Abstract
Monotherapy with anti-programmed cell death protein 1 (PD-1) monoclonal antibody has been approved for the treatment of advanced non-small cell lung cancer with positive programmed cell death-ligand 1 (PD-L1) expression and oncogene wild type, which revealed survival benefit compared with chemotherapy. Nevertheless, certain patients develop rapid progression on anti-PD-1 inhibitor monotherapy. This novel pattern is called hyperprogressive disease (HPD), and the underlying mechanism and molecular characteristics still leaves not clear. Here, we reported two heavily pretreated advanced lung adenocarcinoma cases with HER2 exon 20 insertion who presented HPD after two cycles of anti-PD-1 inhibitor sintilimab monotherapy, and they both carried co-alterations in the PI3K/AKT/mTOR and cell cycle signaling pathway. We speculated that HER2 exon 20 insertion might be viewed as a potential biomarker to avoid single-agent immunotherapy in certain patients with driver mutations, or timely guide proper treatment strategies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-025-01749-3.
Keywords: Hyperprogressive disease, PD-1 inhibitor, HER2, Exon 20 insertion, Non-small cell lung cancer, Case report
Introduction
As the most lethal malignant disease, non-small cell lung cancer (NSCLC) accounts for more than 80% of lung cancer [1, 2]. The current treatment options for patients with advanced NSCLC include chemotherapy, targeted therapy, immunotherapy, and antiangiogenic therapy. With the in-depth research on the tumor immune microenvironment, immunotherapy has become a hot spot in the treatment of NSCLC. In recent years, immunotherapy has been the standard care for treating wild-type advanced NSCLC. However, several studies have reported rapid tumor progression in some patients after utilizing immune checkpoint inhibitor (ICI) [3–5]. These studies uncovered that disease progression within a short period after immunotherapy initiation was a contradictory phenomenon to the expected curative effect of ICI.
Anti-programmed cell death protein 1 (PD-1) inhibitors block the binding of PD-1 to its ligand PD-L1 and restore the activity of effector T cells, thereby reducing the chance of immune escape, which PD-L1 antibody has an anti-tumor effect and tolerance and has revolutionized the therapeutic paradigms for advanced NSCLC and been widely used in clinical practice [6, 7]. However, the safety of anti-PD-1 therapy requires urgent attention because hyperprogressive disease (HPD) might occur in specific populations of patients, which results in rapid disease progression within a short period after ICI initiation. According to the clinical and radiological definition, HPD was defined as progressive disease at the first evaluation in addition to an increase of at least twofold in the tumor growth rate (TGR) during PD-1/PD-L1 inhibitor therapy compared with the TGR before PD-1/PD-L1 immunotherapy, with a more than 50% increase in tumor burden at the time of the first evaluation from treatment initiation, doubling of pace progression, and time to treatment failure (TTF) for less than 2 months [3, 8, 9]. Since HPD is closely associated with poor prognosis, there is an urgency to determine the possible predictors and mechanisms [8, 9].
Human epidermal growth factor receptor 2 (HER2, or ERBB2) is identified as an oncogenic driver, promoting oncogenesis and participates in the acquired drug resistance in NSCLC [10, 11]. There are similar HER2 alterations in many other tumors, including breast and gastric cancers, associated with poor prognosis and inferior overall survival (OS) [12–14]. However, increasing evidence has revealed that HER2-altered NSCLC patients appeared to be insensitive and intrinsically resistant to ICI [15–17]. Furthermore, no evidence of HPD induced by immunotherapy was observed in HER2-mutant NSCLC. Herein, we present two cases of advanced NSCLC with HER2 exon 20 insertion that occurred HPD after anti-PD-1 monotherapy.
