Abstract
Objective
The optimal timing of vasopressin initiation as an adjunctive vasopressor remains unclear. We aimed to study the association between the timing of vasopressin commencement, pre-specified physiological parameters, and hospital mortality.
Design
We conducted a multicentre, retrospective, observational study.
Setting
Twelve ICUs in Queensland, Australia between January 2015 and December 2021.
Participants
Adult patients with septic shock who received vasopressin as an adjunctive vasopressor within 72 hours of ICU admission.
Main Outcome
Hospital mortality.
Results
Overall, 2747 patients fulfilled the inclusion criteria. Of these, 1850 (67%) started vasopressin within six hours of vasopressor therapy start, while 897 (33%) started vasopressin between six hours and 72 hours. APACHE III score, peak lactate, and creatinine were higher in the early start group. Early vasopressin start was independently associated with decreased hospital mortality (aOR = 0.69, 95% CI = 0.57-0.83). Vasopressin infusion start was also associated with an immediate decrease in the noradrenaline-equivalent dose regardless of timing. There was a statistically significant favourable breakpoint at vasopressin start for the course of arterial pH, lactate, heart rate and crystalloid infusion rate (p<0.001).
Conclusions
In patients with septic shock, early adjunctive vasopressin initiation was independently associated with lower hospital mortality. Vasopressin starting at any time was also associated with reduced tachycardia, acidosis, and hyperlactatemia.
Keywords: Vasopressin, Sepsis, Shock, Hypotension, Vasodilation, Critical care, Intensive care unit
1. Introduction
Vasoactive drugs are amongst the most commonly prescribed medications for critically ill adult patients in intensive care units (ICU).1 Noradrenaline is the universally recommended first-line agent for septic shock patients2 and this is consistent with clinician practice globally.3 Vasopressin (arginine vasopressin – AVP or antidiuretic hormone – ADH), a nonapeptide hormone stored and released from the posterior pituitary gland, is also utilised around the world with broad consensus for its use as a second-line vasopressor.[2], [3], [4]
Questions remain around the timing of vasopressin initiation as a second-line vasopressor. The VASST5 and VANISH6 trials, the only large, multicentre, double-blind randomised controlled trials of adjunctive vasopressin administration in septic shock, found no significant difference in 28-day mortality. However, there were signals from these studies and subsequent post hoc and meta-analyses7,8 of potential benefit when commencing vasopressin in patients with less severe shock states (i.e. lower lactate levels, lower noradrenaline dose) and in the absence of established acute kidney injury. Acknowledging this, the most recent iteration of the Surviving Sepsis Campaign guidelines provided a weak recommendation for commencing vasopressin when the dose of noradrenaline is in the range of 0.25–0.5 μg kg−1.min−1.2 This dose range, however, is broad and ignores the duration of septic shock as a consideration for vasopressin initiation. Earlier initiation of an adjunctive non-catecholamine vasopressor may have complementary effects and warrants further investigation.15
Accordingly, we aimed to test the primary hypothesis that the timing of vasopressin infusion initiation would be associated with mortality in a cohort of patients with septic shock; and the secondary hypothesis, that the physiological changes seen after vasopressin start would also differ according to the timing of vasopressin initiation.
2. Methods
2.1. Study design
This was a multicentre, retrospective, observational study using routinely collected electronic medical record clinical data from twelve ICUs in Queensland, Australia.
2.2. Ethical considerations
The study was approved by the Metro South Hospital and Health Service Human Research Ethics Committee (HREC/2022/QMS/82024) with an individual waiver of consent granted.
2.3. Study sites and study population
The study sites comprised five tertiary, three outer metropolitan and four regional ICUs. The centres include most of the state-wide ICU capacity and include all the state-wide referral centres for cardiothoracic, neurosurgical, obstetric and trauma patients, as well as outer metropolitan and regional ICUs. We included all adult patients (age ≥18 years) admitted to the ICU who received vasopressin as an adjunctive vasopressor infusion between January 1st, 2015 and December 31st, 2021. We excluded all patients admitted post-operatively, those who did not have a diagnosis of septic shock, those who started vasopressin after 72 h from ICU admission, patients transferred from another participating ICU and those whose electronic medical records were not retrievable.
2.4. Data access and study population
We obtained hospital administrative data and intensive care data, such as patient characteristics, medications, vital sign observations, fluid balance, laboratory tests and therapies required, as well as patient-centred outcomes including mortality, from the clinical information systems eCritical MetaVision™ (iMDsoft, Boston, MA, USA), and the Australia and Zealand Intensive Care Society (ANZICS), Centre for Outcome and Resource Evaluation (CORE) and Adult Patient Database (APD). Data were stored in a password-protected file in a non-identifiable format.
