Abstract
Introduction
Utilizing 38% silver diamine fluoride (SDF) has been demonstrated in clinical trials to prevent and halt early childhood caries (ECC). Based on a research evaluation, it has been found that 38% SDF can effectively prevent new tooth decay and stop existing tooth decay in children's primary teeth.
Objective
This study aimed to assess the survival of teeth treated with repeated applications of 38% SDF in children with ECC and to compare the outcomes between single and multiple applications.
Materials and methods
A split-mouth, double-blind, active-control, parallel-arm randomized controlled trial was conducted following Consolidated Standards of Reporting Trials (CONSORT) guidelines. Children aged 4–5 years with bilateral ECC were recruited from a primary government school in Maharashtra, India. Treatment involved applying 38% SDF to carious lesions, with lesions randomized into two groups: single application and double application. Follow-ups were conducted at 6 and 12 months to assess lesion progression, depth, and survival.
Results
Eighty-four primary carious teeth from 42 female participants were included. Lesions were predominantly occlusal or proximal, with varying sizes and depths. After 12 months, 12 lesions failed in group I (single application) and 3 in group II (double application). The intergroup comparison of mean levels at 12 months showed a statistically significant difference (p = 0.01). Intragroup comparisons showed an increase in lesion depth and level from baseline to 6 months, with a subsequent rise from 6 to 12 months in group I. The mean survival rate of teeth after SDF application at the end of 12 months was around 94% for group II, while for group I it further decreased from 70 to 58%.
Conclusion
Repeated applications of 38% SDF showed improved survival rates of teeth in children with ECC compared to single applications. Double application of SDF demonstrated superior efficacy in maintaining tooth survival over 12 months.
How to cite this article
Kharat PB, Rajpurohit L, Mathur A, et al. Survival of Silver Diamine Fluoride Varnish Treated Teeth among Children with Single/Multiple Applications: A Split-mouth Randomized Clinical Trial. Int J Clin Pediatr Dent 2024;17(11):1211–1218.
Keywords: Children, Dental caries, Early childhood caries, Primary teeth, Randomized clinical trial, Silver diamine fluoride, Survival
Introduction
Dental caries is a highly prevalent condition of the stomatognathic system, with a prevalence rate ranging from 49 to 83% across different countries.1 An across-the-country investigation conducted in India revealed the following rates of dental caries: 50% in children aged 5 years, 52.5% in children aged 12 years, 61.4% in children aged 15 years, 79.2% in individuals aged 35–44 years, and 84.7% in individuals aged 65–74 years.2 Early childhood caries (ECC) is a common, untreated disease that affects infants and young children. The occurrence of ECC in India was found to be 44% among children aged 8–48 months and 40.6% among children aged 0–3 years in rural South India.3 In the past, the treatment for dental caries involved the removal of damaged and impacted regions of teeth through excavation and drilling. This procedure required specialized skills and produced significant noise, a severe drawback when treating young children. Given that a significant proportion of the affected children are young, frightened, and from low-income families, it is imperative to devise less invasive methods to combat the progression of ECC. Unaddressed dental caries will ultimately penetrate the tooth pulp and give rise to a dental abscess.4 Moreover, premature tooth loss resulting from ECC significantly affects oral function and tooth arrangement. Poor dental health substantially impacts children's nutritional status, thereby impeding growth, development, and overall well-being. ECC is prevalent in children who face social disadvantages, including those from low-income households and those whose parents have limited educational attainment.5 Nevertheless, current preventive and therapeutic approaches for ECC remain inaccessible or expensive for these young individuals.6 Caregivers’ apprehension toward dental procedures involving drilling, along with their own unpleasant dental experiences in childhood, hinders the effective administration of dental therapy to children. Limited financial resources are the primary barrier to accessing preventive dental care. Additional factors include demanding caregiver work schedules, fear of dental procedures, transportation obstacles, and overbooked dental clinic appointments.7 Early identification and intervention can halt the progression of dental caries, a chronic disorder, and improve the long-term survival of the affected tooth. As our understanding of the carious process grows, dental caries therapy is transitioning from surgically removing tooth structure to focusing on preventing and inhibiting its progression.8
Silver diamine fluoride (SDF) has become increasingly popular among dental public health and oral health experts due to its ability to prevent and treat tooth decay. This procedure is considered safe, simple, efficient, affordable, minimally invasive, and adaptable for usage in different community settings.9,10 SDF therapy is an example of nonrestorative caries treatment. Based on a research evaluation, it has been found that 38% SDF can effectively prevent new tooth decay and stop existing tooth decay in children's primary teeth.6 The SDF treatment, as described by Milgrom and Chi, is an important caries control approach focused on prevention during critical early infancy phases.11 In SDF, silver possesses antibacterial properties and effectively inhibits the formation of cariogenic biofilm. Fluoride facilitates the process of restoring minerals to teeth and inhibits the process of mineral loss when teeth come into contact with acid.12 The SDF treatment is simple, non-intrusive, painless, cost-effective, and does not generate aerosols. It can be used in individuals who are not suitable for conventional restorative therapy. Additionally, it can provide treatment for neonates who are not yet suitable candidates for conventional dental rehabilitation procedures performed in a dental chair. It can also be utilized to provide dental care for older individuals who may face challenges in accessing dental facilities, as well as for individuals with special needs who are unable to effectively participate in dental treatment.13
The implementation of SDF treatment holds the capacity to fulfill the objectives set by the World Health Organization's Millennium Goals and meet the requirements for 21st-century medical care established by the United States Institute of Medicine. In 2020, the British Society of Paediatric Dentistry officially supported using SDF as a treatment for dental caries. In 2021, the World Health Organization classified SDF as a crucial drug for the health system, fulfilling the vital needs of both adults and children. Recent bibliometric research indicates a significant increase in global interest in SDF from 2016 to 2021. SDF has also attracted the attention of medical professionals and scholars seeking to employ it to enhance the efficacy of dental care.14 In 2017, Health Canada granted certification to SDF for dental therapy. Multiple manufacturers developed a dental solution containing SDF with concentrations varying from 3.8 to 38%.13 SDF is recommended for challenging lesions and patients with a high risk of tooth decay, such as those with medical or behavioral conditions, requiring multiple treatment sessions, or lacking access to dental services.15 This study aims to assess the survival of teeth treated with multiple applications of SDF in children with ECC. Since there is no published literature on the survival of teeth treated with SDF, this research intends to investigate the survival of SDF-treated teeth with both single and multiple applications.
