Abstract
Bcl-2, a key regulator of cellular apoptosis, is typically linked to adverse prognosis in solid tumors due to its inhibition of apoptotic cell death and promotion of cellular proliferation, leading to tumor progression. However, studies on Bcl-2 in breast cancer have shown inconsistent results, with some indicating favorable outcomes. This study aims to determine the subtype-specific role of Bcl-2 in breast cancer. Female breast cancer patients who completed primary treatment at Wonju Severance Hospital, Korea, from 2004 to 2018 were included. Clinicopathological characteristics, including Bcl-2 expression, were collected, and patients were classified based on Bcl-2 expression in more than or less than 10% of tumor cells. Kaplan–Meier curves compared recurrence-free interval (RFI) and overall survival (OS). The final cohort of 617 patients, with a mean age of 54.79 ± 11.2 years, showed no overall survival difference by Bcl-2 status (p = 0.616). In HER2-overexpressed patients, high Bcl-2 expression was linked to poor prognosis (p = 0.0021). This trend appeared in ER-positive (p = 0.297) and ER-negative (p = 0.029) subgroups. Conversely, in HER2-negative patients, Bcl-2 overexpression indicated better survival (p = 0.009), consistent in ER-positive (p = 0.259) and ER-negative (p = 0.010) subgroups. Bcl-2’s impact on survival varies with HER2 status, showing poor prognosis in HER2-overexpressed and better prognosis in HER2-negative patients.
Keywords: Bcl-2, Breast cancer, HER2 status, Apoptosis, Prognosis, Tumor progression
Subject terms: Breast cancer, Oncogenes, Biomarkers
Introduction
Breast cancer is one of the leading causes of death worldwide, including in Korea1–3. Advances in understanding breast cancer biology have allowed for the definition of different subtypes based on estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) overexpression status. This understanding has also led to improved patient survival through the use of appropriate targeted agents. Further exploration of additional factors beyond ER, PR, and HER2 status, such as BRCA1/2 and PIK3 mutation status, has refined treatment options and contributed to the development of new targeted therapies. Therefore, a comprehensive understanding of tumor biology is critical for improving patient outcomes.
B-cell lymphoma 2 (Bcl-2) is a key regulator of cellular apoptosis. Its overexpression in solid tumors is expected to be associated with an adverse prognosis, because it inhibits apoptotic cell death and promotes cellular proliferation, leading to tumor progression. Unlike in hematologic malignancies, studies on Bcl-2 in breast cancer have reported inconsistent results, with some indicating favorable outcomes, particularly in ER-positive disease4. Recent research has highlighted the heterogeneity in ER-positive breast cancer based on varying levels of positivity, ranging from 1–100%5,6. In contrast, HER2 positivity requires strict adherence to a certain set of criteria, reducing heterogeneity in pathological diagnosis. Given that confounding factors can interfere with accurate analysis, understanding the role of Bcl-2 in the context of subtypes, particularly HER2 status rather than ER positivity, could provide valuable insights.
This study aimed to determine the subtype-specific role of Bcl-2 in breast cancer, with a particular focus on patient survival in the context of HER2 positivity.
Materials and methods
Study population
This retrospective cohort study included female patients diagnosed with stage I–III breast cancer between January 2004 and December 2018 who had completed all phases of primary treatment as indicated. The institutional review board of Wonju Severance Christian Hospital, Wonju, Korea, approved this study (CR323024), and the requirement for informed consent was waived due to its retrospective nature.
Inclusion and exclusion criteria
Given the retrospective nature of the study, all patients diagnosed within the study period were considered candidates. Patients were excluded if they had stage IV disease, refused or could not complete treatment, or had insufficient clinical data, including patient age, tumor size, lymph node status (number of positive lymph nodes and all lymph nodes if axillary lymph nodes were dissected), ER, PR, and HER2 statuses, Bcl-2 status, and survival data appropriate for statistical analysis.
