Abstract
Aim and background
Glass ionomer cement (GIC) serves as a widely used restorative dental material, known for its direct bonding to tooth structures and fluoride-releasing properties. This study aims to investigate the enhancement of GIC through the incorporation of a green-mediated nanocomposite comprising chitosan, titanium, zirconium, and hydroxyapatite, with a focus on evaluating the wear resistance of the modified GIC.
Materials and methods
A one-pot synthesis technique was utilized to prepare a green-mediated nanocomposite incorporating chitosan, titanium, zirconium, and hydroxyapatite nanoparticles. Forty extracted teeth fulfilling the inclusion criteria were chosen for the study. Each tooth received a class I cavity preparation, and then they were divided into groups. Each group, comprising 10 teeth, received a restoration using green-mediated nanocomposite-modified GIC in varying concentrations: 3% for group I, 5% for group II, and 10% for group III. Additionally, there was a control group (group IV) consisting of conventional GIC without any modifications. To assess the wear resistance of the samples, they underwent a testing protocol, followed by placement in a chewing simulator for 30,000 cycles. Surface scans before and after chewing simulation were conducted, and deviations were superimposed using Geomagic software. The interim of root mean square (RMS), maximum deviation, and average deviation were analyzed to quantify the wear levels. Then the data obtained were subjected to statistical analysis, one-way analysis of variance (ANOVA), followed by Tukey's post hoc analysis to identify any significant differences among the groups.
Results
The least deviation of RMS (0.292 ± 0.063), maximum deviation (0.664 ± 0.076), and average deviation (0.263 ± 0.049) were observed in the 5% nanocomposite-based GIC group, followed by the 10 and 3% groups. The nanocomposite-modified GIC groups exhibited superior wear resistance compared to the conventional group. This outcome addressed the limitations of traditional GIC, signifying a substantial advancement in dental restorative solutions.
Conclusion
The incorporation of green-mediated chitosan, titanium, zirconium, and hydroxyapatite nanocomposite into GIC demonstrated a remarkable improvement in wear resistance. This study paves the way for future advancements in dental materials, representing a significant stride toward the creation of environmentally conscious and efficacious dental restorations.
How to cite this article
Sitaram SS, Paulraj J, Maiti S, et al. Enhancing Wear Resistance in Glass Ionomer Cement through Green-mediated Chitosan-, Titanium-, Zirconium-, and Hydroxyapatite-based Nanocomposites: An Analysis before and after Chewing Simulator Endurance. Int J Clin Pediatr Dent 2024;17(11):1229–1235.
Keywords: Chewing simulator, Modified glass ionomer cement, Nanocomposite, Restorative dentistry, Software, Wear resistance
Introduction
Glass ionomer cement (GIC) has long been recognized and utilized in the field of dentistry for its unique properties and versatile applications. Originally introduced in the 1970s, GIC has found widespread use in restorative dentistry due to its ability to bond directly to tooth structure.1,2 Its applications range from dental fillings and sealants to luting agents for crowns and orthodontic brackets.3,5 However, despite these advantages, the inherent limitations of traditional GIC, such as relatively low mechanical strength and susceptibility to wear, have prompted researchers to explore modifications that could enhance its performance and extend its applications.6 The restorative materials must exhibit substantial wear resistance to endure the mechanical forces involved in activities like chewing and bruxism. GIC finds extensive application in pediatric dentistry, especially in cases with a heightened risk of caries, and are often preferred for patients requiring more complex treatments, such as the elderly or those with underlying health conditions.7,8 Notably, the World Health Organization (WHO) has recently added GIC to the list of essential medicines, recognizing its significance in addressing minimal medication requirements for a basic healthcare system.9
In the pursuit of enhancing restorative dental materials, particularly GIC, the incorporation of nanoparticles such as chitosan, titanium, zirconium, and hydroxyapatite brings diverse advantages. Chitosan, derived from crustacean exoskeletons, introduces biocompatibility and antimicrobial properties, fostering potential tissue regeneration. Titanium and zirconium, metallic elements known for their strength and corrosion resistance, contribute mechanical reinforcement to GIC, enhancing its durability. Hydroxyapatite, a bioceramic mimicking natural bone composition, promotes bioactivity and compatibility with dental tissues.10,11 Moreover, using eco-friendly methods like green-mediated synthesis to produce these nanoparticles reflects a commitment to sustainability in dental material improvement. This unique blend of natural and environmentally conscious elements shows potential for developing dental solutions that are not only more compatible with the body but also stronger and kinder to the environment.