Materials and Methods
This study is a split-mouth, double-blind, active-control, parallel-arm randomized controlled trial. The study was conducted in consensus with the Consolidated Standards of Reporting Randomized Controlled Trials (CONSORT) guidelines. It was registered in the Clinical Trial Registry of India under register no. CTRI/2023/03/050924. Approval for the study was obtained from the Institutional Ethics Committee with reference no. DYPDCH/DPU/EC/465/96/2022.
Hypothesis
The study's null hypothesis is that there is no difference in the survival of teeth between single and multiple applications of 38% SDF.
Setting
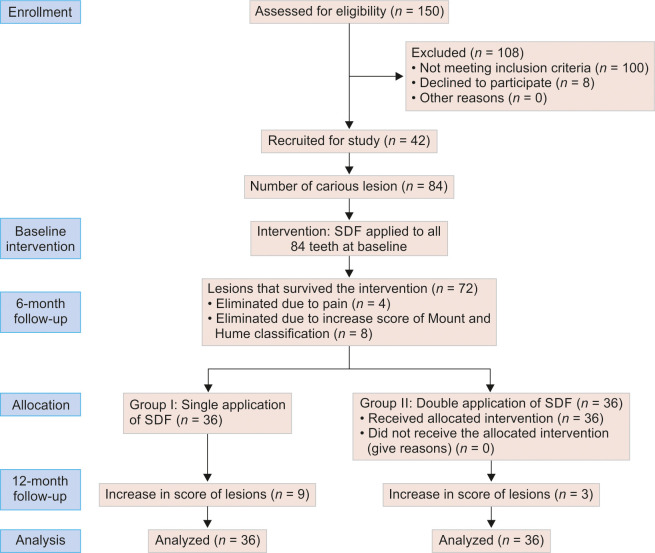
This clinical trial was implemented in a primary government school in the city of Maharashtra. An invitation letter outlining the objective and protocols of the trial was sent to the school administration. After receiving confirmation of participation from the school authorities, the children's parents received consent through the school. The parents were provided with an information sheet regarding the purpose of the research, duration of follow-up, possible advantages, and common adverse effects of SDF treatment, pictures of the lesion before and after treatment, treatment group assignment, and expected treatment outcomes on the information sheet. The dental examination and SDF treatment were administered only to the children whose parents had reviewed the information sheet and signed the parental consent form. Figure 1 shows the CONSORT flow diagram.
Fig. 1:
CONSORT 2010 flow diagram
Participants
Children between the ages of 4–5 years were recruited to participate in this clinical trial. The inclusion criteria were children who (1) have signed informed consent from parents, (2) are generally in good health, and (3) have bilateral early childhood dentinal caries according to the WHO criteria (bilateral ECC participants were selected to illuminate the bias occurring due to different oral conditions). Children who (1) are not ready to accept oral examination or treatment, (2) have substantial systemic diseases, or those on long-term medications that may influence oral health, such as those with autoimmune diseases, etc., are excluded. Primary teeth with irreversible pulpitis, teeth close to exfoliation, and the presence of pulp involvement, mobility, abscess, sinus, fistula, etc., are also excluded.
Recruitment and Examination
A dentist (PK) was trained and calibrated by an expert (LR) before the trial. With the consent of parents, the dentist examined all children enrolled in the participating school. The study children were instructed to sit on a chair while they underwent clinical examinations. Both tactile and visual inspections were used to conduct the dental examination. A dental mirror was used for the visual inspection, and a UNC-15 probe (GDC) was used for the tactile inspection. The WHO-recommended criteria for diagnosing caries were implemented,16 and the number of decayed, missing (due to caries), and filled tooth surfaces (dmfs) was recorded. The following data were recorded at baseline and at the time of each follow-up for the recruited carious tooth surfaces: (1) the status of caries activity (active/arrested), (2) the extent of the caries lesion (by Mount and Hume),17 and (3) the depth of the carious lesion was recorded using the UNC-15 probe. The tooth surface was diagnosed as active if any softened area on the caries lesion was detected through probing.
