Abstract
Cancer therapy continues to face critical challenges, including drug resistance, recurrence, and severe side effects, which often compromise patient outcomes and quality of life. Exploring novel, cost-effective approaches, this review highlights the potential of Piper nigrum (black pepper) extract (PNE) as a complementary anticancer agent. Piper nigrum, a widely available spice with a rich history in traditional medicine, contains bioactive compounds such as piperine, which have demonstrated significant anticancer activities including cell cycle arrest, apoptosis induction, and inhibition of tumor growth and metastasis. The review evaluates the recent findings from in vitro, in vivo, and clinical studies, emphasizing PNE's capacity to enhance the efficacy of conventional chemotherapeutic agents while mitigating their side effects. Key mechanisms underlying these effects include oxidative stress modulation, suppression of pro-metastatic factors, and synergistic interactions with established drugs like doxorubicin and paclitaxel. These interactions suggest that PNE could play a pivotal role in overcoming chemoresistance and improving therapeutic outcomes. Furthermore, this review highlights the potential benefits of PNE in resource-limited settings, where the cost of cancer treatments often restricts access. However, challenges such as compositional variability, limited bioavailability, and the need for standardization and clinical validation need to be addressed to advance the integration of PNE into basic oncology. By providing a comprehensive analysis of the anticancer mechanisms of PNE and its potential as a cost-effective adjuvant therapy, this review provides new insight into the exploitation of Piper nigrum to improve cancer treatment efficacy while reducing side effects. Future research directions are discussed to address current limitations and facilitate clinical translation.
Keywords: Anticancer, Apoptosis, Black pepper, Chemotherapy, Piper nigrum, Synergistic therapy
Introduction
Cancer is the leading cause of death globally and negatively impacts an individual’s quality of life. An estimated 19.3 million new cases contributed to 10.0 million deaths in 2020 [1]. The disease burden in low- and middle-income countries (LMICs) is high, with the number of new cases being 12,122,139 reported in compared to 7,775,879 in high-income countries in 2022. Additionally, LMICs accounted for 6,869,816 deaths compared to 2,834,224 in high-income countries [2]. Several factors contribute to the high proportion of deaths seen in LMICs, including limited resources, limited access to health services, ineffective screening programs, delays in diagnosis, and high treatment costs [3]. Of these the high cost and limited availability of anticancer drugs are the factors that are most likely to influence patients with low-income levels to abandon treatment [3]. In addition, the adverse side effects associated with chemotherapy drugs (e.g., hair loss, diarrhea, fatigue, vomiting, and quality of life reductions) may influence patients to refuse or discontinue treatment [4]. Herbal medicines have been used in traditional treatments for many years [5–9]. The World Health Orgaization (WHO) reported that approximately 80% of people in developing countries rely on herbal medicines for their health needs. Herbal medicine’s popularity and acceptance have increased over the years due to perceptions about its safety, lower prices, and increased availability [10]. A study involving hospitals, health centers, and independent medical practices in seven provinces in Indonesia reported that 79.6% of patients experienced improved quality of life following the use of herbal plants as a complementary cancer therapy [11]. Many studies have evaluated herbal drugs [10]. Such studies have reported that these drugs can kill cancer cells, inhibit cancer cell growth, and improve the cell microenvironment and immunity [12]. Several studies have also sought to clarify the mechanisms underpinning the synergistic interactions between herbal and chemotherapy drugs [13]. The genus Piper (Piperaceae) is widely used in traditional medicines. Globally, 10 species of Piper are known to be used as anticancer agents [14]. In India, Piper longum L., the root of Piper boehmeriifolium Wall, and Piper sylvaticum Roxb. are used to treat tumors [15]. Piper capense L.f, the seeds of Piper cubeba L, the juice of Piper gibbilimbum C.DC, and the seeds of Piper guineense Schum and Thonn are used as cancer treatments in Cameroon, Morocco, Papua New Guinea, and Nigeria, respectively [16]. The root of the “King of spices,” Piper nigrum, is used to treat abdominal tumors in Thailand [17]. In China, the fruit of P. nigrum is used to treat respiratory and gastric cancer [18]. Piper nigrum is also one of the ingredients used in traditional Indian medicine to control tumors [19]. Piper nigrum is a tropical woody vine that produces small, round, green fruits that turn red when ripe. The fruit of the plant is commonly referred to as peppercorn. Propagation can be achieved through seeds or stem cuttings. Although the plant requires a considerable amount of time to produce its first flowers and berries, once they appear, it can yield a significant quantity of peppercorns under suitable conditions. Currently, Vietnam, Brazil, and Indonesia are the leading producers of pepper globally, followed by Burkina Faso, India, Sri Lanka, China, Malaysia, and Tajikistan [20]. Due to the prevalence of this plant in numerous tropical regions, its adaptability to a broad range of environmental conditions, and its suitability for cultivation by rural farmers, it is a promising herbal medicine option [20]. This study focused on P. nigrum’s phytochemicals in the context of the anticancer activities of its extracts. In vitro and in vivo evaluation studies should be conducted to scientifically prove the safety and efficacy of herbal drugs and meet regulations [21]. Therefore, we included anticancer studies of P. nigrum that used both cellular and animal models, as well as clinical studies to assess its potential use in standardized herbal or phytopharmaceutical drugs. The production cost of these drugs is cheaper than pharmaceutical drugs, which are associated with extremely high prices, particularly for pure plant-derived compounds. The production of pure compounds requires lengthy isolation steps, such as extraction, fractionation, and further purification, which, in turn, necessitates large quantities of expensive organic solvents, product development, and the pharmaceutical industry for product development and production [22]. When compared to a pure drug, an extract typically exhibits a higher degree of activity due to synergistic and positive interactions between its components [23]. Additionally, many studies have reported that plant extracts contain substances that inhibit multidrug resistance in cancer [24]. Various compounds can enhance the bioavailability, pharmacokinetic properties, and pharmacodynamic effects of the extracts they are present in [25].
This review provides a novel perspective by emphasizing Piper nigrum extract (PNE) synergistic potential with chemotherapy to enhance efficacy and reduce side effects, a focus not comprehensively addressed in recent literature. Unlike other reviews, it examines clinical applicability, bioavailability-enhancing strategies and its economic advantages in resource-limited settings, offering a translational approach to its use as an integrative cancer therapy.
Review methodology
This updated review systematically analyzed the anticancer properties of P. nigrum (black pepper) extracts by searching multiple electronic databases, including PubMed, MedLine, Scopus, Web of Science, Google Scholar, and Cochrane Library. The search utilized terms such as “Piper nigrum,” “black pepper,” “anticancer properties,” “complementary therapy,” and “phytochemicals”. The literature review for this study covered a time period ranging from 2000 to 2024. This range was chosen to encompass both foundational studies and the most recent advancements in research related to Piper nigrum and its anticancer mechanisms. The inclusion criteria focused on peer-reviewed original research articles, reviews, clinical trials, in vitro, in vivo, and in silico studies that investigated the anticancer properties of Piper nigrum, reported in English, involving various cancer cell lines, animal models, or human clinical studies, and those that demonstrated outcomes such as apoptosis induction, cell cycle arrest, inhibition of cell proliferation and metastasis, or enhancement of conventional chemotherapeutic efficacy. Exclusion criteria included studies unrelated to cancer, non-peer-reviewed sources such as conference abstracts, editorials, and commentaries, and articles lacking clear methodologies or results regarding the anticancer effects of Piper nigrum. The most important and relevant data from these studies have been summarized in comprehensive tables and figures, detailing bioactive compounds, experimental models, doses and observed anticancer activities, to present a clear and systematic understanding of the potential role of PNE in cancer therapy.
Bioactive phytochemicals and anticancer potential of different parts of Piper nigrum
The anticancer properties of P. nigrum are attributed to a diverse range of bioactive compounds. Through the analysis of the plant's chemical composition, researchers have gained insights into its pharmacological mechanisms. Numerous studies have concentrated on the secondary metabolites found in various parts of the plant, including the roots, seeds, as well as dried and fresh fruit. Notably, the fruit is the most commonly utilized part in anticancer research, with both ripe and unripe fruit serving as valuable sources of raw materials for anticancer applications. Deng et al. investigated a piperine-free extract (PFPE) of dried fruits of P. nigrum and reported significant cancer prevention effects in NMU-treated Sprague–Dawley rats. PFPE inhibited VEGF expression, suppressed tumor progression, and induced ROS generation. These findings suggest its potential as an angiogenesis inhibitor and ROS activator for cancer therapy [26].
Saetang et al. identified caryophyllene as a key component (25%) in a low-piperine extract of P. nigrum. Their study demonstrated enhanced antitumor immunity via Th1/Th2/Treg modulation in NMU-induced mammary tumor models, highlighting its immunomodulatory role [27]. De Souza Grinevicius et al. focused on ethanolic extracts enriched in piperamides (piperine and piperyline). The extracts induced oxidative stress and apoptosis in MCF-7 and HT-29 cells, reduced tumor growth in vivo, and modulated apoptosis markers, including Bax, p53, and Bcl-xL. This dual mechanism highlights its therapeutic potential against both breast and colorectal cancers [28]. Kiranmayee et al. synthesized tin oxide nanoparticles (SnO2 NPs) using aqueous extracts of P. nigrum. The nanoparticles exhibited cytotoxicity against MCF-7 cells and favorable pharmacokinetics in silico, interacting effectively with EGFR. These findings support the application of P. nigrum-based nanoparticles in targeted cancer therapy [29]. Buranrat and Boontha demonstrated that ethanolic extracts of P. nigrum inhibited MCF-7 proliferation by suppressing cyclin D1 and NF-κB and inducing ROS production and apoptosis. This study highlights its dual role in regulating cell cycle and promoting cancer cell death [30]. Buranrat further explored phenolic and flavonoid-rich extracts, which inhibited HeLa cell proliferation and migration. The extracts induced mitochondrial dysfunction and ROS-mediated apoptosis. This reinforces the importance of phenolic compounds in cancer inhibition [31]. Kanniah et al. synthesized silver nanoparticles (AgNPs) from P. nigrum seeds, which showed broad-spectrum cytotoxicity against cancer cell lines, including breast, pancreatic, and ovarian cancers. The integration of nanotechnology with P. nigrum extracts broadens their applicability in cancer treatment [32].
Black pepper essential oil (BPEO), rich in terpenes like sabine, D-limonene, and 3-carene, has demonstrated significant anticancer activity, particularly against triple-negative breast cancer. The complementary effects of its bioactive components, including piperine, further enhance its therapeutic value. Research continues to uncover the vast anticancer potential of P. nigrum through its ability to target multiple pathways, including oxidative stress, immune modulation, and apoptosis. Detailed experimental data, including IC50 values and doses, are presented in Table 1.
Table 1.
