Abstract
Introduction
It is reasonable to assume that lumbar spinal stenosis (LSS) affects the cauda nerve roots also at night.
Research question
Does microsurgical decompression influence sleep quality and position?
Materials and methods
A study nurse interviewed 140 patients scheduled for LSS decompression using the Pittsburgh Sleep Quality Index (PSQI), Spinal Stenosis Measure (SSM), Numeric Rating Scale (NRS) for back and leg pain, Douleur Neuropathique (DN4), and Charlson Comorbidity Index. Epidemiologic and MRI data were collected along with self-reported rankings of preferred sleep positions (prone, supine, side, and fetal). Follow-up interviews were conducted by telephone six and 18 months after discharge. Statistical analysis was performed using SSPS 24, with significance set at p < 0.05.
Results
132 patients (55% female, mean age 73 years) were evaluated. Preoperatively, 45 (34.1%) patients were classified as good sleepers (GS: PSQI ≤5, range 1–21 (worst)) and 87 (65.9%) as poor sleepers (PS: PSQI ≥6). Decompression surgery reversed the relationship between PS (31.8%) and GS (68.2%, recovered/improved). Protective fetal sleeping position was the most common (≥70%) before and after surgery for both PS and GS. Risk factors for PS included female sex (p = 0.03), obesity (p = 0.03), high NRS back pain score (p = 0.008), and high SSM symptom score (p = 0.004). MRI imaging did not differ between PS and GS.
Discussion and conclusion
LSS had a negative effect on sleep quality, whereas surgical decompression had a positive effect. The protective fetal sleeping position was the preferred position both before and after surgery.
Keywords: Lumbar spinal stenosis, Sleep quality, Sleep position, Surgical decompression, Prospective cohort study
Graphical abstract
Highlights
-
•
Sleep disorders affect two-thirds of lumbar spinal stenosis patients.
-
•
Fetal sleep position widens lumbar spinal canal the most.
-
•
The fetal sleep position is preferred by three-quarters of patients.
-
•
Surgical decompression has been shown to significantly improve sleep quality.
-
•
Patients continue to prefer the fetal position after surgery.
1. Introduction
In daily practice, the diagnosis of lumbar spinal stenosis (LSS) is based on symptoms such as decreased walking distance, pain relief with anteflexed posture, and MRI images showing a narrowed lumbar spinal canal. However, the impact of LSS on sleep quality and sleep position at night is less well understood. Recent studies have shown that approximately two-thirds of patients with LSS suffer from sleep disturbances (Lee et al., 2020; Kim et al., 2020a). In a survey of 10,000 cardiovascular patients, only 19% reported experiencing sleep disturbance (Michal et al., 2014). In contrast, the meta-analysis of 1936 patients presenting with low back pain (LBP) showed that each one-point increase in LBP on a ten-point visual analog scale (VAS) was associated with a 10% increase in the likelihood of reporting sleep disturbance (Alsaadi et al., 2011). Patients with LSS typically experience less pain when leaning forward, especially when sitting. Leaning forward has been shown to increase the cross-sectional area of the lumbar dura, which provides more space for the cauda nerve roots (CNR) (Papavero et al., 2022). It is worth considering whether patients also unconsciously prefer a protective sleeping position at night.
The objective of this prospective cross-sectional study was to assess the prevalence of sleep disturbances in a cohort of surgical candidates with symptomatic LSS, as well as to investigate the impact of LSS and microsurgical decompression on sleep quality and sleep position. The authors hypothesized that symptomatic LSS would negatively affect sleep quality, while surgical decompression would lead to an improvement. Furthermore, the stenotic spine would favor a fetal sleeping position that maximizes lumbar intradural capacity. It was anticipated that following surgery, the frequency of the protective position would decrease.
