Abstract
The pathogenesis of metabolic dysfunction-associated steatotic liver disease (MASLD) remains unclear due to the complexity of its etiology. The emerging field of the epitranscriptome has shown significant promise in advancing the understanding of disease pathogenesis and developing new therapeutic approaches. Recent research has demonstrated that N4-acetylcytosine (ac4C), an RNA modification within the epitranscriptome, is implicated in progression of various diseases. However, the role of ac4C modification in MASLD remains unexplored. Herein, we performed acRIP-ac4c-seq and RNA-seq analysis in free fatty acids-induced MASLD model cells, identifying 2128 differentially acetylated ac4C sites, with 1031 hyperacetylated and 1097 hypoacetylated peaks in MASLD model cells. Functional enrichments analysis showed that ac4C differentially modified genes were significantly involved in processes related to MASLD, such as nuclear transport and MAP kinase (MAPK) signaling pathway. We also identified 341 differentially expressed genes (DEGs), including 61 lncRNAs and 280 mRNAs, between control and MASLD model cells. Bioinformatics analysis showed that DEGs were significantly enriched in long-chain fatty acid biosynthetic process. Notably, 118 genes exhibited significant changes in both ac4C modification and expression levels in MASLD model cells. Among these proteins, JUN, caveolin-1 (CAV1), fatty acid synthase (FASN), and heterogeneous nuclear ribonucleoprotein A1 (hnRNPA1) were identified as core proteins through protein–protein interaction (PPI) network analysis using cytoscape software. Collectively, our findings establish a positive correlation between ac4C modification and the pathogenesis of MASLD and suggest that ac4C modification may serve as a therapeutic target for MASLD.
Keywords: MASLD, Epitranscriptome, ac4C
Subject terms: Cell biology, Molecular biology, Gastroenterology
Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD), previously known as non-alcoholic fatty liver disease (NAFLD), is regarded as the liver manifestation of metabolic syndrome. MASLD affects approximately 1.7 billion individuals worldwide, with a global prevalence approximately 25%. In developed regions, the prevalence rate surpasses 30% and reaches 27% in Asia, with 10–30% of MASLD cases advancing to metabolic dysfunction-associated steatohepatitis (MASH), previously known as non-alcoholic steatohepatitis (NASH)1–4. The rising incidence of obesity worldwide has contributed to an increase in MASLD cases, affecting increasingly younger populations. Despite its prevalence, few effective clinical treatments for MASLD are available due to the uncertain pathogenesis. Consequently, it is crucial to deepen our understanding of MASLD’s underlying mechanisms to identify novel preventive and therapeutic strategies.
With the in-depth exploration in the field of epigenetics, numerous emerging epigenetic regulatory mechanisms have been discovered. Epitranscriptome modification, also known as RNA modification, is an important component of epigenetic regulation. Over 170 chemical modifications have been identified in RNA, primarily in non-coding RNAs5. Advances in genomic microarray technology and genome and transcriptome sequencing have enabled the identification of diverse RNA modifications, including N6-methyladenine (m6A), N1-methyladenine (m1A), 5-methylcytosine (m5C), 5-hydroxymethylcytosine (hm5C), N7-methylguanine (m7G), and N4-acetylcytosine (ac4C), which impact mRNA expression, metabolism, and function5,6. These modifications are usually regulated by a series of functional proteins, including one or more of modification enzymes (writers), demodification enzymes (erasers) and modification recognition proteins (readers)7. With the continuous discovery of new functional proteins, the reversible changes in dynamic regulation of mRNA modifications have received extensive attention and investigation in the prevention and treatment of human diseases.
ac4C, the first acetylation identified in mRNAs, is abundantly present in the human transcriptome, predominantly in coding sequence (CDS) regions. Given its extensive acetylation targets in the human genome, ac4C has emerged as a critical component of the epitranscriptome8,9. Current research indicates that ac4C modification is regulated exclusively by the enzyme N-acetyltransferase 10 (NAT10), the only known enzyme with both acetylation catalytic activity and RNA-binding capacity10. Altered ac4C levels have been observed in some diseases, such as systemic lupus erythematosus (SLE) and human immunodeficiency virus 1 (HIV-1) infection11,12. However, the role of ac4C modification in MASLD remains largely unstudied.
In this study, we employed transcriptome sequencing (RNA-seq) and acetylated RNA immunoprecipitation sequencing (acRIP-seq) to characterize ac4C mRNA modifications in MASLD cells. The results demonstrated notable ac4C modifications in MASLD cells. Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis indicated that the modified and differentially expressed mRNAs were significantly enriched in the lipid synthesis and metabolism pathways. These findings highlight the involvement of ac4C RNA acetylation in MASLD development, offering novel insights into MASLD pathogenesis and potential prevention strategies.
Materials and methods
Cell experiments and sample collection
The hepatic stem cell line HepaRG was purchased from Shanghai Honsun Biological Technology Co., Ltd (Shanghai, China). The cells, at a density of 2.5 × 104 cells/cm, were cultured in Roswell Park Memorial Institute 1640 medium (HyClone, USA) supplemented with 100 units/mL penicillin, 100 μg/mL streptomycin, 5 μg/ml insulin, 50 μM hydrocortisone hemisuccinate, and 10% FBS at 37 ℃ in a humidified atmosphere with 5% CO2. Cells were differentiated by incubating in 1.7% dimethyl sulfoxide (DMSO) for 2 weeks. The cultivation of MASLD model cells were conducted as previously described13. In brief, the differentiated cells were treated with serum-free medium containing free fatty acids (FFAs), 0.25 mmol/L palmitic acid (PA), 0.5 mmol/L oleic acid (OA), for 24 h. Then, the cells were harvested with TRNzol (TIANGEN, China) for further analysis.
Oil red O staining
Lipid accumulation in MASLD model cells was evaluated via oil red O staining using Modified Lipid Staining Kit (Beyotime, China) according to the manufacturer’s protocols. Briefly, cells were rinsed with PBS for 3 times, and then fixed with 4% paraformaldehyde for 10 min. The cells were then covered with staining wash solution for 20 s, followed by immersion in oil red O staining solution for 20 min. The staining solution was removed by washing, and the samples were rinsed with PBS. The stained cells were observed by microscope and photographed.
