Abstract
Background
Patients in the intensive care unit (ICU) frequently develop hyperactive delirium, which may be accompanied by behaviour that increases clinical risks to themselves as well as other patients and staff. There is a paucity of evidence to inform the urgent enteral administration of antipsychotic drugs to treat such hyperactive delirium and behavioural disturbances.
Objective
The aim of this study is to test the efficacy and safety of administering enteral olanzapine when compared to quetiapine in critically ill patients with hyperactive delirium.
Design, setting, participants, and interventions
This is a cluster-randomised, double-crossover, clinical trial. Critically ill adult patients admitted to three tertiary Australian intensive care units over a 12-month period will be eligible. Randomisation will occur at the site level, with allocation to open-label olanzapine or quetiapine use over four treatment periods of 3-month duration.
Main outcome measure
The primary outcome and days alive and delirium-/coma-free (censored at 14 days post enrolment) will be analysed using median quantile regression accounting for clustering at sites' level and time period and treatment order.
Results and conclusion
This trial will compare the effect of enteral olanzapine to quetiapine in critically ill adults with hyperactive delirium on an important indicator of patient outcome.
Keywords: Delirium, Critical illness, Intensive care units, Antipsychotic agents, Olanzapine, Quetiapine
1. Introduction
Delirium occurs frequently in patients admitted to the intensive care unit (ICU). The reported prevalence varies widely, depending on the method of diagnosis and the geographical region of study, with reports ranging between 16% and 89%.1
Delirium is categorised into three types based on symptoms: hyperactive, hypoactive, or a combination of the two. While delirium is associated with a marked increase in mortality and impaired long-term cognitive function,2 it remains uncertain whether acute treatment reduces these outcomes.[3], [4], [5] Nonetheless, hyperactive delirium and marked behavioural disturbances are particularly problematic as patients may become impulsive and/or aggressive and remove essential interventions, posing a risk to themselves and to staff.6,7 This suggests that a reduction in duration of hyperactive delirium and/or severity of agitation remain important patient-centred outcomes.
Patients experiencing hyperactive delirium and behavioural disturbances frequently receive antipsychotic drugs to control their symptoms.2 Despite such widespread use, there is limited evidence to suggest that the enteral administration of antipsychotic drugs reduces symptoms or improves outcomes in critically ill patients. In 2010, Devlin et al. conducted a small trial of 36 critically ill patients with delirium and compared enterally administered quetiapine to placebo (with ‘as needed’ intravenous [IV] haloperidol) and reported a shorter time to resolution of delirium symptoms.8 More recently, placebo-controlled trials using IV drugs and much larger cohorts have been conducted with haloperidol/ziprasidone in the United States9 and haloperidol in Europe.10 Both of these larger trials revealed neutral results. However, more than half of the patients included in these more recent trials had hypoactive delirium.
Whilst there is a lack of evidence to guide the use of antipsychotics in critical illness, international data suggest that these drugs are still frequently administered. In the United States, across 71 medical centres, antipsychotic drugs were reported to be administered to 17,764 of 164,996 (11%) patients who had at least 1 day in the ICU, with haloperidol being the most common.11 Australian and New Zealand (ANZ) data are similar, with 12% of 627 patients admitted to one of 44 ANZ ICUs receiving at least one antipsychotic in a point prevalence study.12 Of the antipsychotic drugs given in this region, quetiapine and olanzapine (usually via the enteral route) are the most frequently administered.12
The proposed trial is a multicentre, cluster-randomised, double-crossover pragmatic clinical trial of enteral olanzapine with quetiapine in critically ill patients with hyperactive delirium (CALM-ICU). The aims of the CALM-ICU trial are to test the comparative safety and efficacy of the two most frequently administered enteral drugs to critically ill patients with hyperactive delirium in ANZ.
2. Methods
The primary aim of the CALM-ICU trial is to compare days alive and free of delirium/coma between enteral olanzapine and enteral quetiapine in critically ill patients with hyperactive delirium by 14 days after enrolment.
