 5.69 log10 RNA viral copies/μL at 7 and 14 dpe, respectively (Table S3). The medians of RNA copies in the head–wings–legs were higher than those in the abdomen–thorax, measuring 6.13 and 6.68 log10 RNA viral copies/μL at 7 and 14 dpe, respectively. Multiple comparisons of proportions using Kruskal–Wallis tests showed significant difference (p < .05 between two collection time points, with 14 dpe being higher when compared to 7 dpe (Figure 2(B)).
Figure 2.
Viral load comparisons between all four colonies based on the RT–qPCR detection in abdomen–thorax (A) and head–wings–legs (B). Bars represent median with 95% confidence interval (CI). Multiple comparisons of proportions using One-way ANOVA non parametric Kruskal–Wallis corrected with Dunn test were applied. Significant differences are shown; *p < .01.
Virus titres, quantified using the plaque assay, ranged between 3.00 and 4.22 log10 PFU/mL at both 7 and 14 dpe (Fig S1, detailed in Table S4). When using the focus-forming assay, virus titres ranged from 2.70 to 5.85 log10 FFU/mL at 7 dpe (Table S4). The median viral titre from both methods were comparable, at 3.57 log10 PFU/mL and 3.74 log10 FFU/mL at 7 dpe. The only sample at 14 dpe exhibited a slightly higher viral titre in saliva, at 4.22 log10 PFU/mL, compared to the earlier time point.
Ae. albopictus
The Ae. albopictus colony presented 54% (13/24) and 80% (20/25) IRs at 7 and 14 dpe, respectively, with DRs of 92% (12/13) and 100% (20/20) at 7 and 14 dpe, respectively (Table 2). Transmissibility was observed at both time points, with 21% (5/24) and 52% (13/25) at 7 and 14 dpe, respectively.
The viral load in the abdomen/thorax and head–wings–legs samples of Ae. albopictus varied considerably (Figure 2). In the abdomen/thorax samples, median RNA copies were 5.97 and 7.23 log10 RNA viral copies/μL at 7 and 14 dpe, respectively (Table S3). For the head–wings–legs samples, median RNA copies were 6.08 and 6.59 log10 RNA viral copies/μL at 7 and 14 dpe, respectively. The observed fold increase between the two time points ranged from 100 to 10,000-fold, indicating an increase over time. Virus titres in harvested saliva, quantified using the plaque assay, were 3.70 and 5.54 log10 PFU/mL at 7 dpe, and 4.40 log10 PFU/mL at 14 dpe (Table S4).
Cx. pipiens form molestus
The Cx. pipiens form molestus colony presented IRs of 25% (5/20) and 45% (10/22), and DRs of 60% (3/5 and 6/10) at both collection time points. The TEs were 10% (2/20) at 7 dpe and 18% (4/22) at 14 dpe.
The viral load in the abdomen/thorax samples of Cx. pipiens form molestus varied, with some samples showing titres above 5.00 log10 RNA viral copies/μL at both collection time points (Figure 2(A), Table S5). In the head–wings–legs samples, there was a clear distinction between high (>5 log10) and low (<5 log10) titres at both collection time points (Figure 2(B)).
In the abdomen/thorax samples, median RNA copies were 5.69 and 5.79 log10 RNA viral copies/μL at 7 and 14 dpe, respectively (Table S3). In the head–wings–legs samples, median RNA copies were 6.51 and 3.81 log10 RNA viral copies/μL at 7 and 14 dpe, respectively. Virus titres in harvested saliva, quantified using the plaque assay, were 4.06 and 4.19 log10 PFU/mL at 7 and 14 dpe, respectively (Table S4).
Cx. quinquefasciatus
The Cx. quinquefasciatus colony presented IRs of 73% (22/30) and 47% (16/34), and DRs of 64% (14/22) and 75% (12/16) at 7 and 14 dpe, respectively. The TEs were 27% (8/30) at 7 dpe and 35% (12/34) at 14 dpe.
The viral load in the abdomen/thorax samples of Cx. quinquefasciatus varied widely, with titres ranging from 2.85 to 7.47 log10 RNA viral copies/μL at both collection time points (Figure 2(A), Table S5). In the head–wings–legs samples, a clear distinction between high (>5 log10) and low (<3 log10) titres could be observed (Figure 2(B)). Multiple comparisons of proportions using Kruskal–Wallis tests showed significant difference (p < .01) between 7 and 14 dpe.
The median RNA copies in the abdomen/thorax samples were 6.27 and 6.017 log10 RNA viral copies/μL at 7 and 14 dpe, respectively (Table S3). In the head–wings–legs samples, median RNA copies were 6.12 and 6.81 log10 RNA viral copies/μL at 7 and 14 dpe, respectively. Virus titres of harvested saliva were 3.72 and 4.20 log10 PFU/mL at 7 and 14 dpe, respectively, reflecting an overall increase over time (Table S4).