2. Materials and Methods
2.1. Study design and patient selection
A prospective cross-sectional cohort study enrolled 140 consecutive surgical candidates for microsurgical decompression of LSS between October 2021 and June 2022. The inclusion criteria were symptomatic single/multi-level LSS refractory to conservative therapy for six months, clear MRI findings, and informed consent. Exclusion criteria included previous lumbar spine surgery, mobile vertebral slip, revision surgery, abnormal coagulation, and infection. The study protocol (2021-100701-BO-ff) was approved by the Ethics Committee of the State Hamburg. The study was conducted in accordance with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines (von Elm et al., 2007).
2.2. The interview
Upon admission to the ward, the study nurse conducted interviews with the patients. The interview covered demographic data, education, occupation, lifestyle, body mass index (BMI), medical comorbidities (Charlson Comorbidity Index), and the presence of chronic joint pain in the upper or lower extremities. In addition, the patients completed several validated questionnaires (Table 1). Finally, the patients were shown four drawings depicting different sleep positions (prone, supine, side, and fetal) and asked to rank their preferred ones.
Table 1.
Questionnaires used in the interview.
| Questionnaire | Content | Subcategories/total No. of questions | Score |
|---|---|---|---|
| Pittsburgh Sleep Quality Index (PSQI) | Sleep quality | 7/10 | 0–21 (worst) ≤ 5: Good sleeper |
| ≥6: Poor sleeper | |||
| 3 points = MCID | |||
| Spinal Stenosis Measure (SSM) (Formerly: Zuerich Claudication Questionnaire) | Symptoms Function Satisfaction | 3/7 | 7–35 (worst) |
| 3/5 | 5–20 (worst) | ||
| 3/6 | 6–24 (worst) | ||
| Douleur Neuropathique 4 | Neuropathic pain | 2/10 | 0–10 (worst) ≥ 4: Neuropathic pain |
| Numeric Rating Scale (NRS) back/leg pain | Intensity of pain | 1/1 | 1–10 (worst) |
| Sleep posture | Sleep posture | 1/4 | Prone – supine – side – fetal |
| Charlson-Comorbidity-Index | Number of comorbidities | 1/19 | 0–33 (worst) |
MCID: Minimal Clinically Important Difference.
The interview was repeated by phone six and 18 months after discharge from the hospital, and the question 'Satisfaction with the surgery' was added.
2.3. Measurement of sleep disturbance
The study assessed sleep disturbance in the past month by means of the Pittsburgh Sleep Quality Index (PSQI) questionnaire in the local language version. (Buysse et al., 1989). The questionnaire consists of 19 questions that evaluate seven modules: sleep quality, latency, duration, disturbance, habitual sleep efficiency, use of sleep medications, and daytime dysfunction. The sum of the seven components yields a global score ranging from 0 to 21 points (worst). A PSQI score greater than 5 points demonstrated a diagnostic sensitivity of 89.6% and specificity of 86.5% (к = 0.75, p < 0.001) in distinguishing Poor Sleepers (PS) from Good Sleepers (GS). Minimal Clinically Important Difference (MCID) is the difference in PSQI scores between patients with no sleep quality changes and patients with “small” improvements. Previous reports have defined a change in global PSQI score of ≥3 points as the MCID (Hughes et al., 2009).
2.4. MRI - imaging
The severity and morphology of LSS were defined using the following MRI parameters: number of affected levels, facet joint effusion (yes or no), vertebral slip (Meyerding classification, Meyerding, 1932), dural cross-sectional area (DCSA, mm2; Schizas classification, Schizas et al., 2010), and redundant nerve roots (ASED classfication, Papavero et al., 2020). Findings were classified independently by three spine surgeons with varying levels of experience: three, ten, and 35 years. Any borderline cases were discussed and resolved by consensus.
2.5. Surgical procedure
The stenotic levels were decompressed using a unilateral laminotomy and bilateral decompression (ULBD), also known as the 'over the top' or 'cross-over' technique, under general anesthesia. The procedures were performed in a standardized, minimally invasive manner with the aid of a microscope, from skin to skin, by board-certified surgeons (Mayer and Heider, 2016).