Dental restorations are subjected to various mechanical stresses in the oral environment, including wear from mastication forces. Understanding and improving the wear resistance of dental materials are crucial for ensuring the longevity and functionality of dental restorations. A material that withstands the wear of daily mastication would contribute to the longevity of dental restorations, reducing the need for frequent replacements and enhancing patient satisfaction. The main objective of this study was to contribute valuable insights into dental materials, offering implications for the development of more resilient and effective restorative solutions in clinical practice. Hence, the aim of this study was to compare the wear resistance of the chitosan titanium zirconium hydroxyapatite nanocomposite-modified GIC with that of traditional GIC when subjected to simulated chewing forces. The null hypothesis stated that the nanomodified GIC would not significantly enhance its wear resistance and that there would be no difference in durability compared to conventional GIC. By systematically examining the null hypothesis, we seek to provide a thorough understanding of the possible advancements or limitations associated with this modification.
Materials and Methods
Study Design and Ethical Approval
The research was carried out at the Research Center, University Hospital. Ethical clearance for this in vitro investigation was obtained from the Institutional Review Board (SRB SDC UG-1909 23 PEDO 134).
Sample Size Determination
To determine the sample size, the effect size was estimated at dz = 1.5004, assuming a normal distribution with a significance level of α = 0.05 and a power of 0.90. Sample size calculations were conducted using G*Power 3.1.9.3 for Mac OS X®, based on data from an earlier study.12,13 The results indicated that to reach a power of 0.95 and a 95% confidence interval, each group should consist of 10 samples, totaling 40 samples overall.
Inclusion and Exclusion Criteria
Permanent mandibular healthy molars were selected for the study. The presence of carious lesions occlusally or proximally and teeth with aberrant surface morphology were excluded from the study. Additionally, teeth with cracks or fracture lines, endodontically treated teeth, teeth with restorations, and root caries were excluded.
Grouping
The teeth were grouped into four groups, each consisting of 10 teeth based on different concentrations of materials used to restore them, namely, group I (3% nanocomposite incorporated GIC), group II (5% nanocomposite incorporated GIC), group III (10% nanocomposite incorporated GIC), and group IV (conventional GIC without any modification).
Green Synthesis of Chitosan, Titanium, Zirconia and Hydroxyapatite Nanoparticles
Eucalyptus-based chitosan nanoparticles were prepared by mixing 50 mL of a 1 gm eucalyptus solution with 50 mL of chitosan. The chitosan solution consisted of 0.5 gm chitosan powder, 0.5 gm glacial acetic acid, and 49 mL distilled water, which was stored after being stirred continuously.
Neem-based titanium oxide nanoparticles were made by mixing 50 mL of a 1 gm neem solution with 50 mL of a 50 mmol TiO2 solution, which was blended using a magnetic stirrer.
Aloe vera-based zirconium oxide nanoparticles: Zirconium oxide nanoparticles were synthesized by mixing 50 mL of aloe vera with 50 mL of a 20 mmol zirconium oxide solution, which was stirred continuously at 340–350°C and stored overnight.
Hydroxyapatite nanoparticles synthesized from eggshell: Hydroxyapatite nanoparticles were obtained by mixing 50 mL of a 1 gm Moringa oleifera solution with 50 mL of a 0.1 gm hydroxyapatite solution derived from eggshells. After continuous stirring, orthophosphoric acid was added dropwise (with a molar ratio of 1.67 Ca/P), and the mixture was stirred and left overnight.
Synthesis of Green-mediated Chitosan, Titanium, Zirconia and Hydroxyapatite Nanocomposites
Using the one-pot synthesis method by Rahman et al.,14 the four solutions (chitosan, titanium, zirconia, and hydroxyapatite nanoparticles) were stirred vigorously at 80°C for 30 minutes. Ethanol (1.08 mL) was added, and the mixture was refluxed at 80°C for 90 minutes. After removing ethanol at 80°C for 30 minutes, the solution was lyophilized in a freeze dryer for 48 hours at –92°C.
Preparation of Green-mediated Chitosan, Titanium, Zirconia, Hydroxyapatite Nanocomposite-modified Glass Ionomer Cement
Green-mediated (Ch-Ti-Zr-HA) nanocomposites were incorporated into GIC at 3, 5, and 10% levels in the powder component, designated as group I, group II, and group III, respectively. Group IV served as the control with unmodified conventional GIC. The powder was then mixed with a polyacrylic acid-based liquid to create restorative cement.