Vector competence analysis between colonies tested
The Cx. tritaeniorhynchus colony exhibited the highest susceptibility to the virus among the colonies or species investigated in this study. The IRs were greater than or equal to 95% at both 7 and 14 dpe, demonstrating significant differences with the other three colonies (p < .05), as indicated in Table 2 and Table S2. Ae. albopictus and Cx. quinquefasciatus displayed relatively moderate IRs at both collection time points, ranging from 47% to 80%. Meanwhile, Cx. pipiens form molestus exhibited a low IR at 7 dpe, 25%, with significant differences from other colonies tested. At 14 dpe, however, the IR was comparable to Cx. quinquefasciatus.
Meanwhile, DRs of Cx. pipiens form molestus and Cx. quinquefasciatus demonstrated somewhat lower at 14 dpe, ranging from 65% to 75%, with significant differences observed between the fully disseminated group (Cx. tritaeniorhynchus–Ae. albopictus) and moderately disseminated group (Cx. pipiens form molestus–Cx. quinquefasciatus) (Table S2).
All colonies showed transmissibility at both time points, with obvious increases by 14 dpe (Table 2). At 14 dpe, the highest TE was demonstrated by Cx. tritaeniorhynchus (74%), followed by Ae. albopictus (52%), Cx. quinquefasciatus (35%), and Cx. pipiens form molestus (18%). At this collection time point, Cx. tritaeniorhynchus showed a significantly higher TE than Cx. pipiens form molestus and Cx. quinquefasciatus (p < .0005), but not Ae. albopictus (p = .07), while Ae. albopictus exhibited a significantly higher TE than Cx. pipiens form molestus (p < .05), but not Cx. quinquefasciatus (p-value = .28) (Table S2). These data indicated comparable TEs between Cx. tritaeniorhynchus and Ae. albopictus, as well as between Ae. albopictus and Cx. quinquefasciatus.
Viral loads and titres of JEV GIV recovered from specimens
The viral load results for the abdomen/thorax samples exhibited non-significant differences between colonies tested (Figure 2(A)). In contrast, the viral load results for the head/wings/legs revealed statistically significant differences between two collection time points in Cx. tritaeniorhynchus and Cx. quinquefasciatus colonies, primarily indicating a propagation.
The median RNA copies showed consistent results, with the Cx. tritaeniorhynchus and Cx. quinquefasciatus values above the average, particularly at 14 dpe (Table S3). Virus titres of harvested saliva exhibited non-significant differences among the colonies (Fig. S1, S2, detailed in Table S4).
Discussion
JEV GIV was first identified in mosquitoes collected from several areas on Indonesian islands between 1980 and 1981 [19]; later, it was isolated in Bali from pigs in 2017 [43], from Cx. vishnui in 2019 [38], and from an Australian tourist in 2019 [21]. JEV GIV was considered confined to Indonesia and was the least frequently observed genotype, until recent outbreaks in Australia, which reported the first animal and human cases of JEV GIV outside endemic areas [44]. While primary Asian JEV vectors, such as Cx. tritaeniorhynchus and Cx. vishnui, are absent from or limited in Australia, several other species are acknowledged as competent JEV vectors, including Cx. annulirostris, Culex sitiens, and Cx. quinquefasciatus [20,30]. While the entry point and the spread mechanism of JEV GIV in Australia remain uncertain, specific ecological contexts may enhance the role of particular species as vectors [20,30]. This study therefore aimed to examine the vector status of specific local mosquito species susceptible to JEV GIV, leading to the discovery that four mosquito species exhibited susceptibility to GIV.
Our findings revealed that Ae. albopictus and Cx. quinquefasciatus were nearly comparable to Cx. tritaeniorhynchus, which is considered the primary JEV vector in Asia. Additionally, the JEV GIV titres in saliva from all tested colonies, including Cx. pipiens form molestus, were similar at both collection time points, suggesting the potential for JEV transmission. The high susceptibility and transmission exhibited by Cx. tritaeniorhynchus in the current study align with the results of previous studies, which indicated its high susceptibility to JEV GI and GIII [23,26,45–47]. Our observations confirmed complete infection or dissemination (∼100%) in all individuals tested, similar to the results of many studies [26,45,47].
The presumed ability of Ae. albopictus to transmit JEV has long been supported by virus isolations from wild-caught mosquitoes [48–50] and by established vector competence in laboratory experiments [51–54]. Our findings in a Japanese population of Ae. albopictus align with the results from a population in France, which demonstrated a susceptibility of approximately 100% and a transmissibility of JEV GIII and GV of approximately 63% [52]. This implies an efficient transmission ability of Ae. albopictus for JEV, irrespective of the genotype.