Intervention at Baseline
Thirty-eight percent SDF (Kids-e-Dental LLP, India) solution was used to treat all tooth surfaces that had carious lesions. The SDF application was implemented based on the suggested clinical protocols18 as follows: no attempt was made to remove caries or unsupported enamel for caries treatment. Initially, the cavity was cleaned with a small cotton pellet. A piece of gauze or a cotton roll was used to isolate the decaying teeth. After that, a single drop (0.1 mL) of 38% SDF was applied using a disposable micro-applicator tip for 60 seconds. One tooth from each arch, that is, maxilla or mandible, from the left and right sides was selected for intervention. The cavity was then sealed with a cotton pellet for ten minutes, and the child was instructed not to eat or drink anything for at least 45 minutes. Each lesion was treated with a single micro-applicator tip, which was disposed of after use. All examinations and interventions were conducted in school settings.
Randomized Allocation, Concealment, and Blinding at 6-month Follow-up
The extent of the treated carious lesion was assessed based on Mount and Hume's classification of carious lesions. The presence or absence of pain was recorded using tactile examination and a verbal questionnaire. Any treated carious lesions that showed an increase in size/level and experienced pain were considered not surviving the intervention and were excluded from further treatment. The remaining treated carious lesions were considered for further random allocation. This trial used the simple randomization method with computer-generated numbers to allocate treated carious lesions randomly into the right and left arches into two intervention groups.
Group I: Single application of 38% SDF.
Group II: Double application of 38% SDF.
Lesions that were randomly allocated to group II were treated with one more application of 38% SDF. The investigator and statistician were blinded to the treatment modalities and treatment outcomes.
Follow-up Evaluation at 12 Months
The treated carious lesions were evaluated clinically using visual and tactile examination by the trained blind investigator. The extent of carious lesions and the presence or absence of pain were assessed in the same manner as at the 6-month follow-up. Treatment for the corresponding groups’ failures involved pulpectomy and complete coverage restoration.
Sample Size Calculation
The sample size was calculated based on the previous literature considering a 95% confidence interval and 80% power of the study using the formula:
The total sample size was estimated to be 72 carious teeth, 36 in each group. Considering an attrition rate of 15%, a total sample of 84 carious teeth was finalized.
Data Analysis
An assistant entered the collected data into an Excel file and then proofread the data entry. IBM SPSS 26.0 was used to impute the proofread data for statistical analysis. For all tests, a statistical significance level of 0.05 was set. Descriptive statistics were used to assess caries lesion progression, depth, and score of the lesion. Intragroup comparison was performed using the Student's paired t-test or Wilcoxon sum rank test, and intergroup comparisons were made using the independent t-test or Mann–Whitney U test.
Results
Overall, 84 teeth from 42 children were selected for the study. The study was conducted in a girls’ school, so all the participants were female, with a mean age of 4.86 ± 0.44. The mean dmfs (decayed, missing, filled) at baseline was 3.85 ± 1.65.
Eighty-four primary carious teeth involving dentin were treated with SDF. All the carious lesions involved were selected from the mandibular arch to meet the criteria of the split-mouth design. Throughout the entire trial, there was very good intra-examiner reproducibility. For both the baseline and follow-up exams, the Kappa statistic values for the duplicate examinations were at least 0.95.
Site: Out of 42 (right) selected lesions, 21 (50%) were occlusal, 17 (40.5%) were proximal, and 4 (9.5%) were proximo-occlusal. Out of 42 (left) selected lesions, 23 (54.8%) were occlusal, 15 (35.7%) were proximal, and 4 (9.5%) were proximo-occlusal.
Size: Carious primary molars included in the research were categorized as minimal, moderate, or enlarged based on their size at the baseline visit and again recorded at each follow-up visit. On the right side of the mandibular arch, 22 (52.4%) were minimal, 18 (42.9%) were moderate, and 2 (4.8%) were enlarged lesions. On the left side of the mandibular arch, 21 (50%) were minimal, 18 (42.9%) were moderate, and 3 (7.1%) were enlarged carious lesions.
Depth: At each visit, the carious lesions’ depth was measured with a UNC-15 probe, and intra- and intergroup comparisons were made.
Level: At each visit, the carious lesions’ level was also measured. The overall score of the site and size of the lesion was considered the level. Intra- and intergroup comparisons were made at baseline and each follow-up visit.
The first application of SDF was done at baseline and the second application at 6 months. There was no loss of participants or teeth due to exfoliation or extraction. After 6 months of follow-up, a total of 12 carious lesions did not survive (4 due to pain; 8 due to an increase in the level of Mount and Hume classification). At 12 months, there were 12 more losses: 9 from group I and 3 from group II (Fig. 1).
The distribution and comparison of Mount and Hume mean scores at baseline and 6 months did not show any statistically significant difference (Table 1), while mean depth and level increased from baseline to 6 months, with mean differences of 0.155 and 0.238, respectively (p = 0.032, 0.038, respectively) (Tables 2 and 3).