Biochemical analysis
The malondialdehyde (MDA), triglyceride (TG), and total cholesterol levels in cells were determined using commercial test kits (Jiancheng, China). Cell samples were processed following the manufacturer’s instructions. Optical density (OD) values were determined by a VICTOR Nivo Multimode Microplate Reader (Revvity, USA) and the contents of biochemical indicators in cells were calculated according to the kits’ protocols. The content of reactive oxygen species (ROS) was analyzed by flow cytometer CytoFLEX (Beckman coulter, USA). The data for 10,000 cells were collected and evaluated using FlowJo V10.4 software (Tree Star, Inc., USA).
acRIP-ac4c-seq and RNA-seq analysis
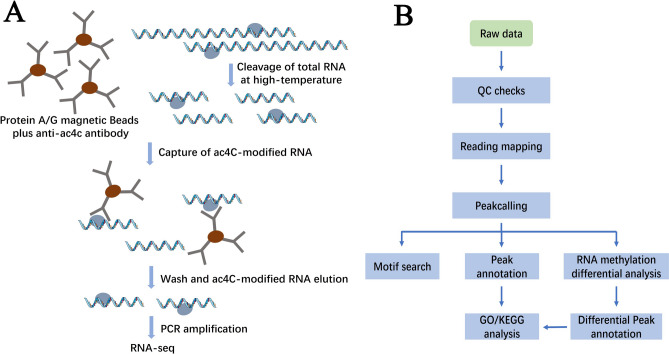
The acRIP-ac4c-seq and RNA-seq services were provided by Genechem Co.,Ltd. (Shanghai, China). The overall experimental process and analytical workflow are shown in Fig. 1A-B. Briefly, Qubit RNA HS kit (Invitrogen, USA) was used to determine the concentration of total RNA extracted with TRNzol Reagent. RNA was digested with the fragmentation buffer into fragments of approximately 100–200 nt. Acetylated RNA enrichment was performed using EpiTM ac4C immunoprecipitation kit according to the commercial protocol (Epibiotek, R1815). The digested RNA samples were then incubated with an anti-ac4C antibody (Abcam, ab252215) for 3 h at 4 ℃. Antibody-protein-RNA complexes were enriched by incubation with protein A/G beads for 2 h at 4 ℃ and then eluted using RNA clean kit (Zymo Research, R1017) to gain the ac4C-enriched RNA. RNA libraries for input and the ac4C IP samples were constructed by EpiTM mini long RNA-seq kit (Epibiotek, E1802). All samples were subjected to 150-bp paired-end sequencing on an Illumina NovaSeq 6000 sequencer. After adapter-trimming and sequence-filtering using cutadapt (v2.5), clean reads were then aligned to the human Ensemble genome GRCh38 by Hisat2 (v2.1.0). Acetylated sites and differential ac4C peaks were identified by exomePeak R package (v2.13.2). The input samples were used for RNA-seq analysis, and featureCounts (v1.6.3) was used to map reads to the genome. The DESeq2 R-package and clusterprofile R package (v3.6.0) were used for differential gene expression analysis and GO/KEGG analysis.
Fig. 1.
Schematic of the acRIP-ac4c-seq procedure. (A) Experimental process. (B) Analytical workflow.
Protein–protein interaction (PPI) network analysis
The information of a total of 118 genes which both significantly changed in ac4C modification and expression levels in MASLD model cells (Pvalue < 0.05) was imported into the string website (https://cn.string-db.org/) for gene interaction analysis, and the results were then imported into cytoscape software (v3.9.1) to obtain the PPI network diagram.
Quantitative real-time polymerase chain reaction
Total RNA was extracted from the HepaRG cells using TRNzol (TIANGEN, China) following commercial instructions. The preparation of complementary DNA (cDNA) was performed by a reverse transcription kit (Vazyme, China). mRNA amplification was conducted by the SYBR qPCR Master Mix (Vazyme, China). The primer sequences are listed in Table 1. Gene expression was normalized to that of GAPDH. The fold changes of mRNA quantification of the genes were calculated using the 2—△△CT method.
Table 1.
Oligonucleotide primers for gene expression.
| Oligo name | Sequence (5' → 3') |
|---|---|
| Human-JUN-forward | AACAGAGCATGACCCTGAAC |
| Human-JUN-reverse | CCGTTGCTGGACTGGATTAT |
| Human-CAV1-forward | CAACATCTACAAGCCCAACAAC |
| Human-CAV1-reverse | TCCCTTCTGGTTCTGCAATC |
| Human-FASN-forward | CTGAAGGACCTGTCTAGGTTTG |
| Human-FASN-reverse | CGGAGTGAATCTGGGTTGAT |
| Human-hnRNPA1-forward | GGATGGCTATAATGGATTTGGTAATG |
| Human-hnRNPA1-reverse | AAATTTCCTCCCTTCATGGGT |
| Human-GAPDH-forward | GTCAACGGATTTGGTCGTATTG |
| Human-GAPDH-reverse | TGTAGTTGAGGTCAATGAAGGG |
Western blotting
Protein was extracted from the HepaRG cells using Radio-Immunoprecipitation Assay (RIPA) buffer (Wanlei, China). The protein concentration was determined by the bicinchoninic acid (BCA) kit (Wanlei, China). The protein was separated on dodecylsulphate polyacrylamide gel electrophoresis and transferred onto the polyvinylidene fluoride membrane (Millipore, USA). After incubation with the primary antibodies (anti-JUN (Proteintech, 24,909–1-AP), anti-CAV1(Proteintech, 16,447–1-AP), anti-FASN (Proteintech, 10,624–2-AP), anti-hnRNPA1 (Proteintech, 11,176–1-AP), and anti-GAPDH antibodies (Proteintech, 60,004–1-Ig)) overnight at 4 ℃, the membranes were further incubated with secondary antibodies for 1 h at room temperature. The quantification of immunoblotting was achieved by Image J software.
Statistical analysis
Experimental data were displayed as mean ± SD and were statistically analyzed in GraphPad Prism 8 software (GraphPad, USA) with the two-tailed Student’s t-test. The P value < 0.05 was defined to be statistically significant.
Results
MASLD model cells construction
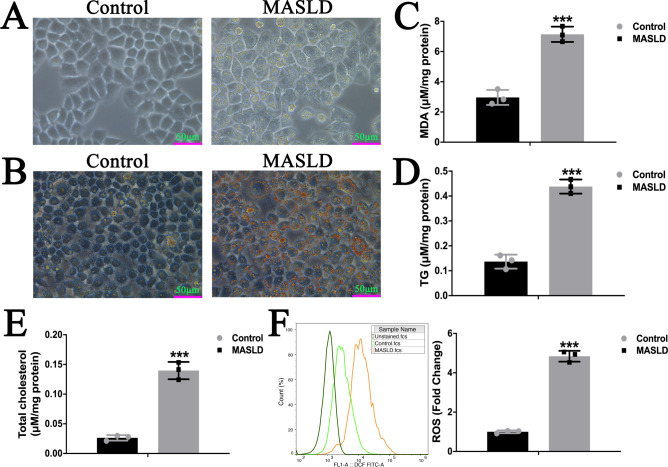
The successful establishment of the MASLD model cells was verified through optical microscopy, oil red O staining, and biochemical indicators analysis. Cells in control group exhibited normal morphology (Fig. 2A), whereas those in the model group cells showed significant lipid droplet accumulation. Furthermore, oil red O staining revealed extensive red lipid droplets in the cytoplasm of the model group, indicating high lipid content and large droplet size (Fig. 2B). Compared with those in normal group, the levels of MDA, TG, total cholesterol, and ROS in cells were significantly increased in model group (Fig. 2C–F).