The secondary objectives are to evaluate whether there are any between-treatment differences for mortality (ICU and hospital), days of delirium or behaviour disturbance, length of stay (ICU and hospital), duration of mechanical ventilation, and adverse drug events.
CALM-ICU is an investigator-initiated, multicentre, cluster-randomised pragmatic clinical trial, with double-crossover cluster periods.
There are four treatment periods, each of 3-month duration, within three hospital ICU clusters in Melbourne, Australia. Each ICU cluster will be allocated a treatment sequence from randomised permuted blocks containing equal replication sequences with the possible order being ABAB, BABA, ABBA, or BAAB (with A and B corresponding to olanzapine and quetiapine, respectively).13 The lead statistician will be blinded to the treatment allocation, but there will be no blinding of study drug to participants, investigators, or clinicians.
The protocol design is in accordance with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) recommendations, with the checklist provided in the supplemental material.14,15
This trial is supported by an Australian and New Zealand Intensive Care Foundation Project Grant ($40,000 AUD). The Foundation is not involved in the design of the study, data collection/interpretation, or the writing of the final manuscript.
Trial registration: The trial was prospectively registered with the Australian New Zealand Clinical Trials Registry: ACTRN12622001532796.
Ethical approval: The CALM-ICU protocol (version 2, dated 23 September 2021) was approved by The Royal Melbourne Hospital Human Research Ethics Committee on 26 October 2021 with an amendment to the trial protocol (version 3, dated 6 June 2023) approved 31 July 2023. The first patient was enrolled on 14 December 2022, with the final patient expected to be enrolled in April 2024.
2.1. Eligibility criteria
All adult patients aged 18 years or older admitted to a participating ICU, who require enteral pharmacological treatment for hyperactive delirium, will be eligible for inclusion. Because systematic identification of patients in the ICU with hyperactive delirium remains challenging, several methodologies, such as Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) and the Intensive Care Delirium Screening Checklist, have been proposed to assist bedside healthcare workers to ascertain a patient has delirium.[16], [17], [18] More recently, interrogation of medical records alongside documentation of sedation score, when compared to using the CAM-ICU screening tool, has been reported to increase the sensitivity of identifying patients with behavioural disturbances and agitation who receive antipsychotic drugs.[19], [20], [21], [22], [23], [24] For the purposes of this study, hyperactive delirium will be defined as a Richmond Agitation–Sedation Scale (RASS) score of +1 or greater and/or language descriptors/clinical notation of agitation (or synonyms) and/or being CAM-ICU-positive.18,19,22 The requirement for pharmacological treatment will be at the discretion of the treating ICU physician. Patients will be excluded if they regularly take an antipsychotic medication before admission25 or if they have an allergy to either olanzapine or quetiapine.
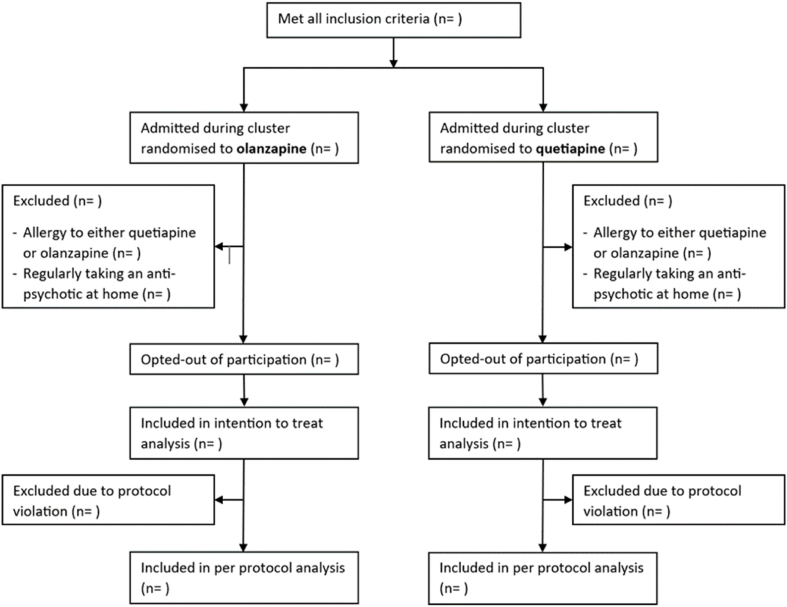
The proposed patient flow diagram for the CALM-ICU trial is provided (Fig. 1).