Different PNE and their anticancer mechanisms
| P. nigrum plant part used | Sample used | Phytochemical/Compounds identified | Experimental model | IC50/Doses | Anticancer activity | Refs. |
|---|---|---|---|---|---|---|
| Dried fruit | Piperine-free P. nigrum fruits extract (PFPE) | n.d. |
In vitro: Breast cancer cells MCF-7 ZR-75-1 In vivo: NMU-treated female Sprague–Dawley mammary tumor rats |
MCF-7 (IC50 = 7.45 µg/mL), ZR-75-1 (IC50 = 7.45 µg/mL) (Dose = 100, 200, 400 mg/kg BW) |
Showed cancer prevention effects through ROS generation in vivo ↓ MMP-2, ↓MMP-9, ↓VEGF in vivo ↓ cancer cells proliferation activity by downregulated trancription factor, c-Myc in vitro |
[26] |
| Dried fruits of black peppercorn |
Low piperine fractional P. nigrum extract (PFPE) |
Fifteen compounds were detected, and caryophyllene is a majority with 25% | In vivo: NMU-Induced Sprague–Dawley mammary tumor rats | (Dose = 200 mg/kg BW) |
↓ tumor progression ↑ the antitumor immunity by regulating the Th1/Th2/Treg |
[27] |
| Dried fruits | Ethanolic extract rich in piperamides | Two major compounds of piperamides: piperine and piperyline |
In vitro: MCF-7 cells human breast cancer HT-29 human colorectal adenocarcinoma In vivo: Ehrlich ascites carcinoma-bearing mice |
MCF-7 human breast cancer cells (IC50 = 27.1 µg/mL), HT-29 colorectal adenocarcinoma (IC50 = 80.5 µg/mL) (Dose = 100 mg/kg BW) |
↓ cell proliferation in MCF-7 and HT-29 cells ↓ Tumor growth in vivo ↑ oxidative stress and apoptosis in vitro and in vivo ↑ Bax, ↑p53, Bcl-xL expression associated with apoptosis Tumor cell death related to oxidative stress in MCF-7 |
[28] |
| Dried fruits | Aqueous water | P. nigrum tin oxide nanoparticle (SnO2 NPs) |
In vitro: human breast cancer cell MCF-7 In silico: EGFR receptor |
Dose = 75–200 µg/mL |
The favorable pharmacokinetics pharmacodynamics, and toxicity profiles of three promising compounds in silico Exhibited cytotoxicity against cancer cell in vitro |
[29] |
| Dried unripe fruits of black pepper | Ethanolic extract | Phenolic group |
In vitro: Human colorectal cancer cells line HCT-116 HCT-15 HT-29 |
HCT-116: IC50 = 4.0 µg/mL, HCT-15: IC50 = 3.2 µg/mL, HT-29: IC50 = 7.9 µg/mL |
Inhibited cell proliferation | [33] |
| Dried fruits | Methanol extract or dichloromethane extract | Alkaloids group |
In vitro: Human Breast cancer cells MCF-7 MDA-MB-231 MDA-MB-468 |
MCF-7: IC50 = 20.25 µg/mL, MDA-MB-231: IC50 = 22.37 µg/mL, MDA-MB-468: IC50 = 9.04 µg/mL |
Inhibited cell proliferation | [34] |
| Unripe fruit | Ethanolic extract | Alkaloids gropus including piperine as a major constituent |
In vitro: PANC-1 human pancreatic cancer |
IC50 = 54.2 µg/mL |
Inhibited proliferation of PANC-1 through G0/G1 arrest ↓ protein levels of cell cycle regulators such as cyclin B1, cyclin D1, survivin, and Forkhead box M1 (FoxM1) ↓ cell migration and invation by decreasing FoxM1 protein level |
[35] |
| Black pepper | Piperine enriched supercritical extract | Piperine is major compound was identified |
In vitro: MCF-7 human breast cancer cells In vivo: Balb/c mice-bearing Ehrlich Ascites Carcinoma (EAC) In silico: piperine binding to CDK2-Cyclin A and BCl-xL |
IC50 = 27.8 µg/mL Dose = 100 mg/kg BW |
↓ growth of breast cancer cell line MCF-7 that confirmed by interaction of piperine and protein targets CDK2-Cyclin A and BCl-xL ↑ apoptosis in MCF-7 breast cancer cells Inhibited tumor growth and showed lower levels of CDK2 and Cyclin A ↑ cell cycle arrest in G2/M phase in vivo ↑apoptotic cells and upregulated of pro apoptotic proteins (p53 and Bax) Hydrophobic interactions between piperine and residue Ser5 in CDK2; with residue Lys8 in Cyclin A; and Bcl-xL receptor |
[36] |
| Dried young fruit | Ethanolic extract | n.d. | In vitro: Human breast cancer cells MCF‑7 | IC50 = 15.6 μg/mL |
Stimulated growth inhibition by increasing the G1 phase arrest and inhibiting cyclin D1 and NF‑κB Inhibited cell migration and reduced MMP-2, MMP 9, VEGFA, and ICAMP1 genes level Activated the ROS formation, increase caspase‑3 activity, and induced breast cancer cell death |
[30] |
| Dried young fruit | Ethanolic extract | Phenolic and flavonoid contents including Piperine | In vitro: HeLa human cervical cancer cells | IC50 = 22.71 µg/mL |
Inhibited cell ploriferation and induced cell cycle arrest at G0/G1 phase Triggered apoptosis by inhibiting mitochondrial function and amplifying ROS production Suppressed cancer cells migration |
[31] |
| Dried young fruit | Ethanolic extract | n.d. |
In vitro: Cholangiocarcinoma (CCA) /bile duct cancer KKU‑100 KKU‑M452 |
KKU-100 IC50 = 12.76 µg/mL, KKU-M452 IC50 = 38.32 µg/mL |
Inhibited cell viability and colony formation Decreased cell growth by cell cycle arrest at G0/G1 phase in KKU‑100 cells and S to G2/M phase in KKU‑M452 cells Induced apoptosis by decreasing mitochondrial function and increasing ROS production Suppressed cell migration |
[37] |
| Black pepper seed | Aqueous extract | PNE silver nanoparticles (AgNPs): nineteen phytochemicals such as piperine, piperanine, ecuramide, pipecolic acid, betaine, salsolinol, hexadecanamide, oleamide, quinine |
In vitro: MDA-MB-231 human breast cancer PANC-1 human pancreatic cancer SKOV-3 human ovarian cancer PC-3 human prostate cancer HeLa human servical cancer |
MDA-MB-231: IC50 = 10 µg/mL PANC-1: IC50 = 10 µg/mL, SKOV-3: IC50 = 10 µg/mL PC-3: IC50 = 10 µg/mL HeLa: IC50 = 10 µg/mL |
Showed potent cytotoxicity against human cancer cell lines | [32] |
| Black pepper seed | Aqueous extract | P. nigrum tin oxide nanoparticle (SnO2 NPs) |
In vitro: HCT-116 human colorectal cancer A549 human lung cancer |
HCT-116: IC50 = 0.165 µg/mL A549: IC50 = 0.135 µg/mL |
Demonstrated toxicity towards HCT 116 and A549 cells through the generation of ROS | [38] |
| Black pepper seed | Ethanolic extract | Characterized piperine |
In vitro: Human metastatic melanoma SK-MEL 19 cells Intestinal adenocarcinoma malignant ascites AGP-01 Intestinal adenocarcinoma with an inactivated PIWIL1 gene AGP-01 PIWIL1−/− Neoplastic human pulmonary fibroblast cell line MCR5 |
SK-MEL 19: IC50 = 14.94 µg/mL AGP-01: IC50 = 13.52 µg/mL AGP-01 PIWIL1−/− IC50 = 21.26 µg/mL MCR5: IC50 = 14.17 µg/mL |
Induced cancer cells toxicity | [39] |
| Dried fruit | Aqueous extract | P. nigrum AgNPS | In vitro: Human hepatocyte carcinoma HepG2 | IC50 = 4.98 µg/mL | Induced cancer cells toxicity | [40] |
| Dried fruit | Aqueous extract | P. nigrum AgNPs |
In vitro: Human breast cancer cells MCF-7 Human larynx carcinoma cancer Hep-2 |
MCF-7: IC50 = 52 µg/mL Hep-2: IC50 = 54 µg/mL |
Induced cancer cells toxicity | [41] |
| Dried black pepper |
Aqueous extract Methanolic extract |
Mainly piperine and alkyl amides |
In vitro: Human colon carcinoma HCT-116 Human breast cancer MCF-7 Human glioblastoma SF-268 Human lung carcinoma NCI-H460 |
HCT-116, MCF-7, SF-268, NCI-H460: IC50 = 200 µg/mL |
Induced cancer cells toxicity | [42] |
| Dried fruit | CHCl3 extract | Alkaloids group (piperidine present) | In vitro: Human servical cancer HeLa | IC50 = 17.47 µg/mL | Induced cancer cells toxicity | [43] |
| Dried fruit | Extract of methanol:water 1:1 | n.d. | In vivo: DMBA-induced skin tumorigenesis in Swiss albino mice | Dose = 150 mg/kg BW | Reduced the number of tumours in vivo test model | [44] |
| Dried fruit | Ethanolic extract | n.d. |
In vitro: Murine Ehrlich Ascites Carcinoma EAC Murine melanoma-B16 Human servical cancer HeLa |
Murine Ehrlich Ascites Carcinoma EAC: IC50 = 8 µg/mL, Murine melanoma-B16: IC50 = 10 µg/mL, HeLa: IC50 = 17 µg/mL |
Induced cancer cells toxicity | [45] |
| Dried fruit | n-hexane, chloroform, methanol and water extracts | Phenolic content | In vitro: Human cervical cancer cell line CaSki | IC50 = 36 µg/mL | Induced cancer cells toxicity | [46] |
| Dried black pepper | Dichloromethane extract | Piperine free P. nigrum extract (PFPE), rich in pipercitine alkaloid and caryophyllene terpene |
In vitro: Human cholangiocarcinoma KKU-100 KKU-M213 KKU-M055 |
KKU 100: IC50 = 17.79 µg/mL, KKU M213: IC50 = 13.70 µg/mL, KKU M055: IC50 = 16.74 µg/mL |
Induced cancer cells toxicity | [47] |
| Dried leaves | Methanolic extract |
Major: Tannin, flavonoid, steroid, polyphenol Minor: saponin, terpenoid, triterpenoid, alkaloid |
Human lung carcinoma A549 | Dose = 200–500 µg/mL | Induced cancer cells toxicity | [48] |
| Dried root |
Extracts: petroleum ether and CHCl3 petroleum ether CHCl3 |
Alkaloids group | In vitro: Human leukemia cells HL 60 |
Petroleum ether: IC50 = 30 µg/mL, CHCl3: IC50 = 11.2 µg/mL, Combined: IC50 = 9.8 µg/mL |
Induced cancer cells toxicity | [49] |
| Black pepper | Supercritical carbon dioxide extracts rich in piperine (SFE) | Piperine is major compound was identified |
In vivo: Balb/c mice bearing-Ehrlich ascites carcinoma cells (EAC) In silico: dGlutathione Peroxidase |
Dose = 100 mg/kg BW |
Inhibited EAC cells viability Enhanced EAC pro-oxidative status to induced oxidative stress Decreased glutatione peroxidase/GPx activity and GSH depletion The GPx-piperine poses hydrogen and hydrophobic bonds that contribute to inhibitionof GPx |
[50] |
| n.d |
Five lead compounds: Clarkinol A Isodihydrofutoquinol B Burchellin Kadsurin B Lancifolin C |
In silico: Five lead compounds to Epidermal growth factor receptor (EGFR) |
Binding score: − 7.304 to − 6.342 kcal/mol |
Interacted well with EGFR receptor | [51] | |
| n.d |
Campesterol Cholesterol Piperine Linoleic acid |
In silico: PPARγ, a glucose metabolism regulator factor that responsible for colorectal cancer |
Binding score: Campesterol (− 8.8 kcal/mol), Cholesterol (− 8.2 kcal/mol), Piperine (− 8.6 kcal/mol), Linoleic acid (− 6.2 kcal/mol) |