Case presentation
Case 1
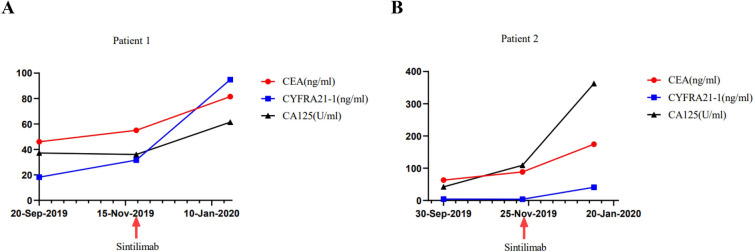
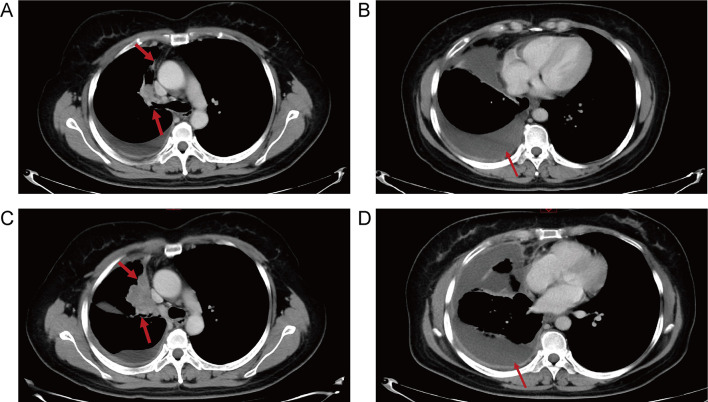
A 59-year-old Chinese man has examined with stage IVB (AJCC/TNM 8th Staging System, c.T2aN0M1c) disease of lung cancer with concomitant vertebral metastases due to gradual chest pain and aggravated cough on April 7, 2018. He then performed palliative middle lobe wedge resection of the right lung, and the pathology revealed poorly differentiated lung adenocarcinoma (Fig. 1A) with a maximum diameter of 3.0 cm and invasion of the visceral pleura. Next-generation sequencing (NGS) via tumor tissue identified HER2 exon 20 insertion (p.A775_G776insYVMA, Fig. 1B), co-existing amplification of HER2, FGF3, FGF4, FGF19, CDK12, CCND1, and mTOR splice mutation, with PD-L1 expression of 20% (Dako 22C3 pharmDx) and low tumor mutational burden (TMB) of 4.0 Muts/Mb, and 22 driver genes were mutated (Supplementary Table 1). He received pemetrexed/platinum chemotherapy combined with anti-HER2 antibody trastuzumab as first-line therapy from May 30, 2018 to January 21, 2019, owing to new lesions of inguinal lymph node metastases, with the best response of stable disease (SD) and progression-free survival (PFS) of 7.9 months. Afterwards, he participated in a single-arm clinical trial (Registration number: ChiCTR1900021684), which was evaluating the activity of HER2-targeted inhibitor pyrotinib with angiogenesis inhibitor apatinib for HER2 amplification or mutations. Then, pyrotinib (400 mg once daily) and apatinib (250 mg once daily) was performed between February 6, 2019 and September 20, 2019, showing the best response of SD and PFS of 7.5 months. Afterwards, he developed pleural and multiple lung metastases after second-line targeted therapy, and the computed tomography (CT) scan on December 3, 2019 demonstrated increased lung metastases but no pleural effusion. The biggest left lung metastasis was 0.6 cm (Fig. 2A, B). Then, he was recruited in another clinical trial (Registration number: ChiCTR1900023074), aiming to assess the activity of PD-1 inhibitor sintilimab for EGFR and HER2 exon 20 insertions. Then, third-line monotherapy of sintilimab (200 mg per 3 weeks) was performed on December 12, 2019. Unfortunately, after two treatment cycles of immunotherapy, the CT scan on January 23, 2020 revealed HPD (Fig. 2C, D). The amount of lung metastases kept increased, and the target lesion at left lung achieved a diameter of 1.8 cm. In addition, massive pleural effusion at the right lung appeared, and the pleural thickening was more obvious than before. He got HPD only after two treatment cycles of immunotherapy with a short PFS of 1.4 months, with rapidly deteriorating physical condition. The tumor markers during the treatment of PD-1 inhibitor sintilimab was depicted in Fig. 3A. This patient administered poziotinib (16 mg once daily) as fourth-line therapy, but his disease kept progressing, and he eventually died on April 18, 2020, with OS of 24.7 months.