Preparation of Specimens
Forty permanent lower molar teeth meeting specific inclusion criteria were selected and preserved in distilled water at 37°C. For mounting the tooth, an acrylic block with a height and width of 1.5 cm was initially crafted, followed by creating a rubber base mold using an impression material. Positive replicas were generated by pouring cold-cure acrylic resin into the mold, embedding tooth roots (starting 2 mm below the cementoenamel junction) to create 40 test samples. Subsequently, class I cavities were created in 40 healthy extracted mandibular molars. These cavities were then restored as per the groups with the restorative materials, and the teeth were kept in distilled water at 37°C for a period of 24 hours postrestoration. Afterward, the samples were subjected to three-dimensional (3D) scanning, followed by the preparation of standard tessellation language (STL) files.
Three-dimensional Scanning
A 3D scanner (Medit Lab Scanner) was utilized to capture detailed surface information of the dental samples after restoration. This created a digital representation of the tooth geometry.
Preparation of Standard Tessellation Language Files
Process the scanned data to create 3D digital models of the dental samples in STL file format. This involves converting the point cloud data obtained from the scanner into a format suitable for geometric analysis.
Chewing Simulation
After the initial scanning and preparation of STL files, the same samples were placed into the chewing simulator chambers (CS-4.8, SD Mechatronik GmbH, Feldkirchen-Westerham, Germany) to undergo the simulation process. The restored teeth were attached to designated holders using silicone putty and a two-part adhesive. In the chewing simulation, a steatite cone with a tip radius of 1.5 mm and a 30° cone angle served as the antagonist, applying the load to the restored tooth. Steatite, known for its reliability, served as an effective substitute for enamel in chewing simulations.15,17 This property makes it ideal as a standardized antagonist, enabling a quantitative evaluation of wear behavior.18,19 The antagonist was visually aligned above the tooth's fissure and brought into contact with the specimen, marking the starting point for the chewing simulation (Fig. 1). The specimens encountered a vertical load of 50 N and experienced cyclic movements that included both vertical and lateral components at a frequency of 1.4 Hz. The vertical motion had a displacement amplitude of 2 mm, while the lateral motion had a displacement amplitude of 1.5 mm. Concurrently, the mounted teeth were subjected to thermal stress with alternating temperatures (5°C/55°C) for 60 seconds in each cycle, utilizing distilled water as a transport medium. This thermal cycling was intended to simulate the conditions of the oral cavity during food consumption, utilizing a testing apparatus that enabled the chamber to be filled and emptied with water at different temperatures. The chewing simulation concluded either upon specimen failure or upon reaching a maximum of 30,000 load cycles. Throughout the chewing simulation, a space between the crosshead and the weight support of the chewing simulator signified the downward movement of the vertical displacement. Visual inspections at regular intervals confirmed that the maximum wear depth was reached when this gap had completely disappeared. After the completion of the simulation, the samples were again subjected to 3D scanning, followed by the preparation of STL files.
Fig. 1:

Placement of samples in chewing simulator
Wear Resistance Analysis
The wear resistance analysis was conducted using superimposition in Geomagic advanced software tools to compare and quantify the volumetric changes in dental samples before and after a wear test. Geomagic is a suite of 3D scanning and modeling software widely employed for such analyses. Its utilization in wear analysis offers a robust and accurate method for evaluating the wear resistance of dental materials. The software's functionalities in aligning, comparing, and quantifying 3D models contribute significantly to gaining a comprehensive understanding of the material's performance under simulated chewing conditions.