The importance of Cx. quinquefasciatus as a JEV vector is supported by the number of virus isolations from wild-caught mosquitoes [50,55] and by proven vector competence studies [28,56–59]. This study affirms that a Cx. quinquefasciatus colony originated from Hanoi, Vietnam, could be infected by JEV and transmit the virus at a relatively moderate level, consistent with findings from other studies [28,56]. Furthermore, the density and longevity of Cx. pipiens form molestus in urban areas are considered additional threats if the mosquitoes can transmit JEV. Previous studies on vector competence in laboratory colonies of Cx. pipiens form molestus yielded varied results. One study indicated a high competence (80–100% transmission) in Cx. pipiens form molestus populations from Taiwan [60], while another study reported a low transmission level (8%) in populations from Uzbekistan [61]. A North American population showed moderate transmission (27%) [59], corresponding to our results (18%) obtained using a Cx. pipiens form molestus colony originated from Turkey. Notwithstanding, the titre of blood meals presented to Cx. pipiens form molestus is notably lower than those presented to the other three species. This discrepancy could potentially trigger a dose–response effect, leading to a lower likelihood of passing the midgut escape barrier and underestimation of the dissemination rate during the earlier time point (7 dpe), as observed in prior studies [62,63].
Other limitations of this study are that only one strain of JEV GIV and one colony of each mosquito species were used in the experiments and that the initial titres in the blood meal vary, which could influence the outcome of the study. Previous studies have reported that mosquito susceptibility to different viral strains could vary [64], the use of different colonies from the same mosquito species could show different results [23,51], and initial dose may impact the ability to overcome midgut escape barriers [65–67]. Therefore, we expect that future studies using more strains/isolates and various colonies of the same mosquito species, other than those used in this study, may demonstrate more detailed differences in the susceptibility to JEV GIV.
In addition, the differing lengths of laboratory colonization among the four laboratory colonies used Cx. tritaeniorhynchus (F30), Ae. albopictus (F14), Cx. pipiens form molestus (F6), and Cx. quinquefasciatus (F4) could have influenced the observed variations in viral titres. Lorenz et al. [68] highlighted how colonization can impact genetic variation within mosquito populations, thereby affecting their susceptibility to arbovirus infections. In our study, the later generations, representing more adapted colonies, showed a more distinct separation between high and low viral titres in abdomen–thorax samples. On the other hand, the earlier generations (Cx. pipiens form molestus and Cx. quinquefasciatus) exhibited less pronounced clustering of viral titre levels, although this pattern was not reflected in the head–wings–legs samples.
Increased virus titres in dissemination samples (head–wings–legs) over infection (abdomen–thorax) were observed in this study, particularly Cx. tritaeniorhynchus samples at both 7 and 14 dpe and Cx. quinquefasciatus at 14 dpe (Fig S2). This observation has also been observed elsewhere [26,65,69–74] but the mechanism behind remains unexplored. Possible explanations include the attribution of viral replication and dissemination dynamics within the host tissues, as was pointed out in Kramer et al. [63] and Salazar et al. [75] where viral titres in mosquito remnants being higher compared to the viral titre in the midgut as virus disseminated out of the midgut and into the legs and salivary glands. Additionally, host immune responses that affect the viral genomic RNA which appear to be more active in the midgut than in the carcass and become increasingly active over time, may contribute to viral load distribution within the host. Notably, this host immune response seems to be varied between mosquitoes [65,69].
Nevertheless, the concept of the vectorial capacity goes beyond the direct biological interaction between a virus and a mosquito [76], and vector competence analysis is just one component shaping the overall vectorial capacity. Various factors, such as mosquito longevity, the age of infection acquisition, and blood-feeding preferences, can markedly influence the vectorial capacity [76,77]. Additionally, the temperature employed in experiments can impact vector competence results [58,78–80]. Specifically, when using a relatively high temperature, such as 28°C in the current experiments, the vector competence analysis results may be elevated.
In conclusion, our first analysis of vector competence in transmitting JEV GIV emphasizes that all tested colonies were susceptible to the infection and capable of transmitting the virus. Japanese Ae. albopictus, Vietnamese Cx. quinquefasciatus, and Turkish Cx. pipiens form molestus exhibited competence nearly comparable to that of Japanese Cx. tritaeniorhynchus. These results suggest a potential risk for sporadic cases in the future if an exotic genotype spreads through initial introduction to new areas. Additionally, the role of other Culex species as vectors for JEV GIV should not be overlooked. In Australia, there is a proposition that Cx. annulirostris could assume this role [20,81]. Finally, the findings contribute to the understanding of JEV transmission dynamics and highlight the importance of considering various mosquito species in the surveillance and control of JEV outbreaks, particularly in regions with a history of JEV circulation.