Interacted well with PPARγ target | [52] | |
| Pure Piperine (97–98%) dissolved in DMSO | Piperine | HT-29 colon carcinoma cells | IC50 = 75–150 μM |
Inhibited HT-29 colon carcinoma cell proliferation by inducing G1 phase cell cycle arrest Triggered apoptosis by producing hydroxyl radical |
[53] | |
| Dried roots | P. nigrum in petroleum ether and chloroform extracts | Several alkaloid groups such as: pellitorine, (E)-1-[30,40-(methylenedioxy) cinnamoyl] piperidine, 2,4-tetradecadienoic acid isobutyl amide, piperine, sylvamide, cepharadione A, piperolactam D and paprazine | HL60 (human promyelocytic leukaemia cells) | IC50 = 30 µg/mL | Inhibitory effect toward HL60 (human promyelocytic leukaemia cells) | [49] |
IC50: half-maximal inhibitory concentration; BW: body weight; PNE: P. nigrum extract; AgNPs: silver nanoparticles; PFPE: Piperine-free P.nigrum extract; PFPE-CH: Piperine-free P. nigrum extract combined with coconut oil and honey ROS; Reactive Oxygen Species: NMU: N-nitroso-N-methylurea; DMBA: 7:12-dimethylbenz[a]anthracene; n.d: not determined
Many other biologically important phytochemicals are extracted from P. nigrum plants, including alkaloids, amides, propenyphenols, lignans, neolignans, terpenes, steroids, kawapyrones, piperolides, chalcones, dihydrochalcones, brachyamide, dihydropipericide, 3,4-dihydroxy-6 (N-ethylamine), benzamide, (2E,4E)-N-eicosadienoyl pereridine, N-trans-feruloyltyramine, N-formyl piperidine, guineensine, (2E,4E)-N-5[(4-Hydroxyphenyl)-pentadienoyl] piperidine, (2E,4E)-N-isobutyldecadienamide, (2E,4E)-N-isobutyl-eicosadienamide, (2E,4E,8Z)-N-isobutyl-eicosatrienamide, (2E,4E)-N-isobutyloctadienamide, piperamide, piperamine, piperettine, pipericide, piperine, piperolein, trichostachine, sarmentine, sarmentosine, tricholein, and retrofractamide [54]. Of these, the most prominent alkaloid compounds in P. nigrum are piperine and piperidine, which are commonly extracted from the root or fruit. Research on alkaloids has recently gained special attention as several have shown promising anti-inflammatory, antioxidant, and anticancer effects. Ethanolic P. nigrum extract has been shown to inhibit tumor growth and enhance the antitumor immune response in murine models of breast cancer and melanoma. Piperine demonstrates anticancer properties against multiple cancer types, including HER overexpressing breast cancer cell lines (SKBR-3 and MCF-7), the MCF-7 cell line, and 7,12-dimethylbenz[a]anthracene (DMBA)-induced carcinogenesis in Syrian golden hamsters. Studies have shown that piperine and piperidine can activate or inhibit several signaling pathways fundamental for cancer regulation, demonstrating their anticancer effects against ovarian, prostate, and lung cancer [55]. Fifteen alkaloid compounds extracted from the dried fruits of P. nigrum L. using MeOH, including piperine, piperolein, pipersintenamide, trichostachine, and piperamide, have demonstrated strong cytotoxic activity against a human cervical cancer cell line, Hela, a breast cancer cell line, MCF-7, and the HL-6 human promyelocytic leukemia cell line [56]. Piper nigrum extracts (PNE) may be interesting alternatives to their respective bioactive components, as they demonstrate anticancer action via complementary pathways. PNE has been investigated for its anti-inflammatory, immunomodulatory, antioxidant, and anticancer properties [57]. The ethanolic extract of P. nigrum contains a substantial amount of piperine and has been found to cause cell cycle arrest [56]. Notably, the raw extract showed greater specificity and was more harmful to cancer cells than the two primary alkaloids, piperine and pellitorine, individually [58]. Black pepper essential oil (BPEO) has demonstrated strong anticancer activity, particularly against triple-negative breast cancer [59]. BPEO-loaded nanoparticles considerably reduce MDA-MB-231 cell migration, invasion, and proliferation [60]. This inhibition is driven by a reduction in the Wnt/β-catenin signaling pathway and GSK-3β activation. Sabine, 3-carene, d-limonene, α-pinene, caryophyllene, β-phellandrene, α-phellandrene, α-thujene, and β-bisabolene are the principal chemical components of BPEO. These substances, in addition to piperine, support BPEO’s anticancer characteristics [60].
Mechanisms underlying anticancer activity
Suppresses cancer cell growth
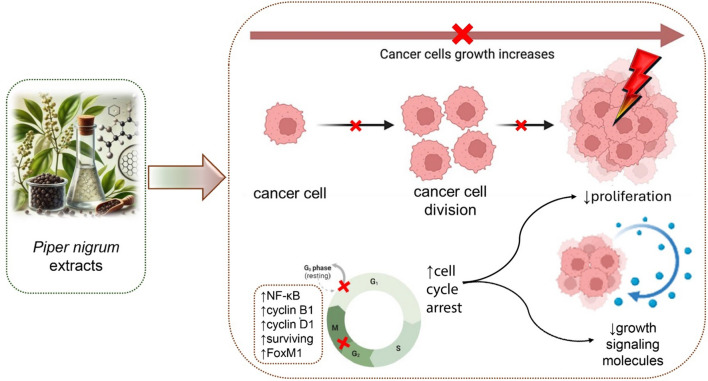
Cancer involves dysregulated cell division, and inducing cell cycle arrest is a key strategy to inhibit tumor growth [61–63]. Most in vitro, in vivo, and in silico studies have shown that P. nigrum extracts (PNE) inhibit cancer cell proliferation (Fig. 1).
Fig. 1.
Mechanism of PNE in inducing cell cycle arrest and reducing tumor growth. PNE inhibit cancer cell proliferation by inducing cell cycle arrest at critical phases (e.g., G₀/G₁), reducing cell division, and downregulating growth-promoting signaling pathways. These extracts regulate key proteins, including NF-κB, cyclin B1, cyclin D1, survivin, and FoxM1, leading to decreased cancer cell division, reduced signaling for proliferation, and suppression of tumor progression. FoxM1: Forkhead Box M1; NF-κB: Nuclear Factor Kappa-light-chain-enhancer of activated B cells
In vitro experiments have shown that PNE results in anti-proliferation activity in the breast cancer models MCF-7, 4T1, MDA-MB-231, MDA-MB-468, and ZR-75-1; the HeLa and CaSki cervical cancer cell lines; colorectal cancer HT-29, SW-620, HCT-116, and HCT-15 cells; pancreatic cancer PANC-1 cells; melanoma B16-F10 and B16 cell lines; neuroblastoma SK-N-SH and LA-N-5 cells; lung carcinoma H-358, A-549, and NCI-H460 cell lines; cholangiocarcinoma (CCA) KKU‑100, KKU‑M452, KKU-M213, and KKU-M055 cells; fibrocarcinoma L-929 cells; ovarian cancer SKOV-3 cells; human prostate cancer PC-3 cells; human hepatocyte carcinoma HepG2 cell line; larynx carcinoma cancer Hep-2 cell line; glioblastoma SF-268 cells; murine Ehrlich ascites carcinoma (EAC); and human leukemia HL 60 cells (Table 1). In general, PNE demonstrates strong toxicity against various cancer cells. Dehydrogenase enzyme activity in the active mitochondria of cancer cells decreases in the presence of PNE (Table 1). Slowed cell growth has shown to result in the inhibition of cell colony formation in HT-29, B16-F10, 4T1, MCF-7, HT-29, KKU‑100, and KKU‑M452 cells and reduced tumor sizes in HT-29 nude mouse xenografts, murine models of 4T1 breast cancer and B16-F10 melanoma, N-nitroso-N-methylurea (NMU) treated Sprague–Dawley mammary tumor rats, EAC, and reduced DMBA-induced skin tumorigenesis in Swiss albino mice [26, 28, 36, 37, 44, 64–66].
Induction of cell cycle arrest
Disruption of the cell cycle is a hallmark of cancer, and inducing cell cycle arrest is a pivotal strategy employed by anticancer agents to halt uncontrolled tumor cell proliferation and promote therapeutic efficacy [67, 68]. PNE’s ability to cause cell cycle arrest has been noted in different cell types and among different tumor behaviors (Fig. 1). For example, in mice carcinoma and KKU-M452 CCA cell models, PNE results in S to G2/M phase arrest, while in MCF-7 breast cancer, HeLa cervical cancer, and CCA KKU-100 CCA cells, it results in G0/G1 phase arrest [30, 37]. G1 phase arrest has been linked to the downregulation of cell cycle regulators, such as nuclear factor κB (NF‑κB), cyclin B1, cyclin D1, survivin, and Forkhead box protein M1 (FoxM1) (Table 1). NF-κB is a pro-inflammatory protein that modulates gene expression, including genes involved in cancer cell growth and progression via cyclin D1 [69]. Cyclin D1, in conjunction with CDK4/6, facilitates the integration of mitogenic signals that control the G1 cell cycle checkpoint in solid tumors [70]. Cyclin B1 is primarily associated with the G2/M phase. High cyclin B1 expression has been observed in benign tumors and is associated with tumor grade and poor survival in patients with solid tumors [71, 72]. Survivin is an apoptosis inhibitor protein that regulates cell division and spindle formation. FoxM1 is a critical proliferation-associated transcription factor that plays a fundamental role in cyclin B1, cyclin D1, and surviving expression. It acts as an upstream regulator of the G1 to S phase transition and the G2 to M phase progression [73]. The overexpression of survivin or FoxM1 is commonly associated with an aggressive cancer phenotype, poor prognosis, and drug resistance in many types of cancer [74]. An in silico study showed that the primary compounds of PNE are potential novel inhibitors of the epidermal growth factor receptor and peroxisome proliferator-activated receptor gamma, which are the receptors involved in G1 cell cycle progression [75]. Various PNE solvents exhibit distinct impacts on the proliferation inhibition pathway of MCF-7 breast cancer cells. For instance, as shown in Table 1, the ethanol extract obtained from young, dried fruit causes cell cycle arrest in the G1 phase. Conversely, the piperine-enriched supercritical extract induces cell cycle arrest in the G2/M phase by lowering the levels of CDK2 and cyclin A both in vitro and in vivo. The findings from in silico studies indicate that piperine exhibits a high affinity for the cyclin A binding site of CDK2, suggesting it may inhibit the cell cycle [30, 36]. In CCA cells, PNE treatment blocks the G0/G1 cell cycle phase in KKU-100 cells, and the S to G2/M phase in KKU-M452 cells [37]. Based on this evidence, it appears that PNE inhibits the progression of cell cycle regulators, leading to a reduction in cancer cell growth through distinct signaling pathways depending on the type of extract and plant part used.