Superimposition Technique
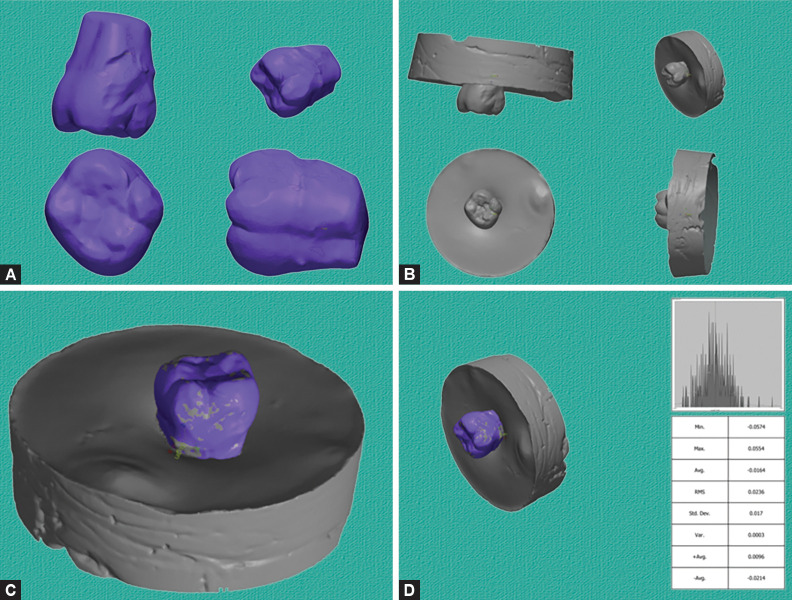
The superimposition contains two steps: alignment and surface comparison to determine the amount of wear. Geomagic was used first to align the STL files of the dental samples taken before and after the chewing simulation. This alignment is a crucial step to ensure accurate comparison, typically achieved through best-fit algorithms or manual alignment of key reference points (Fig. 2). After alignment, surface comparison was done. Geomagic's superimposition tools were employed to compare the surfaces before and after the chewing simulation. This involves overlaying the prewear and postwear STL files and analyzing the differences between corresponding points on the occlusal surfaces.
Figs 2A to D:
(A) Prescan STL file (before chewing simulation); (B) Postscan STL file (after chewing simulation); (C) Alignment of prescan over postscan; (D) Superimposition using best-fit algorithm
Quantitative Analysis
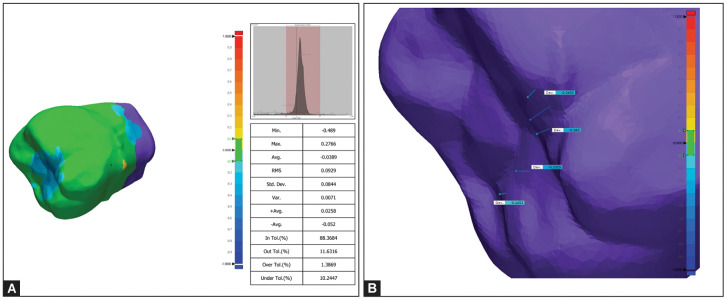
Volumetric measurement was checked by means of maximum deviation, average deviation, and root mean square (RMS) deviation values, in particular selected six points (Fig. 3). Geomagic allows for the calculation of volumetric changes between the two superimposed surfaces. This quantifies the amount of wear, providing a numerical measure of the wear experienced by the dental samples during the simulated chewing forces.
Figs 3A and B:
(A) 3D comparison image of surface analysis with RMS values extracted by Geomagic software; (B) 3D comparison image of surface analysis with maximum deviation values extracted by Geomagic software based on selected points
Statistical Analysis
Data collection and organization were conducted using Google Sheets, and subsequently, the information was imported into the statistical software Statistical Package for the Social Sciences (SPSS) (version 26.0) for the purpose of obtaining statistical significance at a significance level of α = 0.05. The normality of the data distribution was assessed through the Shapiro–Wilk test, confirming that the data exhibited a normal distribution. The overall abrasion analysis for each group was conducted using a one-way analysis of variance (ANOVA), followed by Tukey's post hoc analysis to detect any noteworthy distinctions among the groups.
Results
The amount of wear was compared based on deviations observed from the results of superimposition between the prechewing simulation scan and the postchewing simulation scans in surface analysis by analyzing specific points in the molars’ occlusal surface of restoration. One-way ANOVA revealed that there are statistically significant differences in the RMS value, maximum deviation, and average deviation when compared among nanocomposite-modified GIC groups and the conventional GIC group, where green-mediated nanocomposite-modified GIC groups exhibited superior performance with a p-value of 0.001 (p < 0.05). The least deviation of RMS (0.292 ± 0.063), maximum deviation (0.664 ± 0.076), and average deviation (0.263 ± 0.049) were found in group II (5%) followed by group III (10%) and group I (3%) nanocomposite-modified GIC, where the conventional GIC showed maximum deviation (Table 1). Pairwise comparison by Tukey's post hoc analysis revealed that between group IV (conventional GIC) and group I (3%), there was no significant difference (p > 0.05), whereas all other pairs showed significant differences between groups (Table 2).