Supplementary Material
Acknowledgements
This study was partially supported by Japan International Cooperation Agency (JICA) and Japan Agency for Medical Research and Development (AMED) on Science and Technology Research Partnership for Sustainable Development (SATREPS) project entitled One Health approach to control of Neglected Tropical Diseases with special attention on sandfly and mosquito borne infections in Turkey.
Funding Statement
This work was funded by the Japan Agency for Medical Research and Development (AMED) on SATREPS project [grant number 21jm0110024], Research Program on Emerging and Re-emerging Infectious Diseases [grant numbers JP21fk0108613, JP24fk0108693, JP20fk0108123, JP23fk0108656], Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) [grant numbers JP20wm0225007, JP23wm0225030], and Research Project for Promoting Solutions to Global Health Issues [grant number 24jk0210051j0001].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Fischer M, Hills S, Staples E, et al. Japanese encephalitis prevention and control: advances, challenges, and new initiatives. In: Michael Scheld W, Hammer SM, Hughes JM, editors. Emerging infections 8. Washington (DC: ): ASM Press; 2014. p. 93–124. [Google Scholar]
- 2.Walker PJ, Siddell SG, Lefkowitz EJ, et al. Recent changes to virus taxonomy ratified by the International Committee on Taxonomy of Viruses. Arch Virol. 2022;167(11):2429–2440. doi: 10.1007/s00705-022-05516-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollidge BS, González-Scarano F, Soldan SS.. Arboviral encephalitides: transmission, emergence, and pathogenesis. J Neuroimmune Pharmacol. 2010;5:428–442. doi: 10.1007/s11481-010-9234-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halstead SB, Jacobson J.. Japanese encephalitis. Adv Virus Res. 2003;61:103–138. doi: 10.1016/S0065-3527(03)61003-1 [DOI] [PubMed] [Google Scholar]
- 5.Campbell G, Hills S, Fischer M, et al. Estimated global incidence of Japanese encephalitis. Bull World Health Organ. 2011;89:766–774. doi: 10.2471/BLT.10.085233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erlanger TE, Weiss S, Keiser J, et al. Past, present, and future of Japanese encephalitis. Emerg Infect Dis. 2009;15:1–7. doi: 10.3201/eid1501.080311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomon T, Ni H, Beasley DWC, et al. Origin and evolution of Japanese encephalitis virus in Southeast Asia. J Virol. 2023;77:3091–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGuinness SL, Muhi S, Britton PN, et al. Japanese encephalitis: emergence in Australia. Curr Infect Dis Rep. 2023;25:111–122. doi: 10.1007/s11908-023-00804-w [DOI] [Google Scholar]
- 9.Simon-Loriere E, Faye O, Prot M, et al. Autochthonous Japanese encephalitis with yellow fever coinfection in Africa. N Engl J Med. 2017;376:1483–1485. doi: 10.1056/NEJMc1701600 [DOI] [PubMed] [Google Scholar]
- 10.Sumiyoshi H, Mori C, Fuke I, et al. Complete nucleotide sequence of the Japanese encephalitis virus genome RNA. Virology. 1987;161:497–510. doi: 10.1016/0042-6822(87)90144-9 [DOI] [PubMed] [Google Scholar]
- 11.Xu G, Gao T, Wang Z, et al. Re-emerged genotype IV of Japanese encephalitis virus is the youngest virus in evolution. Viruses. 2023;15:626. doi: 10.3390/v15030626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuh AJ, Ward MJ, Leigh Brown AJ, et al. Phylogeography of Japanese encephalitis virus: genotype is associated with climate. PLoS Negl Trop Dis. 2013;7:e2411. doi: 10.1371/journal.pntd.0002411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuh AJ, Ward MJ, Leigh Brown AJ, et al. Dynamics of the emergence and establishment of a newly dominant genotype of Japanese encephalitis virus throughout Asia. J Virol. 2014;88:4522–4532. doi: 10.1128/JVI.02686-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuh AJ, Li L, Tesh RB, et al. Genetic characterization of early isolates of Japanese encephalitis virus: genotype II has been circulating since at least 1951. J Gen Virol. 2010;91:95–102. doi: 10.1099/vir.0.013631-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H, Cha G-W, Jeong YE, et al. Detection of Japanese encephalitis virus genotype V in Culex orientalis and Culex pipiens (Diptera: Culicidae) in Korea. PLoS One. 2015;10:e0116547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee A-R, Song JM, Seo S-U.. Emerging Japanese encephalitis virus genotype V in Republic of Korea. J Microbiol Biotechnol. 2022;32:955–959. doi: 10.4014/jmb.2207.07002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanborn MA, Wuertz KM, Kim H-C, et al. Metagenomic analysis reveals Culex mosquito virome diversity and Japanese encephalitis genotype V in the Republic of Korea. Mol Ecol. 2021;30:5470–5487. doi: 10.1111/mec.16133 [DOI] [PubMed] [Google Scholar]