Apoptosis induction
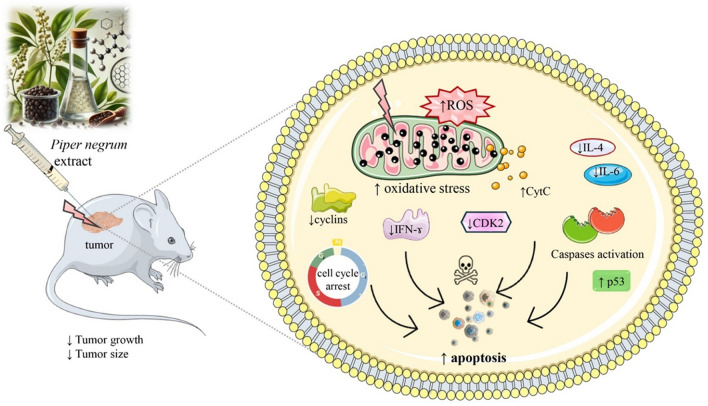
Apoptosis, a programmed cell death mechanism fundamental for maintaining cellular homeostasis, is a key anticancer strategy targeted by bioactive compounds [76, 77]. In vitro and in vivo studies have shown that PNE induces apoptosis in various cancers, including HT-29 human colorectal cancer, B16-F10 melanoma, MCF-7, and 4T1 breast cancer, HeLa cervical cancer, CCA KKU‑100 and KKU‑M452 cells, and EAC (Table 1 and Fig. 1). In MCF-7 cells, PNE induces apoptosis by upregulating p53 and cyt C and increasing caspase-3 activity and oxidative stress generation [28, 30]. In HeLa and CCA cells, PNE induces apoptosis by inhibiting mitochondrial function and amplifying ROS production [31]. Apoptosis induction is associated with increased Bax and p53 levels and reduced Bcl-xL expression in EAC-bearing mice [28, 36]. Therefore, it was concluded that the primary molecular mechanism of P. nigrum compounds is inducing apoptosis by activating ROS formation, which leads to oxidative stress generation. Researchers typically use PNE to provoke apoptosis in cancer cells (Table 1). PFPE and rich piperine extract of PNE exhibit comparable apoptotic activity [36, 66]. This suggests that other active compounds also contribute to inducing cancer cell death in addition to piperine. Grinevicius et al. reported that piperamide-rich PNE exhibits apoptosis-inducing activity both in vitro and in vivo [28]. The ability of other extracts to induce apoptosis in cancer cells has yet to be explored. Further evaluation of these extracts, including methanol, dichloromethane, water, CHCl3, and n-hexane, is necessary, given that these extracts exhibit notable cytotoxicity towards cancer cells (Table 1 and Fig. 2).
Fig. 2.
Apoptosis molecular mechanisms of Piper nigrum extract (PNE). PNE administration leads to decreased tumor size and growth, primarily through the induction of oxidative stress via increased ROS levels, resulting in mitochondrial dysfunction and CytC release. This process activates caspases and p53, promoting apoptosis. PNE also causes cell cycle arrest by reducing the expression of cyclins and CDK2. Additionally, PNE modulates inflammatory responses by lowering IL-4, IL-6, and IFN-γ levels, contributing to its anticancer effects. CDK2: Cyclin-dependent kinase 2, a cell cycle regulatory protein; CytC: Cytochrome c, released from mitochondria during apoptosis; IFN-γ: Interferon-gamma, a cytokine involved in immune regulation; IL-4: Interleukin-4, an anti-inflammatory cytokine; IL-6: Interleukin-6, a pro-inflammatory cytokine; p53: A tumor suppressor protein involved in apoptosis regulation; PNE: Piper nigrum extract; ROS: Reactive oxygen species, molecules causing oxidative stress; ↓decrease, ↑increase
Pro-oxidant activity and oxidative stress generation of PNE
Some studies have reported that oxidative stress may be involved in modulating cancer cell growth, either by suppressing growth or inducing cell death. (Table 1) PNE has been shown to generate ROS in MCF-7 breast cancer cells and HT-29 colorectal adenocarcinoma cells, leading to reduced cell proliferation in both cell types and inducing apoptosis in MCF-7 cells [28]. PNE-synthesized nanoparticles have also demonstrated ROS generation in HCT-116 colorectal cancer and A549 lung cancer cells [38]. PNE has exhibited pro-oxidant activity in 4T1, B16-F10, CCA, and HeLa cervical cancer cells, resulting in mitochondrial dysfunction, which was evaluated by measuring cellular oxidative stress using 2′,7′-dichlorodihydrofluorescein diacetate [31, 37, 65]. In vitro findings have aligned with previous research involving mice models of breast tumors treated with NMU, where PNE elevated lipid peroxidation product, malondialdehyde (MDA) levels. These increased levels were associated with apoptosis induction, as demonstrated by the increased protein levels of p53, Bax, and cytochrome c [26]. Another in vivo report demonstrated that PNE pro-oxidant activity in NMU-induced breast cancer rat models resulted in increased thiobarbituric acid reactive substances (TBARS) levels or in EAC-bearing mice by increasing lipid peroxidation, carbonyl protein content, and the activity of antioxidant enzymes, such as glutathione reductase, superoxide dismutase, and catalase [28]. TBARS is a widely recognized method for assessing lipid peroxidation and oxidative stress in biological fluids and has been employed to predict the survival prospects of patients who have not received chemotherapy [78].
PNE’s pro-oxidant activity against cancer cells shows promise for the development of complementary medicines. Many pro-oxidant agent have been employed as anticancer drug, such as mitomycin C, doxorubicin, and geldanamycin, due to their ability to trigger oxidative stress. Drug toxicity has been linked to ROS generation, which, in turn, can cause oxidative stress or result in the modification of cellular components, which depletes cellular thiols, ultimately leading to the depletion of antioxidant defenses [79]. Drug-protein interaction studies have demonstrated that interactions between ligands and oxidative stress-related enzymes, such as cytochrome p450 reductase (CYP450R), glutathione peroxidases (GPX), superoxide dismutases (SODs), and apoptosis-inducing factor (AIF), are associated with toxicity against cancer cells [80–82]. Some active compounds of PNE have been reported to display good binding to oxidative stress-related enzymes. For example, GPx-piperine contains hydrogen bonds and is hydrophobic, which contributes to GPx inhibition, thereby increasing the pro-oxidant status that triggers oxidative stress [50]. This finding is in line with our in silico study that showed that the compounds of PNE play a primary role in targeting monoamine oxidase B (MAO B), which was confirmed by SwissTargetPrediction and a molecular docking study [83]. However, docking studies involving PNE active compounds against the enzymes involved in oxidative stress remain limited. Therefore, future research should involve in silico studies to elucidate the mechanisms behind this at the molecular level.
Metastases inhibition
Some studies have demonstrated an antimetastatic effect of PNE on cancer cell lines or cancer models. At a dose of 15.67 mg/kg, PNE decreased the occurrence of macrometastases in 4T1 model BALB/cAnNCrl mice [65]. Pancreatic cancer metastases overexpress FoxM1. PNE inhibits PANC-1 cell migration and suppresses the protein levels of FoxM1 [35]. PNE ethanol extract also inhibits the migration of the CCA/bile duct cancer KKU‑100 and KKU‑M452 cell lines [37]. Furthermore, PNE inhibits MCF‑7 cell migration by reducing matrix metalloproteinase MMP 9 expression, as well as MMP 2, MMP 9, VEGFA, and ICAMP1 gene expression [30]. PFPE was found to inhibit metastases by down-regulating estrogen receptor, E-cadherin (E-cad), MMP-9, MMP-2, c-Myc, and vascular endothelial growth factor (VEGF) levels in breast cancer rats, as well as protein levels of E-cad, c-Myc, and VEGF in MCF-7 cells [26].
Modulation of inflammatory factors
Chronic inflammation is a key contributor to the development and progression of various chronic diseases, including cancer, where it plays a pivotal role in tumor initiation, progression, and metastasis, making it an essential target for cancer prevention strategies [84]. PNE have shown potent anti-inflammatory properties by modulating cytokine levels in both in vitro and in vivo cancer models [85]. Studies have demonstrated that PNE reduces the expression of pro-inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), which are often associated with tumor-promoting inflammation. Simultaneously, PNE increases levels of anti-inflammatory cytokines, such as interleukin-10 (IL-10), contributing to an anti-tumor microenvironment. For example, in NMU-treated Sprague–Dawley mammary tumor models, PNE significantly reduced IL-4 and IL-6 levels while inhibiting tumor growth through oxidative stress induction [65]. Moreover, PNE’s ability to modulate inflammatory pathways not only suppresses tumor progression but also enhances the efficacy of concurrent chemotherapeutic agents [65]. These findings highlight the dual role of PNE in targeting both inflammation and cancer cell proliferation, making it a promising candidate for integrative cancer therapies.