Fig. 1.
Patient flow diagram of the CALM-ICU trial.
2.2. Participant safety
The Royal Melbourne Hospital is the sponsor of the trial. The protocol has been approved by the Royal Melbourne Hospital Research Ethics Committee (Reference number: 2021.013) with governance approval at each site. Patients who are eligible for enrolment into the study will be included with a waiver of consent and an opt-out process for ongoing participation and/or retention of data. The protocol was prospectively registered with the Australian New Zealand Clinical Trials Registry: ACTRN12622001532796. The trial has a Trial Safety Committee that meets quarterly to review compliance with the trial protocol and Riskman and Serious Adverse Event reports from all patients.
2.3. Interventions
CALM-ICU will compare the efficacy of enteral olanzapine and quetiapine. The ICU physician caring for the patient will be responsible for the dose and frequency of the allocated (trial) drug, and the investigators have not provided additional instructions regarding dosing regimen.
During each 3-month period, the ICU will have an ‘allocated’ antipsychotic drug (either enteral olanzapine or quetiapine) as the standard treatment of hyperactive delirium and behavioural disturbances.
All participants will exclusively receive the allocated enteral antipsychotic for the first three doses of their pharmacological management of hyperactive delirium (including any doses administered as part of a regular regimen and ‘as required’) before other agents are considered as part of usual care. If further treatment is required after the first three doses, use of additional drugs is permitted. At any stage, if the treating ICU physician believes that an IV drug is required for patient or staff safety, this can be administered. Administration of the alternative antipsychotic drug will be strongly discouraged during each treatment period.
Participants will remain on the study treatment until they experience any of the following: death, discharge from the ICU, completion of 14 days from enrolment, or when the treating clinician determines that the patient no longer requires the use of antipsychotics to treat hyperactive delirium, whichever occurs first.
Patients who remain in the ICU at a point of ICU cluster crossover will continue their original assigned treatment.26 There is no washout interval before the cluster crosses over so that all new patients receive the treatment allocated to the new cluster period.
If the treating team believes that either olanzapine or quetiapine is clearly preferable, the treating ICU physician can override the treatment allocation according to cluster period. This will be recorded as a protocol violation, with data retained for an intention to treat analysis. Given the open-label design, an exploratory per-protocol analysis will also be performed.
2.4. Outcomes
The primary outcome for this trial is the number of days alive and delirium-/coma-free at 14 days after enrolment.
The rationale for using this composite outcome primary outcome is based on the trial by Girard et al.9 This outcome adjusts for death occurring, so additional days of delirium are not recognised; it also incorporates two of the seven core outcomes listed in the core outcome set for delirium prevention or treatment trials.27
A patient will be regarded as delirium-/coma-free for that calendar day if:
-
-
They are alive and discharged from the ICU or
-
-They are alive in the ICU with all the following:
-
a)No CAM-ICU-positive outcome recorded at any timepoint.
-
b)The RASS score being −2 to 0 at all timepoints throughout the day.
-
c)No documentation of agitation (or any synonym) in the medical records.
-
a)
Note:
-
-
All patients admitted to these ICUs have CAM-ICU/RASS scores recorded at least twice a day as part of standard clinical care at all three sites.
-
-
‘Delirium-/coma-free days' commence once the patient is not delirious or in coma again throughout the 14-day study period.
-
-
If clinicians deem the study drug (olanzapine or quetiapine) should continue after the ICU, this is allowed according to clinical judgement.