2.6. Statistical analysis
The study required 131 patients with a power of 0.90 (alpha = 0.05) to detect a difference in means of one-point PSQI score, assuming a standard deviation of 3.5. The patients were divided into two groups: PS (PSQI score ≥6) and GS (PSQI score ≤5). Multivariate logistic regression analysis was used to assess the effect of surgical treatment on the improvement of PSQI score at the six and 18-month interviews. Continuous variables were presented as means ± standard deviation, while discontinuous measures were presented as standard deviations and quartiles. Categorical and nominal variables were reported as absolute and relative frequencies. The tested variables did not follow a normal distribution (Kolmogorov-Smirnov test: p < 0.05). For dependent and independent samples of continuous data, the Wilcoxon and Mann-Whitney U tests were used, respectively. The tests were two-tailed, and a p-value <0.05 indicated statistical significance. Statistical analyses were performed using IBM SPSS software version 24 for Macintosh (IBM Corp. Armonk, New York).
3. Results
3.1. Comparison of poor sleepers with good sleepers
LSS considerably affected the sleep quality of patients. According to the PSQI, 87 patients (65.9%) were classified as poor sleepers, while 45 (34.1%) were classified as good sleepers. These data confirm the findings of Kim et al., 2020a, Kim et al., 2020b). Risk factors for poor sleep included female sex, BMI >29 kg/m2, NRS of back pain >7, and high SSM-Symptoms scores. The severity of leg pain and neurogenic claudication were also more severe in poor sleepers, but not significantly so. Furthermore, there were no differences in workload, painful peripheral joints, neuropathic pain, general comorbidities, or satisfaction with surgery between PS and GS. Contrary to expectations, the MRI-imaging parameters that depict the extension and severity of lumbar stenosis did not differ significantly between the two groups (Table 2).
Table 2.
Poor sleepers (PS) and good sleepers (GS).
| PS | Mean ± SD | 95%CI | GS | Mean ± SD | 95% CI | P | |
|---|---|---|---|---|---|---|---|
| Female sex | 46% | 26.7% | 0.039 | ||||
| PSQI* | 9.71 ± 2.97 | [ 9.08, 10.35] | 3.58 ± 1.23 | [3.21, 3.95] | < 0.001 | ||
| BMI [kg/m2] | 29.26 ± 5.04 | [28.18, 30.33] | 27.09 ± 4.51 | [25.73, 28.44] | 0.033 | ||
| NRS back pain | 7.14 ± 2.27 | [6.65, 7.62] | 5.42 ± 3.43 | [4.39, 6.45] | 0.008 | ||
| Pain medication | 0.59 ± 1.11 | [0.35, 0.82] | 0.09 ± 0.47 | [0.05, 0.23] | 0.003 | ||
|
SSM-Symptoms |
24.05 ± 4.26 |
[23.14, 24.95] |
21.96 ± 3.15 |
[21.01, 22.90] |
0.003 |
||
| NRS leg pain | 6.75 ± 2.93 | [6.12, 7.37] | 6.13 ± 2.66 | [5.33, 6.93] | 0.070 | ||
|
SSM-Function |
14.09 ± 2.35 |
[13.59,14.59] |
13.24 ± 2.39 |
[12.53, 13.96] |
0.078 |
||
| Occupation | (none/sed/phys) | 0.915 | |||||
| DN4** | 2.21 ± 1.81 | [ 1.82, 2.59] | 1.69 ± 1.29 | [1.30, 2.08] | 0.152 | ||
| Charlson-Index |
0.80 ± 1.46 |
[ 0.49, 1.12] |
0.64 ± 1.25 |
[0.27, 1.02] |
0.476 |
||
| DCSA (mm2) | 50.26 ± 19.43 | [45.94, 54.59] | 53.07 ± 19.22 | [0.27, 1.02] | 0.253 | ||
| RNR positive | 62.7% | 61.4% | 1.000 | ||||
| Satisfaction with surgery*** | 6 mos | 2.15 ± 0.85 | 2.22 ± 0.88 | 0.346 | |||
| 18 mos | 1.91 ± 1.09 | 1.89 ± 1.08 | 0.454 | ||||
Bold values (significant), Cursive values (trend). Values are represented as mean ± SD. *PS was defined as ≥ 6 of global PSQI score. ** DN4 ≥ 4 indicates neuropathic pain. Pain medication: 0 = none, 1 = non-opioid, 2 = opioid. *** Range from 1 (very satisfied) to 4 (very dissatisfied).