Table 1:
Distribution and comparison of study participants based on Mount and Hume score at baseline and 6 months
| N | Mean | SD | Mean difference | SE | t-value | p-value | |
|---|---|---|---|---|---|---|---|
| Score at baseline | 84 | 1.726 | 0.6715 | −0.1702 | 1.0446 | −1.494 | 0.139 |
| Score at 6 months | 84 | 1.896 | 1.2914 |
N, number of samples; SD, standard deviation; SE, standard error
Table 2:
Distribution and comparison of study participants based on depth at baseline and 6 months
| N | Mean | SD | Mean difference | SE | t-value | p-value | |
|---|---|---|---|---|---|---|---|
| Depth at baseline | 84 | 3.61 | 0.695 | −0.155 | 0.649 | −2.185 | 0.032* |
| Depth at 6 months | 84 | 3.76 | 1.071 |
N, number of samples; SD, standard deviation; SE, standard error; *(p < 0.05) analyzed using Student's paired t-test
Table 3:
Distribution and comparison of study participants based on Mount and Hume level at the baseline and 6 months
| N | Mean | SD | Mean difference | SE | Z-value | p-value | |
|---|---|---|---|---|---|---|---|
| Level at baseline | 84 | 1.55 | 0.609 | −0.238 | 1.037 | −2.105 | 0.038* |
| Level at 6 months | 84 | 1.79 | 1.318 |
N, number of samples; SD, standard deviation; SE, standard error; *(p < 0.05) analyzed using Mann–Whitney U test
The mean score of the carious lesions treated with SDF at 6 months and 12 months between groups I and II did not show any significant difference. There was a significant difference in the mean depth of the carious lesion at 6 months and 12 months between groups I and II, with group I having a higher mean and mean difference of 0.19 and 0.415, respectively (p < 0.05) (Table 4). The intergroup comparison of mean levels at 12 months showed a statistically significant difference (p = 0.01) (Table 5).
Table 4:
Distribution and comparison of study participants based on Mount and Hume score and depth at 6 and 12 months
| Group | N | Mean | SD | Mean difference | t-value | p-value | |
|---|---|---|---|---|---|---|---|
| Score at 6 months | 1 | 31 | 1.742 | 0.6392 | 0.0590 | 0.388 | 0.971 |
| 2 | 41 | 1.683 | 0.6379 | ||||
| Depth at 6 months | 1 | 31 | 3.68 | 0.945 | 0.190 | 1.041 | 0.003* |
| 2 | 41 | 3.49 | 0.597 | ||||
| Score at 12 months | 1 | 31 | 1.771 | 0.6278 | 0.0832 | 0.551 | 0.835 |
| 2 | 41 | 1.688 | 0.6384 | ||||
| Depth at 12 months | 1 | 31 | 3.90 | 0.831 | 0.415 | 2.470 | 0.055* |
| 2 | 41 | 3.49 | 0.597 |
N, number of samples; SD, standard deviation; SE, standard error; *(p < 0.05) Student's unpaired t-test
Table 5:
Distribution and comparison of study participants based on Mount and Hume level at the 6 and 12 months
| Group | N | Mean | SD | Mean difference | Z-value | p-value | |
|---|---|---|---|---|---|---|---|
| Level at 6 months | 1 | 31 | 1.61 | 0.615 | 0.149 | −1.020 | 0.308 |
| 2 | 41 | 1.46 | 0.552 | ||||
| Level at 12 months | 1 | 31 | 1.90 | 0.651 | 0.391 | −2.545 | 0.011* |
| 2 | 41 | 1.51 | 0.637 |
N, number of samples; SD, standard deviation; SE, standard error; *(p < 0.05) analyzed using Mann–Whitney U test
A total of 31 lesions were analyzed and evaluated at the end of 12 months in group I. The effectiveness of the SDF application decreased over time for a single application, as the mean score and mean level of the lesion increased from 6 months to 12 months, with statistically significant differences of 0.001 and 0.003, with mean differences of 0.029 and 0.29, respectively (Tables 6 and 7).
Table 6:
Comparison of Mount and Hume score and depth at 6–12 months in group I
| Mean | N | SD | Mean difference | t-value | p-value | |
|---|---|---|---|---|---|---|
| Score at 6 months | 1.742 | 31 | 0.6392 | −0.0290 | −3.503 | 0.001* |
| Score at 12 months | 1.771 | 31 | 0.6278 | |||
| Depth at 6 months | 3.68 | 31 | 0.945 | −0.226 | −1.880 | 0.070 |
| Depth at 12 months | 3.90 | 31 | 0.831 |
N, number of samples; SD, standard deviation; SE, standard error; *(p < 0.05) Student's unpaired t-test
Table 7:
Comparison of Mount and Hume level at 6–12 months in group I
| Mean | N | SD | Mean difference | Z-value | p-value | |
|---|---|---|---|---|---|---|
| Level at 6 months | 1.61 | 31 | 0.615 | −0.290 | −3.000b | 0.003* |
| Level at 12 months | 1.90 | 31 | 0.651 |
N, number of samples; SD, standard deviation; SE, standard error; *(p < 0.05) analyzed using Mann–Whitney U test
A total of 41 lesions were analyzed and evaluated at the end of 12 months in group II. The mean score, depth, and level of the lesion for double application of SDF did not show a statistically significant difference, with p-values > 0.05 and mean differences of 0.0049 and 0.049, respectively (Tables 8 and 9).