- 18.Takhampunya R, Kim H-C, Tippayachai B, et al. Emergence of Japanese encephalitis virus genotype V in the Republic of Korea. Virol J. 2011;8:449. doi: 10.1186/1743-422X-8-449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuh AJ, Guzman H, Tesh RB, et al. Genetic diversity of Japanese encephalitis virus isolates obtained from the Indonesian archipelago between 1974 and 1987. Vector-Borne Zoonotic Dis. 2013;13:479–488. doi: 10.1089/vbz.2011.0870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van den Hurk AF, Skinner E, Ritchie SA, et al. The emergence of Japanese encephalitis virus in Australia in 2022: existing knowledge of mosquito vectors. Viruses. 2022;14:1208. doi: 10.3390/v14061208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pyke AT, Choong K, Moore F, et al. A case of Japanese encephalitis with a fatal outcome in an Australian who traveled from Bali in 2019. Trop Med Infect Dis. 2020;5:133. doi: 10.3390/tropicalmed5030133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mourya DT, Mishra AC, Soman RS.. Transmission of Japanese encephalitis virus in Culex pseudovishnui & C. tritaeniorhynchus mosquitoes. Indian J Med Res. 1991;93:250–252. [PubMed] [Google Scholar]
- 23.Takahashi M. Differential transmission efficiency for Japanese encephalitis virus among colonized strains of Culex tritaeniorhynchus. Med Entomol Zool. 1982;33:325–333. doi: 10.7601/mez.33.325 [DOI] [Google Scholar]
- 24.Phillips D, Poidinger M, Mackenzie J, et al. Isolation of Japanese encephalitis virus from Culex annulirostris in Australia. Am J Trop Med Hyg. 1997;56:80–84. doi: 10.4269/ajtmh.1997.56.80 [DOI] [PubMed] [Google Scholar]
- 25.Auerswald H, Maquart P-O, Chevalier V, et al. Mosquito vector competence for Japanese encephalitis virus. Viruses. 2021;13:1154. doi: 10.3390/v13061154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faizah AN, Kobayashi D, Amoa-Bosompem M, et al. Evaluating the competence of the primary vector, Culex tritaeniorhynchus, and the invasive mosquito species, Aedes japonicus japonicus, in transmitting three Japanese encephalitis virus genotypes. PLoS Negl Trop Dis. 2020;14:e0008986. doi: 10.1371/journal.pntd.0008986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hameed M, Liu K, Anwar MN, et al. The emerged genotype I of Japanese encephalitis virus shows an infectivity similar to genotype III in Culex pipiens mosquitoes from China. PLoS Negl Trop Dis. 2019;13:e0007716. doi: 10.1371/journal.pntd.0007716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karna AK, Bowen RA.. Experimental evaluation of the role of ecologically-relevant hosts and vectors in Japanese encephalitis virus genotype displacement. Viruses. 2019;11:32. doi: 10.3390/v11010032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han N, Adams J, Chen P, et al. Comparison of genotypes I and III in Japanese encephalitis virus reveals distinct differences in their genetic and host diversity. J Virol. 2014;88:11469–11479. doi: 10.1128/JVI.02050-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackenzie JS, Williams DT, van den Hurk AF, et al. Japanese encephalitis virus: the emergence of genotype IV in Australia and its potential endemicity. Viruses. 2022;14:2480. doi: 10.3390/v14112480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thenmozhi V, Paramasivan R, Samuel PP, et al. Japanese encephalitis virus isolation from mosquitoes during an outbreak in 2011 in Alappuzha district, Kerala. J Vector Borne Dis. 2013;50:229–231. Available from: https://journals.lww.com/jvbd/fulltext/2013/50030/japanese_encephalitis_virus_isolation_from.12.aspx [PubMed] [Google Scholar]