Synergistic effects of PNE with conventional therapies or other bioactive compounds
The combination of seeds from Alpinia galanga, P. nigrum, Citrus aurantifolia, Themeda triandra, and Cannabis sativa has demonstrated antiproliferative and antimigration activity against human colorectal and lung cancer cell lines [86]. The combination of P. nigrum seeds and the root bark of Azadirachta indica has shown similar actions against the epidermoid carcinoma A 431 cell line [87]. Regarding the molecular mechanism of action, the combinations of P. nigrum fruit and dried leaves of Andrographis paniculata, dried leaves of Ziziphus spina-christi, and dried leaves of Gymnanthemum extensum and the combination of the plant extract of Ocimum sanctum and P. nigrum have been found to cause apoptosis in human breast cancer MCF-7 [88, 89]. Some combinations also exhibit immunomodulatory activity. For example, the combination of the seeds of black pepper and cardamom enhances the cytotoxic capacity and exhibits a synergistic stimulatory impact on the cytotoxic activity of Natural Killer cells against YAC-1 tumor cells [90]. A combination of PFPE and turmeric has been found to significantly boost IL-10 levels but not IL-4, IFN-γ, or IL-6 levels in NMU-induced mammary rats [25]. Several studies have investigated the combination of PNE with established cancer drugs, such as doxorubicin and paclitaxel. PFPE was found to suppress cancer-related cytokines when combined with doxorubicin and diminished the systemic immune response side effects associated with doxorubicin in NMU-induced mammary tumors in female Sprague Dawley rats [27]. The combination of dried black pepper and doxorubicin demonstrated synergistic activity, enhanced the efficacy and antigenotoxic effect of doxorubicin, and provided protection against the toxic and genotoxic effects induced by doxorubicin in hamster ovary (CHO-K1) cells [91]. A combination of P. nigrum stems and paclitaxel improves the anticancer effect of paclitaxel in apoptotic cervical cancer cells [92]. Piperine significantly suppresses the growth of androgen-dependent and androgen-independent prostate cancer cells [93]. Makhov et al. noted enhanced anticancer activity associated with the co-administration of piperine and docetaxel for human prostate cancer treatment [94]. Additionally, piperine induces DNA damage and apoptosis in tumor cells and is a potential therapeutic agent for osteosarcoma treatment. Piperine also stimulates antioxidative protective enzyme activity and decreases lipid peroxidation, resulting in lung cancer reduction. Therefore, piperine exhibits potential anticancer activities [95]. However, limited studies have investigated the antitumor potential of piperine and BPEO, with most using animal models. Therefore, future studies should focus on the bioactivity of BPEO in clinical investigations with humans. The use of P. nigrum as a dietary supplement, namely a low piperine fractional P. nigrum extract (PFPE) combined with cold-pressed coconut oil and honey (PFPE-CH) in distilled water, was studied to assess its potential to reduce tumor risk and mitigate chemotherapy side effects during breast cancer treatment. At 5000 mg/kg, PFPE-CH caused no toxicity or deleterious effects in rats after 14 days, while a dose of 86 mg/kg body weight/day for six months did not impair kidney or liver function. In rats with breast tumors, PFPE-CH reduced the tumor incidence by up to 71.4%, led to improved immune responses, and reduced chemotherapy-induced toxicity while maintaining doxorubicin’s anticancer activity [25]. The effect of PFPE-CH on tumor formation, reactive oxygen species (ROS), and cancer-related cytokines has also been evaluated. Cytokines regulate the critical immunological cells involved in cancer genesis and progression, making them viable therapeutic targets. Some findings suggest that the anticancer effects of P. nigrum could be mediated by enabling synergy between the numerous active components acting in complementary pathways [25]. The exploration of alternative sources of piperine could be beneficial for the large-scale production and development of new pharmaceuticals [96]. Endophytic fungi isolated from P. nigrum or P. longum could be a potential source of piperine. These fungi, such as Colletotrichum gloeosporioides and Periconia sp, can produce piperine under suitable conditions, such as liquid cultures [97]. Optimizing their growing conditions may enable the large-scale biosynthesis of piperine. Piperine is also well-known for its potential to enhance the bioavailability of various natural compounds, including resveratrol [98]. This effect is achieved through the inhibition of cytochrome P450 enzymes and p-glycoprotein, which reduces the metabolism and efflux of these compounds. The co-administration of piperine or PNE with resveratrol has demonstrated a synergistic effect, improving the pharmacokinetics and therapeutic efficacy of resveratrol. This synergy enhances anticancer activity by promoting apoptosis induction, inhibiting cancer cell proliferation, and modulating key inflammatory pathways. These bioavailability-enhancing properties highlight the potential of PNE and piperine as adjuvants in combination therapies involving other natural compounds. Current combination therapies that combine PNE with another plant extract or cancer drug are shown in Table 2.
Table 2.
Combination treatment of PNE and chemotherapy drugs or other compounds
| Combination | Extract type | Experimental model | Dose/Concentration | Anticancer activity | References |
|---|---|---|---|---|---|
| Seed of A. galanga, P. nigrum, C. aurantifolia, T. triandra, and C. sativa | Ethanolic extract | In vitro: Human colorectal cancer cells (SW620, HCT116), human lung cancer cells (A549, NCI-H460) | IC50 = 12.15–19.72 µg/mL | Exhibited antiproliferative and antimigration activities | [86] |
| Seed of P. nigrum and root bark of A. indica | Combination of methanolic extracts | In vitro: Epidermoid carcinoma cell line (A431) | IC50 = 208 µg/mL | Inhibited cancer cell proliferation | [87] |
| Dried fruits of P. nigrum and doxorubicin | Piperine-Free P. nigrum extract (PFPE) + doxorubicin | In vivo: NMU-induced mammary tumor in female Sprague Dawley rats | Dose = 200 mg/kg BW | PFPE did not affect doxorubicin's anticancer properties; suppressed cancer-related cytokines; reduced immune side effects | [27] |
| Dried fruits of P. nigrum and extracts from: A. paniculata, Z. spina-christi, G. extensum | PFPE + dichloromethane extracts | In vitro: colorectal adenocarcinoma (HT-29), colorectal cancer (SW620), breast cancer (MCF-7), ovarian cancer (A2780) | IC50 = 8.82–21.12 µg/mL | Antagonism with A. paniculata (HT-29, SW620, MCF-7); Synergy with Z. spina-christi in MCF-7; Apoptosis induction | [88] |
| Plant extract of O. sanctum and P. nigrum | Combination of aqueous extracts | In vitro: human breast cancer (MCF-7); In silico: ADMET analysis | IC50 = 78.61 µg/mL (combination) | Synergistic inhibition of cell proliferation, Apoptosis induction, ADMET properties for eugenol and piperine | [89] |
| Dried black pepper and doxorubicin | Ethanolic extract + doxorubicin | In vitro: Hamster ovary (CHO-K1) cells | IC50 = 8.5 µg/mL | Synergistic activity with doxorubicin; Enhanced efficacy and antigenotoxic effect; protection against genotoxicity | [91] |
| Stem of P. nigrum and paclitaxel | Ethanolic extract + paclitaxel | In vitro: paclitaxel-resistant cervical cancer cells (HeLa/PTX) | IC50 = 5.61–33.2 µM | Improved anticancer effect; enhanced apoptosis in resistant cancer cells | [92] |
| Black peppercorn of P. nigrum with turmeric or doxorubicin | PFPE + turmeric; PFPE + doxorubicin; PFPE + turmeric + doxorubicin | In vivo: NMU-induced mammary tumors in rats | PFPE-CH Dose = 100 mg/kg BW Turmeric Dose = 25 mg/kg BW | PFPE + turmeric/doxorubicin increased oxidative stress (TBARS); inhibited tumor growth; PFPE + turmeric enhanced IL-10 | [25] |
IC50: half-maximal inhibitory concentration; PFPE: Piperine-free P. nigrum extract; PTX: paclitaxel; NK: natural killer; ADMET: absorption: distribution: metabolism: excretion: and toxicity; NMU: N-nitroso-N-methylurea; BPE: black pepper extract
Challenges, limitations and comparative advantages of Piper nigrum extracts as potential adjuvant in oncology
Despite the promising anticancer potential of Piper nigrum extracts (PNE), several challenges hinder their clinical application. Variability in the phytochemical composition of PNE is a major concern, as it depends on factors like plant origin, harvesting time, extraction methods, and environmental conditions. This variability complicates standardization and makes it difficult to ensure consistent efficacy and safety in preclinical and clinical studies. For instance, variations in piperine and other alkaloid concentrations have been shown to impact anticancer effects. Additionally, the lack of standardized extraction and purification processes poses challenges in determining optimal dosages for therapeutic use. Piperine, the primary bioactive compound in PNE, exhibits low bioavailability due to poor solubility, rapid metabolism, and quick clearance from the body. Advanced delivery systems such as nanoparticles and liposomes have shown promise in enhancing its bioavailability; however, these approaches are not yet fully validated in clinical settings. For example, studies have demonstrated improvements in piperine's pharmacokinetics using these technologies, but their therapeutic potential in humans remains unexplored. Another limitation is the scarcity of clinical evidence. Preclinical studies have demonstrated apoptosis induction, inhibition of metastasis, and enhanced efficacy of chemotherapeutic agents, but the lack of well-designed clinical trials restricts the translation of these findings into clinical practice. For example, PNE's ability to reduce tumor progression in rodent models requires further validation in human trials to establish safety and efficacy profiles. Concerns regarding potential toxicity and safety are also significant. Piperine has been associated with gastrointestinal irritation, hepatotoxicity, and interactions with drug-metabolizing enzymes, potentially altering the pharmacokinetics of co-administered medications. For instance, its interactions with cytochrome P450 enzymes underscore the need for comprehensive toxicological studies, particularly in cancer patients undergoing complex treatment regimens. PNE's interactions with cytochrome P450 enzymes further complicate its use in oncology. These interactions can either enhance or inhibit the metabolism of other drugs, increasing the risk of adverse effects or reduced therapeutic efficacy. This is particularly problematic for cancer patients on polypharmacy, highlighting the need for careful evaluation of drug interactions before considering PNE in combination therapies. Regulatory challenges and quality control issues also hinder the integration of PNE into mainstream oncology. The lack of established guidelines for standardizing PNE production and certification affects consistency and quality, making it difficult to develop reliable formulations for clinical use. Furthermore, the precise mechanisms underlying PNE's anticancer effects and its potential synergistic interactions with other therapies are not fully understood. For example, while piperine has shown promising synergy with chemotherapeutic agents like doxorubicin, optimizing these combinations requires further research. Finally, the integration of PNE into complementary and alternative medicine (CAM) practices is underdeveloped. Despite its historical use in traditional medicine, the absence of standardized protocols and guidelines limits its application alongside conventional cancer treatments. In summary, PNE holds significant promise as a complementary anticancer therapy, but challenges such as variability in composition, low bioavailability, limited clinical evidence, toxicity concerns, and regulatory hurdles must be addressed. Addressing these limitations through rigorous research, standardization, and clinical validation is crucial to realizing the full potential of PNE in oncology.The anticancer potential of PNE positions it as a promising candidate in integrative cancer therapies, yet its contextualization alongside existing treatments provides deeper insight into its utility. Compared to conventional chemotherapeutic agents such as doxorubicin or paclitaxel, PNE offers unique advantages. For instance, standard chemotherapy is associated with severe side effects, such as cardiotoxicity and immune suppression, and PNE has been shown to mitigate these effects when used as an adjunct. In NMU-induced mammary tumor models, Piperine-free P. nigrum extracts (PFPE) reduced the systemic immune suppression caused by doxorubicin without compromising its efficacy, indicating its immunomodulatory potential [27]. Cost-wise, PNE exhibits greater affordability than synthetic chemotherapeutic agents, particularly in low- and middle-income countries (LMICs), where the high cost of treatment often limits access. The production of herbal extracts like PNE relies on relatively simple processing techniques compared to the complex synthesis pathways for drugs like docetaxel. This makes PNE not only cost-effective but also sustainable for resource-constrained settings. Regarding efficacy, PNE shows comparable cytotoxicity against various cancer cell lines with IC50 values often aligning with those of synthetic drugs. For example, PNE’s IC50 values range from 4 µg/mL to 52 µg/mL across colorectal, breast, and cervical cancer models, rivaling first-line chemotherapeutic agents. Moreover, its synergistic potential when combined with established drugs like paclitaxel enhances apoptosis and overcomes drug resistance in cervical cancer cells [92]. In terms of integration, the versatility of PNE as a dietary supplement, nanoparticle formulation, or combined therapy enhances its adaptability. However, challenges such as variability in phytochemical content and limited clinical trials necessitate further research. Nonetheless, the dual role of PNE in reducing side effects and augmenting anticancer efficacy underscores its value in integrative cancer treatment regimens.