3.2. The impact of surgical decompression on neurogenic claudication and on sleep quality
Table 3 displays the effect of surgical decompression on symptoms (SSM-Symptoms) and neurogenic claudication (SSM-Function) resulting from LSS. The noteworthy improvement after surgery confirms the appropriateness of both the indication and surgical procedure.
Table 3.
The surgical impact on Symptoms and Function.
| Cohort: n = 132 |
Preoperative |
Postoperative* |
P value** | ||
|---|---|---|---|---|---|
| Mean ± SD | 95%CI | Mean ± SD | 95%CI | ||
| SSM-Symptoms | |||||
| Points: 7–35 (worst) |
23.33 ± 4.03 |
[22.64, 24.03] |
16.98 ± 5.75 |
[15.99, 17.98] |
< 0.001 |
| SSM-Function | |||||
| Points: 5–20 (worst) | 13.80 ± 2.39 | [13.39,14.21] | 9.54 ± 3.68 | [8.90, 10.17] | < 0.001 |
*Six months after surgery ** Wilcoxon signed ranks test.
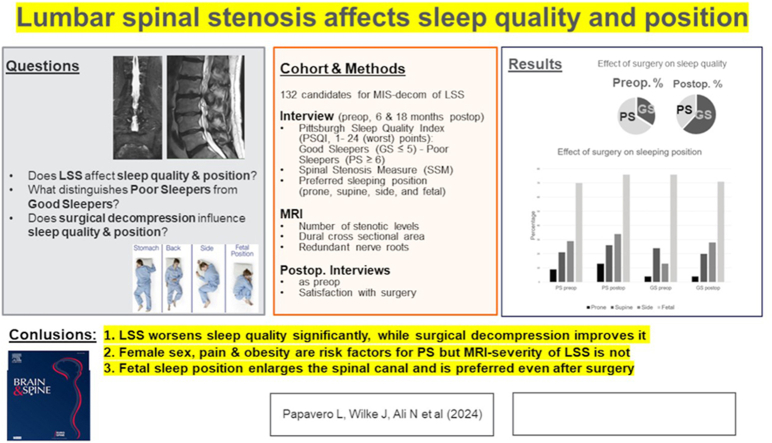
The study defined postoperative improvement of sleep quality as a decrease of ≥3 points, the MCID, in the PSQI global score. The transition from poor sleep (cutoff PSQI ≥6) to good sleep corresponded to the complete 'recovery' of sleep quality. Out of the 45 preoperative good sleepers, two (4.4%) became poor sleepers postoperatively. Conversely, out of the 87 preoperative poor sleepers, eight (9.2%) improved in terms of MCID and 39 (44.8%) became good sleepers postoperatively. In other words, the preoperative distribution of 34% GS and 66% PS was reversed by surgery to 62% GS, 6% improved sleepers, and 32% PS (Fig. 1).
Fig. 1.
The impact of surgery on sleep quality.
Surgical decompression leads to an inversion of the PS/GS ratio within the first six months after surgery. Although the positive effect on sleep quality decreases slightly after 18 months, it remains highly significant.