Fig. 2.
FFA induced steatosis in control and MASLD model cells. Representative bright field microscopy images of control and MASLD model cells in native (A) and Oil red O stained conditions(B). The levels of MDA (C), TG (D), total cholesterol (E), and ROS (F) in control and MASLD model cells. (Data are expressed as mean ± SD, *p < .05, **p < .01, ***p < .001 compared with the controls using two-tailed Student’s t-test, n = 3).
Whole-transcriptome profiling of ac4C modifications in control and MASLD model cells
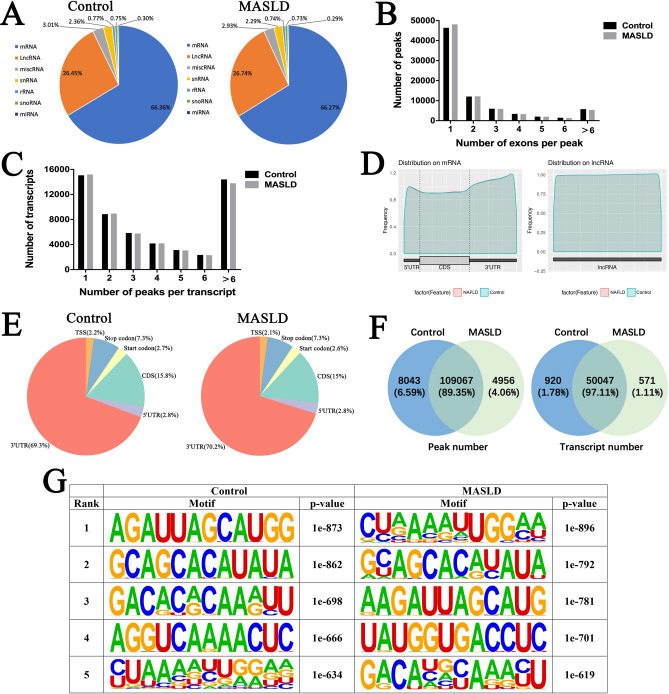
Using the exomePeak R package for peak calling and analysis, we identified 60,651 and 61,434 ac4C peaks on transcriptome in control and MASLD model cells, respectively, including mRNA, lncRNA, miscellaneous RNA (miscRNA), small nuclear RNA (snRNA), ribosomal RNA (rRNA), small nucleolar RNA (snoRNA), and microRNA (miRNA), and most of them are distributed on mRNA and lncRNA (Fig. 3A). Besides, among ac4C-modified transcripts, a significant number of RNA acetylation sites spanning 2 or more exons were identified (40% in the control group, 38.6% in the model group) (Fig. 3B). According to the results of the single base site analysis, the majority of ac4c-modified transcripts were found to have one ac4C modification site in both of two groups (28% for control group, 28.5% for model group). Intriguingly, another large proportion of the ac4c-modified transcripts were found to have more than six ac4C modification sites (26.7% for control group, 25.8% for model group) (Fig. 3C).
Fig. 3.
Overview of ac4C peaks in control and MASLD model cells. (A) Distribution of ac4C sites on transcriptome in control and MASLD model cells. (B) The number of ac4C peaks spanning different number of exons in control and MASLD model cells. (C) The number of transcripts containing different number of ac4C peaks in control and MASLD model cells. (D) Distribution of ac4C peaks occurring within CDS or UTRs in mRNA and lncRNA regions in control and MASLD model cells. (E) The distribution of ac4Cs on mRNA in control and MASLD model cells within the 5′ UTR, CDS, start codon, stop codon, TSS, and 3′ UTR. (F) The overlaps and differences of ac4C peaks and ac4C modified transcripts between control and MASLD model cells. (G) The sequence logo of the top 5 differential mode motifs of ac4Cs from control and MASLD model cells.
The acetylome analysis showed that most of ac4C sites were located in 3’UTR (69.3% for control group, 70.2% for model group) and CDS (15.8% for control group, 15% for model group) on mRNAs (Fig. 3D–E). Besides, ac4c modification showed no distribution specificity on lncRNAs (Fig. 3D right panel). The results of the intersection analysis between control and MASLD model groups revealed that 109,067 ac4C peaks were overlapped between the two groups, whereas 50,047 ac4C modified transcripts were overlapped. In addition, 4956 unique ac4C peaks correspond to 571 unique ac4C modified transcripts were observed in MASLD model cells (Fig. 3F). Homer de novo motif analysis results showed that the top five ac4C peak motifs in both groups comprised the typical ac4c motif “CXX” (Fig. 3G).
Distribution and functional pathways of differentially acetylated ac4C sites in control and MASLD model cells
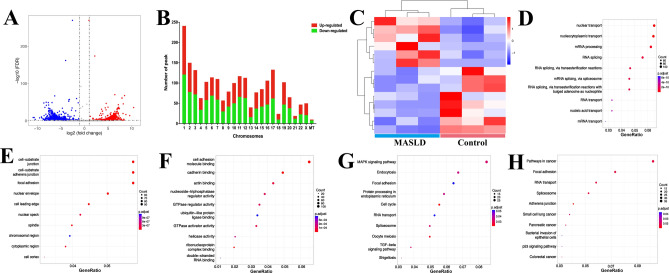
Among all ac4C peaks found in two groups, a total of 2128 differentially acetylated ac4C sites were identified (fold change > 2; FDR < 0.05), of which 1031 hyperacetylated and 1097 hypoacetylated peaks were detected in MASLD model cells comparing with those in control cells (Fig. 4A). The number of up- and down-regulated acetylated ac4C sites on chromosomes is shown in Fig. 4B, with the top ten hyper- and hypoacetylated peaks listed in Table 2. Cluster analysis revealed a clear distinction in the ac4C acetylation patterns between control and MASLD model cells (Fig. 4C).
Fig. 4.