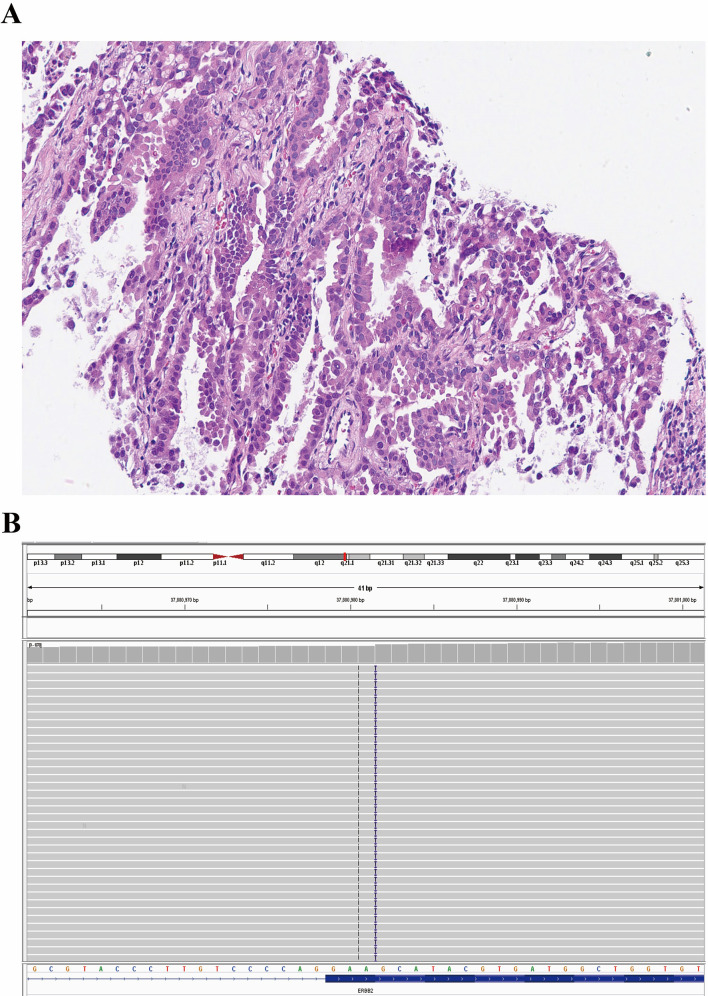
Fig. 1.
Optical image of lung adenocarcinoma (Case 1) in Hematoxylin and Eosin staining with 200 × magnification ratio in paraffin-embedded tissue after palliative resection of right lung (A). The integrative genomics viewer of HER2 exon 20 insertion p.A775_G776insYVMA detected by the next-generation sequencing (B)
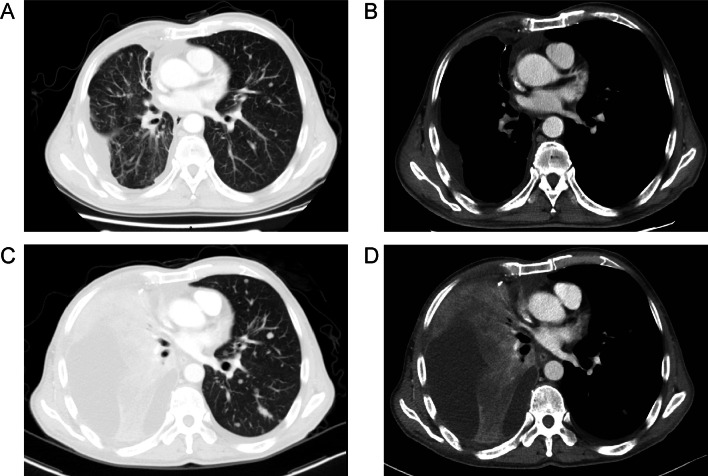
Fig. 2.
The relapsed disease after targeted therapy with pleural and lung metastases (A, B). Hyperprogressive disease after two treatment cycles of PD-1 inhibitor sintilimab monotherapy (C, D)
Fig. 3.
The tumor markers during the monotherapy of PD-1 inhibitor sintilimab in these two cases (A, B)
Case 2
A 45-year-old Chinese woman was identified with a lung tumor measured of 6.1 × 5.2 cm in the right upper lobe, with confirmed pleural metastasis by pleural effusion cytology on November 12, 2018, revealing a stage IVA disease (AJCC/TNM 8th Staging System, c.T3N0M1a). The biopsy revealed lung adenocarcinoma (Fig. 4A) and detection of HER2 exon 20 insertion (p.P780_Y781insGSP, Fig. 4B), amplification of AKT1, CCND3, VEGFA, MYC, and TP53 splice mutation, with PD-L1 expression of 1% (Dako 22C3 pharmDx) and TMB of 2.4 Muts/Mb, and 41 driver genes were mutated (Supplementary Table 1). She received first-line chemotherapy (pemetrexed/carboplatin) plus bevacizumab for 6 treatment cycles, showing a partial response (PR) and PFS of 4.4 months. When her disease progressed with elevated blood tumor markers, she received second-line afatinib (30 mg once daily), but blood tumor markers still kept increasing, though the tumor size was stable after one month. Owing to the unfavorable efficacy, she self-administered poziotinib (16 mg once daily) plus angiogenesis inhibitor anlotinib (8 mg once daily for two weeks and stopped for one week) in the third-line setting and observed tumor shrinkage and reduction of blood tumor markers, with the best response of SD and PFS of 5.0 months. On November 13, 2019, her disease progressed with enlarged lesion at the right lung measured 2.4 cm (Fig. 5A, B). Subsequently, she joined the clinical trial (Registration number: ChiCTR1900023074) of PD-1 inhibitor sintilimab for EGFR and HER2 exon 20 insertions, and received fourth-line sintilimab (200 mg per 3 weeks) monotherapy from November 29, 2019 to December 20, 2019. As well, after two treatment cycles of immunotherapy, the CT scan on January 7, 2020 demonstrated her disease developed HPD (Fig. 5C, D). The target lesion at the right lung achieved a diameter of 5.9 cm, with more obvious pleural thickening and increased massive right pleural effusion. She got HPD only after two treatment cycles of immunotherapy with a short PFS of 1.3 months. The tumor markers during the treatment of PD-1 inhibitor sintilimab was depicted in Fig. 3B. She then received fifth-line pyrotinib plus apatinib, achieving the best response of SD and PFS of 10.1 months. However, due to emerging liver metastasis and de novo drug resistance to dacomitinib within one month, she accepted fam-trastuzumab deruxtecan-nxki (T-DXd, DS8201), and achieved PR with PFS of 7.2 months. Then, she was treated with mobocertinib (160 mg once daily) when her disease relapsed on T-DXd, and achieved SD with PFS of 6.8 months. Eventually, her disease progressed and she died on May 13, 2022, with OS of 42.6 months.