Clinicopathologic data
Clinicopathologic characteristics, as described above, were collected. ER, PR, and HER2 statuses were obtained from pathology reports, which were determined based on the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) 2010 and 2013 guidelines7,8. Briefly, tumors were classified as ER-negative and PR-negative if they showed < 10% reactivity until 2010; the thresholds for ER and PR positivity decreased to < 1% since June 2010. HER2 status was assessed using immunohistochemical (IHC) staining and was defined as positive if the score was 3. Fluorescent in situ hybridization was selectively performed if the IHC staining score was 2, based on the cut-off value proposed by the ASCO/CAP 2013 guidelines7,8. The combination of ER, PR, and HER2 statuses yielded eight subgroups. Patients were stratified into two groups based on Bcl-2 expression levels: Bcl-2-high, defined as equal to or greater than 10% of tumor cells expressing Bcl-2, and Bcl-2-low, defined as less than 10% of tumor cells expressing Bcl-29–11. Patient survival data were retrieved from the breast cancer database at Wonju Severance Christian Hospital and the Korean National Cancer Center database.
Statistical analysis
The χ2 test or Fisher’s exact test was used to compare the frequencies of categorical variables depending on the expected frequency. Continuous variables were analyzed with the Student’s t-test. The primary endpoints were recurrence-free interval (RFI) and overall survival (OS). In accordance with the standardized definitions for efficacy endpoints in adjuvant breast cancer trial criteria12, RFI was defined as the time from the date of primary diagnosis to the date of one of the following events: invasive ipsilateral breast tumor recurrence, local or regional invasive recurrence, distant recurrence, or death from breast cancer. OS was defined as the time from the date of primary diagnosis to the date of death from any cause (breast cancer, non-breast cancer, or an unknown cause)12. To compare survival rates, Kaplan–Meier (KM) curves were generated and compared using the log-rank test. The Cox proportional hazards model was used to identify risk factors for poor survival. Impact of Bcl-2 on RFI was adjusted for potential confounding variables, including patients age, tumor size, axillary node metastasis, ER, PR, and HER2. Statistical analyses were performed using SPSS (version 25.0; IBM, Armonk, NY, USA). Statistical significance was set at p < 0.05.
Results
Baseline characteristics: clinicopathological and Bcl-2 perspective
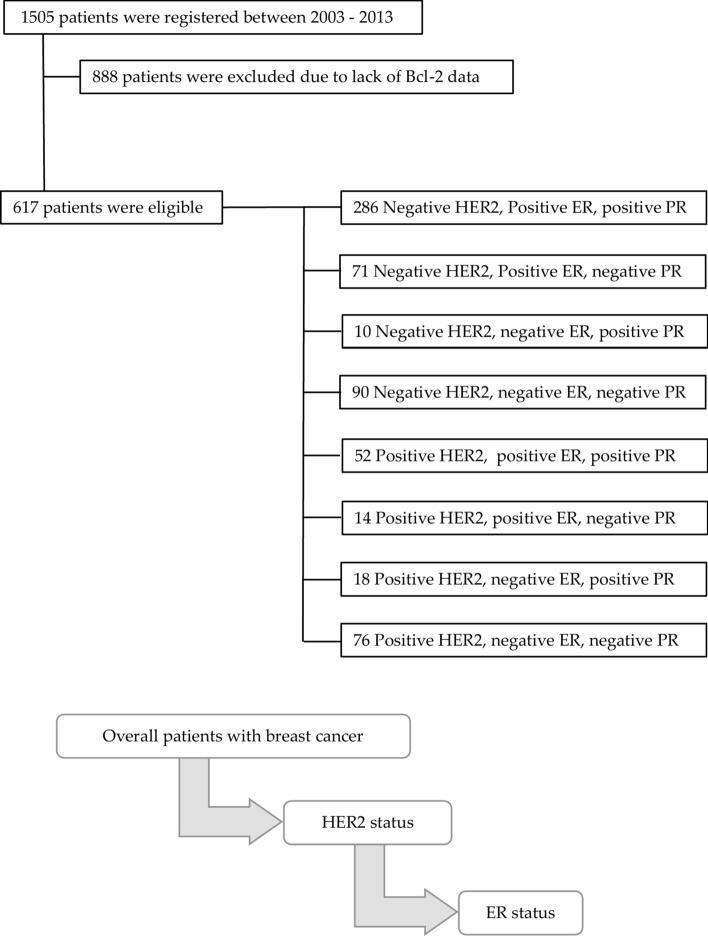
The final cohort comprised 617 patients (Fig. 1). The mean age was 54.8 ± 11.2 years, and the mean tumor size was 2.17 ± 1.49 cm. More than two-thirds of the patients had node-negative disease (70.0%). Approximately two-thirds of the patients had ER-positive disease (68.9%), and a quarter had HER2-overexpressed disease (74.2%). Over three-quarters of the patients exhibited high Bcl-2 expression (84.3%). There were no significant differences in age, tumor size, or nodal positivity when patients were stratified by Bcl-2 status (Table 1). However, patients with high Bcl-2 expression were more likely to have ER-positive disease (p = 0.012) and HER2-negative disease (p < 0.001) compared to those with low Bcl-2 expression (Table 1).