Overview of the distribution of differentially acetylated ac4C sites in control and MASLD model cells. (A) Volcano plots of differential acetylated ac4C sites with statistical significance (fold change > 2; FDR < 0.05). Blue points indicate significantly down-regulated transcripts and red points indicate significantly up-regulated transcripts. (B) The distribution of differential ac4C peaks on chromosomes in control and MASLD model cells. (C) Heatmap of different ac4C modification patterns between control and MASLD model cells. GO enrichment map of ADMGs in (D) biological processes (BP), (E) cellular components (CC), (F) molecular functions (MF) categories. KEGG enrichment map of hyperacetylated (G) and hypoacetylated (H) ADMGs between control and MASLD model cells.
Table 2.
Top 10 hyperacetylated and hypoacetylated peaks in MASLD model cells.
| Ensembl gene ID | chromosome | thickStart | thickEnd | Gene name | Gene biotype | Acetylation status | Fold change |
|---|---|---|---|---|---|---|---|
| ENSG00000123560 | X | 103773717 | 103792619 | PLP1 | protein_coding | hyperacetylated | 1448 |
| ENSG00000163466 | 2 | 218253995 | 218254176 | ARPC2 | protein_coding | hyperacetylated | 776 |
| ENSG00000170265 | 7 | 149225259 | 149225440 | ZNF282 | protein_coding | hyperacetylated | 443 |
| ENSG00000117632 | 1 | 25900864 | 25901454 | STMN1 | protein_coding | hyperacetylated | 383 |
| ENSG00000183691 | 17 | 56593698 | 56595590 | NOG | protein_coding | hyperacetylated | 340 |
| ENSG00000118922 | 13 | 73687285 | 73687705 | KLF12 | protein_coding | hyperacetylated | 338 |
| ENSG00000166848 | 16 | 75648443 | 75648623 | TERF2IP | protein_coding | hyperacetylated | 302 |
| ENSG00000095787 | 10 | 28619877 | 28620088 | WAC | protein_coding | hyperacetylated | 292 |
| ENSG00000161960 | 17 | 7578616 | 7578826 | EIF4A1 | protein_coding | hyperacetylated | 254 |
| ENSG00000179431 | 11 | 35619687 | 35620010 | FJX1 | protein_coding | hyperacetylated | 241 |
| ENSG00000139329 | 12 | 91102628 | 91111831 | LUM | protein_coding | hypoacetylated | 1783 |
| ENSG00000164187 | 5 | 36100419 | 36100599 | LMBRD2 | protein_coding | hypoacetylated | 1552 |
| ENSG00000136104 | 13 | 50,970,092 | 50,973,506 | RNASEH2B | protein_coding | hypoacetylated | 1351 |
| ENSG00000177640 | 10 | 118099095 | 118210153 | CASC2 | antisense_RNA | hypoacetylated | 695 |
| ENSG00000162695 | 1 | 100980615 | 100980796 | SLC30A7 | protein_coding | hypoacetylated | 549 |
| ENSG00000268043 | 1 | 146969592 | 146971286 | NBPF12 | protein_coding | hypoacetylated | 501 |
| ENSG00000006451 | 7 | 39707527 | 39707767 | RALA | protein_coding | hypoacetylated | 437 |
| ENSG00000253967 | 8 | 70471133 | 70485687 | LINC03020 | lincRNA | hypoacetylated | 393 |
| ENSG00000160789 | 1 | 156139249 | 156139430 | LMNA | protein_coding | hypoacetylated | 388 |
| ENSG00000249709 | 19 | 12525908 | 12526238 | ZNF564 | protein_coding | hypoacetylated | 377 |
GO enrichment analysis was applied to reveal the potential functions of ac4C differentially modified genes (ADMGs) in MASLD model cells. In biological processes (BP), we found that ADMGs were significantly enriched in 710 GOs, including nuclear transport, nucleocytoplasmic transport, and mRNA processing (Fig. 4D). In cellular components (CC), 158 GO terms were significantly enriched, including cell − substrate junction, cell − substrate adherens junction, and focal adhesion (Fig. 4E). In molecular functions (MF), 76 GO terms were enriched, such as cell adhesion molecule binding, cadherin binding, and actin binding (Fig. 4F). KEGG enrichment analysis was further utilized to analyze the pathways associated with ADMGs in MASLD model cells. For hyperacetylated ADMGs enrichment, 184 pathways were identified, of which 27 pathways were significantly enriched. For hypoacetylated ADMGs enrichment, there were 182 pathways discovered, with 30 pathways terms significantly enriched. Moreover, the hyperacetylated ADMGs were mostly enriched in MAPK signaling pathway, endocytosis, and focal adhesion (Fig. 4G), while the hypoacetylated ADMGs were mainly enriched in pathways in cancer, focal adhesion, and RNA transport (Fig. 4H).
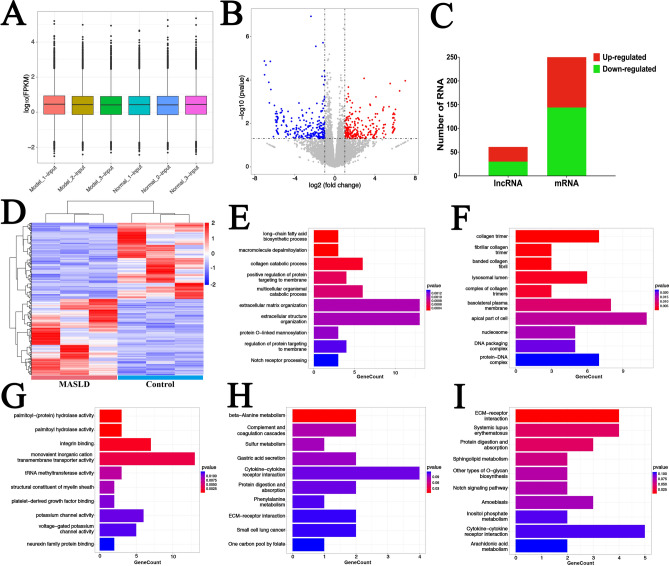
Whole-transcriptome profiling of gene expression and functional enrichment of differentially expressed genes (DEGs) in control and MASLD model cells
To investigate the expression of phenotype-related genes, the DEGs between control and MASLD model groups were analyzed. Gene counts were standardized by fragments per kilo base million reads (FPKM). The box plot indicated that standardization was good with no significant bias among groups (Fig. 5A). As illustrated in Fig. 5B, 61 differentially expressed lncRNAs and 280 differentially expressed mRNAs were identified (fold change > 2, P < 0.05), with 31 lncRNAs and 136 mRNAs up-regulated, and 30 lncRNAs and 144 mRNAs down-regulated in MASLD model cells (Fig. 5C). The top ten up- and down-regulated genes in MASLD model cells are listed in Table 3. The cluster plot showed a clear expression pattern difference between control and MASLD model cells (Fig. 5D).