2.5. Definitions
According to previously published conversion tables, vasopressors were converted to noradrenaline-equivalent doses.9,10 In accordance with the VANISH randomised clinical trial,6 we defined ‘early’ start of vasopressin as a continuous infusion started within 6 h of any vasopressor initiation. The ‘late’ start group was defined as a continuous infusion of vasopressin started between 6 and 72 h after any vasopressor initiation. The Sequential Organ Failure Assessment (SOFA) score11 and the Acute Physiology and Chronic Health Evaluation III (APACHE III) score12 as previously defined, were recorded. Sepsis was defined according to the Sepsis-3 consensus definition.13 Proven or suspected infection was defined as the commencement or escalation of antimicrobial therapy and microbiological sampling within 1 day of a SOFA score increase.23 Septic shock was defined as the administration of a vasopressor medication and at least one blood lactate greater than 2 mmol L−1 on the day of vasopressin start. When ‘day one’ is mentioned, it relates to the day of vasopressor commencement.
2.6. Outcomes
The primary outcome of this study was hospital mortality. Secondary outcomes related to the evolution of physiological parameters according to the timing of vasopressin commencement such as change in noradrenaline dose, arterial pH, creatinine, lactate and haemodynamic observations.
2.7. Statistical analysis
Continuous variables are reported as mean with standard deviation or median with interquartile range and categorical variables as absolute numbers and percentages. Logistic regression was used to assess the risk factors of hospital mortality. The variable selection was conducted using a LASSO regularisation. Numerical variables were standardised and categorical variables were one-hot encoded. The dataset was split into training test sets (80:20). The optimal lambda values were determined using 10-fold cross-validation. Model performance was assessed using accuracy. Assumptions were checked for significant outliers and multicollinearity in the final model. Type I error was adjusted by the false discovery rate method. As a sensitivity analysis, an entropy-balanced propensity score was used. A standardised mean difference (SMD) > 0.1 was considered a significant imbalance. We assessed the propensity score distribution before and after weighting to check its quality. For the exploratory physiological analysis, significant breakpoints and the parameters evolutions across time were searched with the Davies test. Statistical analyses were performed using R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria) with the packages ‘dplyr,’ ‘ggplot2′, ‘ggpubr,’ ‘tableone,’ ‘WeightIt,’ survey,’ ‘cobalt,’ ‘mice,’ ‘jskm,’ ‘glmnet,’ ‘caret,’ ‘broom’ and ‘segmented.'
2.8. Missing data management
To handle missing data within our dataset, we used multiple imputations to create ten plausible substitute datasets and combined their estimates in a way that maintained statistical power and reduced bias. The amount of missing data for key variables is shown in Supplementary Table S2.
3. Results
3.1. Baseline characteristics
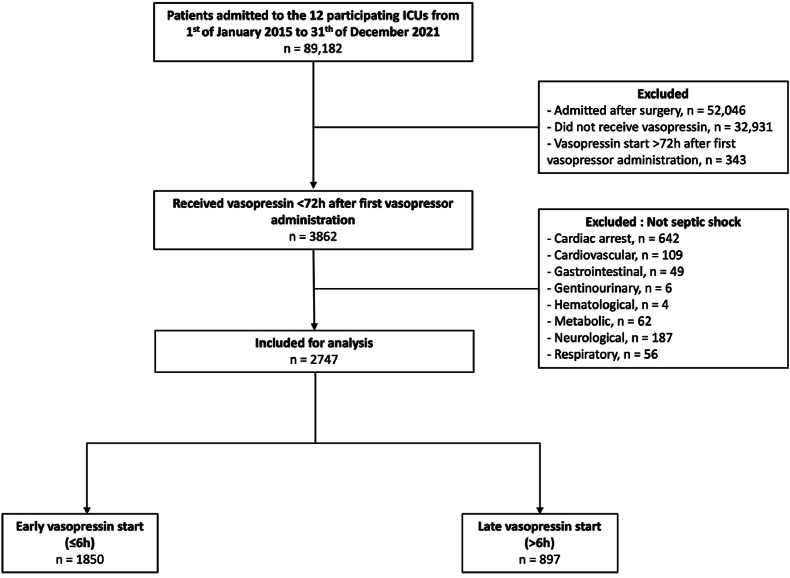
Within the 7-year study period, there were 89,182 admissions to the twelve ICUs. Among them, 2747 patients who presented with septic shock and received vasopressin within 72 h of vasopressor start were included for analysis (Fig. 1). The main baseline characteristics of each group are summarised in Table 1. Characteristics of septic shock patients who did not receive vasopressin are displayed in Supplementary Table S1.