3.3. Sleep position
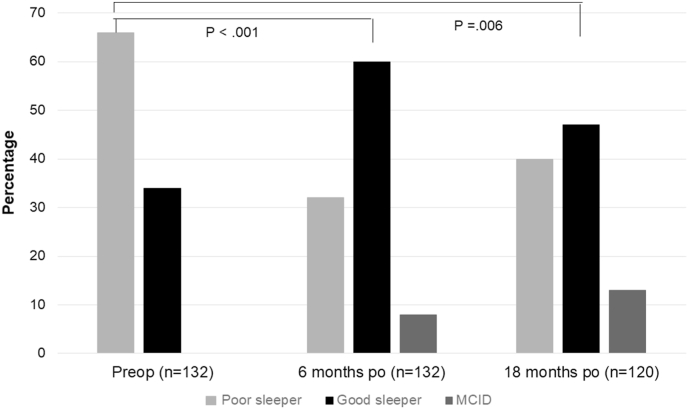
The fetal sleep position (F) and the side sleep position (S) decrease lumbar lordosis. That enlarges the dural cross-sectional area and mitigates compression of stenosis on the cauda nerve roots (CNR). Therefore, these positions are considered 'protective' or 'supportive'. During the interview, patients were asked to rank their preferred postures. If multiple postures were mentioned, the final percentage was greater than 100. Before surgery, 75% of GS (64% F and 11% S) and 79% of PS (55% F and 24% S) chose supportive sleep positions. The prevalence of the maximum supportive position, which is the fetal one, was particularly high, exceeding the prevalence in the normal population, with 38% in males and 51% in females (Fig. 2).
Fig. 2.
The preoperative distribution of sleep position.
Both good and poor sleepers prefer a supportive or protective sleeping position that reduces lumbar lordosis, increases dural cross-sectional area, and reduces cauda nerve roots compression. The prevalence of the fetal position far exceeds that of the normal population.
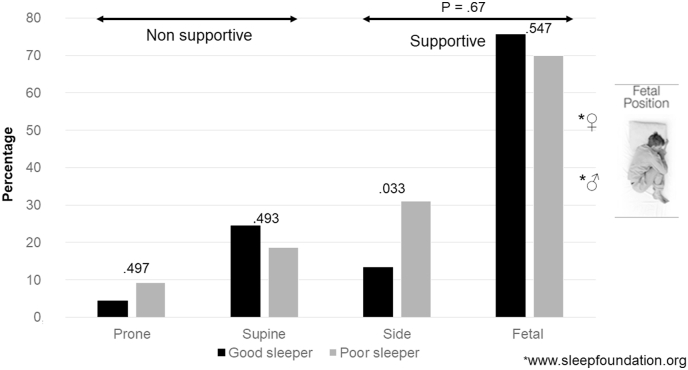
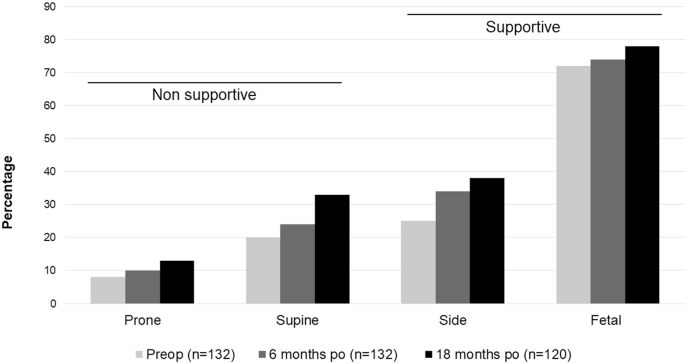
4. The impact of surgery on sleep position
Most patients maintained their supportive sleep position, specifically the fetal position, even 18 months after surgery. This result was somewhat surprising. One possible explanation is that symptoms of lumbar spinal stenosis develop slowly over many years, even decades. It is possible that during this long-time span, the fetal sleep position became a protective reflex for minimizing the compression of the CNR and became engraved as a cerebral engram. This could explain why the posture persisted even after surgery. However, we observed an increased tendency to switch positions after decompression over time, which we interpreted as a higher degree of freedom from pain constraints (Fig. 3).
Fig. 3.
The impact of surgery on sleep position.
Although supportive sleep positions are still preferred after surgery, the patients are more free to move between the positions than before decompression.