Fig. 1.
Study Profile: A total of 617 patients were diagnosed with primary breast cancer and completed their planned treatment between 2004 and 2018. Data from 617 patients were analyzed after excluding 888 patients without Bcl 2 data. Breast cancer subtyping data and study flow were described.
Table 1.
Baseline characteristics of the patients.
| Overall (n = 617) | BCL2 low (n = 97) | BCL2 high (n = 520) | p value | |
|---|---|---|---|---|
| Mean age ± deviation, yr | 54.8 ± 11.22 | 52.86 ± 11.62 | 55.15 ± 11.12 | 0.65 |
| Mean tumor size ± standard deviation, cm | 2.17 ± 1.49 | 2.09 ± 1.21 | 2.19 ± 1.53 | 0.58 |
| Nodal disease (n, %) | 0.054 | |||
| Negative | 432(70) | 76(78.4) | 356(68.5) | |
| Positive | 185(30) | 21(21.6%) | 164(31.5) | |
| ER (n, %) | 0.012 | |||
| Positive | 425(68.9) | 56(57.7) | 369(71.0) | |
| Negative | 192(31.1) | 41(42.3) | 151(29.0) | |
| PR (n, %) | 0.573 | |||
| Positive | 368(59.6) | 42(43.4) | 207(39.8) | |
| Negative | 249(40.4) | 55(56.7) | 313(60.2) | |
| HER2 (n, %) | < 0.001 | |||
| Negative | 458(74.2) | 55(56.7) | 403(77.5) | |
| Positive | 158(25.6) | 42(43.3) | 116(22.3) | |
| Missing | 1(0.2) | 0 | 1(0.2) |
Bcl-2 status and RFI
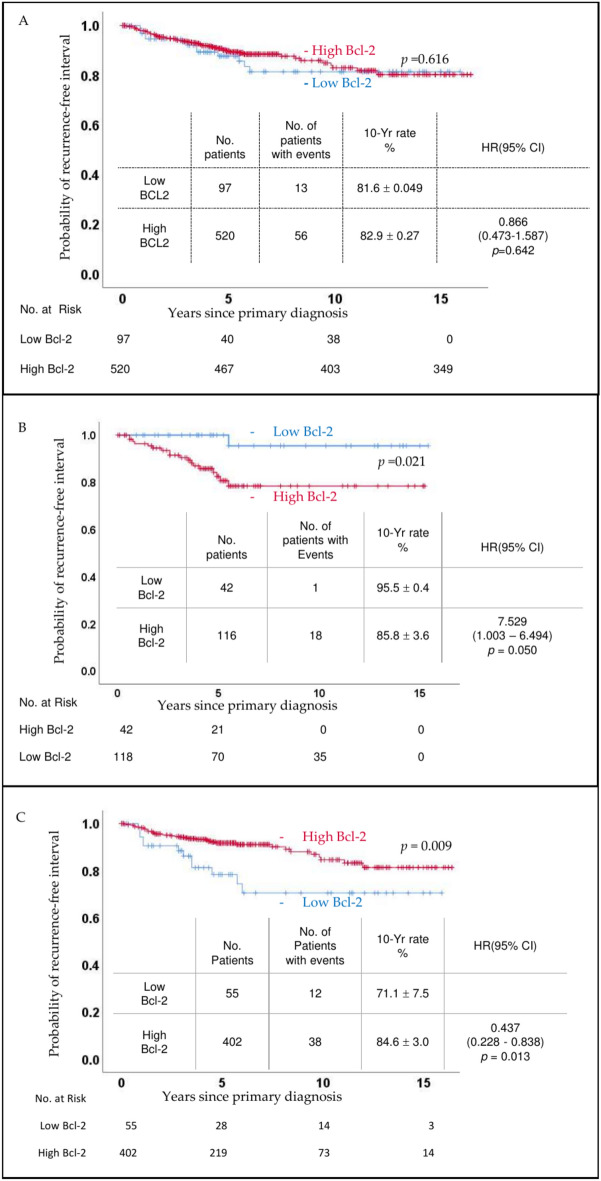
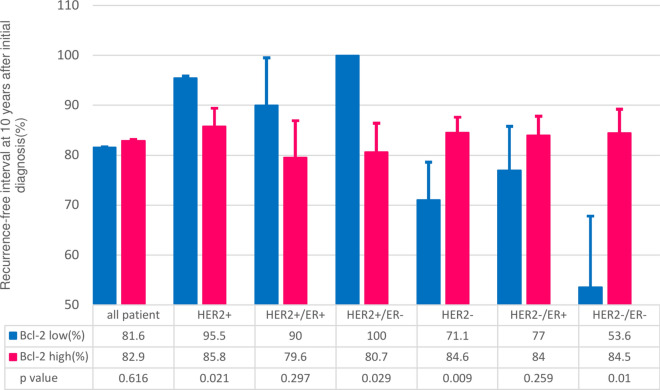