Fig. 5.
Overview of gene expression and differentially expressed genes (DEGs) in control and MASLD model cells. (A) The distribution of gene expression in each cell sample after FPKM standardization. (B) Volcano plots of DEGs with statistical significance (fold change > 2; Pvalue < 0.05). Blue points indicate significantly down-regulated genes and red points indicate significantly up-regulated genes. (C) The distribution of differentially expressed lncRNAs and mRNAs in MASLD model cells. (D) Heatmap of DEGs patterns between control and MASLD model cells. GO enrichment map of DEGs in (E) biological processes (BP), (F) cellular components (CC), (G) molecular functions (MF) categories. KEGG enrichment map of up-regulated (H) and down-regulated (I) DEGs between control and MASLD model cells.
Table 3.
Top 10 up-regulated and down-regulated genes in MASLD model cells.
| Ensembl gene ID | LncRNA | mRNA | |||||
|---|---|---|---|---|---|---|---|
| Gene name | Expression status | Fold change | Ensembl gene ID | Gene name | Expression status | Fold change | |
| ENSG00000262898 | AC139099.2 | up | 66 | ENSG00000117586 | TNFSF4 | up | 88 |
| ENSG00000273568 | AC131009.3 | up | 63 | ENSG00000149968 | MMP3 | up | 64 |
| ENSG00000249584 | LINC02225 | up | 59 | ENSG00000087495 | PHACTR3 | up | 62 |
| ENSG00000259457 | AC100826.1 | up | 55 | ENSG00000149452 | SLC22A8 | up | 61 |
| ENSG00000278991 | AC090181.3 | up | 43 | ENSG00000122176 | FMOD | up | 60 |
| ENSG00000260949 | AP006545.1 | up | 28 | ENSG00000137726 | FXYD6 | up | 60 |
| ENSG00000235314 | LINC00957 | up | 19 | ENSG00000203942 | C10orf62 | up | 60 |
| ENSG00000266538 | AC005838.2 | up | 18 | ENSG00000198719 | DLL1 | up | 58 |
| ENSG00000253633 | AP002852.2 | up | 17 | ENSG00000131668 | BARX1 | up | 58 |
| ENSG00000224318 | CHL1-AS2 | up | 16 | ENSG00000181634 | TNFSF15 | up | 57 |
| ENSG00000280311 | AC131212.4 | down | 57 | ENSG00000204262 | COL5A2 | down | 130 |
| ENSG00000251161 | AC020661.1 | down | 52 | ENSG00000168542 | COL3A1 | down | 118 |
| ENSG00000214548 | MEG3 | down | 50 | ENSG00000177076 | ACER2 | down | 71 |
| ENSG00000273162 | AL133215.2 | down | 43 | ENSG00000128482 | RNF112 | down | 57 |
| ENSG00000261167 | AC107027.3 | down | 34 | ENSG00000072182 | ASIC4 | down | 57 |
| ENSG00000242767 | ZBTB20-AS4 | down | 29 | ENSG00000117400 | MPL | down | 57 |
| ENSG00000270673 | YTHDF3-AS1 | down | 28 | ENSG00000165125 | TRPV6 | down | 57 |
| ENSG00000224167 | AL390729.1 | down | 26 | ENSG00000143845 | ETNK2 | down | 56 |
| ENSG00000170161 | AL512625.1 | down | 18 | ENSG00000170381 | SEMA3E | down | 56 |
| ENSG00000279873 | LINC01126 | down | 16 | ENSG00000116254 | CHD5 | down | 56 |
GO analysis was applied for functional classification of DEGs. In BP, 159 GOs were significantly enriched, mainly included in long-chain fatty acid biosynthetic process, macromolecule depalmitoylation, and collagen catabolic process (Fig. 5E). In CC, 18 GOs were significantly enriched, including collagen trimer, fibrillar collagen trimer, and banded collagen fibril (Fig. 5F). In MF, 33 GOs were significantly enriched, mainly involving palmitoyl − (protein) hydrolase activity, palmitoyl hydrolase activity, and integrin binding (Fig. 5G). KEGG pathway analysis linked up-regulated DEGs to beta-Alanine metabolism (Fig. 5H), and down-regulated DEGs to ECM − receptor interaction, systemic lupus erythematosus, and protein digestion and absorption (Fig. 5I).
Association analysis of differential ac4C modifications with differential gene expression in control and MASLD model cells
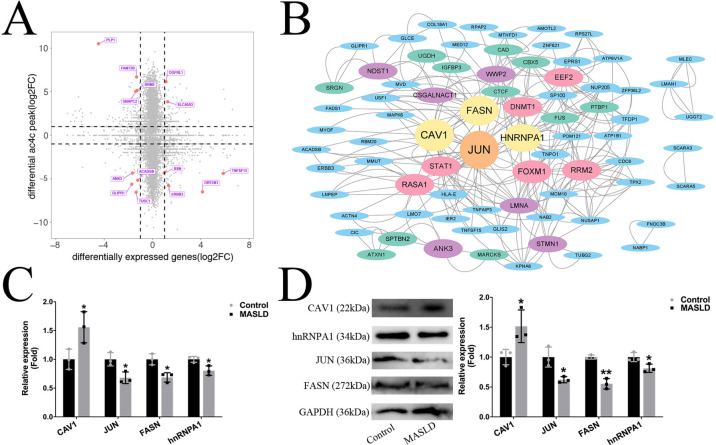
To explore the association of ac4C modification with gene expression, we cross-analyzed the acRIP-ac4c-seq and RNA-seq data. There are 4 types of association analysis results: Hyperacetylated-up (hyperacetylated ac4C modification and up-regulated RNA expression); hyperacetylated-down (hyperacetylated ac4C modification and down-regulated RNA expression); hypoacetylated-up (hypoacetylated ac4C modification and up-regulated RNA expression); hypoacetylated-down (hypoacetylated ac4C modification and down-regulated RNA expression). As illustrated in Fig. 6A a total of 118 genes were found both significantly changed in ac4C modification and expression levels in MASLD model cells (Pvalue < 0.05). The PPI network analysis by cytoscape software showed that JUN, CAV1, FASN, and hnRNPA1 were core proteins among these 118 genes (Fig. 6B)14. Furthermore, consistent with the sequencing data, the mRNA and protein expression levels of CAV1 were significantly increased, whereas those of JUN, FASN, and hnRNPA1 were significantly decreased in MASLD model cells (Fig. 6C, D). These genes were reported to be involved in the lipid synthesis and metabolism15,16. Moreover, the top 14 genes (red points in Fig. 6A), which were significantly changed in ac4C modification and expression levels with the highest fold change in MASLD model cells, are listed in Table 4.