5. Discussion
The symptoms of LSS during the day are well-known. Patients often adopt forward-bent postures while walking, sitting, or squatting to relieve symptoms, as these postures are believed to increase the cross-sectional area of the spinal canal. Given that sleep constitutes approximately one-third of our lifetime, it is plausible that LSS could also cause symptoms during the night. Only a few recent studies have examined the impact of LSS on sleep disturbance (Kim et al., 2020a, 2020b; Lee et al., 2020). However, none of these studies have investigated the relationship between LSS and the preferred sleep position of affected patients. A study using postural upright-MRI has shown that the DCSA is maximally increased in the flexed sitting position compared to the supine and neutral sitting positions. Additionally, the postural change disproportionately enlarges the intradural capacity at the stenotic level compared to the non-stenotic levels in the same spine (Papavero et al., 2022).
The present study found that 65.9% of patients with LSS reported sleep disturbances. It is important to note that various factors, such as sex, socioeconomic status, medical risk factors, depression, and anxiety, can contribute to the occurrence of sleep disorders. However, two studies conducted in different countries also found that 63.5% and 66.1% of patients with LSS experienced sleep disturbances (Lee et al., 2020); Kim et al., 2020a, 2020b. Risk factors for PS include female sex, overweight, high NRS of back pain, and high SSM-Symptoms scores. The severity of leg pain and neurogenic claudication was slightly more severe in PS, but not significantly (p = 0.07). Surprisingly, the MRI-imaging parameters that defined the extension and severity of lumbar stenosis did not differ significantly between PS and GS (refer to Table 2). A possible explanation for this counterintuitive fact is that, like to daytime, there is not a strict correlation between the severity of MRI-imaging and clinical disturbances.
In a cross-sectional study conducted in Denmark (Skarpsno et al., 2017), accelerometers were used to record the sleep positions (front, back, and side) of 363 men and 301 women from the general population for six consecutive nights. The data was stratified by sex, age group, and BMI. The overweight participants aged 55–65 years with a BMI of 25–29.9 kg/m2 were found to be the most comparable to our cohort (mean age: 73 years; BMI 28.6 ± 4.8 kg/m2). The study found that as age and BMI increased, there was a higher prevalence of side sleep position (58.3%), compared to back (32.8%) and front (7.7%) positions. In our study, patients were asked to differentiate between side (S) and fetal (F) sleep positions, as the latter can be seen as the nighttime equivalent of the daytime flexed sitting. Both protective postures minimize the compressive effect of stenosis on the cauda nerve roots. In the GS and PS positions, 75% (64% F + 11% S) and 79% (55% F + 24% S) respectively, were found to be protective. Even when considering the influence of aging and BMI, in our study the prevalence of protective positions exceeded by far the prevalence in general population., The fetal sleep position can be considered a powerful unconscious mechanism that protects the CNR.
Several factors, including female sex, overweight, depression, and foraminal stenosis, have been reported to be associated with sleep disturbance in patients with LSS (Finan and Smith, 2013). Additionally, improvement in sleep quality following surgical treatment can be defined as a decrease of at least three points (MCID) in the PSQI global score or as the transition from poor sleep (cutoff PSQI ≥6) to good sleep, which corresponds to the 'recovery' of sleep quality. Finally, the invasiveness of surgery may also play a role, ranging from a single-level microsurgical decompression to a four-level open decompression with fixation. These factors explain the wide range of postoperative improvement reported in studies, from 17.5% (Lee et al., 2020) to 85% (Kim et al., 2020a, Kim et al., 2020b). The patients in the present study underwent microsurgical single- or multi-level decompressions using a microscope, from skin to skin. The study found that 45% of patients with preoperative poor sleep quality due to LSS experienced normalization, while 9% experienced improvement. This aspect should be taken into consideration when counseling patients in daily practice.