There was no overall difference in RFI based on Bcl-2 status (p = 0.616, Fig. 2A). However, when categorized by HER2 status, cl-2 status was associated with a statistically significant difference in prognosis (Fig. 2B,C). Interestingly, the impact of Bcl-2 on RFI was reversed depending on HER2 status. In cases of HER2 overexpression, high Bcl-2 was associated with an adverse prognosis (p = 0.021, Fig. 2B). Conversely, in HER2-negative cases, high Bcl-2 correlated with a favorable prognosis (p = 0.009, Fig. 2C). This trend persisted even after further stratification by ER status (Fig. 3 and supplementary Figs. 1 and 2). In patients with HER2-overexpressed disease, high Bcl-2 status showed a statistically significant survival disadvantage in ER-negative disease (p = 0.029), while a non-significant trend was noted in ER-positive disease (p = 0.297). Similarly, in HER2-negative disease, the RFI advantage in high.
Fig. 2.
Recurrence Free Interval (RFI): (A) Kaplan Meier graphs comparing Bcl 2 low (blue line) and Bcl 2 high (red line) groups failed to show a significant difference (p = 0.616). However, when patients were stratified into HER2 overexpressed (B) and HER2 negative (C) groups, distinct patterns emerged.
Fig. 3.
Recurrence-Free Interval (RFI) in Stratified Patient Groups: The inverse relationship between HER2 overexpressed and HER2 negative disease persisted after patients were further stratified according to ER status.
Bcl-2 status was significant in ER-negative patients (p = 0.010) and showed a favorable trend in ER-positive patients (p = 0.259) (Fig. 3).