Fig. 1.
Flow chart. ICU: Intensive care unit. Early vasopressin start refers to a start within 6h since the first vasopressor administration. Late vasopressin start refers to a start after 6h since the first vasopressor administration.
Table 1.
Baseline characteristic of the overall cohort according to the vasopressin start time.
| Overall (n = 2747) | Early start (n = 1850) | Late start (n = 897) | P value | |
|---|---|---|---|---|
| Demographic | ||||
| Age – yr | 60 (15) | 60 (15) | 60 (15) | |
| Sex female – n (%) | 1104 (40) | 734 (40) | 370 (41) | |
| Body mass index – kg.m−2 | 30.0 (8.8) | 29.5 (8.7) | 29.8 (9.0) | |
| Admission source – n (%) | ∗∗∗ | |||
| Emergency department | 1487 (54) | 1056 (57) | 431 (48) | |
| Ward | 809 (30) | 485 (26) | 324 (36) | |
| Other hospital | 225 (8) | 143 (8) | 82 (9) | |
| Other ICU | 226 (8) | 166 (9) | 60 (7) | |
| Comorbidities | ||||
| Respiratory – n (%) | 133 (5) | 82 (4) | 51 (6) | |
| Chronic heart failure – n (%) | 117 (4) | 83 (5) | 34 (4) | |
| End stage kidney disease – n (%) | 80 (3) | 41 (2) | 39 (4) | ∗∗ |
| Chronic liver disease – n (%) | 173 (6) | 122 (7) | 51 (6) | |
| Any immunosuppression – n (%) | 420 (15) | 305 (17) | 115 (13) | ∗∗∗ |
| Hemopathy – n (%) | 193 (7) | 147 (8) | 46 (5) | ∗∗∗ |
| Cancer with metastasis – n (%) | 72 (3) | 55 (3) | 17 (2) | |
| Prognostic scores | ||||
| APACHE III Score | 93 (29) | 95 (30) | 90 (28) | ∗∗∗ |
| APACHE III risk of death – % | 49 (27) | 50 (27) | 47 (26) | ∗∗∗ |
| Total SOFA score | 8 (3) | 8 (3) | 8 (3) | |
| Biological parameters | ||||
| Max serum creatinine at day 1 – μmol.L−1 | 202 (156) | 206 (153) | 196 (161) | . |
| Max white count cells at day 1 – x109.L−1 | 18 (15) | 18 (14) | 17 (17) | |
| Max serum lactate at day 1 – mmol.L−1 | 5.1 (4.6) | 5.5 (4.8) | 4.3 (4.2) | ∗∗∗ |
| Minimum pH at day 1 | 7.23 (0.15) | 7.22 (0.15) | 7.26 (0.14) | ∗∗∗ |
| Max bilirubin at day1 — μmol.L−1 | 43 (68) | 45 (68) | 41 (65) | . |
| Organ support | ||||
| Hydrocortisone – n (%) | 1799 (66) | 1247 (67) | 552 (62) | ∗∗ |
| Vasopressin start time – median (Q1-Q3), hr | 3 (0–9) | 1 (0–3) | 15 (9–26) | ∗∗∗ |
| Noradrenaline equivalent dose at vasopressin start time – μg.kg−1.min−1 | 0.26 (0.21) | 0.26 (0.22) | 0.24 (0.19) | ∗∗ |
| Max noradrenaline equivalent dose at day one – μg.kg−1.min−1 | 0.29 (0.29) | 0.34 (0.31) | 0.20 (0.21) | ∗∗∗ |
| Noradrenaline – n (%) | 2739 (99) | 1843 (99) | 896 (99) | |
| Adrenaline – n (%) | 863 (31) | 634 (34) | 229 (26) | ∗∗∗ |
| Dopamine – n (%) | 99 (4) | 60 (3) | 39 (4) | . |
| Phenylephrine – n (%) | 25 (1) | 12 (1) | 13 (1) | . |
| Metaraminol – n (%) | 817 (30) | 468 (25) | 349 (39) | ∗∗∗ |
| Dobutamine – n (%) | 355 (13) | 201 (11) | 154 (17) | ∗∗∗ |
| Milrinone – n (%) | 144 (5) | 103 (6) | 41 (5) | |
| Invasive ventilation at day 1 – n (%) | 1938 (71) | 1280 (69) | 658 (73) | ∗ |
| Renal replacement therapy at day 1 – n (%) | 97 (4) | 73 (4) | 24 (3) | ∗ |
| Anti-infective therapy | ||||
| Anti-infective agents – n, median (Q1-Q3) | 2 (1–3) | 2 (1–3) | 2 (2–3) | |
| Penicillin – n (%) | 1537 (60) | 987 (53) | 550 (63) | ∗ |
| Carbapenem | 786 (31) | 555 (30) | 231 (27) | ∗∗ |
| Aminoglycoside | 267 (10) | 178 (10) | 89 (10) | |
| Glycopeptide | 1019 (40) | 695 (38) | 324 (36) | . |
Continuous values are presented as mean (SD) unless otherwise specified.