- 32.Liu W, Fu S, Ma X, et al. An outbreak of Japanese encephalitis caused by genotype Ib Japanese encephalitis virus in China, 2018: a laboratory and field investigation. PLoS Negl Trop Dis. 2020;14:e0008312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tao Z, Liu G, Wang M, et al. Molecular epidemiology of Japanese encephalitis virus in mosquitoes during an outbreak in China, 2013. Sci Rep. 2014;4:4908. doi: 10.1038/srep04908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulvey P, Duong V, Boyer S, et al. The ecology and evolution of Japanese encephalitis virus. Pathogens. 2021;10:1534. doi: 10.3390/pathogens10121534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Y-JS, Hettenbach SM, Park SL, et al. Differential infectivities among different Japanese encephalitis virus genotypes in Culex quinquefasciatus mosquitoes. PLoS Negl Trop Dis. 2016;10:e0005038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benedict MQ, Levine RS, Hawley WA, et al. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector-Borne Zoonotic Dis. 2007;7:76–85. doi: 10.1089/vbz.2006.0562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Longbottom J, Walekhwa AW, Mwingira V, et al. Aedes albopictus invasion across Africa: the time is now for cross-country collaboration and control. Lancet Glob Health. 2023;11:e623–e628. doi: 10.1016/S2214-109X(23)00046-3 [DOI] [PubMed] [Google Scholar]
- 38.Faizah AN, Kobayashi D, Maekawa Y, et al. Identification and isolation of Japanese encephalitis virus genotype IV from Culex vishnui collected in Bali, Indonesia in 2019. Am J Trop Med Hyg. 2021;105:813–817. doi: 10.4269/ajtmh.20-1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobayashi D, Kai I, Faizah AN, et al. Comparative analysis of the susceptibility of Aedes aegypti and Japanese Aedes albopictus to all dengue virus serotypes. Trop Med Health. 2023;51:61. doi: 10.1186/s41182-023-00553-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ejiri H, Kuwata R, Tsuda Y, et al. First isolation and characterization of a mosquito-borne orbivirus belonging to the species Umatilla virus in East Asia. Arch Virol. 2014;159:2675–2685. doi: 10.1007/s00705-014-2117-0 [DOI] [PubMed] [Google Scholar]
- 41.Bharucha T, Sengvilaipaseuth O, Vongsouvath M, et al. Development of an improved RT-qPCR assay for detection of Japanese encephalitis virus (JEV) RNA including a systematic review and comprehensive comparison with published methods. PLoS One. 2018;13:e0194412. doi: 10.1371/journal.pone.0194412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azerigyik FA, Faizah AN, Kobayashi D, et al. Evaluating the mosquito host range of Getah virus and the vector competence of selected medically important mosquitoes in Getah virus transmission. Parasit Vectors. 2023;16:99. doi: 10.1186/s13071-023-05713-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuwata R, Torii S, Shimoda H, et al. Distribution of Japanese encephalitis virus, Japan and Southeast Asia, 2016–2018. Emerg Infect Dis. 2020;26:125–128. doi: 10.3201/eid2601.190235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waller C, Tiemensma M, Currie BJ, et al. Japanese encephalitis in Australia—a sentinel case. N Engl J Med. 2022;387:661–662. doi: 10.1056/NEJMc2207004 [DOI] [PubMed] [Google Scholar]
- 45.Doi R, Shirasaka A, Sasa M, et al. Studies on the susceptibility of three species of mosquitoes to Japanese encephalitis virus. J Med Entomol. 1977;13:591–594. doi: 10.1093/jmedent/13.4-5.591 [DOI] [PubMed] [Google Scholar]
- 46.Gresser I, Hardy JL, Hu SMK, et al. Factors influencing transmission of Japanese B encephalitis virus by a colonized strain of Culex tritaeniorhynchus Giles, from infected pigs and chicks to susceptible pigs and birds. Am J Trop Med Hyg. 1958;7:365–373. doi: 10.4269/ajtmh.1958.7.365 [DOI] [PubMed] [Google Scholar]
- 47.Turell MJ, Mores CN, Dohm DJ, et al. Laboratory transmission of Japanese encephalitis, West Nile, and Getah viruses by mosquitoes (Diptera: Culicidae) collected near camp greaves, Gyeonggi Province, Republic of Korea, 2003. J Med Entomol. 2006;43:1076–1081. doi: 10.1093/jmedent/43.5.1076 [DOI] [PubMed] [Google Scholar]
- 48.Vythilingam I, Oda K, Chew TK, et al. Isolation of Japanese encephalitis virus from mosquitoes collected in Sabak Bernam, Selangor, Malaysia in 1992. J Am Mosq Control Assoc. 1995;11:94–98. [PubMed] [Google Scholar]