Fig. 6.
Correlation of differential ac4C peaks with DEGs in control and MASLD model cells. (A) Scatter plot of genes with significant changes in ac4C modifications and RNA expression. (B) PPI network of genes with significant changes in ac4C modifications and RNA expression. (C) mRNA expression of JUN, CAV1, FASN, and HNRNPA1 in control and MASLD model cells. (D) Protein expression of JUN, CAV1, FASN, and HNRNPA1 in control and MASLD model cells. The full-length membranes were cropped prior to hybridisation with antibodies. The original images of all blots are presented in Supplementary information file. (Data are expressed as mean ± SD, *p < .05, **p < .01, ***p < .001 compared with the controls using two-tailed Student’s t-test, n = 3).
Table 4.
Top 14 genes with significant changes in ac4C modification and expression levels in MASLD model cells.
| Ensembl gene ID | Chromosome | thickStart | thickEnd | Gene name | Gene biotype | Acetylation and expression status |
|---|---|---|---|---|---|---|
| ENSG00000119900 | 6 | 71301967 | 71302148 | OGFRL1 | protein_coding | Hyperacetylated-up |
| ENSG00000139508 | 13 | 28700243 | 28700424 | SLC46A3 | protein_coding | Hyperacetylated-up |
| ENSG00000104976 | 19 | 7922414 | 7922594 | SNAPC2 | protein_coding | Hyperacetylated-down |
| ENSG00000123560 | X | 103773717 | 103792619 | PLP1 | protein_coding | Hyperacetylated-down |
| ENSG00000171848 | 2 | 10127105 | 10127255 | RRM2 | protein_coding | Hyperacetylated-down |
| ENSG00000188610 | 1 | 121181322 | 121183938 | FAM72B | protein_coding | Hyperacetylated-down |
| ENSG00000065361 | 12 | 56095416 | 56095794 | ERBB3 | protein_coding | Hypoacetylated-up |
| ENSG00000151150 | 10 | 60026297 | 60029777 | ANK3 | protein_coding | Hypoacetylated-up |
| ENSG00000181634 | 9 | 114784634 | 114806126 | TNFSF15 | protein_coding | Hypoacetylated-up |
| ENSG00000184698 | 11 | 5390151 | 5393263 | OR51M1 | protein_coding | Hypoacetylated-up |
| ENSG00000139278 | 12 | 75499790 | 75500090 | GLIPR1 | protein_coding | Hypoacetylated-down |
| ENSG00000164061 | 3 | 49667825 | 49670975 | BSN | protein_coding | Hypoacetylated-down |
| ENSG00000196177 | 10 | 123057263 | 123058311 | ACADSB | protein_coding | Hypoacetylated-down |
| ENSG00000198680 | 9 | 25677220 | 25677400 | TUSC1 | protein_coding | Hypoacetylated-down |
Discussion
It is estimated that approximately 1.7 billion people globally suffer from MASLD, with an overall prevalence of approximately 25%4. Although the current research on MASLD has been in-depth and comprehensive, the exact pathogenesis of MASLD remains unclear due to its complex etiology.
FFAs, key lipotoxic factors, can cause insulin resistance, disorders of glucose-lipid metabolism and lipid peroxidation, and contribute to fatty liver induction17,18. Esterification of excess FFA to triglyceride in hepatocytes leads to lipid accumulation in liver, resulting in hepatic steatosis19. In our study, a large accumulation of intracellular lipid droplets in HepaRG cells suggested that FFA successfully induced steatosis, which can be further used in the investigation of the pathogenesis of MASLD.
ac4C, similar to N6-methyladenosine (m6A), is a conserved RNA modification among eukaryotes. Previous studies have indicated that ac4C is primarily present on tRNA and 18S rRNA20, but recent research has revealed its presence on mRNAs, where it plays a crucial role in promoting RNA stability and protein translation, positioning it as a promising area in epitranscriptomics8,21. Advances in understanding ac4C have demonstrated its involvement in the progression of various human diseases22. Much of the current research has focused on the role of ac4C in cancers, such as colon, bladder, breast, and gastric cancers23–26. Other studies have evidenced that ac4C modification also influences autoimmune, chronic, and infectious diseases. Guo et al. found that ac4C modification and the expression of acetyltransferase NAT10 was decreased in CD4+ T cells of SLE patients. Meanwhile, functional enrichments indicated that ac4C modification was involved in biological process of SLE progression11. Another study documented that NAT10 increased the replication of HIV-1 by adding ac4C to HIV-1 RNA, thus facilitating HIV-1 disease progression12. Dodson et al. reported that ac4C levels on murine tRNAIni(CAU) were increased in heart tissues from type II diabetic mice model, indicating that ac4C modification was associated with diabetes27. Additionally, the studies on RNA chemical modifications in disease has demonstrated that ac4C is involved in sepsis, myocardial infarction, and Alzheimer’s disease28–30. However, whether ac4C modification plays a role in MASLD remains unclear. In this study, using an in vitro MASLD model induced by FFA, we identified that ac4C modification plays a vital role in biological processes of MASLD progression. ac4Cs were mainly distributed in 3’UTR and CDS of transcripts in both control and MASLD model cells, which indicates that ac4C is a highly conserved and hardwired epitranscriptomic modification. Although we detected the aberrant ac4C modification in MASLD model cells, RNA-seq analysis revealed no significant differences in NAT10 expression between control and MASLD model cells, suggesting the potential involvement of other “writer(s)” in ac4C regulation in MASLD. Notably, we also identified 8 and 5 ac4C peaks on mitochondrial DNA (mtDNA) of control and MASLD model cells, respectively. Research has confirmed that mitochondrial dysfunction and dynamics are closely related to neurodegenerative and metabolic diseases, including MASLD31. Mposhi et al. reported that the methylation levels of mitochondrial NADH dehydrogenase subunit 6 (ND6) gene were increased in mice fed a high-fat diet (HFD) and in MASLD patients, and the induction of mtDNA methylation promoted mitochondrial dysfunction and interfered the lipid metabolism in liver cells32,33. Therefore, whether the changes in ac4C level on the mtDNA can initiate MASLD progression by affecting mitochondrial function in liver remains to be determined.