Early start: ≤6h after noradrenaline start, Late start: >6h after noradrenaline start.
ICU: Intensive care unit; AIDS: Acquired immunodeficiency syndrome; APACHE: Acute Physiology and Chronic Health Evaluation, SOFA: Sequential Organ Failure Assessement.
P value: 0 ‘∗∗∗’ 0.001 ‘∗∗’ 0.01 ‘∗’ 0.05 ‘.’ 0.1 ‘’ 1.
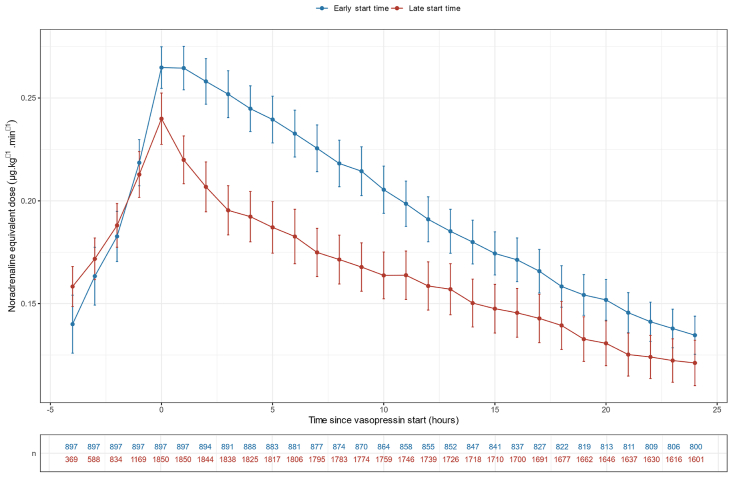
The overall cohort comprised 1643 (60%) males and 1104 (40%) females with a mean age of 60. More than half of the cohort was admitted from the emergency department (n = 1487, 54%) and one-quarter from the wards (n = 809, 30%). Most patients were invasively ventilated (n = 1938, 71%) and 4% received continuous renal replacement therapy on day one of vasopressor start. In total, 1850 (67%) started vasopressin within 6 h from vasopressor start, while 897 (33%) started between 6 h and 72 h. The median (Q1 – Q3) time from vasopressor start to vasopressin infusion was 1 (0–3) h in the early start group and 15 (9–26) hours in the late start group. Vasopressin was administered for a median duration of 2 (1–4) days at a starting dose of 0.04 (0.02–0.04) units/min−1. The mean noradrenaline equivalent infusion dose at vasopressin start was 0.26 ± 0.22 μg kg−1.min−1 in the early start group versus 0.24 ± 0.19 μg kg−1.min−1 in the late start group (Fig. 2). The APACHE III score was higher in the early start group (95 ± 30 vs 90 ± 28, p < 0.001) as was the day one peak lactate (5.5 ± 4.8 vs 4.3 ± 4.2 mmol L−1, p < 0.001).
Fig. 2.
Noradrenaline equivalent dose evolution in the overall population according to vasopressin start.