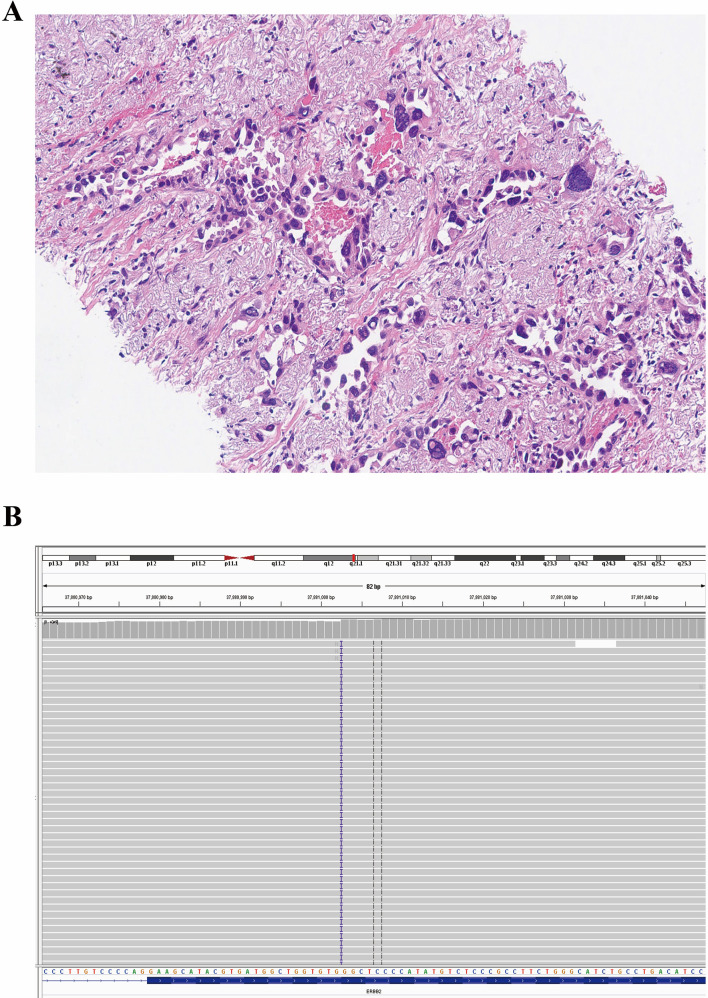
Fig. 4.
Optical image of lung adenocarcinoma (Case 2) in Hematoxylin and Eosin staining with 200 × magnification (A). The integrative genomics viewer of HER2 exon 20 insertion p.P780_Y781insGSP detected by the next-generation sequencing (B)
Fig. 5.
The relapsed disease before PD-1 inhibitor sintilimab monotherapy with pleural and lung metastases (A, B). Hyperprogressive disease after two treatment cycles of PD-1 inhibitor sintilimab monotherapy (C, D)
Discussion
ICI has been considered a breakthrough in NSCLC, which innovated the systemic therapy for the advanced disease. Compared with conventional chemotherapy, patients treated with ICI have better survival benefits and unprecedented cure rates [18]. Nevertheless, with the widespread use of ICI, tumor progression has an unexpected acceleration rate and volume in a subset of patients. Chubachi et al. provided the first clue to the existence of HPD when they described the NSCLC case characterized by accelerated tumor growth during anti-PD-1 immunotherapy and named this phenomenon "disease flare" [4]. Champion et al. were the first to describe and clearly define this new type of tumor response called HPD [3]. It is worth noting that HPD represents a different progression model that does not overlap with traditional progressive disease.