Concluding remarks
In the context of anticancer research, alkaloids represent the primary phytochemical components found in P. nigrum extracts (PNE). PNE has demonstrated the ability to inhibit the proliferation of various cancer cells, induce cell cycle arrest, and suppress the expression of critical cell cycle proteins such as cyclin, NF‑κB, FoxM1, and survivin. Furthermore, PNE has been shown to generate oxidative stress, contributing to cell death in cancer cells. Its compounds interact with oxidative stress-related enzymes, including CYP450R, GPX, SODs, AIF, and MAO B, suggesting its potential as an oxidative stress modulator. In addition to its antiproliferative effects, PNE has exhibited antimetastatic activity by inhibiting the expression of MMP and VEGF, which are key regulators of tumor invasion and angiogenesis. Studies have also highlighted its synergistic effects when combined with plant extracts such as A. galanga, C. aurantifolia, T. triandra, C. sativa, A. indica, A. paniculata, Z. spina-christi, G. extensum, and O. sanctum. These combinations enhance its ability to inhibit cell proliferation, induce apoptosis, and modulate the immune response. Furthermore, synergistic interactions between PNE and established anticancer drugs like doxorubicin and paclitaxel have shown promise in enhancing therapeutic efficacy while potentially reducing the side effects of chemotherapy. Compared to isolated compounds, PNE offers unique advantages due to the synergistic interactions among its bioactive constituents. These benefits include cost-effectiveness, broad-spectrum anticancer activity, and potential to address drug resistance. However, challenges such as limited bioavailability, variability in composition, and a lack of comprehensive clinical evidence remain. Advanced drug delivery systems, including nanoparticles and liposomal formulations, present opportunities to improve PNE’s bioavailability and therapeutic application. Future research should prioritize standardizing extraction methods, conducting well-designed clinical trials, and elucidating molecular mechanisms to facilitate the integration of PNE into clinical oncology. This review highlights the potential of PNE as a complementary cancer therapy, with the ability to improve treatment outcomes, minimize adverse effects, and provide a cost-effective option for patients, particularly in resource-constrained settings.
Acknowledgements
The authors would like to express their gratitude to Dr. Irina Zamfir, MD, RCP London, Basildon University Hospital, UK for providing professional English editing of this manuscript and for editorial support. This research was funded by the Directorate of Research and Community Service and Innovation of Universitas Padjadjaran.
Author contributions
HLW, IFM, NAH, JR, DC, JS-R made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas that is, revising or critically reviewing the article; giving final approval of the version to be published; agreeing on the journal to which the article has been submitted; and confirming to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hesti Lina Wiraswati, Daniela Calina and Javad Sharifi-Rad have contributed equally to this work.
Contributor Information
Hesti Lina Wiraswati, Email: hesti.lina@unpad.ac.id.
Daniela Calina, Email: calinadaniela@gmail.com.
Javad Sharifi-Rad, Email: javad.sharifirad@gmail.com.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer WHO. Global cancer observatory: cancer today, Globocan. 2022. https://gco.iarc.fr/today/en/fact-sheets-populations#groupshttps://gco.iarc.fr/today/en/fact-sheets-populations#groups. Accessed 19 Jul 2023.
- 3.Ocran Mattila P, Ahmad R, Hasan SS, Babar ZU. Availability, affordability, access, and pricing of anti-cancer medicines in low- and middle-income countries: a systematic review of literature. Front Public Health. 2021;9:628744. 10.3389/fpubh.2021.628744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang N-T, Chang Y-H, Huang Y-T, Chen S-C. Factors associated with refusal or discontinuation of treatment in patients with bladder cancer: a cohort population-based study in Taiwan. Int J Environ Res Public Health. 2021;18:618. 10.3390/ijerph18020618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sajid A, Manzoor Q, Iqbal M, Tyagi AK, Sarfraz RA, Sajid A. Pinus roxburghii essential oil anticancer activity and chemical composition evaluation. EXCLI J. 2018;17:233–45. 10.17179/excli2016-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sajid A, Manzoor Q, Imran M, Aslam F, Gondal T, Ahmad R, Arshad M, Imran A, Hussain G. Essential oil and leaves from lantana camara significantly ameliorate different cancer cell lines by suppressing the NF-κB pathway. 2024.
- 7.Imran M, Saeed F, Alsagaby SA, Imran A, Ahmad I, El Ghorab AH, Abdelgawad MA, Qaisrani TB, Mehmood T, Umar M, Mumtaz MA, Sajid A, Manzoor Q, Hussain M, Al Abdulmonem W, Al Jbawi E. Curcumin: recent updates on gastrointestinal cancers. CyTA J Food. 2023;21(1):502–13. 10.1080/19476337.2023.2245009. [Google Scholar]
- 8.Sajid A, Manzoor Q, Sajid A, Nazir A, Mumtaz MA, Fatima N, Alshawwa SZ, Iqbal M, Younas U. Downregulation of NF-κB activation pathways using essential oil derived from Citrus pseudolimon: anticancer and anti-inflammatory potential. Biocatal Agric Biotechnol. 2023;47:102599. 10.1016/j.bcab.2022.102599. [Google Scholar]
- 9.Sajid A, Manzoor Q, Sajid A, Mehrun N, Imtiaz F, Mumtaz MA, Fatima N, Mohammed OA, Abdel-Reheim MA, Iqbal M. In vitro and in silico studies of terpenes extracted from Cymbopogon flexuous leaves against human myeloid leukemia as an inhibitor of NF-κB activation signaling pathway. J Mol Struct. 2025;1321:139675. 10.1016/j.molstruc.2024.139675. [Google Scholar]
- 10.Khan MSA, Ahmad I. Chapter 1-Herbal medicine: current trends and future prospects. In: Khan MSA, Ahmad I, Chattopadhyay D, editors. New look to phytomedicine. Cambridge: Academic Press; 2019. 10.1016/B978-0-12-814619-4.00001-X. [Google Scholar]
- 11.Nur Hasanah SW. Herbal as a compelementary therapy for tumor/cancer patients. J Kefarmasian Indones. 2016;6:49–59. [Google Scholar]
- 12.Azzam M, Fauziah N, Wiraswati H. The anticancer effect of phytochemicals and potential of Breynia cernua: an overview. Biomed Pharmacol J. 2022;15:2259–78. 10.13005/bpj/2564. [Google Scholar]
- 13.Changou CA, Shiah HS, Chen LT, Liu S, Luh F, Liu SH, Cheng YC, Yen Y. A phase II clinical trial on the combination therapy of PHY906 plus capecitabine in hepatocellular carcinoma. Oncologist. 2021;26(3):e367–73. 10.1002/onco.13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang YH, Morris-Natschke SL, Yang J, Niu HM, Long CL, Lee KH. Anticancer principles from medicinal piper (hú jiāo) plants. J Tradit Complement Med. 2014;4(1):8–16. 10.4103/2225-4110.124811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holdsworth D. Traditional medicinal plants of Rarotonga, Cook Islands part I. Pharm Biol. 2008;28:209–18. 10.3109/13880209009082815. [Google Scholar]
- 16.Soladoye MO, Amusa N, Raji-Esan SO, Chukwuma E, Taiwo AA. Ethnobotanical survey of anti-cancer plants in Ogun State, Nigeria. Ann Biol Res. 2010;1:261–73. [Google Scholar]
- 17.Chaveerach PDA, Mokkamul P, Runglawan S, Tanee T. Ethnobotany of the genus Piper (Piperaceae) in Thailand. Ethnobot Res Appl. 2008. 10.17348/era.4.0.223-231. [Google Scholar]
- 18.Chen T. Observation of the medicine made by oneself in treating with 97 cases with gastric diseases. J Pract Med Technol. 2008;15:593–4. [Google Scholar]
- 19.Bezerra DP, Pessoa C, de Moraes MO, Saker-Neto N, Silveira ER, Costa-Lotufo LV. Overview of the therapeutic potential of piplartine (piperlongumine). Eur J Pharm Sci. 2013;48(3):453–63. 10.1016/j.ejps.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Al-Zarliani WO, Muzuna M, Sugianto S. Behavior and marketing analysis of pepper (Piper nigrum L.): a comparative study of farmers, trading districts and retailers in southeast Sulawesi, Indonesia. Univ Sebel Maret. 2023;38(1):14. [Google Scholar]
- 21.Moreira DdL, Teixeira SS, Monteiro MHD, De-Oliveira ACAX, Paumgartten FJR. Traditional use and safety of herbal medicines1. Rev Bras. 2014;24(2):248–57. 10.1016/j.bjp.2014.03.006. [Google Scholar]
- 22.Vincent Rajkumar S. The high cost of prescription drugs: causes and solutions. Blood Cancer J. 2020;10(6):71. 10.1038/s41408-020-0338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh S, Sarkar B, Ranadheera CS, Thongmee S. Chapter 6-Synergistic effects of plant extracts and nanoparticles for therapy. In: Kaneria M, Rakholiya K, Egbuna C, editors. Nanotechnology and in silico tools. Amsterdam: Elsevier; 2024. 10.1016/B978-0-443-15457-7.00003-4. [Google Scholar]
- 24.Mangoato IM, Mahadevappa CP, Matsabisa MG. Cannabis sativa L. extracts can reverse drug resistance in colorectal carcinoma cells in vitro. Synergy. 2019;9:100056. 10.1016/j.synres.2019.100056. [Google Scholar]
- 25.Mad-Adam N, Madla S, Lailerd N, Hiransai P, Graidist P. Piper nigrum extract: dietary supplement for reducing mammary tumor incidence and chemotherapy-induced toxicity. 2023. Foods. 10.3390/foods12102053. [DOI] [PMC free article] [PubMed]
- 26.Deng Y, Sriwiriyajan S, Tedasen A, Hiransai P, Graidist P. Anti-cancer effects of Piper nigrum via inducing multiple molecular signaling in vivo and in vitro. J Ethnopharmacol. 2016;188:87–95. 10.1016/j.jep.2016.04.047. [DOI] [PubMed] [Google Scholar]
- 27.Saetang J, Tedasen A, Sangkhathat S, Sangkaew N, Dokduang S, Prompat N, Taraporn S, Graidist P. Low piperine fractional Piper nigrum extract enhanced the antitumor immunity via regulating the Th1/Th2/Treg Cell subsets on NMU-induced tumorigenesis rats. Planta Med. 2022;88(7):527–37. 10.1055/a-1458-5646. [DOI] [PubMed] [Google Scholar]