Table 1:
Comparison among groups based on deviation happened between pre- and post-chewing simulation, p-value was derived from one-way ANOVA test
| 95% CI | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group | Mean ± SD | SE | Lower | Upper | F-value | p-value | Null hypothesis | |
| RMS | Group I (3%) | 0.589 ± 0.72 | 0.025 | 0.529 | 0.650 | 44.736 | 0.001* | Rejected |
| Group II (5%) | 0.292 ± 0.063 | 0.022 | 0.238 | 0.345 | ||||
| Group III (10%) | 0.390 ± 0.056 | 0.020 | 0.343 | 0.437 | ||||
| Group IV (control) | 0.595 ± 0.061 | 0.021 | 0.544 | 0.647 | ||||
| Maximum deviation | Group I (3%) | 0.951 ± 0.069 | 0.024 | 0.892 | 1.009 | 45.353 | 0.001* | Rejected |
| Group II (5%) | 0.664 ± 0.076 | 0.027 | 0.600 | 0.728 | ||||
| Group III (10%) | 0.767 ± 0.070 | 0.024 | 0.709 | 0.826 | ||||
| Group IV (control) | 1.013 ± 0.051 | 0.018 | 0.970 | 1.056 | ||||
| Average deviation | Group I (3%) | 0.570 ± 0.056 | 0.019 | 0.524 | 0.617 | 51.167 | 0.001* | Rejected |
| Group II (5%) | 0.263 ± 0.049 | 0.017 | 0.222 | 0.302 | ||||
| Group III (10%) | 0.399 ± 0.059 | 0.021 | 0.349 | 0.449 | ||||
| Group IV (control) | 0.581 ± 0.072 | 0.025 | 0.520 | 0.642 | ||||
*Statistically significant value of p < 0.05; CI, confidence interval; SD, standard deviation; SE, standard error
Table 2:
Pairwise comparison between groups based on deviation happened between pre- and post-chewing simulation, p-value was derived from Tukey's post hoc analysis
| Groups | RMS | Maximum deviation | Average deviation | |||
|---|---|---|---|---|---|---|
| MD | p-value | MD | p-value | MD | p-value | |
| Control vs 3% | 0.006 | 0.998 | 0.062 | 0.278 | 0.010 | 0.984 |
| Control vs 5% | 0.303 | 0.001* | 0.348 | 0.001* | 0.318 | 0.001* |
| Control vs 10% | 0.205 | 0.001* | 0.245 | 0.001* | 0.181 | 0.001* |
| 3 vs 10% | 0.199 | 0.001* | 0.183 | 0.001* | 0.171 | 0.001* |
| 3 vs 5% | 0.297 | 0.001* | 0.286 | 0.001* | 0.307 | 0.001* |
| 5 vs 10% | 0.098 | 0.022* | 0.103 | 0.024* | 0.136 | 0.001* |
*Statistically significant value of p < 0.05; MD, mean difference
Discussion
The study underscores the efficacy of nanocomposite modifications, specifically the 5% and 10% nanocomposites, in improving the wear resistance of GIC. The persistent trend of conventional GIC demonstrated elevated deviations, highlighting the urgency for advancements in restorative materials capable of withstanding the mechanical stresses inherent in the oral environment. These results provide crucial insights for the dental materials field, laying the groundwork for continued exploration and fine-tuning of nanocomposite modifications to enhance clinical effectiveness in restorative dentistry. The superior wear resistance observed in chitosan, titanium, zirconium, and hydroxyapatite composite-based GIC, particularly in the 5 and 10% incorporated groups, can be attributed to the unique properties of the nanocomposite. Chitosan, derived from chitin, is known for its biocompatibility and antimicrobial characteristics, and it has the potential to enhance wear resistance through its interaction with the dental structure. The incorporation of titanium and zirconium, recognized for their reinforcing properties in materials, likely contributes to the overall strength and durability of the GIC.20 Hydroxyapatite, resembling the mineral composition of natural bone, not only adds bioactivity but also enhances the material's performance in dental applications. The 5 and 10% incorporation levels may represent an optimal balance, where the nanocomposite imparts additional strength and resistance to wear without compromising the overall properties of the GIC. This suggests that these concentrations strike a favorable synergy among the nanocomposite components, resulting in a nanocomposite GIC with enhanced wear resistance. The uniform dispersion of these elements in the GIC matrix could contribute to better load distribution and resistance to abrasive forces encountered during mastication. The findings highlight the potential of this nanocomposite modification to address the limitations of conventional GIC, providing a basis for further exploration and refinement of these nanocomposite concentrations for enhanced wear resistance in practical dental applications.