Secondary outcomes include mortality (ICU and hospital), days of delirium or behaviour disturbance, duration of admission (ICU and hospital), duration of mechanical ventilation in those that are ventilated at randomisation, and adverse drug events.
Compliance and exposure to concomitant medications will be reported including:
-
-
The number of enteral doses of the allocated study drug per patient and the total amount of study drug on the calendar day
-
-
Use of ‘nonstudy’ antipsychotic drug (i.e., if the cluster period is quetiapine, then any olanzapine administered for the 3-month period)
-
-
The number of doses of IV or intramuscular olanzapine administered and the total amount on the calendar day
-
-
The number of doses of haloperidol administered per patient (enteral or IV) and the total amount on the calendar day
-
-
The number of doses of clonidine administered per patient (enteral or IV bolus) or the number of hours (infusion) on the calendar day; the total amount of clonidine will also be recorded for the day
-
-
The number of hours per patient that dexmedetomidine infusion is administered on the calendar day and the total amount for the day
-
-
The number of doses of benzodiazepine (specify individual agent) administered per patient (enteral or IV bolus) or the number of hours (infusion) on the calendar day; the total amount of benzodiazepine will also be recorded for the day
The corrected QT (QTc) will be calculated from the daily electrocardiogram, with the longest QTc per patient per day recorded.28
Table 1 provides a detailed summary of the data collection and assessment of primary and secondary outcomes. Demographic data, relevant medical history, and admission status will be collected on admission from the patients' medical records.
Table 1.
Data collection.
| Baseline data |
|---|
| Age (years) |
| Sex (female/male) |
| Weight (kg) |
| Mechanically ventilated at inclusion |
| Source of admission to the ICU |
| Patient type (elective, emergency, or nonsurgical) |
| APACHE III score and diagnostic code |
| Daily data—clinicala |
| Described as agitated, delirious, confused, or restless |
| CAM-ICU positive at any timepoint |
| Highest and lowest RASS score |
| Mechanically ventilated at any timepoint |
| Longest QTc recorded |
| Event related to self-injury or staff assault |
| Use of mechanical restraints |
| Daily data—drug administrationa |
| Number of enteral doses of allocated study drug and the total amount |
| Number of enteral doses of ‘nonstudy’ antipsychotic administered (i.e., olanzapine if the ICU has been allocated quetiapine for the 3-month period) and the total amount |
| Number of doses of IV or IM olanzapine administered and the total amount |
| Number of doses of haloperidol (enteral or IV) administered and the total amount |
| Number of hours of dexmedetomidine infusion and the total amount |
| Number of doses of clonidine (enteral or IV) administered OR the number of hours of clonidine infusion; the total amount of clonidine will also be recorded |
| Number of doses of benzodiazepine (enteral or IV) administered OR the number of hours of benzodiazepine infusion; the total amount of benzodiazepine will also be recorded (and the specific benzodiazepine will be noted) |
| Patient exclusively received the allocated enteral antipsychotic for the first three doses of pharmacological hyperactive delirium treatment |
| New adverse drug reaction potentially related to study drug |
| Discharge data |
| Duration of ICU and hospital admission |
| ICU and hospital discharge status |
Abbreviations: ICU: intensive care unit; IV: intravenous; IM: intramuscular; APACHE: Acute Physiology and Chronic Health Evaluation.
All ‘daily data’ will be collected until death, discharge from ICU, or 14 days post enrolment—whichever comes first.
The duration of delirium before administration of quetiapine or olanzapine is not recorded, and the duration before commencing an intervention may influence the response to the study drug.