PD-1 is a transmembrane immune checkpoint receptor that expresses in activated T cells, B cells, natural killer cells, and some myeloid cells, which restricts the function of T cell effectors in tissues by binding to PD-L1 expressed on the surface of tumor cells [19]. Tumor cells overexpress PD-L1, which further blocks the anti-tumor immune response. PD-1 inhibitors could effectively block the binding of PD-1 and enhance the killing effect of immune cells on tumors. Anti-PD-1 therapy increases endogenous anti-tumor immune responses and promotes the OS, confirmed in multiple cancers, such as melanoma, lung cancer, and renal cell carcinoma [20–22]. Anti-PD-1 therapy tended to recover the immune effect of T cells against tumor cells [23]. It is worth that blocking PD-1 might induce the compensatory mechanism that controls the immunity of anti-tumor T cells, which might give rise to the disadvantages of anti-PD-1 therapy in T cell activation and inducing an immunosuppressive environment dedicated to the development of HPD [24–26]. For instance, RY Huang et al. reported that multiple immune inhibitory receptors expressed by tumor-associated lymphocytes and blocked one of these receptors leads to upregulation of other receptors that invalidate the effectiveness of ICIs therapy in ovarian cancer [24]. Another study showed the IL-10 receptor (IL-10R) level up-regulated in PD-1 high tumor antigen (TA)-specific CD8+ T cells, and the expression of IL-10R was enhanced by PD-1/ PD-L1 axis blockade [27]. Anti-PD-1 therapy might magnify the TA-specific CD8+ T cells sensitivity to IL-10 immunosuppression.
The duty of Treg cells is a part of immune regulation, which plays the role of maintaining self-immunity tolerance. Treg cells might be a potential adverse factor for cancer patients due to impeding the effective anti-tumor immune response of the patients, thereby promoting tumorigenesis and progression [28]. Zhang et al. found that Treg cells that lack PD-1 confermore powerful immunosuppressive activity than that with intact PD-1 expression. For efficacious protection against autoimmune diseases, Treg cells and PD-1 mutually influence to maintain immune tolerance [29]. It is noteworthy that some tumor-infiltrating Treg cells express not only PD-1 but also PD-L1, thus increasing the possibility of PD-1/PD-L1 signal interaction, thereby finely regulating the homeostasis of the immune microenvironment of the tumor tissues [30, 31]. These data implicate that Treg cells possibly participate in the mechanism of HPD after anti-PD-1 therapy.
HPD occurred following ICI administration, and this unexpected acceleration of disease may reflect the immune response to ICI but is not associated with other clinical characteristics [32]. However, considering the unpredictable characteristics and rapid development, both the unanticipated immune system response and intrinsic features of cancer cells might facilitate the occurrence of HPD after ICI administration. In recent years, researchers have paid attention to whether particular tumor genomic alterations are related to HPD. Kato et al. analyzed the gene spectrum of 155 advanced cancer patients who received ICI and revealed the amplification of MDM2/4 might associate with the occurrence of HPD [9]. In addition, they found that 6 of 155 patients had MDM2/MDM4 amplification, and 4 of 6 patients were present with significant tumor growth and poor clinical outcome after PD-1/PD-L1 inhibitor immunotherapy, indicating MDM2/4 might be a potential HPD inductor, and 2 out of 10 patients with epidermal growth factor receptor (EGFR) aberrations who identified as HPD in this cohort [9]. EGFR is a member of the human epidermal receptor family that involves multiple signaling pathways to regulate the internal homeostasis of cells. Notably, aberrant EGFR enhances the secretion of metabolites with immunosuppressive properties and triggers immune escape through different mechanisms. It speculates that EGFR possibly connects with HPD, but more studies are still needed to certify this potential mechanism. Additionally, other genomic alterations, such as BRCA2, KRAS, and STK11, possibly linked with HPD, but mechanisms are still the enigma [33–36]. In this report, whole-exome sequencing (WES) is performed to detect the variations in patients. Notably, gene mutations as aforesaid were unfound in these two cases. However, both patients harbored HER2 exon 20 insertion, mutations in the PI3K/AKT, and cell cycle signaling pathways.