3.2. The association between vasopressin timing and mortality
Hospital mortality in the early start group was 35%, compared with 40% in the late start group. Variables associated with hospital mortality by unadjusted logistic regression are presented in Supplementary Table S3. There was no interaction between the vasopressin timing start and steroid use (Supplementary Table S4). No site-specific effect was detected across the twelve ICUs (Supplemental Table S5). Among the 31 variables included for analysis, the LASSO regression identified seven variables for computing the multivariable logistic regression: APACHE III score (aOR = 1.03, 95% CI 1.02–1.03), peak lactate on day 1 (aOR = 1.12, 95% CI = 1.10–1.15), invasive ventilation on day 1 (aOR 1.30, 95% CI = 1.07–1.59), chronic respiratory disease (aOR = 2.17, 95% CI = 1.48–3.18) and chronic liver disease (aOR = 2.12, 95% CI = 1.48–3.05). These variables were independently associated with increased hospital mortality. In contrast, early vasopressin start was associated with decreased hospital mortality (aOR = 0.69, 95% CI = 0.57–0.83) (Table 2, Supplementary Figs. S1 and S2).
Table 2.
Factors associated with hospital mortality by multivariable logistic regression in the overall population.
| aOR | CI 95% | padj | |
|---|---|---|---|
| Chronic respiratory disease | 2.17 | 1.48–3.18 | <0.001 |
| APACHE III score (per point) | 1.03 | 1.02–1.03 | <0.001 |
| Peak lactate at day 1 (per mmol.L−1) | 1.12 | 1.10–1.15 | <0.001 |
| Vasopressin early start groupa | 0.69 | 0.57–0.83 | <0.001 |
| Invasively ventilated at day 1 | 1.30 | 1.07–1.59 | 0.008 |
| Hydrocortisone | 0.86 | 0.72–1.04 | 0.117 |
| Chronic liver disease | 2.12 | 1.48–3.05 | <0.001 |
a OR: Adjusted Odds Ratio, CI 95%: 95% Confidence Interval, padj: p value adjusted by false discovery rate method, APACHE: Acute Physiology and Chronic Health Evaluation.
Early start group refers to patients who received vasopressin within 6h from vasopressor start.
The entropy balanced propensity score accounting for differences between early vs late vasopressin start also showed an association between early start and decreased hospital mortality (OR = 0.65, 95% CI = 0.50–0.95) (Supplementary Tables S6 and S7, Supplementary Figs. S3 and S4). The sensitivity analysis performed without patients admitted from other ICUs showed similar results (Supplementary Table S8). Analysing the delay from vasopressor start to vasopressin start as a continuous variable showed consistent results regarding hospital mortality (aOR 1.02, 95% CI = 1.02–1.03 for each hour's delay) (Supplementary Table S9,Supplementary Fig. S5).
3.3. Physiological changes after vasopressin start
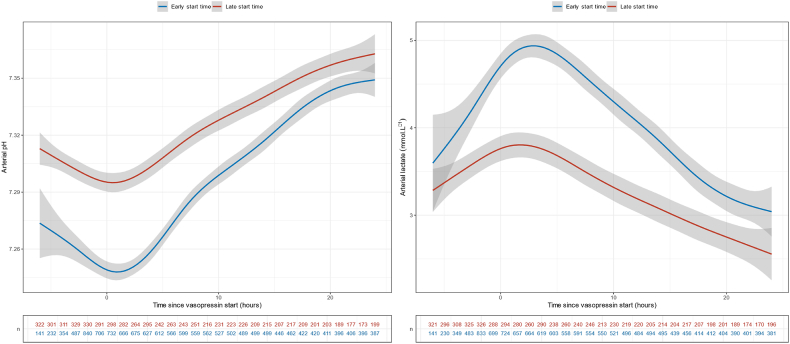
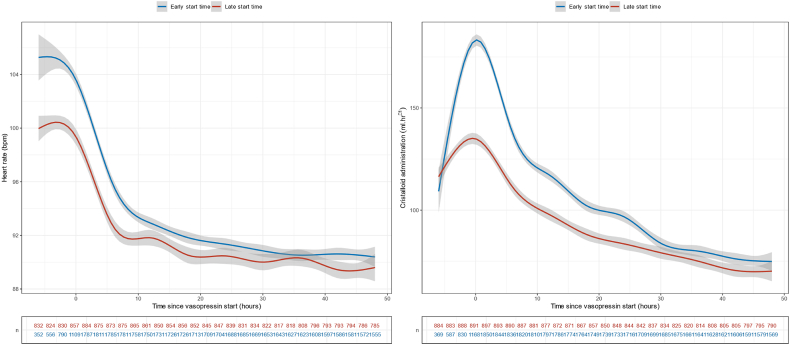
The start of vasopressin infusion was associated with an immediate decrease in noradrenaline-equivalent dose. This change was not affected by the timing of vasopressin start nor by the noradrenaline-equivalent dose at vasopressin start (Fig. 2). There was also a statistically significant favourable turning point at vasopressin infusion start time for arterial pH, lactate, heart rate and crystalloid infusion rate (p < 0.001) (Fig. 3, Fig. 4, Supplementary Table S10). No such turning point was found for urine output (Supplementary Table S10, Supplementary Fig. S6).