2.5. Sample size
Power calculations have been performed in accordance with Hemming29 and are based on an intracluster correlation (ICC) of 0.01 between patients from the same cluster in the same time period and an intracluster correlation of 0.005 for the correlation between two patients in differing time periods. These two important correlations have been conservatively estimated based on Casamento et al.30 A standard deviation of 4.1 for coma- and delirium-free days to day 14 was derived from the SPICE III trial.31 Based on an approximate period cluster of 100 patients per 3-month treatment period, the CALM-ICU study was designed to have an 80% power (two-sided p-value of 0.05) to detect a clinically relevant 1-day difference in coma- and delirium-free alive days to day 14. To account for the likelihood that coma- and delirium-free days will not be normally distributed, calculations include a 15% inflation for non-normality.32
Using annual ICU admission data for the contributing hospitals and an observed rate of psychotropic drug usage in Australia of 13.5%,31 we anticipate 1200 patients will be recruited from the three sites over the four study periods.
2.6. Statistical methods
All data will be assessed for normality. Baseline comparisons will be performed using Chi-squared tests for equal proportion, Student's t-tests for normally distributed data, and Wilcoxon rank-sum tests otherwise, with results reported as n (%), mean (standard deviation), or median (interquartile range), respectively.
The primary outcome, days alive and free of delirium/coma (censored at 14 days), will be analysed using median quantile regression with clustering at an ICU level and adjustment for randomisation time period and order of administration of treatment, with results reported as differences of medians (95% confidence interval [CI]). Sensitivity analysis will be adopted using mixed linear modelling adjusted for period and order with robust standard errors clustered at an ICU level and results reported as difference of means (95% CI).
Binomial secondary outcomes (mortality and any adverse event) will be compared between treatment arms using generalised estimating equations with adjustment for time period and treatment order, with an exchangeable working correlation matrix and robust standard errors using the ICU as the clustering unit. Duration of mechanical ventilation along with ICU and hospital length of stay will be compared between treatment arms using Cox regression with robust standard errors clustered at ICU level to estimate cause-specific hazard ratios (95% CI), with results summarised as medians (interquartile range) and presented as cumulative incidence functions with death treated as a competing risk.
All analyses will be performed using SAS version 9.4 (SAS Institute Inc., Cry, NC, USA) using the intention-to-treat principle. A two-sided p-value will be used to indicate statistical significance for the primary outcome. A per-protocol analysis will also be provided. No adjustment will be made for multiplicity in secondary outcomes, and as such, all secondary outcome findings will be considered hypothesis generating.
Every effort will be made to ensure missing data are limited. However, for any missing data, exploratory analyses will be conducted to test the effects of missing data,33 including:
-
1.
Best-worst and worst-best sensitivity analyses
-
2.
Standard appropriate multiple imputation method.
2.7. Data sharing
Nonidentifiable individual participant data that underlie the results reported in this trial will be made available after 3 years following publication and ending 7 years after publication. Availability will only be made to researchers who provide a written proposal for data evaluation that is judged to be methodologically sound by a committee approved by investigators. Proposals should be directed to Melissa.Ankravs@mh.org.au. If the proposal is approved, access data requestors will be required to sign a data-access agreement before accessing data.
3. Discussion
This multicentre, cluster-randomised, double-crossover clinical trial will test the comparative safety and efficacy of the two most frequently administered enteral drugs that are administered to treat critically ill patients with hyperactive delirium in ANZ.
Funding
This trial is supported by an Australian and New Zealand Intensive Care Foundation Project Grant ($40,000 AUD).
Conflict of interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Melissa Ankravs reports financial support was provided by The Australian and New Zealand Intensive Care Foundation (in the form of a grant as outlined in the manuscript). CCR Editor-in-Chief – Rinaldo Bellomo; Associate Editor – Andrew Udy; Editorial Board – Yasmine Ali Abdelhamid, Adam Deane, Mark Plummer. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to acknowledge the input of Nathaniel Adamson and Yasmin Ramage for their insights utilised in the conception of the trial and Brianna Tascone for her assistance in the preparation of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ccrj.2024.08.003.
Contributor Information
Melissa J. Ankravs, Email: melissa.ankravs@mh.org.au.
Adam M. Deane, Email: adam.deane@mh.org.au.