HER2 is the second most important member of the ErbB family. In this family, EGFR was well known for oncogenesis regulation. Unlike EGFR, HER2 is rarely considered to be responsible for HPD occurrence in the current ICI therapies. Previous studies revealed the immunosuppressive ability of HER2 [37, 38]. IL-8 could recruit neutrophils to infiltrate into the tumor microenvironment, which diminishes the effectiveness of PD-1/PD-L1 inhibitors in several types of cancer [39]. HER2 aberration enhances IL-8 secretion in the tumor microenvironment by activating PI3K/AKT/mTOR pathway [40]. Herrmann F et al. reported that HER2 expression is negatively correlated with MHC-I expression and other components of the antigen processing mechanism [41]. Repression of the level of HER2 enhances the expression of MHC-I and regulates the interaction between PD-1 and PD-L1 [42, 43]. This report presents two NSCLC cases occurring HPD who carried HER2 exon 20 insertion and underwent ICIs monotherapy. Our two cases and a previous POLISH study revealed that upregulation of PI3K/AKT signaling was commonly observed in HER2-mutant or amplified NSCLC, which also had been reported in other HER2-positive solid tumors [17, 44–46]. It has been observed that HER2 could abolish phosphorylation of TANK-binding kinase 1 (TBK1) and attenuate stimulator of interferon genes (STING) signaling, thus suppressing immune response of type I and II interferon [47, 48]. Our POLISH study observed that ICIs plus chemotherapy combination failed to obtain a better survival benefit than sole chemotherapy for HER2-altered NSCLC due to activation of PI3K/AKT signaling might mediate immunosuppression in HER2-altered NSCLC. Significantly, AKT1 recruitment could impair TBK1 phosphorylation and suppress interferon regulatory factor 3 (IRF3), thus disrupting the STING signaling for antitumor immune response [17]. Therefore, there possibly existed a tight causality between HER2 exon 20 insertion and HPD, but more investigation is required for this potential role.
Finally, there were several limitations to our study. Although a rapidly increased pace of progression was observed in these two cases within two months after PD-1 inhibitor monotherapy, the main drawback in correlating the results with the mutational profile might be the high tumor burden before ICI treatment or the low expression of the PD-L1. In addition, more cases of HER2 exon 20 insertion receiving PD-1/PD-L1 immunotherapy would be required to have an absolutely unbiased conclusion.
In conclusion, the present report describes two cases of NSCLC with HER2 exon 20 insertion developing HPD after sintilimab monotherapy administration. The molecular mechanism of HPD is still unclear, but genomic analysis may help clarify it. To lessen the untoward reaction of ICI, developing biomarkers for HPD prediction to further improve the efficiency and safety of immunotherapy in tumor treatment is necessary.
Supplementary Information
Acknowledgements
The authors acknowledge the patients for their participation.
Abbreviations
- CT
Computed tomography
- EGFR
Epidermal growth factor receptor
- HER2
Human epidermal growth factor receptor 2
- HPD
Hyperprogressive disease
- ICI
Immune checkpoint inhibitor
- IL-10R
IL-10 receptor
- IRF3
Interferon regulatory factor 3
- NGS
Next-generation sequencing
- NSCLC
Non-small cell lung cancer
- OS
Overall survival
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed cell death-ligand 1
- PFS
Progression-free survival
- PR
Partial response
- SD
Stable disease
- STING
Stimulator of interferon genes
- TA
Tumor antigen
- TBK1
TANK-binding kinase 1
- T-DXd
Fam-trastuzumab deruxtecan-nxki
- TGR
Tumor growth rate
- TMB
Tumor mutational burden
- TTF
Time to treatment failure
- WES
Whole-exome sequencing
Author contributions
G Yang contributed to the design of the study, the acquisition of data, drafting the article and critically revising its important intellectual content. L Tian contributed to the data analysis, picture production, interpretation of data, and drafting the article. Y Wang contributed to the scientific review and final approval of the version submitted. All authors read and approved the final manuscript.
Funding
The authors have no funding sources to report.
Data availability
All data generated or analysed are included in this published article.
Declarations
Ethics approval and consent to participate
This study was granted an exemption from requiring ethics approval from the Ethics Committee of National Cancer Center/Cancer Hospital, as it was an observational report. All the human data was in accordance with the guidelines of the 1964 Declaration of Helsinki, and written informed consent for study participation from these two patients was obtained.
Consent for publication
Written informed consent was obtained from these two patients for the publication of their case report and any accompanying images.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guangjian Yang and Linyan Tian contributed equally to this work and shared first authorship.