The utilization of a naturally derived, green-mediated composite in this study, composed of chitosan, titanium, zirconium, and hydroxyapatite in GIC, presents distinct advantages. The green synthesis approach not only aligns with sustainable and eco-friendly practices but also harnesses the inherent properties of these natural elements. Chitosan, as a biopolymer derived from chitin, enhances biocompatibility and brings antimicrobial benefits to the composite. Titanium and zirconium, incorporated through green synthesis, contribute to the mechanical reinforcement of the GIC, providing strength and durability. The inclusion of hydroxyapatite, a biomimetic material resembling the composition of natural bone, adds both bioactivity and compatibility with dental tissues. This naturally derived, green-mediated composite offers a holistic approach, combining environmental sustainability with improved material performance. Such an approach is promising for dental applications and aligns with the broader trend in materials science toward sustainable and biocompatible solutions. This study sets the stage for further exploration of green-mediated composites in restorative dentistry, emphasizing the potential for eco-friendly and effective dental materials.
The present study results proved that greater wear resistance of nanocomposite-modified GIC is in line with the study done by Mohammadi et al.21 who proved that nanocellulose-modified GIC increased wear resistance when compared to conventional GIC. Another study, which is in accordance with our results, concluded that GIC exhibited poor mechanical performance, characterized by high porosity and wear rates, in comparison to resin composite.22 Also, the study done by Sainath Reddy et al.23 concluded that conventional GIC exhibited low wear resistance when compared with hybrid GIC. As the surface roughness of a restoration material has a great influence on wear and longevity, zirconia was reinforced to GIC in the current study, as zirconia reinforced GIC has proved to have less surface roughness with increased microhardness and greater wear resistance.24 Another work done by Poorzandpoush et al.25 proved that incorporation of nanohydroxyapatite increases the wear resistance, which is in accordance with our current results. Addition of nanoparticles improves the wear resistance,26 but in other ways, it has a detrimental effect when an increase in concentration occurs, which is similar to the present study where 5% nanocomposite-modified GIC gave a better result compared to 10% nanocomposite-modified GIC.
Considering that traditional glass ionomer restorative materials are not typically designed for long-term resistance to high occlusal loads, a relatively low load of 50 N was considered suitable for this study. However, establishing direct correlations between the wear characteristics observed in this study and in vitro data or other chewing simulator tests poses challenges due to significant methodological differences. These variations include factors like the type of opposing material, the intensity of the loading force, and the patterns of movement, all of which are crucial in designing wear tests that simulate oral conditions. Wear, being a nonintrinsic property, lacks standardized methods for determining the wear resistance of restorative materials.27 In this study's chewing simulation test, a steatite cone acted as the antagonist, directly applying force to the restoration. Although steatite may not be as clinically relevant as enamel or an opposing tooth for load transfer, employing standardized steatite antagonists in chewing simulation tests allows for quantitative result evaluation and comparison of the modified and unmodified glass ionomer restorative materials.19,28 Due to the relatively limited surface area of the prepared cavities, a cone-shaped antagonist was deemed optimal for precise placement within the fissure. Another constraint in this experimental arrangement is the utilization of water, as opposed to artificial saliva, as a transport medium for chewing simulation.29,32 Moreover, an escalation in the wear rate might occur when acidic substances interact with high occlusal loads.33,35 Subsequent research should address corrosive wear to offer a comprehensive exploration of the various wear mechanisms in the oral cavity. While this study offers valuable insights into the wear characteristics of glass ionomer restorative materials under specific conditions, it is crucial to interpret the results mindful of the specified constraints. Future research initiatives should strive to overcome these limitations, aiming for a more thorough understanding of wear mechanisms in the oral environment and exploring innovative methodologies to enhance clinical relevance and applicability.
Conclusion
A groundbreaking outcome was revealed in our investigation into the enhancement of GIC through the utilization of a green-mediated nanocomposite consisting of chitosan, titanium, zirconium, and hydroxyapatite. A remarkable improvement in wear resistance was observed with the incorporation of these nanoparticles, particularly in 5 and 10% concentrations. The limitations of traditional GIC were effectively addressed, marking a significant advancement in the realm of dental restorative solutions. The advantageous properties of chitosan, titanium, zirconium, and hydroxyapatite, coupled with the eco-friendly approach of green synthesis, were underscored as multifaceted benefits of this innovation. As dentistry progresses toward sustainable and biocompatible materials, our study lays the groundwork for future advancements, representing a significant stride in the creation of dental restorations that excel in both efficacy and environmental responsibility.