Bcl-2 as an independent risk factor
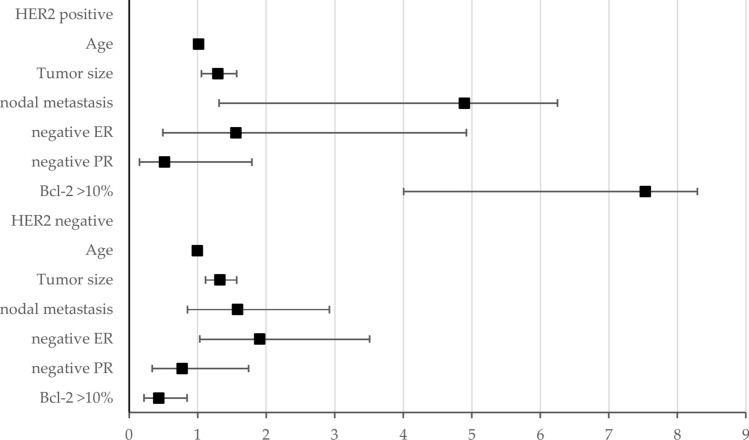
The Cox hazard model revealed varying results regarding the impact of high Bcl-2 expression on patient survival. While tumor size, metastatic nodal disease, and ER and PR positivity consistently influenced recurrence-free survival, the effect of high Bcl-2 expression significantly depended on HER2 status (Fig. 4). Specifically, Bcl-2 expression equal to or higher than 10% of tumor cells (high Bcl-2) demonstrated divergent outcomes based on HER2 status. In HER2-positive patients, high Bcl-2 expression was associated with a significantly adverse prognosis for RFI (Hazard ratio (HR) 7.53, 95% Confidence interval (CI) 4.03–8.29). Conversely, in HER2-negative patients, high Bcl-2 expression correlated with a favorable RFI (HR 0.43, 95% CI 0.22–0.84).
Fig. 4.
BCL2 as an Independent Prognostic Factor: This forest plot illustrates the hazard ratio (HR) and 95% confidence intervals (CIs) for various prognostic factors. While conventional prognostic factors such as age, tumor size, nodal metastasis, and ER negativity were significant poor prognostic factors, high BCL2 levels had an opposite impact in HER2-positive and HER2-negative breast cancer. Detailed data regarding HR and CIs are available in the supplementary table.
Discussion
This study demonstrated that Bcl-2 status exhibited opposing effects on patient survival based on HER2 status in breast cancer patients. Furthermore, our observations suggested that ER positivity may act as a confounding factor when investigating Bcl-2.
Variations in ER- and HER2-mediated pathways have been extensively documented and investigated in breast cancer research13,14. However, their clinical applications have evolved differently based on available systemic treatments. Determining ER positivity has been the initial step in identifying the biological characteristics of breast cancer in clinical practice. Indeed, clinical guidelines developed by the NCCN, ASCO, and ESMO have endorsed this approach for some time15–18. Conversely, HER2 was not recommended as a biomarker until trastuzumab was incorporated into clinical practice19,20. Interestingly, the recently published ASCO guidelines propose evaluating HER2 status as the first step in managing early breast cancer21. In this study, we also applied HER2 status as the primary biomarker to identify the biological characteristics of tumors, revealing statistically significant results in patient survival.
Focusing the analysis on HER2 status clarified statistically significant results that are not always consistent in existing literature, particularly in the context of ER status. HER2 status appeared to be associated with conflicting results regarding high Bcl-2 status and patient survival, showing a significantly unfavorable prognosis in HER2-overexpressed tumors and vice versa. These discrepant results were not altered by ER status, suggesting that HER2 status could serve as a biomarker to determine whether Bcl-2 is associated with a favorable or unfavorable prognosis in breast cancer.
The inconsistent results regarding the role of Bcl-2 in the survival of patients with breast cancer underscore the importance of biomarkers. Biomarkers are measurable indicators of some biological state or condition22. In medicine, biomarkers are intended to measure characteristics of normal biological processes, pathogenic processes, or responses to exposure or interventions, including therapeutic interventions in the context of a disease of interest; thus, they provide valuable information that may reduce uncertainty in practice22,23. However, Bcl-2 has shown discrepant and inconsistent results in breast cancer, often indicating mixed outcomes or even favorable prognosis in ER-positive breast cancer. In this study, although high Bcl-2 expression did not demonstrate a significant survival difference in the general cohort, it showed statistical significance when patients were stratified based on HER2 status. This indicates its potential as a biomarker, particularly in HER2-positive breast cancer. Therefore, considering the distinct subtypes contributing to the heterogeneity of breast cancer, careful and appropriate patient selection, such as HER2-based categorization, is critical for the successful identification of relevant biomarkers.