To elucidate the potential functions of ADMGs and DEGs in MASLD model cells, we performed the GO and KEGG analysis for functional enrichment classification of these genes. GO analysis can be divided into three major groups: BP, CC, and MF. BP, being closest to phenotype, often reflects the biological state of the samples; CC describes gene localization within cells, which is significant for subcellular localization studies; MF explains the functional role of proteins, which is valuable when focusing on protein action changes within biological events34. KEGG analysis has a more complete pathway annotation35,36. The genes identified via the KEGG analysis are related to signaling pathways37. Therefore, the purpose of KEGG analysis in this study was to find the signaling pathways that are significantly enriched in ac4c-modified gene. Here, KEGG analysis of hyperacetylated ADMGs indicated that MAPK signaling pathway was the most significantly enriched pathway. MAPK activation has been previously linked to exacerbation of hepatic steatosis and mitochondrial dysfunction, making it a notable molecular feature in MASLD progression38,39. In addition, GO analysis of DEGs showed that long-chain fatty acid biosynthetic process was the most significantly enriched, including genes such as very long chain fatty acid-like family member 6 (ELOVL6), proteolipid protein 1 (PLP1), arachidonate 12-lipoxygenase, and 12R type (ALOX12B). ELOVL6 was proved to be positively correlated with MASLD progression and MASH pathology40. The role of PLP1 and ALOX12B in MASLD progression remains unknown. Based on our GO and KEGG results for ADMGs and DEGs, we hypothesize that ac4C modification may regulate the stability and translation of lipid synthesis and metabolism-related genes, resulting in lipid homeostasis imbalance. This hypothesis requires further experimental validation.
The results of the association analysis of ac4C modification with gene expression showed that majority of the ac4C differential peaks has rather no effect on transcript abundance, which reminds us whether ac4C modification has a role in translational level of genes during MASLD progression. Since the regulation of gene expression is complex, in addition to ac4C modification, there may be other chemical modifications (such as m6A) or pathway regulation that may affect the transcription level of genes. Therefore, the exact role of ac4C modification in the MASLD progression requires further in-depth studies.
We further applied cytoscape software to investigate the interaction relationships among the genes both significantly changed in ac4C modification and expression levels in MASLD model cells. JUN, CAV1, FASN, and hnRNPA1 were identified as the core genes after network analysis by cytoscape. JUN, also known as c-Jun and AP-1, can regulate the transcription of many genes through binding to their promoters. Xie et al. has proved that the activation of JUN promoted hepatic steatosis by upregulating lipogenic gene expression41. CAV1, a structural and signaling protein of caveolae on cytoplasmic membrane, was evidenced to be positively associated with development of MASLD42,43. FASN, a key enzyme driving the de novo lipogenesis (DNL) in the liver, was found to be increased in liver of MASLD patients and obese diabetic mice with MASLD44,45. Epitranscriptome analysis has reported that increased FASN expression and m6A modification in MASLD model mice, underscoring its central role in MASLD progression46. Additionally, Wang et al. has found that long noncoding RNA suppressor of hepatic gluconeogenesis and lipogenesis (lncSHGL) repressed hepatic lipogenesis in HFD mice by recruiting hnRNPA116. Therefore, the signaling pathways related to these four core genes are strongly implicated in molecular and PPI network associated with MASLD. However, inconsistent with previous findings, our study observed decreased expression of JUN, FASN, and hnRNPA1, with only CAV1 elevated in MASLD model cells. Therefore, further mechanistic studies on these proteins and MASLD progression are warranted.
Conclusions
The present study elucidates a transcriptome-wide ac4C modified profile of MASLD model cells. The altered expression of genes related to lipid synthesis and metabolism pathways, regulated by aberrant ac4C RNA acetylation, suggests a notable association between ac4C modification and MASLD progression. Collectively, our findings offer new insights into MASLD pathogenesis, and indicating the therapeutic potential of targeting ac4C-modified genes in prevention and management of MASLD.
Supplementary Information
Acknowledgements
We thank Prof. Ting Wang for her help in experiments and her constructive comments.
Abbreviations
- MASLD
Metabolic dysfunction-associated steatotic liver disease
- ac4C
N4-acetylcytosine
- NAT10
N-acetyltransferase 10
- FFAs
Free fatty acids
- DEGs
Differentially expressed genes
- ADMGs
Ac4C differentially modified genes
- PPI
Protein–protein interaction
- CAV1
Caveolin-1
- FASN
Fatty acid synthase
- hnRNPA1
Heterogeneous nuclear ribonucleoprotein A1
- MASH
Metabolic dysfunction-associated steatohepatitis
- MDA
Malondialdehyde
- TG
Triglyceride
- ROS
Reactive oxygen species
Author contributions
Xiqian Zhang designed and directed studies, analyzed and interpreted data, and drafted the manuscript. Yujie Yang and Fangyi Long suggested experiments, supervised the study, and revised the manuscript. Yaxian Zheng, Jing Yang, and Yan Yang performed cell experiments and data bioinformatics analysis. Qin He and Min Xu contributed to the discussion. All authors read and approved the final manuscript.
Funding
This study was financially supported by Technological Innovation Project of Chengdu Science and Technology Department (No. 2024-YF05-00157-SN), Seedling Engineering Project of Sichuan Science and Technology Department (No. 2022JDRC0145), Cultivation Project of Sichuan Science and Technology Department (Nos. 2022088, 2022089), and Chengdu Medical Research Project (No. 2024090).
Data availability
The datasets generated during the current study are available in NCBI SRA accession PRJNA1168032.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fangyi Long, Email: longfangyi@cmc.edu.cn.
Yujie Yang, Email: yangyujie@swjtu.edu.cn.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-84564-0.