Orcid
Jessy Paulraj https://orcid.org/0000-0001-9231-6077
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Gunay A, Celenk S, Adiguzel O, et al. Comparison of antibacterial activity, cytotoxicity, and fluoride release of glass ionomer restorative dental cements in dentistry. Med Sci Monit. 2023;29:e939065. doi: 10.12659/MSM.939065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mustafa HA, Soares AP, Paris S, et al. The forgotten merits of GIC restorations: a systematic review. Clin Oral Investig. 2020;24(7):2189–2201. doi: 10.1007/s00784-020-03334-0. [DOI] [PubMed] [Google Scholar]
- 3.Fricker JP. Therapeutic properties of glass-ionomer cements: their application to orthodontic treatment. Aust Dent J. 2022;67(1):12–20. doi: 10.1111/adj.12888. [DOI] [PubMed] [Google Scholar]
- 4.Dezanetti JMP, Nascimento BL, Orsi JSR, et al. Effectiveness of glass ionomer cements in the restorative treatment of radiation-related caries—a systematic review. Support Care Cancer. 2022;30(11):8667–8678. doi: 10.1007/s00520-022-07168-2. [DOI] [PubMed] [Google Scholar]
- 5.Iaculli F, Salucci A, Di Giorgio G, et al. Bond strength of self-adhesive flowable composites and glass ionomer cements to primary teeth: a systematic review and meta-analysis of in vitro studies. Materials (Basel) 2021;14(21):6694. doi: 10.3390/ma14216694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heintze SD, Loguercio AD, Hanzen TA, et al. Clinical efficacy of resin-based direct posterior restorations and glass-ionomer restorations - an updated meta-analysis of clinical outcome parameters. Dent Mater. 2022;38(5):e109–e135. doi: 10.1016/j.dental.2021.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Messer-Hannemann P, Samadi M, Böttcher H, et al. Evaluation of a method to determine wear resistance of class I tooth restorations during cyclic loading. Materials (Basel) 2022;15(15):5440. doi: 10.3390/ma15155440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berg JH, Croll TP. Glass ionomer restorative cement systems: an update. Pediatr Dent. 2015;37(2):116–124. [PubMed] [Google Scholar]
- 9.World Health Organization . World Health Organization Model List of Essential Medicines—22nd List, 2021. World Health Organization; Geneva, Switzerland: 2021. [Google Scholar]
- 10.Ivanišević A, Rajić VB, Pilipović A, et al. Compressive strength of conventional glass ionomer cement modified with TiO2 nano-powder and marine-derived HAp micro-powder. Materials (Basel) 2021;14(17):4964. doi: 10.3390/ma14174964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moheet IA, Luddin N, Rahman IA, et al. Modifications of glass ionomer cement powder by addition of recently fabricated nano-fillers and their effect on the properties: a review. Eur J Dent. 2019;13(3):470–477. doi: 10.1055/s-0039-1693524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faul F, Erdfelder E, Buchner A, et al. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 13.Saadat M, Moradian M, Mirshekari B. Evaluation of the surface hardness and roughness of a resin-modified glass ionomer cement containing bacterial cellulose nanocrystals. Int J Dent. 2021;2021:8231473. doi: 10.1155/2021/8231473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahman IAB. One-pot synthesis of hydroxyapatite–silica nanopowder composite for hardness enhancement of glass ionomer cement (GIC) Bull Mater Sci. 2014;37(2):213–219. [Google Scholar]
- 15.Weigl P, Sander A, Wu Y, et al. In-vitro performance and fracture strength of thin monolithic zirconia crowns. J Adv Prosthodont. 2018;10(2):79–84. doi: 10.4047/jap.2018.10.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frankenberger R, Dudek MC, Winter J, et al. Amalgam alternatives critically evaluated: effect of long-term thermomechanical loading on marginal quality, wear, and fracture behavior. J Adhes Dent. 2020;22(1):107–116. doi: 10.3290/j.jad.a44001. [DOI] [PubMed] [Google Scholar]
- 17.Baumgart P, Kirsten H, Haak R, et al. Biomechanical properties of polymer-infiltrated ceramic crowns on one-piece zirconia implants after long-term chewing simulation. Int J Implant Dent. 2018;4(1):16. doi: 10.1186/s40729-018-0127-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wassell RW, McCabe JF, Walls AW. A two-body frictional wear test. J Dent Res. 1994;73(9):1546–1553. doi: 10.1177/00220345940730091001. [DOI] [PubMed] [Google Scholar]