The reasons behind the discrepant results of Bcl-2 activity based on HER2 status remain unclear. It is known that not all tumors with HER2-overexpressed biology respond successfully to HER2-targeting agents24. HER2-targeted therapies have significantly improved the prognosis of HER2-positive breast and gastric cancer. Interestingly, while HER2-targeting treatment in breast cancer requires a strict definition of HER2 overexpression, gastric cancer does not20,25,26. Moreover, HER2 overexpression and mutation are pathogenic drivers in non-small cell lung cancer and colorectal cancer; however, to date, no approved HER2-targeted therapies exist for these indications25. These variations in response to targeted agents suggest biological differences among and within tumors24. Therefore, the variability in downstream signaling pathways activated by Bcl-2 and HER2 may lead to different clinical manifestations, warranting further investigations in future studies.
This study has some limitations. First, its retrospective nature may introduce potential biases, such as selection bias and recall bias, which may affect the validity of the findings. We included 617 patients among 1,505 potential candidates, accounting for approximately 41% of all patients. A total of 888 patients were excluded due to insufficient data, primarily related to Bcl-2. Determining the reasons for data incompleteness on Bcl-2 through retrospective chart review is highly limited, making potential selection bias inevitable in this study. The retrospective design also makes it impossible to control for confounding variables, such as treatment variations, comorbid conditions, or lifestyle factors, which could influence patient outcomes and survival. Therefore, results found in this study should be confirmed in well-controlled future studies. Another bias arises from the nature of a single-center study. Conducting the study at a single institution may limit the generalizability of the results to other populations or settings. Despite the relatively large sample size (617 patients), certain subgroup analyses (e.g., based on HER2 and Bcl-2 status) might still be underpowered, potentially limiting the ability to detect significant differences, highlighting the necessity for confirmation in other cohorts. Additionally, changes in the thresholds for ER and PR positivity over the study period, mostly based on ASCO/CAP guidelines, could introduce inconsistencies in the classification of receptor status, affecting the study’s internal validity. Moreover, evolving treatment strategies, which heavily influence patient survival, have also changed over time. In summary, this study has exposed limitations primarily stemming from its retrospective design. However, we also believe that retrospective studies can inspire basic research to answer questions arising from clinical practice. We hope that follow-up studies can address the discrepant behavior of Bcl-2 in breast cancer, leading to the identification of appropriate patient groups suitable for Bcl-2-targeting agents.
In conclusion, HER2-based interpretation of Bcl-2 provided consistent and significant results in the context of survival of breast cancer patients. It underscores the importance of appropriate categorization, HER2-based in this case, for an adequate understanding of tumor biology. Cell signal transduction is a fundamental process in the development and progression of cancer27. While high Bcl-2 has yielded mixed or even discrepant results regarding survival in ER-positive breast cancer, leading to difficulties in interpreting the roles of Bcl-2 in breast cancer4,9,11, HER2-based analysis offered a clearer prognostic value. Therefore, incorporating HER2 status as a primary factor in analyzing Bcl-2 could enhance the accuracy of prognosis and potentially guide targeted therapies. Future studies should focus on further elucidating the molecular mechanisms underlying these interactions and validating these findings in larger, multi-center cohorts to ensure broader applicability and reliability of the results.
Supplementary Information
Acknowledgements
We acknowledge the courage of all patients in the fight against breast cancer. We would also like to thank Editage for their support with English editing.
Author contributions
Airi Han (A.H.) made concepts of this research. Taeyeong Kim (T .K.) and A.H set methodology. A.H set software programs. Seung Taek Lim (S.L.) and Jong-In Lee (J.L.) collected data. Hyangsuk Choi (H.C.), In-Jeong Cho (I.C.), and Hany Noh (H.N.) validated the results. Formal analysis was done by T.K. and A.H. Original draft was prepared by T.K. and A.H. Review and editing was done A.H. All figures and tables were prepared by T.K. and A.H. All authors reviewed the manuscript.
Funding
This research received no external funding.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of of Wonju Severance Christian Hospital, Wonju, Korea (CR323024).