References
- 1.Zhou, J. et al. Epidemiological features of NAFLD From 1999 to 2018 in China. Hepatology71, 1851–1864 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Masoodi, M. et al. Metabolomics and lipidomics in NAFLD: Biomarkers and non-invasive diagnostic tests. Nat. Rev. Gastroenterol. Hepatol.18, 835–856 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Loomba, R., Friedman, S. L. & Shulman, G. I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell184, 2537–2564 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powell, E. E., Wong, V. W. & Rinella, M. Non-alcoholic fatty liver disease. Lancet397, 2212–2224 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Wiener, D. & Schwartz, S. The epitranscriptome beyond m(6)A. Nat Rev Genet22, 119–131 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Dominissini D, Rechavi G. Epitranscriptome regulation. Nat Struct Mol Biol 2018. [DOI] [PubMed]
- 7.Zaccara, S., Ries, R. J. & Jaffrey, S. R. Reading, writing and erasing mRNA methylation. Nat. Rev. Mol. Cell Bio.20, 608–624 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Arango, D. et al. Acetylation of cytidine in mRNA promotes translation efficiency. Cell175, 1872–86.e24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sas-Chen, A. et al. Dynamic RNA acetylation revealed by quantitative cross-evolutionary mapping. Nature583, 638–643 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dominissini, D. & Rechavi, G. N(4)-acetylation of cytidine in mRNA by NAT10 regulates stability and translation. Cell175, 1725–1727 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Guo, G. et al. Epitranscriptomic N4-acetylcytidine profiling in CD4(+) T cells of systemic lupus erythematosus. Front Cell Dev Biol8, 842 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai, K. et al. Acetylation of cytidine residues boosts HIV-1 gene expression by increasing viral RNA Stability. Cell Host Microbe.28, 306–12.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu, Y., Xu, W., Zhai, T., You, J. & Chen, Y. Silibinin ameliorates hepatic lipid accumulation and oxidative stress in mice with non-alcoholic steatohepatitis by regulating CFLAR-JNK pathway. Acta Pharm. Sin. B9, 745–757 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shannon, P. et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res.13, 2498–2504 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nassir, F. NAFLD: Mechanisms, treatments, and biomarkers. Biomolecules12, 824 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang, J. et al. Long Noncoding RNA lncSHGL Recruits hnRNPA1 to Suppress Hepatic Gluconeogenesis and Lipogenesis. Diabetes67, 581–593 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Boden, G. Interaction between free fatty acids and glucose metabolism. Curr. Opin. Clin. Nutr.5, 545–549 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Delarue, J. & Magnan, C. Free fatty acids and insulin resistance. Curr. Opin. Clin. Nutr.10, 142–148 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Zhang, J. et al. Association between serum free fatty acid levels and nonalcoholic fatty liver disease: A cross-sectional study. Sci. Rep.-Uk4, 5832 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong, C. et al. tRNA modification profiles of the fast-proliferating cancer cells. Biochem. Biophys. Res. Commun.476, 340–345 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Castello, A. et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell149, 1393–1406 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Luo, J., Cao, J., Chen, C. & Xie, H. Emerging role of RNA acetylation modification ac4C in diseases: Current advances and future challenges. Biochem. Pharmacol.213, 115628 (2023). [DOI] [PubMed] [Google Scholar]
- 23.Deng, M. et al. Helicobacter pylori-induced NAT10 stabilizes MDM2 mRNA via RNA acetylation to facilitate gastric cancer progression. J. Exp. Clin. Cancer Res.42, 9 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang, G. et al. NAT10-mediated mRNA N4-acetylcytidine modification promotes bladder cancer progression. Clin. Transl. Med.12, e738 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng, X. et al. N-acetyltransferase 10 promotes colon cancer progression by inhibiting ferroptosis through N4-acetylation and stabilization of ferroptosis suppressor protein 1 (FSP1) mRNA. Cancer Commun.42, 1347–1366 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang, X. et al. Revealing the potential markers of N(4)-Acetylcytidine through acRIP-seq in triple-negative breast cancer. Genes13, 2400 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dodson, T. A. et al. Ribonucleosides from tRNA in hyperglycemic mammalian cells and diabetic murine cardiac models. Life Sci.318, 121462 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang, H. et al. NAT10 regulates neutrophil pyroptosis in sepsis via acetylating ULK1 RNA and activating STING pathway. Commun. Biol.5, 916 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, K. et al. PIWI-interacting RNA HAAPIR regulates cardiomyocyte death after myocardial infarction by promoting NAT10-mediated ac(4) C acetylation of Tfec mRNA. Adv. Sci.9, e2106058 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma, Y. et al. Comprehensive analysis of long non-coding RNAs N4-acetylcytidine in Alzheimer’s disease mice model using high-throughput sequencing. J. Alzheimers Dis.90, 1659–1675 (2022). [DOI] [PubMed] [Google Scholar]
- 31.Peng, K. Y. et al. Mitochondrial dysfunction-related lipid changes occur in nonalcoholic fatty liver disease progression. J. Lipid Res.59, 1977–1986 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mposhi, A. et al. Mitochondrial DNA methylation in metabolic associated fatty liver disease. Front. Nutr.10, 964337 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Wijst, M. G., van Tilburg, A. Y., Ruiters, M. H. & Rots, M. G. Experimental mitochondria-targeted DNA methylation identifies GpC methylation, not CpG methylation, as potential regulator of mitochondrial gene expression. Sci. Rep-Uk7, 177 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashburner, M. et al. Gene ontology: tool for the unification of biology the gene ontology consortium. Nat. Genet.25, 25–29 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanehisa, M. & Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res28, 27–30 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanehisa, M., Sato, Y., Kawashima, M., Furumichi, M. & Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res.44, D457–D462 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Draghici, S. et al. A systems biology approach for pathway level analysis. Genome Re17, 1537–1545 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bai, L. et al. Multispecies transcriptomics identifies SIKE as a MAPK repressor that prevents NASH progression. Sci. Transl. Med.16, eade7347 (2024). [DOI] [PubMed] [Google Scholar]
- 39.Ding, J. et al. HADHA alleviates hepatic steatosis and oxidative stress in NAFLD via inactivation of the MKK3/MAPK pathway. Mol. Biol. Rep.50, 961–970 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuzaka, T. et al. Elovl6 promotes nonalcoholic steatohepatitis. Hepatology56, 2199–2208 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Xie, D. et al. High uric acid induces liver fat accumulation via ROS/JNK/AP-1 signaling. Am. J. Physiol-Endoc. M320, E1032–E1043 (2021). [DOI] [PubMed] [Google Scholar]
- 42.Deng, G. H. et al. Caveolin-1 is critical for hepatic iron storage capacity in the development of nonalcoholic fatty liver disease. Military Med. Res.10, 53 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xue, W. et al. Caveolin-1 alleviates lipid accumulation in NAFLD associated with promoting autophagy by inhibiting the Akt/mTOR pathway. Eur. J. Pharmacol.871, 172910 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Mitsuyoshi, H. et al. Analysis of hepatic genes involved in the metabolism of fatty acids and iron in nonalcoholic fatty liver disease. Hepatol. Res.39, 366–373 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Matsukawa, T. et al. Hepatic FASN deficiency differentially affects nonalcoholic fatty liver disease and diabetes in mouse obesity models. JCI Insight8, e161282 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo, Y. et al. Analysis of N6-methyladenosine methylation modification in fructose-induced non-alcoholic fatty liver disease. Front. Endocrinol.12, 780617 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available in NCBI SRA accession PRJNA1168032.