- 28.de Souza Grinevicius VM, Kviecinski MR, Santos Mota NS, Ourique F, Porfirio Will Castro LS, Andreguetti RR, Gomes Correia JF, Filho DW, Pich CT, Pedrosa RC. Piper nigrum ethanolic extract rich in piperamides causes ROS overproduction, oxidative damage in DNA leading to cell cycle arrest and apoptosis in cancer cells. J Ethnopharmacol. 2016;189:139–47. 10.1016/j.jep.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 29.Kiranmayee M, Rajesh N, Vidya Vani M, Khadri H, Mohammed A, Chinni SV, Ramachawolran G, Riazunnisa K, Moussa AY. Green synthesis of Piper nigrum copper-based nanoparticles: in silico study and ADMET analysis to assess their antioxidant, antibacterial, and cytotoxic effects. Front Chem. 2023;11:1218588. 10.3389/fchem.2023.1218588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buranrat B, Boontha S. Effect of Piper nigrum ethanolic extract on human breast cancer cell growth and cell migration. Pharmacogn Mag. 2019;15:538. 10.4103/pm.pm_109_19. [Google Scholar]
- 31.Buranrat B. Proliferative inhibition, apoptotic induction, migratory suppression of Piper nigrum extract on HeLa cervical cell line. Indian J Pharm Sci. 2022;84:1210–7. [Google Scholar]
- 32.Kanniah P, Chelliah P, Thangapandi JR, Gnanadhas G, Mahendran V, Robert M. Green synthesis of antibacterial and cytotoxic silver nanoparticles by Piper nigrum seed extract and development of antibacterial silver based chitosan nanocomposite. Int J Biol Macromol. 2021;189:18–33. 10.1016/j.ijbiomac.2021.08.056. [DOI] [PubMed] [Google Scholar]
- 33.Prashant A, Rangaswamy C, Yadav AK, Reddy V, Sowmya MN, Madhunapantula S. In vitro anticancer activity of ethanolic extracts of Piper nigrum against colorectal carcinoma cell lines. Int J Appl Basic Med Res. 2017;7(1):67–72. 10.4103/2229-516x.198531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sriwiriyajan S, Ninpesh T, Sukpondma Y, Nasomyon T, Graidist P. Cytotoxicity screening of plants of genus piper in breast cancer cell lines. Trop J Pharm Res. 2014;13:921. 10.4314/tjpr.v13i6.14. [Google Scholar]
- 35.Jeong JH, Ryu J, Lee HJ. In vitro inhibition of Piper nigrum and piperine on growth, migration, and invasion of PANC-1 human pancreatic cancer cells. Nat Prod Commun. 2021;16:1934578X2110576. 10.1177/1934578X211057694. [Google Scholar]
- 36.Grinevicius V, Andrade KS, Mota N, Bretanha LC, Felipe KB, Ferreira SRS, Pedrosa RC. CDK2 and Bcl-xL inhibitory mechanisms by docking simulations and anti-tumor activity from piperine enriched supercritical extract. Food Chem Toxicol. 2019;132:110644. 10.1016/j.fct.2019.110644. [DOI] [PubMed] [Google Scholar]
- 37.Buranrat B, Senggunprai L, Prawan A, Kukongviriyapan V. Anticancer effects of Piper nigrum extract against cholangiocarcinoma cells. Pharmacogn Mag. 2022;18:160. [Google Scholar]
- 38.Tammina SK, Mandal BK, Ranjan S, Dasgupta N. Cytotoxicity study of Piper nigrum seed mediated synthesized SnO(2) nanoparticles towards colorectal (HCT116) and lung cancer (A549) cell lines. J Photochem Photobiol B. 2017;166:158–68. 10.1016/j.jphotobiol.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 39.Alves FS. Evaluation of antimicrobial activity and cytotoxicity effects of extracts of Piper nigrum L. and piperine. Separations. 2022;10:1–18. [Google Scholar]
- 40.Mahfouz AY, Daigham GE, Radwan AM, Mohamed AA. Eco-friendly and superficial approach for synthesis of silver nanoparticles using aqueous extract of Nigella sativa and Piper nigrum L. seeds for evaluation of their antibacterial, antiviral, and anticancer activities a focus study on its impact on seed germination and seedling growth of Vicia faba and Zea mays. Egypt Pharm J. 2020;19(4):401. [Google Scholar]
- 41.Krishnan V, Elayaperumal M, Rajagopal K, Maaza M. Green synthesis of silver nanoparticles using Piper nigrum concoction and its anticancer activity against MCF-7 and Hep-2 cell lines. J Antimicro. 2016. 10.4172/2472-1212.1000123. [Google Scholar]
- 42.Liu Y, Yadev VR, Aggarwal BB, Nair MG. Inhibitory effects of black pepper (Piper nigrum) extracts and compounds on human tumor cell proliferation, cyclooxygenase enzymes, lipid peroxidation and nuclear transcription factor-kappa-B. Nat Prod Commun. 2010;5(8):1253–7. [PubMed] [Google Scholar]
- 43.Priya NC, Kumari PS. Antiviral activities and cytotoxicity assay of seed extracts of Piper longum and Piper nigrum on human cell lines. J Pharm Sci Rev Res. 2017;44:197–202. [Google Scholar]
- 44.Raja W, Dubey A, Bandhe L, Pandey S. Anticarcinogenic activity of Piper nigrum extract and its active component piperine against 7, 12- dimethylbenz (a) anthracene induced mouse skin carcinogenesis. Eur J Biochem. 2016;3:101–6. [Google Scholar]
- 45.Roy UB, Vijayalaxmi KK. Evaluation of in vitro antitumor property of ethanolic extract of Piper nigrum seeds. Int J Biotechnol Allied Field. 2013;1:419–37. [Google Scholar]
- 46.Sruthi D, Zachariah JT. In vitro antioxidant activity and cytotoxicity of sequential extracts from selected black pepper (Piper nigrum L.) varieties and Piper species. Int Food Res J. 2017;24:75–85. [Google Scholar]
- 47.Tedasen A, Khoka A, Madla S, Sriwiriyajan S, Graidist P. Anticancer effects of piperine-free Piper nigrum extract on cholangiocarcinoma cell lines. Pharmacogn Mag. 2020;16:28. 10.4103/pm.pm_288_19. [Google Scholar]
- 48.Mahadevi MS, Madhavan A Anticancer activity of Piper nigrum methanolic extract against A549 human lung cancer cell line. UGC Care J. 2021; 43.
- 49.Ee GC, Lim CM, Lim CK, Rahmani M, Shaari K, Bong CF. Alkaloids from Piper sarmentosum and Piper nigrum. Nat Prod Res. 2009;23(15):1416–23. 10.1080/14786410902757998. [DOI] [PubMed] [Google Scholar]
- 50.Grinevicius VMAS, Ourique F, Andrade KS, Mota NSRS, Ferreira SRS, Pedrosa RC. P-25-Docking studies predict GPx inhibitory mechanisms behind antitumor activity of supercritical carbon dioxide black pepper bioactive fractions rich in piperine. Free Rad Biol Med. 2018;120:S52. 10.1016/j.freeradbiomed.2018.04.172. [Google Scholar]
- 51.Elekofehinti OO, Adetoyi IR, Popoola HO, Ayodeji FO, Taiwo FA, Akinjiyan MO, Koledoye OF, Iwaloye O, Adegboyega AE. Discovery of potential epidermal growth factor receptor inhibitors from black pepper for the treatment of lung cancer: an in-silico approach. In Silico Pharmacol. 2024;12(1):28. 10.1007/s40203-024-00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paul R, Devi Y, Saikia R, Gogoi D, Pegu D, Johari S. Exploring the possible mechanism of Piper nigrum against PPAR γ receptor protein responsible for colorectal cancer. 2018. 10.1109/BSB.2018.8770670.
- 53.Yaffe PB, Power Coombs MR, Doucette CD, Walsh M, Hoskin DW. Piperine, an alkaloid from black pepper, inhibits growth of human colon cancer cells via G1 arrest and apoptosis triggered by endoplasmic reticulum stress. Mol Carcinog. 2015;54(10):1070–85. 10.1002/mc.22176. [DOI] [PubMed] [Google Scholar]
- 54.Ashokkumar K, Murugan M, Dhanya MK, Pandian A, Warkentin TD. Phytochemistry and therapeutic potential of black pepper [Piper nigrum (L.)] essential oil and piperine: a review. Clin Phytosci. 2021;7(1):52. 10.1186/s40816-021-00292-2. [Google Scholar]
- 55.Mitra S, Anand U, Jha NK, Shekhawat MS, Saha SC, Nongdam P, Rengasamy KRR, Proćków J, Dey A. Anticancer applications and pharmacological properties of piperidine and piperine: a comprehensive review on molecular mechanisms and therapeutic perspectives. Front Pharmacol. 2021;12:772418. 10.3389/fphar.2021.772418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turrini E, Sestili P, Fimognari C. Overview of the anticancer potential of the “king of spices” Piper nigrum and its main constituent piperine. Toxins. 2020. 10.3390/toxins12120747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akram M, Solosky G, Ali A. Phytochemistry and pharmacology of Piper nigrum. Comp Clin Pathol. 2024;33(2):337–41. 10.1007/s00580-023-03536-4. [Google Scholar]
- 58.Benayad S, Wahnou H, El Kebbaj R, Liagre B, Sol V, Oudghiri M, Saad EM, Duval RE, Limami Y. The promise of piperine in cancer chemoprevention. Cancers. 2023. 10.3390/cancers15225488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guillén-Mancina E, Jiménez-Alonso JJ, Calderón-Montaño JM, Jiménez-González V, Díaz-Ortega P, Burgos-Morón E, López-Lázaro M. Artificial diets with selective restriction of amino acids and very low levels of lipids induce anticancer activity in mice with metastatic triple-negative breast cancer. Cancers. 2023. 10.3390/cancers15051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Azizi M, Ghourchian H, Yazdian F, Dashtestani F, AlizadehZeinabad H. Cytotoxic effect of albumin coated copper nanoparticle on human breast cancer cells of MDA-MB 231. PLoS ONE. 2017;12(11): e0188639. 10.1371/journal.pone.0188639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suski JM, Braun M, Strmiska V, Sicinski P. Targeting cell-cycle machinery in cancer. Cancer Cell. 2021;39(6):759–78. 10.1016/j.ccell.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma C, Gurkan-Cavusoglu E. A comprehensive review of computational cell cycle models in guiding cancer treatment strategies. NPJ Syst Biol Appl. 2024;10(1):71. 10.1038/s41540-024-00397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peron G, Mastinu A, Peña-Corona SI, Hernández-Parra H, Leyva-Gómez G, Calina D, Sharifi-Rad J. Silvestrol, a potent anticancer agent with unfavourable pharmacokinetics: current knowledge on its pharmacological properties and future directions for the development of novel drugs. Biomed Pharmacother. 2024;177:117047. 10.1016/j.biopha.2024.117047. [DOI] [PubMed] [Google Scholar]