- 19.Rosentritt M, Preis V, Behr M, et al. Two-body wear of dental porcelain and substructure oxide ceramics. Clin Oral Investig. 2012;16(3):935–943. doi: 10.1007/s00784-011-0589-9. [DOI] [PubMed] [Google Scholar]
- 20.Nawafleh N, Hatamleh M, Elshiyab S, et al. Lithium disilicate restorations fatigue testing parameters: a systematic review. J Prosthodont. 2016;25:116–126. doi: 10.1111/jopr.12376. [DOI] [PubMed] [Google Scholar]
- 21.Mohammadi N, Fattah Z, Borazjani LV. Nano-cellulose reinforced glass ionomer restorations: an in vitro study. Int Dent J. 2023;73(2):243–250. doi: 10.1016/j.identj.2022.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neves AB, Lopes LIG, Bergstrom TG, et al. Porosity and pore size distribution in high-viscosity and conventional glass ionomer cements: a micro-computed tomography study. Restor Dent Endod. 2021;46(4):e57. doi: 10.5395/rde.2021.46.e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sainath Reddy TH, Venkatesh KV, Mani R. Comparative evaluation of three different glass ionomer cements. Indian J Dent Res. 2021;32(4):485–488. doi: 10.4103/ijdr.ijdr_603_21. [DOI] [PubMed] [Google Scholar]
- 24.Nica I, Stoleriu S, Iovan A, et al. Conventional and resin-modified glass ionomer cement surface characteristics after acidic challenges. Biomedicines. 2022;10(7):1755. doi: 10.3390/biomedicines10071755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poorzandpoush K, Omrani LR, Jafarnia SH, et al. Effect of addition of nano hydroxyapatite particles on wear of resin modified glass ionomer by tooth brushing simulation. J Clin Exp Dent. 2017;9(3):e372–e376. doi: 10.4317/jced.53455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azmy E, Al-Kholy MRZ, Fattouh M, et al. Impact of nanoparticles additions on the strength of dental composite resin. Int J Biomater. 2022;2022:1165431. doi: 10.1155/2022/1165431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heintze SD, Reichl FX, Hickel R. Wear of dental materials: clinical significance and laboratory wear simulation methods—a review. Dent Mater J. 2019;38:343–353. doi: 10.4012/dmj.2018-140. [DOI] [PubMed] [Google Scholar]
- 28.Sindhu JS, Maiti S, Nallaswamy D. Comparative analysis on efficiency and accuracy of parallel confocal microscopy and three-dimensional in motion video with triangulation technology-based intraoral scanner under influence of moisture and mouth opening – a crossover clinical trial. J Indian Prosthodont Soc. 2023;23:234–243. doi: 10.4103/jips.jips_65_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turssi CP, Faraoni JJ, de Menezes M, et al. Analysis of potential lubricants for in vitro wear testing. Dent Mater. 2006;22:77–83. doi: 10.1016/j.dental.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Alvanforoush N, Wong R, Burrow M, et al. Fracture toughness of glass ionomers measured with two different methods. J Mech Behav Biomed Mater. 2019;90:208–216. doi: 10.1016/j.jmbbm.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 31.R S, Maiti S, P J. Comparative analysis of abrasion resistance in relation to different temporary acrylic crown material using toothbrush simulator—an in vitro study. Int J Dentistry Oral Sci. 2021;8(4):2153–2215. [Google Scholar]
- 32.Neha N, Maiti S, Jessy P. Adhesion of microflora and the role of dentifrices in color stability on provisional crowns: an in vitro study. Int J Dentistry Oral Sci. 2021;8(8):3805–3809. doi: 10.19070/2377-8075-21000780. [DOI] [Google Scholar]
- 33.Pavithra AS, Paulraj J, Rajeshkumar S, et al. Comparative evaluation of antimicrobial activity and compressive strength of conventional and thyme-modified glass ionomer cement. Ann Dent Spec. 2023;11(1):70–77. doi: 10.51847/FrmCSw6TqP. [DOI] [Google Scholar]
- 34.Paulraj J, Nagar P. Antimicrobial efficacy of triphala and propolis-modified glass ionomer cement: an in vitro study. Int J Clin Pediatr Dent. 2020;13(5):457–462. doi: 10.5005/jp-journals-10005-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou ZR, Zheng J. Tribology of dental materials: a review. J Phys D Appl Phys. 2008;41:1130. [Google Scholar]