Table 8:
Comparison of Mount and Hume score and Depth at 6–12 months in group I
| Mean | N | SD | Mean difference | t-value | p-value | |
|---|---|---|---|---|---|---|
| Score at 6 months | 1.683 | 41 | 0.6379 | −0.0049 | −1.432 | 0.160 |
| Score at 12 months | 1.688 | 41 | 0.6384 | |||
| Depth at 6 months | 3.49a | 41 | 0.597 | – | – | – |
| Depth at 12 months | 3.49a | 41 | 0.597 |
N, number of samples; SD, standard deviation; SE, standard error
Table 9:
Comparison of Mount and Hume level at 6–12 months in group II
| Mean | N | SD | Mean difference | Z-value | p-value | |
|---|---|---|---|---|---|---|
| Level at 6 months | 1.46 | 41 | 0.552 | −0.049 | −1.414b | 0.157 |
| Level at 12 months | 1.51 | 41 | 0.637 |
N, number of samples; SD, standard deviation; SE, standard error
The mean survival for group I was 11 months and for group II it was 12 months. There was a statistically significant difference in the mean survival of both groups (Table 10).
Table 10:
Comparison of mean survival time
| Meana | |||||
|---|---|---|---|---|---|
| 95% confidence interval | |||||
| Groups | Meana estimate | Standard error | Lower bound | Upper bound | p-value |
| Single application | 11.014 | 0.267 | 10.491 | 11.536 | 0.001 |
| Double application | 12.000 | 0.000 | 12.000 | 12.000 | |
| Overall | 11.538 | 0.131 | 11.282 | 11.795 | |
The mean survival rate of teeth after application of SDF at the end of 6 months was around 70%; however, the mean survival rate of teeth after SDF application at the end of 12 months was around 94% for group II, while for group I it further decreased from 70 to 58% (Fig. 2).
Fig. 2:
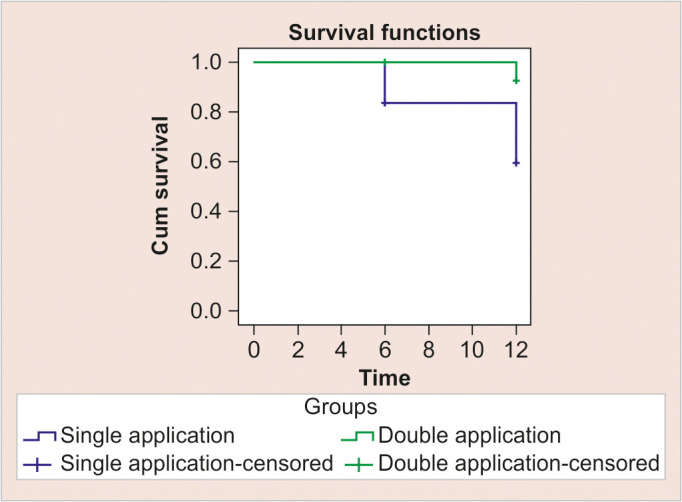
Survival plot at 12 months
Discussion
The present study is a split-mouth, double-blind, active control, parallel-arm randomized controlled trial that aims to assess the survival of teeth treated with multiple applications of SDF in children with ECC. Since there is no published literature on this topic, this research intended to investigate the survival of SDF-treated teeth with both single and multiple applications.
ECC is a common condition that affects young children and infants in India. Conventional therapy entails the extraction and drilling of decayed teeth, which may be bothersome and challenging for young children.4,19 Timely detection and prevention may halt the advancement of ECC and enhance the tooth's long-lasting viability. With the advancement of our knowledge of the carious process, dental caries treatment is shifting from the surgical removal of tooth tissue to a greater emphasis on preventing and prohibiting its progression.11
In our study, the participants’ demographic characteristics, comprising females with a mean age of 4.86 years, reflect the targeted population for ECC prevention interventions. This aligns with existing literature emphasizing the importance of addressing dental caries at a young age to mitigate its long-term impact on oral health.20
The anticariogenic properties of SDF are rooted in its two active components: silver and fluoride. Silver ions act as a potent antimicrobial agent that inhibits the growth of bacteria like Streptococcus mutans, a key pathogen in dental caries development. This action is supported by research done by Zhao et al., which demonstrated the bactericidal effects of silver against oral pathogens.21 Fluoride, on the other hand, aids in the remineralization of enamel and makes the tooth surface more resistant to acid attack. The combined effect not only halts the progression of existing lesions but also prevents new caries formation. This dual mechanism underscores SDF's effectiveness as reported in the literature, including a systematic review by Gao et al., which found a significant reduction in caries progression with SDF application.22
Traditional dental treatments for ECC typically involve mechanical removal of decay followed by restoration. However, these methods can be challenging for young children due to the need for cooperation and possible discomfort. SDF offers a noninvasive alternative that is particularly advantageous in pediatric populations and resource-constrained environments. This aligns with the findings of Crystal et al., who advocate for the use of SDF as a practical approach to managing caries in young children and special needs populations due to its ease of application and effectiveness.23
Extensive research has been conducted on a 38% SDF solution, which has shown significant efficacy in halting the progression of dental caries. The synergistic impact of silver and fluoride may effectively arrest the advancement of tooth decay and inhibit the formation of new cavities by reducing the number of colony- forming units (CFU) of S. mutans and Actinomyces naeslundii. Additionally, SDF improves the mineral composition of tooth hard tissues and speeds up calcium absorption. As a result, it is known that carious lesions treated with SDF have noticeably higher surface microhardness. Despite the ongoing significance of ECC in pediatric dentistry, there has been little focus on using 38% SDF in primary teeth.24 The present study focuses on the survival of primary teeth treated with 38% SDF to overcome the lacunae in literature, as there is no clinical investigation that has shown the survival analysis of teeth after applying 38% SDF to primary teeth.