- 49.Su C-L, Yang C-F, Teng H-J, et al. Molecular epidemiology of Japanese encephalitis virus in mosquitoes in Taiwan during 2005-2012. PLoS Negl Trop Dis. 2014;8:e3122. doi: 10.1371/journal.pntd.0003122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weng MH, Lien JC, Wang YM, et al. Isolation of Japanese encephalitis virus from mosquitoes collected in Northern Taiwan between 1995 and 1996. J Microbiol Immunol Infect. 1999;32:9–13. [PubMed] [Google Scholar]
- 51.Weng M-H, Lien J-C, Wang Y-M, et al. Susceptibility of three laboratory strains of Aedes albopictus (Diptera: Culicidae) to Japanese encephalitis virus from Taiwan. J Med Entomol. 1997;34:745–747. doi: 10.1093/jmedent/34.6.745 [DOI] [PubMed] [Google Scholar]
- 52.de Wispelaere M, Desprès P, Choumet V.. European Aedes albopictus and Culex pipiens are competent vectors for Japanese encephalitis virus. PLoS Negl Trop Dis. 2017;11:e0005294. doi: 10.1371/journal.pntd.0005294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hernández-Triana LM, Folly AJ, Sewgobind S, et al. Susceptibility of Aedes albopictus and Culex quinquefasciatus to Japanese encephalitis virus. Parasit Vectors. 2022;15:210. doi: 10.1186/s13071-022-05329-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicholson J, Ritchie SA, Van Den Hurk AF.. Aedes albopictus (Diptera: Culicidae) as a potential vector of endemic and exotic arboviruses in Australia. J Med Entomol. 2014;51:661–669. doi: 10.1603/ME13204 [DOI] [PubMed] [Google Scholar]
- 55.Mourya DT, Ilkal MA, Mishra AC, et al. Isolation of Japanese encephalitis virus from mosquitoes collected in Karnataka state, India from 1985 to 1987. Trans R Soc Trop Med Hyg. 1989;83:550–552. doi: 10.1016/0035-9203(89)90288-5 [DOI] [PubMed] [Google Scholar]
- 56.Chen W-J, Dong C-F, Chiou L-Y, et al. Potential role of Armigeres subalbatus (Diptera: Culicidae) in the transmission of Japanese encephalitis virus in the absence of rice culture on Liu-Chiu Islet, Taiwan. J Med Entomol. 2000;37:108–113. doi: 10.1603/0022-2585-37.1.108 [DOI] [PubMed] [Google Scholar]
- 57.Hurlbut HS, Thomas JI.. Potential vectors of Japanese encephalitis in the Caroline Islands. Am J Trop Med Hyg. 1949;s1-29:215–217. doi: 10.4269/ajtmh.1949.s1-29.215 [DOI] [PubMed] [Google Scholar]
- 58.Mackenzie-Impoinvil L, Impoinvil DE, Galbraith SE, et al. Evaluation of a temperate climate mosquito, Ochlerotatus detritus (Aedes detritus), as a potential vector of Japanese encephalitis virus. Med Vet Entomol. 2015;29:1–9. doi: 10.1111/mve.12083 [DOI] [PubMed] [Google Scholar]
- 59.Reeves WC, Hammon WM, Assistance of T, et al. Laboratory transmission of Japanese B encephalitis virus by seven species (three genera) of North American mosquitoes. J Exp Med. 1946;83:185–194. doi: 10.1084/jem.83.3.185 [DOI] [PubMed] [Google Scholar]
- 60.Weng M-H, Lien J-C, Lin C-C, et al. Vector competence of Culex pipiens molestus (Diptera: Culicidae) from Taiwan for a sympatric strain of Japanese encephalitis virus. J Med Entomol. 2000;37:780–783. doi: 10.1603/0022-2585-37.5.780 [DOI] [PubMed] [Google Scholar]
- 61.Turell MJ, Mores CN, Dohm DJ, et al. Laboratory transmission of Japanese encephalitis and West Nile viruses by molestus form of Culex pipiens (Diptera: Culicidae) collected in Uzbekistan in 2004. J Med Entomol. 2006;43:296–300. doi: 10.1093/jmedent/43.2.296 [DOI] [PubMed] [Google Scholar]
- 62.Weaver SC, Scherer WF, Cupp EW, et al. Barriers to dissemination of Venezuelan encephalitis viruses in the Middle American enzootic vector mosquito, Culex (Melanoconion) Taeniopus. Am J Trop Med Hyg. 1984;33:953–960. doi: 10.4269/ajtmh.1984.33.953 [DOI] [PubMed] [Google Scholar]
- 63.Kramer LD, Hardy JL, Presser SB, et al. Dissemination barriers for Western equine encephalomyelitis virus in Culex tarsalis infected after ingestion of low viral doses. Am J Trop Med Hyg. 1981;30:190–197. doi: 10.4269/ajtmh.1981.30.190 [DOI] [PubMed] [Google Scholar]