References
- 1.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):252–71. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 3.Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23(8):1920–8. [DOI] [PubMed] [Google Scholar]
- 4.Chubachi S, Yasuda H, Irie H, Fukunaga K, Naoki K, Soejima K, et al. A case of non-small cell lung cancer with possible “disease flare” on nivolumab treatment. Case Rep Oncol Med. 2016;2016:1075641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saada-Bouzid E, Defaucheux C, Karabajakian A, Coloma VP, Servois V, Paoletti X, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2017;28(7):1605–11. [DOI] [PubMed] [Google Scholar]
- 6.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19(7):813–24. [DOI] [PubMed] [Google Scholar]
- 7.Chen R, Tao Y, Xu X, Shan L, Jiang H, Yin Q, et al. The efficacy and safety of nivolumab, pembrolizumab, and atezolizumab in treatment of advanced non-small cell lung cancer. Discov Med. 2018;26(143):155–66. [PubMed] [Google Scholar]
- 8.Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated With PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4(11):1543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23(15):4242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazieres J, Peters S, Lepage B, Cortot AB, Barlesi F, Beau-Faller M, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol. 2013;31(16):1997–2003. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Jiang T, Qin Z, Jiang J, Wang Q, Yang S, et al. HER2 exon 20 insertions in non-small-cell lung cancer are sensitive to the irreversible pan-HER receptor tyrosine kinase inhibitor pyrotinib. Ann Oncol. 2019;30(3):447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abrahao-Machado LF, Scapulatempo-Neto C. HER2 testing in gastric cancer: an update. World J Gastroenterol. 2016;22(19):4619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomizawa K, Suda K, Onozato R, Kosaka T, Endoh H, Sekido Y, et al. Prognostic and predictive implications of HER2/ERBB2/neu gene mutations in lung cancers. Lung Cancer. 2011;74(1):139–44. [DOI] [PubMed] [Google Scholar]
- 14.Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. [DOI] [PubMed] [Google Scholar]
- 15.Saalfeld FC, Wenzel C, Christopoulos P, Merkelbach-Bruse S, Reissig TM, Lassmann S, et al. Efficacy of immune checkpoint inhibitors alone or in combination with chemotherapy in NSCLC harboring ERBB2 mutations. J Thorac Oncol. 2021;16(11):1952–8. [DOI] [PubMed] [Google Scholar]
- 16.Riudavets M, Sullivan I, Abdayem P, Planchard D. Targeting HER2 in non-small-cell lung cancer (NSCLC): a glimpse of hope? An updated review on therapeutic strategies in NSCLC harbouring HER2 alterations. ESMO Open. 2021;6(5): 100260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang G, Yang Y, Liu R, Li W, Xu H, Hao X, et al. First-line immunotherapy or angiogenesis inhibitor plus chemotherapy for HER2-altered NSCLC: a retrospective real-world POLISH study. Ther Adv Med Oncol. 2022;14:17588359221082340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gettinger S, Horn L, Jackman D, Spigel D, Antonia S, Hellmann M, et al. Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the CA209-003 study. J Clin Oncol. 2018;36(17):1675–84. [DOI] [PubMed] [Google Scholar]
- 19.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Queirolo P, Spagnolo F. Atypical responses in patients with advanced melanoma, lung cancer, renal-cell carcinoma and other solid tumors treated with anti-PD-1 drugs: a systematic review. Cancer Treat Rev. 2017;59:71–8. [DOI] [PubMed] [Google Scholar]
- 21.Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–86. [DOI] [PubMed] [Google Scholar]
- 23.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang RY, Francois A, McGray AR, Miliotto A, Odunsi K. Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer. Oncoimmunology. 2017;6(1): e1249561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawakami Y, Ohta S, Sayem MA, Tsukamoto N, Yaguchi T. Immune-resistant mechanisms in cancer immunotherapy. Int J Clin Oncol. 2020;25(5):810–7. [DOI] [PubMed] [Google Scholar]
- 26.Shi H, Lan J, Yang J. Mechanisms of resistance to checkpoint blockade therapy. Adv Exp Med Biol. 2020;1248:83–117. [DOI] [PubMed] [Google Scholar]
- 27.Sun Z, Fourcade J, Pagliano O, Chauvin JM, Sander C, Kirkwood JM, et al. IL10 and PD-1 cooperate to limit the activity of tumor-specific CD8+ T cells. Cancer Res. 2015;75(8):1635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression—implications for anticancer therapy. Nat Rev Clin Oncol. 2019;16(6):356–71. [DOI] [PubMed] [Google Scholar]