The mechanism of action of SDF is based on its dual action provided by fluoride and silver. The presence of fluoride stimulates remineralization, while the silver it contains offers antibacterial activity, resulting in a dual impact against caries. In addition, when bacteria that have been killed by the silver ions are introduced to the live bacteria, the silver is reactivated, resulting in the effective elimination of the live bacteria, causing a zombie-like effect. Therefore, SDF is used as a chemotherapeutic agent to halt the advancement of dental caries in cavitated lesions of ECC.25
The research conducted by Fung et al. showed that the administration of 38% SDF resulted in a caries arrest rate increasing by 18–20% compared to the control group treated with 12% SDF. It was shown that applying 38% SDF after 12 months and 6 months resulted in a caries arrest rate of 66.9 and 75.7%, respectively. The caries arrest achieved by 12% SDF was much lower compared to this.26 Another research conducted by Yee et al. in Nepal investigated the effectiveness of arresting caries of 12 and 38% SDF with a single application. It was observed that the single application of 38% SDF, with or without the use of a reducing agent, was significantly more effective in the short-term arresting of caries than 12% SDF. However, the long-term caries arrest rate of 38% SDF was insignificant. A single application of 38% SDF was useful in preventing only 50% of the arrested surfaces at 6 months from reverting to active lesions again over 24 months. The study also examined the average number of caries that were treated with SDF. The results showed that the mean number of arrested caries was considerably higher in 38% of the cases when SDF was used.27
The American Dental Association (ADA) guidelines advise using 38% SDF twice a year to treat cavitated caries coronal lesions in primary and permanent teeth. This is preferred over using 5% NaF varnish once a week for 3 weeks. Regular treatment of SDF annually was much more effective in preventing carious lesions compared to the use of fluoride varnish four times per year.28 Research is now examining different frequencies (such as three times a year, weekly, twice a year, once a year, five times a week each year, etc.) of SDF. However, the findings are inconclusive and not consistent. According to the research conducted by Tirupathi et al., using SDF once a year has a good percentage of ceasing cavities, but the caries arrest rate decreases over time. The application of SDF two times annually was shown to be more effective than applying it once a year in stopping the progression of cavitated carious lesions. These results were similar to the present study, where the biannual application of SDF was done.29
In the present study, the distribution of carious lesions based on site, size, depth, and level underscores the comprehensive evaluation conducted, providing insights into the varied presentations of dental caries in primary molars. Such detailed characterization aids in understanding the efficacy of SDF treatment across different lesion types and locations, enhancing the study's clinical relevance, which is similar to the study conducted by Tirupathi et al.29,30
In the present study, there was a significant difference in the mean depth of the carious lesion at 6 months and 12 months between groups I (single application of SDF) and 2 (double application of SDF), with group I having a higher mean. The intergroup comparison of the mean score at 12 months in group I showed a statistically significant difference, and these results were similar to the results of Fung et al.26 The total arrest rate was reported as 75%, with the greatest benefit shown at lower anterior teeth (91.7%), followed by upper anterior teeth (85.6%), lower posterior teeth (62.4%), and upper posterior teeth (57.0%). They also suggested that increasing the frequency of application from once a year to twice a year may enhance the caries arrest rate in children with inadequate oral hygiene.
The primary outcome measure, survival of treated teeth, revealed intriguing findings. While both single and multiple applications of SDF demonstrated efficacy in arresting carious lesions, notable differences emerged over the 12 months. Group II, receiving double applications, exhibited significantly higher mean survival rates (94%) compared to group I (58% at the end of 12 months), suggesting a dose-response relationship with SDF treatment. This observation corroborates previous research advocating for multiple applications of SDF to optimize its therapeutic effects.31 The decline in survival rates observed in group I between 6 and 12 months underscores the temporal limitations of single-application SDF treatment in maintaining long-term lesion stability. Conversely, group II demonstrated consistent survival rates over the study period, indicating the potential for prolonged efficacy with multiple applications. This underscores the importance of longitudinal assessments to capture the dynamic nature of caries progression and treatment response.32
SDF can be an effective treatment option for managing early-stage cavities, particularly in children and individuals with special needs who may have difficulty receiving traditional dental treatments. When comparing the effectiveness of a single application of SDF vs multiple applications, research suggests that multiple applications provide better long-term survival in terms of cavity arrest and tooth preservation. Multiple applications of SDF over time may provide greater protection against cavity progression and improve the likelihood of tooth survival. Dentists may recommend periodic reapplication of SDF to ensure ongoing protection and monitor the status of treated teeth.10
The integration of SDF into public health programs could be transformative, especially in underprivileged areas where access to dental care is limited. Deploying SDF in schools and community health centers could significantly lower the incidence of ECC. This public health strategy is supported by the World Health Organization's endorsement of SDF as an essential medicine for dental care, reflecting its potential to impact global health significantly. Rosenblatt et al. described SDF as a “silver-fluoride bullet,” emphasizing its potential to provide equitable dental care solutions.33 Regardless of the number of SDF applications, proper oral hygiene practices, including regular brushing, flossing, and routine dental check-ups, are essential for maintaining the health of treated teeth and preventing new cavities from forming.