- 64.Weger-Lucarelli J, Rückert C, Chotiwan N, et al. Vector competence of American mosquitoes for three strains of Zika virus. PLoS Negl Trop Dis. 2016;10:e0005101. doi: 10.1371/journal.pntd.0005101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carpenter A, Clem RJ.. Factors affecting arbovirus midgut escape in mosquitoes. Pathogens. 2023;12:220. doi: 10.3390/pathogens12020220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson SL, Richards SL, Tabachnick WJ, et al. Effects of West Nile virus dose and extrinsic incubation temperature on temporal progression of vector competence in Culex pipiens quinquefasciatus. J Am Mosq Control Assoc. 2010;26:103–107. doi: 10.2987/09-5926.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Richards SL, Mores CN, Lord CC, et al. Impact of extrinsic incubation temperature and virus exposure on vector competence of Culex pipiens quinquefasciatus Say (Diptera: Culicidae) for West Nile virus. Vector Borne Zoonotic Dis. 2007;7:629–636. doi: 10.1089/vbz.2007.0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lorenz L, Tabachnick WJ, Wallis GP, et al. The effect of colonization upon Aedes aegypti susceptibility to oral infection with yellow fever virus. Am J Trop Med Hyg. 1984;33(4):690–694. pmid:6476217. doi: 10.4269/ajtmh.1984.33.690 [DOI] [PubMed] [Google Scholar]
- 69.Hodoameda P, Ebel GD, Mukhopadhyay S, et al. Extreme infectious titer variability in individual Aedes aegypti mosquitoes infected with Sindbis virus is associated with both differences in virus population structure and dramatic disparities in specific infectivity. PLoS Pathog. 2024;20:e1012047. doi: 10.1371/journal.ppat.1012047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mutsaers M, Engdahl CS, Wilkman L, et al. Vector competence of Anopheles stephensi for O’nyong-nyong virus: a risk for global virus spread. Parasit Vectors. 2023;16:133. doi: 10.1186/s13071-023-05725-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Forrester NL, Guerbois M, Seymour RL, et al. Vector-borne transmission imposes a severe bottleneck on an RNA virus population. PLoS Pathog. 2012;8:e1002897. doi: 10.1371/journal.ppat.1002897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patterson EI, Khanipov K, Rojas MM, et al. Mosquito bottlenecks alter viral mutant swarm in a tissue and time-dependent manner with contraction and expansion of variant positions and diversity. Virus Evol. 2018;4:vey001. doi: 10.1093/ve/vey001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Faizah AN, Kobayashi D, Azerigyik FA, et al. Vector competence of two globally distributed mosquito species originated from Japan in transmitting Japanese encephalitis virus—analyses according to their respective insect-specific virus status. The Microbe. 2024;2:100037. doi: 10.1016/j.microb.2024.100037 [DOI] [Google Scholar]
- 74.Quiner CA, Parameswaran P, Ciota AT, et al. Increased replicative fitness of a dengue virus 2 clade in native mosquitoes: potential contribution to a clade replacement event in Nicaragua. J Virol. 2014;88:13125–13134. doi: 10.1128/JVI.01822-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salazar MI, Richardson JH, Sánchez-Vargas I, et al. Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol. 2007;7:9. doi: 10.1186/1471-2180-7-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kenney JL, Brault AC.. The role of environmental, virological and vector interactions in dictating biological transmission of arthropod-borne viruses by mosquitoes. Adv Virus Res. 2014;89:39–83. doi: 10.1016/B978-0-12-800172-1.00002-1 [DOI] [PubMed] [Google Scholar]
- 77.Mayton EH, Tramonte AR, Wearing HJ, et al. Age-structured vectorial capacity reveals timing, not magnitude of within-mosquito dynamics is critical for arbovirus fitness assessment. Parasit Vectors. 2020;13:310. doi: 10.1186/s13071-020-04181-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Folly AJ, Dorey-Robinson D, Hernández-Triana LM, et al. Temperate conditions restrict Japanese encephalitis virus infection to the mid-gut and prevents systemic dissemination in Culex pipiens mosquitoes. Sci Rep. 2021;11:6133. doi: 10.1038/s41598-021-85411-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kay BH, Fanning ID, Mottram P.. Rearing temperature influences flavivirus vector competence of mosquitoes. Med Vet Entomol. 1989;3:415–422. doi: 10.1111/j.1365-2915.1989.tb00249.x [DOI] [PubMed] [Google Scholar]
- 80.Kilpatrick AM, Meola MA, Moudy RM, et al. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 2008;4:e1000092. doi: 10.1371/journal.ppat.1000092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klein MJ, Jackson SA, Suen WW, et al. Australian Culex annulirostris mosquitoes are competent vectors for Japanese encephalitis virus genotype IV. Emerg Microbes Infect. 2024;13:2429628. doi: 10.1080/22221751.2024.2429628 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.