CRediT authorship contribution statement
MA, AU, RB, JP, YA, MB, KB, MG, MP, LS, MY and AD all contributed to the conception and design of the trial. MA and AD drafted the manuscript. All authors reviewed and approved the final manuscript. MA, AU, RB, KB and AD were responsible for funding acquisition.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Reade M.C., Finfer S. Sedation and delirium in the intensive care unit. N Engl J Med. 2014;370(5):444–454. doi: 10.1056/NEJMra1208705. PubMed PMID: 24476433. Epub 2014/01/31. eng. [DOI] [PubMed] [Google Scholar]
- 2.Stollings J.L., Kotfis K., Chanques G., Pun B.T., Pandharipande P.P., Ely E.W. Delirium in critical illness: clinical manifestations, outcomes, and management. Intensive Care Med. 2021;47(10):1089–1103. doi: 10.1007/s00134-021-06503-1. PubMed PMID: 34401939. PMCID: PMC8366492. Epub 2021/08/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reade M.C., Eastwood G.M., Bellomo R., Bailey M., Bersten A., Cheung B., et al. Effect of dexmedetomidine added to standard care on ventilator-free time in patients with agitated delirium: a randomized clinical trial. JAMA. 2016;315(14):1460–1468. doi: 10.1001/jama.2016.2707. PubMed PMID: 26975647. Epub 2016/03/16. eng. [DOI] [PubMed] [Google Scholar]
- 4.Andersen-Ranberg N.C., Poulsen L.M., Perner A., Hästbacka J., Morgan M., Citerio G., et al. Haloperidol vs. placebo for the treatment of delirium in ICU patients: a pre-planned, secondary Bayesian analysis of the AID-ICU trial. Intensive Care Med. 2023;49(4):411–420. doi: 10.1007/s00134-023-07024-9. PubMed PMID: 36971791. Epub 2023/03/28. eng. [DOI] [PubMed] [Google Scholar]
- 5.Devlin J.W., Duprey M.S., Girard T.D. How does haloperidol influence the long-term outcomes of delirium? Intensive Care Med. 2024;50(2):269–271. doi: 10.1007/s00134-024-07321-x. [DOI] [PubMed] [Google Scholar]
- 6.Northcott M.H., Johnston G., Presneill J.J., Fazio T.N., Adamson N., Ankravs M.J., et al. Aggression, violence and threatening behaviour during critical illness. Crit Care Resusc. 2023;25(2):65–70. doi: 10.1016/j.ccrj.2023.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaworska N., Makuk K., Krewulak K.D., Niven D.J., Ismail Z., Burry L.D., et al. A national modified delphi consensus process to prioritize experiences and interventions for antipsychotic medication deprescribing among adult patients with critical illness. Crit Care Explor. 2022 Dec;4(12) doi: 10.1097/CCE.0000000000000806. PubMed PMID: 36506828. PMCID: PMC9722588. Epub 2022/12/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devlin J.W., Roberts R.J., Fong J.J., Skrobik Y., Riker R.R., Hill N.S., et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010;38(2):419–427. doi: 10.1097/CCM.0b013e3181b9e302. PubMed PMID: 19915454. Epub 2009/11/17. eng. [DOI] [PubMed] [Google Scholar]
- 9.Girard T.D., Exline M.C., Carson S.S., Hough C.L., Rock P., Gong M.N., et al. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506–2516. doi: 10.1056/NEJMoa1808217. PubMed PMID: 30346242. PMCID: PMC6364999. Epub 2018/10/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen-Ranberg N.C., Poulsen L.M., Perner A., Wetterslev J., Estrup S., Hästbacka J., et al. Haloperidol for the treatment of delirium in ICU patients. N Engl J Med. 2022;387(26):2425–2435. doi: 10.1056/NEJMoa2211868. PubMed PMID: 36286254. Epub 2022/10/27. eng. [DOI] [PubMed] [Google Scholar]