The interaction between sleep disturbance and pain is reciprocal and bidirectional. Sleep problems can exacerbate pain, and pain can interfere with sleep. According to Koffel et al. (2016), improvements in self-reported sleep measures within three months predicted improvements in pain over the course of a year. Conversely, worsening sleep predicted worsening pain over time. However, there is growing evidence to suggest that sleep has a greater impact on pain than pain has on sleep (Koffel et al., 2016; Finan et al., 2013). An analysis of data from a randomized controlled trial involving 250 chronic pain patients indicated that changes in sleep were a stronger predictor of changes in pain (p < 00.001) than changes in pain predicting changes in sleep (p < 00.05). It is important to keep in mind these findings when advising patients on surgical decompression of lumbar stenosis. Improving the quality of life should not only focus on daytime activities, such as walking without constraints, but also consider the relationship between sleep quality and pain perception. There are limitations to this study. The self-reported sleep positions may be uncertain due to unconscious reporting, but the goal of the interview was to assess differences between the preferred sleep postures of both PS and GS, rather than the absolute reliability of the answers. Therefore, any bias was expected to be equally distributed between the two groups. Kim et al. (2020a) reported that anxiety and depression are significant risk factors for sleep disturbances. The interview in this study lasted at least 45 min. To save time, we did not include additional specific scores, such as the Hamilton Depression Rating Scale.
Equally, obstructive sleep apnea (OSA), a common sleep-related breathing disorder, was not investigated. Its prevalence and severity of sleep disturbances increase with advanced age and body weight. In the general adult population, the prevalence ranges between 41% and 49%. However, in people older than 65 years, the condition affects as many as 90% of men and 78% of women, whether severe or mild (Senaratna et al., 2017; Ghavami et al., 2023). In this study, there was no significant difference in age (p = 0.45) or body weight (p = 0.59) between the patients who improved after decompression and those who did not. Thus, it can be inferred that OSA did not have an impact on the varied clinical outcome. To the best of our knowledge, we investigated sleep disturbances and sleep position in the largest cohort of patients affected by LSS requiring surgical decompression to date. The study was monocentric, which provided homogeneity of indications and surgical technique, increasing the reliability of postoperative interviews.
6. Conclusions
This prospective cross-sectional cohort study investigated the influence of LSS on sleep quality and sleep position. The effect of microsurgical decompression on both parameters was analyzed at six and 18 months after surgery. Two-thirds of patients with symptomatic LSS experienced sleep disturbances. Like the bent-forward sitting position during the day, the fetal sleeping position appeared to be a protective posture that increases the lumbar intradural capacity, reducing the compression of the CNR. Surgical decompression had a clear positive effect on postoperative sleep quality. This effect decreased over time but remained significant at 18 months after surgery. The fetal sleeping position remained the preferred position after surgery, but patients switched between positions more freely than before decompression.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics approval
This study has been approved by the Ethics Committee of the State of Hamburg (2021-100701-BO-ff) and was conducted according to principles enshrined in the Declaration of Helsinki and in accordance with the Medical Research Involving Human Subjects Act (WMO). The patients provided a written informed consent.
Author contributions
All authors contributed to the study conception and design. Material preparation and data collection and analysis were performed LP, JW, NA, KS, AH and KSch. The first draft of the manuscript was written by LP and all authors commented on previous versions of the manuscript. All authors revised the manuscript and approved the version to be published.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author upon reasonable request.
Declaration of Generative AI and AI-assisted technologies in the writing process
Nothing to disclose.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank former patient Mr. Dieter Witt for sponsoring the Open Access APC. The authors thank the surgeons Janina Isabell Bergmann, Christina Gibbert, Ralph Kothe, Justus Oehm, Markus Pietrek, Gregor Schmeiser, and Moritz Schreiber for their cooperation.