Informed consent
Patient consent was waived due to retrospective nature of the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-83302-w.
References
- 1.Harbeck, N. & Gnant, M. Breast cancer. Lancet389, 1134–1150 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin.71, 209–249 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Lim, S. T. et al. Hounsfield units predict survival of patients with estrogen receptor-positive and human epithelial growth factor receptor 2-negative breast cancer. Clin. Breast Cancer23, e424–e433 (2023). [DOI] [PubMed] [Google Scholar]
- 4.Hwang, K. T. et al. Prognostic influences of BCL1 and BCL2 expression on disease-free survival in breast cancer. Sci. Rep.11, 11942 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu, K. D., Cai, Y. W., Wu, S. Y., Shui, R. H. & Shao, Z. M. Estrogen receptor-low breast cancer: Biology chaos and treatment paradox. Cancer Commun. (Lond)41, 968–980 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoder, R. et al. Impact of low versus negative estrogen/progesterone receptor status on clinico-pathologic characteristics and survival outcomes in HER2-negative breast cancer. NPJ Breast Cancer8, 80 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolff, A. C. et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol.31, 3997–4013 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Hammond, M. E. et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch. Pathol. Lab. Med.134, e48-72 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Eom, Y. H., Kim, H. S., Lee, A., Song, B. J. & Chae, B. J. BCL2 as a subtype-specific prognostic marker for breast cancer. J. Breast Cancer19, 252–260 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hellemans, P. et al. Prognostic value of bcl-2 expression in invasive breast cancer. Br. J. Cancer72, 354–360 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callagy, G. M. et al. Bcl-2 is a prognostic marker in breast cancer independently of the Nottingham Prognostic Index. Clin. Cancer Res.12, 2468–2475 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Hudis, C. A. et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J. Clin. Oncol.25, 2127–2132 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Li, X. et al. Uncovering the subtype-specific molecular characteristics of breast cancer by multiomics analysis of prognosis-associated genes, driver genes, signaling pathways, and immune activity. Front. Cell Dev. Biol.9, 689028 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li, Y., Tang, X. Q., Bai, Z. & Dai, X. Exploring the intrinsic differences among breast tumor subtypes defined using immunohistochemistry markers based on the decision tree. Sci. Rep.6, 35773 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardoso, F. et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up dagger. Ann. Oncol.30, 1194–1220 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Gradishar, W. J. et al. NCCN Guidelines(R) Insights: Breast Cancer, Version 4.2023. J. Natl. Compr. Cancer Netw.21, 594–608 (2023). [DOI] [PubMed] [Google Scholar]
- 17.Harris, L. N. et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol.34, 1134–1150 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andre, F. et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: ASCO clinical practice guideline update-integration of results from TAILORx. J. Clin. Oncol.37, 1956–1964 (2019). [DOI] [PubMed] [Google Scholar]
- 19.American Society of Clinical Oncology. 1997 update of recommendations for the use of tumor markers in breast and colorectal cancer. Adopted on November 7, 1997 by the American Society of Clinical Oncology. J. Clin. Oncol.16, 793–795 (1997). [DOI] [PubMed] [Google Scholar]
- 20.Bast, R. C. Jr. et al. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: Clinical practice guidelines of the American Society of Clinical Oncology. J. Clin. Oncol.19, 1865–1878 (2001). [DOI] [PubMed] [Google Scholar]
- 21.Loibl, S. et al. Early breast cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol.35, 159–182 (2024). [DOI] [PubMed] [Google Scholar]
- 22.Fidock, M. & DeSilva, B. Bioanalysis of biomarkers for drug development. Bioanalysis4, 2425–2426 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther.69, 89–95 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Swain, S. M., Shastry, M. & Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov.22, 101–126 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Indini, A., Rijavec, E. & Grossi, F. Trastuzumab Deruxtecan: Changing the destiny of HER2 expressing solid tumors. Int. J. Mol. Sci.22, 4774–4791 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allison, K. H. et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J. Clin. Oncol.38, 1346–1366 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: The next generation. Cell144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.