- 11.Swan J.T., Fitousis K., Hall J.B., Todd S.R., Turner K.L. Antipsychotic use and diagnosis of delirium in the intensive care unit. Crit Care. 2012;16(3):R84–R. doi: 10.1186/cc11342. PubMed PMID: 22591601. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ankravs M.J., Udy A.A., Byrne K., Knowles S., Hammond N., Saxena M.K., et al. A multicentre point prevalence study of delirium assessment and management in patients admitted to Australian and New Zealand intensive care units. Crit Care Resusc. 2020;22(4):355–360. doi: 10.51893/2020.4.OA8. PubMed PMID: 38046881. PMCID: PMC10692531. Epub 2023/12/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemming K., Taljaard M., Weijer C., Forbes A.B. Use of multiple period, cluster randomised, crossover trial designs for comparative effectiveness research. BMJ. 2020;371 doi: 10.1136/bmj.m3800. PubMed PMID: 33148538. Epub 2020/11/06. eng. [DOI] [PubMed] [Google Scholar]
- 14.Chan A.-W., Tetzlaff J.M., Gøtzsche P.C., Altman D.G., Mann H., Berlin J.A., et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. Br Med J. 2013;346 doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell M.K., Piaggio G., Elbourne D.R., Altman D.G. Consort 2010 statement: extension to cluster randomised trials. Br Med J. 2012;345 doi: 10.1136/bmj.e5661. [DOI] [PubMed] [Google Scholar]
- 16.Kotfis K., van Diem-Zaal I., Williams Roberson S., Sietnicki M., van den Boogaard M., Shehabi Y., et al. The future of intensive care: delirium should no longer be an issue. Crit Care. 2022;26(1):200. doi: 10.1186/s13054-022-04077-y. PubMed PMID: 35790979. PMCID: PMC9254432. Epub 2022/07/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inouye S.K., van Dyck C.H., Alessi C.A., Balkin S., Siegal A.P., Horwitz R.I. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. doi: 10.7326/0003-4819-113-12-941. PubMed PMID: 2240918. Epub 1990/12/15. eng. [DOI] [PubMed] [Google Scholar]
- 18.Ely E.W., Inouye S.K., Bernard G.R., Gordon S., Francis J., May L., et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. PubMed PMID: 11730446. Epub 2001/12/26. eng. [DOI] [PubMed] [Google Scholar]
- 19.Young M., Holmes N., Kishore K., Marhoon N., Amjad S., Serpa-Neto A., et al. Natural language processing diagnosed behavioral disturbance vs confusion assessment method for the intensive care unit: prevalence, patient characteristics, overlap, and association with treatment and outcome. Intensive Care Med. 2022;48(5):559–569. doi: 10.1007/s00134-022-06650-z. PubMed PMID: 35322288. PMCID: PMC9050783. Epub 2022/03/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmes N.E., Amjad S., Young M., Berlowitz D.J., Bellomo R. Using language descriptors to recognise delirium: a survey of clinicians and medical coders to identify delirium-suggestive words. Crit Care Resusc. 2019;21(4):299–302. PubMed PMID: 31778637. Epub 2019/11/30. eng. [PubMed] [Google Scholar]
- 21.Young M., Holmes N.E., Kishore K., Amjad S., Gaca M., Serpa Neto A., et al. Natural language processing diagnosed behavioural disturbance phenotypes in the intensive care unit: characteristics, prevalence, trajectory, treatment, and outcomes. Crit Care. 2023;27(1):425. doi: 10.1186/s13054-023-04695-0. PubMed PMID: 37925406. PMCID: PMC10625294. Epub 2023/11/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young M., Holmes N., Robbins R., Marhoon N., Amjad S., Neto A.S., et al. Natural language processing to assess the epidemiology of delirium-suggestive behavioural disturbances in critically ill patients. Crit Care Resusc. 2021;23(2):144–153. doi: 10.51893/2021.2.oa1. PubMed PMID: 38045514. PMCID: PMC10692527. Epub 2023/12/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielsen A.H., Larsen L.K., Collet M.O., Lehmkuhl L., Bekker C., Jensen J.F., et al. Intensive care unit nurses' perception of three different methods for delirium screening: a survey (DELIS-3) Aust Crit Care. 2023;36(6):1035–1042. doi: 10.1016/j.aucc.2022.12.008. PubMed PMID: 36774292. Epub 2023/02/12. eng. [DOI] [PubMed] [Google Scholar]