While the benefits of SDF are well documented, the esthetic concern of tooth staining after SDF application remains a drawback. Future research should focus on formulations or adjunctive treatments that minimize the effect of black staining while maintaining efficacy. Additionally, long-term studies are needed to assess the durability of SDF treatments over several years and to establish optimal reapplication intervals. Ongoing studies like those by Mei et al. explore novel SDF formulations with enhanced esthetic outcomes.34
The findings support the integration of SDF into routine dental care protocols, especially within pediatric and community health settings. This would involve training healthcare providers in SDF application and educating the community about its benefits and limitations.
Limitations of the Study
The primary limitation associated with using SDF for treating ECC centers primarily around esthetic concerns, sensory reaction, and potential tissue irritation. The most prominent issue is the black staining of carious lesions treated with SDF. This discoloration can have a significant impact on a child's self-esteem. Alongside esthetic drawbacks, SDF is known for its distinctive metallic taste, which can be off-putting to children and may affect their willingness to undergo or repeat the treatment. Developing better application methods to protect soft tissues could reduce risks of irritation, making SDF a more widely accepted option in dental practice.
Conclusion
Silver diamine fluoride is a cost-effective and durable treatment option for anxious children and adolescents at high risk of developing dentin caries. Teeth treated with multiple applications of 38% SDF exhibited enhanced survival rates compared to those treated with a single application. This suggests that a multiple application approach offers improved outcomes in terms of the survival of teeth. The study concludes that there is a significant difference in the mean survival rate of SDF-treated teeth biannually as compared to the annual application of SDF. Further research with longer follow-up durations is required in order to improve approaches to SDF treatment.
Orcid
Priyanka B Kharat https://orcid.org/0000-0001-6002-693X
Ladusingh Rajpurohit https://orcid.org/0000-0001-7424-9755
Anmol Mathur https://orcid.org/0000-0002-1704-462X
Sneha Kalpe https://orcid.org/0000-0002-7919-1051
Chaitanya S Buddhikot https://orcid.org/0000-0001-9746-9574
Kabir S Dash https://orcid.org/0000-0002-4108-8825
Isha Inamdar https://orcid.org/0009-0009-9789-4832
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Frencken JE, Sharma P, Stenhouse L, et al. Global epidemiology of dental caries and severe periodontitis-a comprehensive review. J Clin Periodontol. 2017;44:S94–S105. doi: 10.1111/jcpe.12677. [DOI] [PubMed] [Google Scholar]
- 2.Bali RK, Mathur VB, Talwar PP, Chanana HB. National Oral Health Survey and Fluoride Mapping, 2002–2003. India: Dental Council of India; 2004. [Google Scholar]
- 3.Henry JA, Muthu MS, Saikia A, et al. Prevalence and pattern of early childhood caries in a rural South Indian population evaluated by ICDAS with suggestions for enhancement of ICDAS software tool. Int J Pediatr Dent. 2017;27:191–200. doi: 10.1111/ipd.12251. [DOI] [PubMed] [Google Scholar]
- 4.Chu CH. Treatment of early childhood caries: a review and case report. Gen Dent. 2000;48:142–148. [PubMed] [Google Scholar]
- 5.Chu CH, Ho PL, Lo EC. Oral health status and behaviors of preschool children in Hong Kong. BMC Public Health. 2012;12:767. doi: 10.1186/1471-2458-12-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu CH, Lo EC. Promoting caries arrest in children with silver diamine fluoride: a review. Oral Health Prev Dent. 2008;6:315–321. [PubMed] [Google Scholar]
- 7.Yon MJY, Chen KJ, Gao SS, et al. Dental fear and anxiety of kindergarten children in Hong Kong: a cross-sectional study. Int J Environ Res Public Health. 2020;17(8):2827. doi: 10.3390/ijerph17082827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shivana V, Raju KR. Minimal intervention and concepts for minimally invasive cavity preparations, techniques, and materials review. J Conserv Dent. 2002;5:101–109. [Google Scholar]
- 9.Yan IG, Zheng FM, Gao SS, et al. Ion concentration of silver diamine fluoride solutions. Int Dent J. 2022;72(6):779–784. doi: 10.1016/j.identj.2022.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raskin SE, Tranby EP, Ludwig S, et al. Survival of silver diamine fluoride among patients treated in community dental clinics: a naturalistic study. BMC Oral Health. 2021;21(1):35. doi: 10.1186/s12903-020-01379-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milgrom P, Chi DL. Prevention-centered caries management strategies during critical periods in early childhood. J Calif Dent Assoc. 2011;39:735–741. [PubMed] [Google Scholar]