- 64.Wu R, Zhao J, Wei P, Tang M, Ma Z, Zhao Y, Du L, Wan L. Piper nigrum extract inhibits the growth of human colorectal cancer HT-29 cells by inducing p53-mediated apoptosis. Pharmaceuticals. 2023. 10.3390/ph16091325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lasso P, Rojas L, Arévalo C, Urueña C, Murillo N, Nossa P, Sandoval T, Chitiva LC, Barreto A, Costa GM, Fiorentino S. Piper nigrum extract suppresses tumor growth and enhances the antitumor immune response in murine models of breast cancer and melanoma. Cancer Immunol Immunother. 2023;72(10):3279–92. 10.1007/s00262-023-03487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sriwiriyajan S, Tedasen A, Lailerd N, Boonyaphiphat P, Nitiruangjarat A, Deng Y, Graidist P. Anticancer and cancer prevention effects of piperine-free Piper nigrum extract on N-nitrosomethylurea-induced mammary tumorigenesis in rats. Cancer Prev Res. 2016;9(1):74–82. 10.1158/1940-6207.Capr-15-0127. [DOI] [PubMed] [Google Scholar]
- 67.Wiraswati HL, Ma’ruf IF, Sharifi-Rad J, Calina D. Piperine: an emerging biofactor with anticancer efficacy and therapeutic potential. BioFactors. 2024. 10.1002/biof.2134. [DOI] [PubMed] [Google Scholar]
- 68.Salanci Š, Vilková M, Martinez L, Mirossay L, Michalková R, Mojžiš J. The induction of G2/M phase cell cycle arrest and apoptosis by the chalcone derivative 1C in sensitive and resistant ovarian cancer cells is associated with ROS generation. Int J Mol Sci. 2024. 10.3390/ijms25147541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pavitra E, Kancharla J, Gupta VK, Prasad K, Sung JY, Kim J, Tej MB, Choi R, Lee JH, Han YK, Raju GSR, Bhaskar L, Huh YS. The role of NF-κB in breast cancer initiation, growth, metastasis, and resistance to chemotherapy. Biomed Pharmacother. 2023;163:114822. 10.1016/j.biopha.2023.114822. [DOI] [PubMed] [Google Scholar]
- 70.Tchakarska G, Sola B. The double dealing of cyclin D1. Cell Cycle. 2020;19(2):163–78. 10.1080/15384101.2019.1706903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ye C, Wang J, Wu P, Li X, Chai Y. Prognostic role of cyclin B1 in solid tumors: a meta-analysis. Oncotarget. 2017;8(2):2224–32. 10.18632/oncotarget.13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nar A, Ozen O, Tutuncu NB, Demirhan B. Cyclin A and cyclin B1 overexpression in differentiated thyroid carcinoma. Med Oncol. 2012;29(1):294–300. 10.1007/s12032-010-9800-0. [DOI] [PubMed] [Google Scholar]
- 73.Katoh M, Katoh M. Human FOX gene family (Review). Int J Oncol. 2004;25(5):1495–500. [PubMed] [Google Scholar]
- 74.Raghuwanshi S, Zhang X, Arbieva Z, Khan I, Mohammed H, Wang Z, Domling A, Camacho CJ, Gartel AL. Novel FOXM1 inhibitor STL001 sensitizes human cancers to a broad-spectrum of cancer therapies. Cell Death Discov. 2024;10(1):211. 10.1038/s41420-024-01929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheng S, Qian K, Wang Y, Wang G, Liu X, Xiao Y, Wang X. PPARγ inhibition regulates the cell cycle, proliferation and motility of bladder cancer cells. J Cell Mol Med. 2019;23(5):3724–36. 10.1111/jcmm.14280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khurram I, Khan MU, Ibrahim S, Ghani MU, Amin I, Falzone L, Herrera-Bravo J, Setzer WN, Sharifi-Rad J, Calina D. Thapsigargin and its prodrug derivatives: exploring novel approaches for targeted cancer therapy through calcium signaling disruption. Med Oncol. 2024;42(1):7. 10.1007/s12032-024-02541-z. [DOI] [PubMed] [Google Scholar]
- 77.Capó X, Jiménez-Garcia M, Sharopov F, Tsouh Fokou PV, Martorell M, Aldahish AA, Pezzani R, Sharifi-Rad J, Calina D. Unveiling the potential of HS-1793: a review of its anticancer properties and therapeutic promise. Future Med Chem. 2024. 10.1080/17568919.2024.2424150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Slopovsky J, Kucharska J, Obertova J, Mego M, Kalavska K, Cingelova S, Svetlovska D, Gvozdjakova A, Furka S, Palacka P. Plasma thiobarbituric acid reactive substances predicts survival in chemotherapy naïve patients with metastatic urothelial carcinoma. Transl Oncol. 2021;14(1):100890. 10.1016/j.tranon.2020.100890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chem Res Toxicol. 2000;13(3):135–60. 10.1021/tx9902082. [DOI] [PubMed] [Google Scholar]
- 80.Nur Mustofa H, Wiraswati H, Ekawardhani S, Fianza P, Windria S. Anticancer activities of saponins and quinones group through oxidative stress and glycolysis inhibition via in silico studies. Biomed Pharmacol J. 2020;13:999–1010. 10.13005/bpj/1969. [Google Scholar]
- 81.Wiraswati H, Martoprawiro M, Akhmaloka A, Warganegara F. Apoptosis inducing factor (AIF) stabilizes menadione-conjugate product in programmed cell death. Int J PharmTech Res. 2017;10:237–45. 10.20902/IJPTR.2017.10430. [Google Scholar]
- 82.Wiraswati H, Warganegara F, Akhmaloka A, Martoprawiro M. Molecular docking studies of ROS agent from quinone family to reductase enzymes: implication in finding anticancer drug candidate. Biomed Pharmacol J. 2021;14:681–9. 10.13005/bpj/2170. [Google Scholar]
- 83.Wiraswati HL, Rohmawaty E, Ramadhanti J, Achadiyani, Khairani S, Berbudi A, Ma’ruf IF. Investigation on anticancer potential of secondary metabolites of Piper nigrum: in silico study. Manuscript draft. 2024.
- 84.Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, Li Y. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6(1):263. 10.1038/s41392-021-00658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bui TT, Piao CH, Song CH, Shin HS, Shon D-H, Chai OH. Piper nigrum extract ameliorated allergic inflammation through inhibiting Th2/Th17 responses and mast cells activation. Cell Immunol. 2017;322:64–73. 10.1016/j.cellimm.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 86.Jongrungraungchok S, Madaka F, Wunnakup T, Sudsai T, Pongphaew C, Songsak T, Pradubyat N. In vitro antioxidant, anti-inflammatory, and anticancer activities of mixture Thai medicinal plants. BMC Complement Med Ther. 2023;23(1):43. 10.1186/s12906-023-03862-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sivaraj D, Shanmugam S, Rajan M, Sasidharan SP, Sathyanarayanan S, Muniyandi K, Thangaraj P, de Souza Araújo AA. Evaluation of Aristolochia indica L. and Piper nigrum L. methanol extract against centipede Scolopendra moristans L. using Wistar albino rats and screening of bioactive compounds by high pressure liquid chromatography: a polyherbal formulation. Biomed Pharmacother. 2018;97:1603–12. 10.1016/j.biopha.2017.11.114. [DOI] [PubMed] [Google Scholar]
- 88.Faisal M, Maungchanburi S, Dokduang S, Rattanaburee T, Tedasen A, Graidist P. Dichloromethane crude extract of Gymnanthemum extensum combined with low piperine fractional Piper nigrum extract induces apoptosis on human breast cancer cells. Indian J Pharm Sci. 2021;83:247–60. 10.36468/pharmaceutical-sciences.770. [Google Scholar]
- 89.Mathiyazhagan J, Madhyastha H, Nalini E, Gothandam KM. Synergistic effect of Ocimum sanctum and Piper nigrum: an in vitro study on type 2 diabetes-related enzymes and MCF-7 breast cancer cell line. Curr Trends Biotechnol Pharm. 2022;16(Suppl 1):56–63. 10.5530/ctbp.2022.2s.31. [Google Scholar]
- 90.Majdalawieh AF, Carr RI. In vitro investigation of the potential immunomodulatory and anti-cancer activities of black pepper (Piper nigrum) and cardamom (Elettaria cardamomum). J Med Food. 2010;13(2):371–81. 10.1089/jmf.2009.1131. [DOI] [PubMed] [Google Scholar]
- 91.Sari N, Lestari B, Saputri D, Fauzia A, Santoso R, Ediati S, Meiyanto E. Reveal cytotoxicity and antigenotoxicity of Piper nigrum L. ethanolic extract and its combination with doxorubicin on CHO-K1 cells. Indones J Cancer Chemoprev. 2018;8:110. 10.14499/indonesianjcanchemoprev8iss3pp110-119. [Google Scholar]
- 92.Xu J, Wei Y, Liu Q, Liu X, Zhu C, Tu Y, Lei J, Yu J. The bioactive amide alkaloids from the stems of Piper nigrum. Food Chem. 2023;405(Pt A):134736. 10.1016/j.foodchem.2022.134736. [DOI] [PubMed] [Google Scholar]
- 93.Ouyang DY, Zeng LH, Pan H, Xu LH, Wang Y, Liu KP, He XH. Piperine inhibits the proliferation of human prostate cancer cells via induction of cell cycle arrest and autophagy. Food Chem Toxicol. 2013;60:424–30. 10.1016/j.fct.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 94.Makhov P, Golovine K, Canter D, Kutikov A, Simhan J, Corlew MM, Uzzo RG, Kolenko VM. Co-administration of piperine and docetaxel results in improved anti-tumor efficacy via inhibition of CYP3A4 activity. Prostate. 2012;72(6):661–7. 10.1002/pros.21469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Selvendiran K, Banu SM, Sakthisekaran D. Protective effect of piperine on benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice. Clin Chim Acta. 2004;350(1–2):73–8. 10.1016/j.cccn.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 96.Khan Z, Moni F, Sharmin S, Al-Mansur M, Gafur A, Rahman O, Afroz F. Isolation of bulk amount of piperine as active pharmaceutical ingredient (API) from black pepper and white pepper (Piper nigrum L.). Pharmacol Pharm. 2017;08:253–62. 10.4236/pp.2017.87018. [Google Scholar]
- 97.Chithra S, Jasim B, Sachidanandan P, Jyothis M, Radhakrishnan EK. Piperine production by endophytic fungus Colletotrichum gloeosporioides isolated from Piper nigrum. Phytomedicine. 2014;21(4):534–40. 10.1016/j.phymed.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 98.Johnson JJ, Nihal M, Siddiqui IA, Scarlett CO, Bailey HH, Mukhtar H, Ahmad N. Enhancing the bioavailability of resveratrol by combining it with piperine. Mol Nutr Food Res. 2011;55(8):1169–76. 10.1002/mnfr.201100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Mad-Adam N, Madla S, Lailerd N, Hiransai P, Graidist P. Piper nigrum extract: dietary supplement for reducing mammary tumor incidence and chemotherapy-induced toxicity. 2023. Foods. 10.3390/foods12102053. [DOI] [PMC free article] [PubMed]
Data Availability Statement
No datasets were generated or analysed during the current study.