- 29.Zhang B, Chikuma S, Hori S, Fagarasan S, Honjo T. Nonoverlapping roles of PD-1 and FoxP3 in maintaining immune tolerance in a novel autoimmune pancreatitis mouse model. Proc Natl Acad Sci USA. 2016;113(30):8490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chevalier MF, Schneider AK, Cesson V, Dartiguenave F, Lucca I, Jichlinski P, et al. Conventional and PD-L1-expressing regulatory T cells are enriched during BCG therapy and may limit its efficacy. Eur Urol. 2018;74(5):540–4. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Carlsson R, Comabella M, Wang J, Kosicki M, Carrion B, et al. FoxA1 directs the lineage and immunosuppressive properties of a novel regulatory T cell population in EAE and MS. Nat Med. 2014;20(3):272–82. [DOI] [PubMed] [Google Scholar]
- 32.Adashek JJ, Subbiah IM, Matos I, Garralda E, Menta AK, Ganeshan DM, et al. Hyperprogression and immunotherapy: fact, fiction, or alternative fact? Trends Cancer. 2020;6(3):181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim Y, Kim CH, Lee HY, Lee SH, Kim HS, Lee S, et al. Comprehensive clinical and genetic characterization of hyperprogression based on volumetry in advanced non-small cell lung cancer treated with immune checkpoint inhibitor. J Thorac Oncol. 2019;14(9):1608–18. [DOI] [PubMed] [Google Scholar]
- 34.Laderian B, Mundi P, Fojo T, Bates SE. Emerging therapeutic implications of STK11 mutation: case series. Oncologist. 2020;25(9):733–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J, Wu Q, Wu S, Xie X. Investigation on potential biomarkers of hyperprogressive disease (HPD) triggered by immune checkpoint inhibitors (ICIs). Clin Transl Oncol. 2021;23(9):1782–93. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Wang F, Zhong M, Yarden Y, Fu L. The biomarkers of hyperprogressive disease in PD-1/PD-L1 blockage therapy. Mol Cancer. 2020;19(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seliger B, Kiessling R. The two sides of HER2/neu: immune escape versus surveillance. Trends Mol Med. 2013;19(11):677–84. [DOI] [PubMed] [Google Scholar]
- 38.Inoue M, Mimura K, Izawa S, Shiraishi K, Inoue A, Shiba S, et al. Expression of MHC Class I on breast cancer cells correlates inversely with HER2 expression. Oncoimmunology. 2012;1(7):1104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Todorovic-Rakovic N, Milovanovic J. Interleukin-8 in breast cancer progression. J Interferon Cytokine Res. 2013;33(10):563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paplomata E, O’Regan R. The PI3K/AKT/mTOR pathway in breast cancer: targets, trials and biomarkers. Ther Adv Med Oncol. 2014;6(4):154–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herrmann F, Lehr HA, Drexler I, Sutter G, Hengstler J, Wollscheid U, et al. HER-2/neu-mediated regulation of components of the MHC class I antigen-processing pathway. Cancer Res. 2004;64(1):215–20. [DOI] [PubMed] [Google Scholar]
- 42.Dirix LY, Takacs I, Jerusalem G, Nikolinakos P, Arkenau HT, Forero-Torres A, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat. 2018;167(3):671–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kubota Y, Kawazoe A, Sasaki A, Mishima S, Sawada K, Nakamura Y, et al. The impact of molecular subtype on efficacy of chemotherapy and checkpoint inhibition in advanced gastric cancer. Clin Cancer Res. 2020;26(14):3784–90. [DOI] [PubMed] [Google Scholar]
- 44.Liu J, Pan C, Guo L, Wu M, Guo J, Peng S, et al. A new mechanism of trastuzumab resistance in gastric cancer: MACC1 promotes the Warburg effect via activation of the PI3K/AKT signaling pathway. J Hematol Oncol. 2016;9(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanker AB, Pfefferle AD, Balko JM, Kuba MG, Young CD, Sanchez V, et al. Mutant PIK3CA accelerates HER2-driven transgenic mammary tumors and induces resistance to combinations of anti-HER2 therapies. Proc Natl Acad Sci USA. 2013;110(35):14372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12(4):395–402. [DOI] [PubMed] [Google Scholar]
- 47.Wu S, Zhang Q, Zhang F, Meng F, Liu S, Zhou R, et al. HER2 recruits AKT1 to disrupt STING signalling and suppress antiviral defence and antitumour immunity. Nat Cell Biol. 2019;21(8):1027–40. [DOI] [PubMed] [Google Scholar]
- 48.Kumagai S, Koyama S, Nishikawa H. Antitumour immunity regulated by aberrant ERBB family signalling. Nat Rev Cancer. 2021;21(3):181–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed are included in this published article.