Handling Editor: Prof F Kandziora
References
- Alsaadi S.M., McAuley J.H., Hush J.M., Maher C.G. Prevalence of sleep disturbance in patients with low back pain. Eur. Spine J. 2011;20(5):737–743. doi: 10.1007/s00586-010-1661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan P.H., Smith M.T. The comorbidity of insomnia, chronic pain, and depression: dopamine as a putative mechanism. Sleep Med. Rev. 2013;17(3):173–183. doi: 10.1016/j.smrv.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse D.J., Reynolds C.F., Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatric Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Finan P.H., Goodin B.R., Smith M.T. The association of sleep and pain: an update and a path forward. J. Pain. 2013;14(12):1539–1552. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghavami T., Kazeminia M., Ahmadi N., Rajati F. Global prevalence of obstructive sleep apnea in the elderly and related factors: a systematic review and meta-analysis study. J. PeriAnesthesia Nurs. 2023 doi: 10.1016/j.jopan.2023.01.018. [DOI] [PubMed] [Google Scholar]
- Hughes C.M., McCullough C.A., Bradbury I., Boyde C., Hume D., Yuan J., et al. Acupuncture and reflexology for insomnia: a feasibility study. Acupunct. Med. 2009;27(4):163–168. doi: 10.1136/aim.2009.000760. [DOI] [PubMed] [Google Scholar]
- Kim J., Lee S.H., Kim T.H. Improvement of sleep quality after treatment in patients with lumbar spinal stenosis: a prospective comparative study between conservative versus surgical treatment. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-71145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Park J., Kim S.W., Oh J.K., Park M.S., Kim Y.W., et al. Prevalence of sleep disturbance in patients with lumbar spinal stenosis and analysis of the risk factors. Spine J. 2020;20:1239–1247. doi: 10.1016/j.spinee.2020.02.008. [DOI] [PubMed] [Google Scholar]
- Koffel E., Kroenke K., Bair M.J., Leverty D., Polusny M.A., Krebs E.E. The bidirectional relationship between sleep complaints and pain: analysis of data from a randomized trial. Health Psychol. 2016;35(1):41–49. doi: 10.1037/hea0000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N.K., Jeon S.W., Heo Y.W., Shen F., Kim H.J., Yoon I.Y., et al. Sleep disturbances in patients with lumbar spinal stenosis. Clin. Spine Surg. 2020;33(4):E185–E190. doi: 10.1097/BSD.0000000000000944. [DOI] [PubMed] [Google Scholar]
- Mayer H.M., Heider F. “Slalom” microsurgical cross-over decompression for multilevel degenerative lumbar stenosis. BioMed Res. Int. 2016;2016 doi: 10.1155/2016/9074257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerding H.W. Spondylolisthesis. Surg. Gynecol. Obstet. 1932;54:371–377. [Google Scholar]
- Michal M., Wiltink J., Kirschner Y., Schneider A., Wild Ps, Münzel T., et al. Complaints of sleep disturbances are associated with cardiovascular disease: results of the Gutenberg Health Study. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0104324. 10.137/journal.pone.0104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papavero L., Marques C.J., Lohmann J., Fitting Th, Schawjinski K., Ali N. Redundant nerve roots in lumbar spinal stenosis: inter- and intra-rater reliability of an MRI-based classification. Neuroradiology. 2020;62:223–230. doi: 10.1007/s00234-019-02337-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papavero L., Ali N., Schawjinski K., Holtdirk A., Maas R., Ebert S. The prevalence of redundant nerve roots in standing positional MRI decreases by half in supine and almost to zero in flexed seated position: a retrospective cross-sectional cohort study. Neuroradiology. 2022;64:2191–2201. doi: 10.1007/s00234-022-03047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizas C., Theumann N., Burn A., Tansey R., Wardlaw D., Smith F., et al. Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine. 2010;35(21):1919–1924. doi: 10.1097/BRS.0b013e3181d359bd. [DOI] [PubMed] [Google Scholar]
- Senaratna C., Perret Jl, Lodge C.J., Lowe A.J., Campbell B.Ε., Matheson M.C., et al. Prevalence of obstructive apnea in the general population: a systematic review. Sleep Med. Rev. 2017;34:70–81. doi: 10.1016/j.smrv.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Skarpsno E.S., Mork P.J., Nilsen T.I.L., Holtermann A. Sleep positions and nocturnal body movements based on free-living accelerometer recordings: association with demographics, lifestyle, and insomnia symptoms. Nat. Sci. Sleep. 2017;1(9):267–275. doi: 10.2147/NSS.S145777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Elm E., Altmann D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author upon reasonable request.