- 12.Gao SS, Zhang S, Mei ML, et al. Caries remineralization and arresting effect in children by professionally applied fluoride treatment - a systematic review. BMC Oral Health. 2016;16:12. doi: 10.1186/s12903-016-0171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan IG, Zheng FM, Gao SS, et al. Effect of application time of 38% silver diamine fluoride solution on arresting early childhood caries in preschool children: a randomized double-blinded controlled trial protocol. Trials. 2022;23:215. doi: 10.1186/s13063-022-06130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang CM, Duangthip D, Chan AKY, et al. Global research interest regarding silver diamine fluoride in dentistry: a bibliometric analysis. J Dent. 2021;113:103778. doi: 10.1016/j.jdent.2021.103778. [DOI] [PubMed] [Google Scholar]
- 15.Horst JA, Ellenikiotis H, Milgrom PL. UCSF protocol for caries arrest using silver diamine fluoride: rationale, indications, and consent. J Calif Dent Assoc. 2016;44(1):16–28. [PMC free article] [PubMed] [Google Scholar]
- 16.Oral Health Surveys: Basic Methods; World Health Organization; Geneva, Switzerland: 2013. [Google Scholar]
- 17.Mount GJ, Hume WR. A revised classification of carious lesions by site and size. Quintessence Int. 1997;28:301–303. [PubMed] [Google Scholar]
- 18.Yan IG, Zheng FM, Gao SS, et al. A review of the protocol of SDF therapy for arresting caries. Int Dent J. 2022;72:579–588. doi: 10.1016/j.identj.2022.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fung MH, Wong MC, Lo EC, et al. Arresting early childhood caries with silver diamine fluoride - A literature review. Oral Hyg Health. 2013;1:117. doi: 10.4172/2332-0702.1000117. [DOI] [Google Scholar]
- 20.American Academy of Pediatric Dentistry Guideline on caries-risk assessment and management for infants, children, and adolescents. Pediatr Dent. 2013;35(5):E157–E164. [PubMed] [Google Scholar]
- 21.Zhao IS, Gao SS, Crystal YO, et al. Effects of silver diamine fluoride on dentin cariogenic bacteria inhibition and tooth hardness increase. J Dent Res. 2018 [Google Scholar]
- 22.Gao SS, Zhao IS, Mei ML, et al. Using silver diamine fluoride for dental caries management. J Dent Res. 2016 [Google Scholar]
- 23.Crystal YO, Zhao IS, Mei ML, et al. Silver diamine fluoride for dental caries management in children and adolescents: a systematic review. Pediatrics. 2017 [Google Scholar]
- 24.Jabin Z, Vishnupriya V, Agarwal N, et al. Effect of 38% silver diamine fluoride on control of dental caries in primary dentition: a systematic review. J Family Med Prim Care. 2020;9(3):1302–1307. doi: 10.4103/jfmpc.jfmpc_1017_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wakshlak RB, Pedahzur R, Avnir D. Antibacterial activity of silver-killed bacteria: the “zombies” effect. Sci Rep. 2015;5(1):9555. doi: 10.1038/srep09555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fung MHT, Duangthip D, Wong MCM, et al. Randomized clinical trial of 12% and 38% silver diamine fluoride treatment. J Dent Res. 2018;97:171–178. doi: 10.1177/0022034517728496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yee R, Holmgren C, Mulder J, et al. Efficacy of silver diamine fluoride for arresting caries treatment. J Dent Res. 2009;88(7):644–647. doi: 10.1177/0022034509338671. [DOI] [PubMed] [Google Scholar]
- 28.Janakiram C, Ramanarayanan V, Devan I. Effectiveness of silver diammine fluoride applications for dental caries cessation in tribal preschool children in India: study protocol for a randomized controlled trial. Methods Protoc. 2021;4(2):30. doi: 10.3390/mps4020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tirupathi S, SVSG N, Rajasekhar S, et al. Comparative cariostatic efficacy of a novel nano-silver fluoride varnish with 38% silver diamine fluoride varnishes a double-blind randomized clinical trial. J Clin Exp Dent. 2019;11(2):e105–e112. doi: 10.4317/jced.54995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crystal YO, Marghalani AA. Uptake and clearance of silver diamine fluoride treatment in caries lesions: a systematic review. J Dent. 2016;51:1–14. [Google Scholar]
- 31.Zhi QH, Lo ECM, Lin HC. Randomized clinical trial on effectiveness of silver diamine fluoride and glass ionomer in arresting dentine caries in preschool children. J Dent. 2012;40(11):962–967. doi: 10.1016/j.jdent.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Chibinski AC, Wambier LM, Feltrin J, et al. Silver diamine fluoride has efficacy in controlling caries progression in primary teeth: a systematic review and meta-analysis. Caries Res. 2017;51(5):527–541. doi: 10.1159/000478668. [DOI] [PubMed] [Google Scholar]
- 33.Rosenblatt A, Stamford TC, Niederman R. Silver diamine fluoride: a caries “silver-fluoride bullet.”. J Dent Res. 2009;88:116–125. doi: 10.1177/0022034508329406. [DOI] [PubMed] [Google Scholar]
- 34.Mei ML, Crystal YO, Gao SS, et al. Novel silver diamine fluoride application strategies for caries prevention. Int J Environ Res Public Health. 2018 [Google Scholar]