- 24.Reade M.C., Eastwood G.M., Peck L., Bellomo R., Baldwin I. Routine use of the confusion assessment method for the intensive care unit (CAM-ICU) by bedside nurses may underdiagnose delirium. Crit Care Resusc. 2011 Dec;13(4):217–224. PubMed PMID: 22129282. Epub 2011/12/02. eng. [PubMed] [Google Scholar]
- 25.Atherton J., Abdrabbo M., Kassab H. Impact of abrupt interruption of home psychotropic medications at ICU admission. J Pharm Technol. 2023;39(4):199–204. doi: 10.1177/87551225231182286. PubMed PMID: 37529150. PMCID: PMC10387813. Epub 2023/08/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Summers M.J., Chapple L.S., Bellomo R., Chapman M.J., Ferrie S., Finnis M.E., et al. Study protocol for TARGET protein: the effect of augmented administration of enteral protein to critically ill adults on clinical outcomes: a cluster randomised, cross-sectional, double cross-over, clinical trial. Crit Care Resusc. 2023;25(3):147–154. doi: 10.1016/j.ccrj.2023.08.001. PubMed PMID: 37876373. PMCID: PMC10581259. Epub 2023/10/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose L., Burry L., Agar M., Campbell N.L., Clarke M., Lee J., et al. A core outcome set for research evaluating interventions to prevent and/or treat delirium in critically ill adults: an international consensus study (Del-COrS) Crit Care Med. 2021;49(9):1535–1546. doi: 10.1097/CCM.0000000000005028. PubMed PMID: 33870914. Epub 2021/04/20. eng. [DOI] [PubMed] [Google Scholar]
- 28.Stollings J.L., Boncyk C.S., Birdrow C.I., Chen W., Raman R., Gupta D.K., et al. Antipsychotics and the QTc interval during delirium in the intensive care unit: a secondary analysis of a randomized clinical trial. JAMA Netw Open. 2024;7(1) doi: 10.1001/jamanetworkopen.2023.52034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hemming K., Kasza J., Hooper R., Forbes A., Taljaard M. A tutorial on sample size calculation for multiple-period cluster randomized parallel, cross-over and stepped-wedge trials using the Shiny CRT Calculator. Int J Epidemiol. 2020;49(3):979–995. doi: 10.1093/ije/dyz237. PubMed PMID: 32087011. PMCID: PMC7394950. Epub 2020/02/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casamento A.J., Serpa Neto A., Young M., Lawrence M., Taplin C., Eastwood G.M., et al. A phase II cluster-crossover randomized trial of fentanyl versus morphine for analgosedation in mechanically ventilated patients. Am J Respir Crit Care Med. 2021;204(11):1286–1294. doi: 10.1164/rccm.202106-1515OC. PubMed PMID: 34543581. Epub 2021/09/21. eng. [DOI] [PubMed] [Google Scholar]
- 31.Shehabi Y., Howe B.D., Bellomo R., Arabi Y.M., Bailey M., Bass F.E., et al. Early sedation with dexmedetomidine in critically ill patients. N Engl J Med. 2019;380(26):2506–2517. doi: 10.1056/NEJMoa1904710. PubMed PMID: 31112380. [DOI] [PubMed] [Google Scholar]
- 32.Lehmann E. Prentice Hall; 1998. Nonparametrics: statistical methods based on ranks. [Google Scholar]
- 33.Jakobsen J.C., Gluud C., Wetterslev J., Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials – a practical guide with flowcharts. BMC Med Res Methodol. 2017;17(1):162. doi: 10.1186/s12874-017-0442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.