Fig. 3.
Arterial pH and lactate evolution across time according to the vasopressin start time.
Fig. 4.
Arterial heart rate and crystalloid administration rate evolution across time according to the vasopressin start time.
4. Discussion
4.1. Key findings
In this study, we found that patients with septic shock commenced on a vasopressin infusion had lower hospital mortality if commenced within 6 h of vasopressor start compared with those who commenced vasopressin after 6 h. Time to adjunctive vasopressin initiation was also independently associated with hospital mortality and this association was robust to entropy balanced propensity score assessment accounting for differences between early vs late vasopressin start. Moreover, patients in our study cohort were twice as likely to be administered vasopressin early and there was a clear separation between the two groups of more than 12 h in the time to vasopressin commencement. Finally, vasopressin was associated with an immediate noradrenaline-sparing effect and a rapid reduction in acidemia, hyperlactatemia, tachycardia and use of crystalloid fluids regardless of the timing of initiation.
4.2. Relationship to previous studies
Prior to this study, there was limited data on the timing of initiation of adjunctive vasopressin therapy. In the VASST trial, the mean time to commencement of vasopressin was nearly 12 h after reaching a noradrenaline inclusion threshold of 5 μg min−1 with a mean noradrenaline dose of 0.26 μg kg−1.min−1 and serum lactate was 3.5 mmol L−1 at the time of vasopressin start. In the VANISH trial, the median time to commencement of vasopressin from the onset of shock was 3.5 h with a lower mean noradrenaline dose of 0.15 μg kg−1.min−1 and serum lactate of 2.3 mmol L−1 at randomisation. In our study, there was an earlier median vasopressin start time in the ‘early’ group at 1 h and a higher mean noradrenaline dose of 0.27 μg kg−1.min−1 and serum lactate of 5.5 mmol L−1. It is plausible that this earlier timepoint of intervention in a critically ill patient's shock state may impact mortality despite the greater severity of shock. The association between early vasopressin and decreased mortality was with a starting dose of 0.04 units/min−1 compared with 0.03 units/min−1 in the VASST study and 0.06 units/min−1 in the VANISH study. Mortality in our ‘early’ cohort was similar to that of the VANISH study (35% compared with 33%). However, it is important to note that our study only included patients who received adjunctive vasopressin. Therefore, in this cohort of patients where clinicians deemed the addition of adjunctive vasopressin necessary, earlier initiation appeared to result in lower hospital mortality.
Our findings complement another recent, large retrospective study on adjunctive vasopressin use in 1610 septic shock patients. Sacha et al.14 reported no independent association between hospital mortality and timing of vasopressin initiation in relation to shock onset but rather a significant association between increasing lactate concentration and higher noradrenaline-equivalent dose and mortality. Our study supports the association of higher lactate levels and noradrenaline-equivalent doses with higher hospital mortality. Our contrasting findings relating to the timing of vasopressin initiation may be explained by the differing median time from shock onset to vasopressin administration (5 h in Sacha's study). Furthermore, in the study by Sacha et al., hospital mortality was very high at 59% suggesting a sicker cohort of patients being selected for adjunctive vasopressin administration.
Other, smaller observational studies analysing the timing of vasopressin initiation and restricted to cohorts of patients who received adjunctive vasopressin, have been similarly promising15 for different thresholds of ‘early’ initiation. Initiation within three,16 four17 and seven18 hours of shock onset were all associated with decreases in time to shock resolution, ICU length of stay, ICU mortality and hospital mortality.
The physiological changes seen after vasopressin start, as demonstrated in our study, are consistent with the literature. Open-label randomised controlled studies of adjunctive vasopressin use have shown similar reductions in noradrenaline dose, lactate, acidosis and heart rate to those achieved in our study.[19], [20], [21], [22] These do not appear to be time-dependent physiological improvements. Whether earlier normalisation of these physiological parameters, such as metabolic acidosis,24 or relative reduction in catecholamine use accounts for lower patient mortality is hypothesis generating.
4.3. Implications of study findings
To our knowledge, this is the largest cohort study of adjunctive vasopressin use in critically ill patients with septic shock. This study provides hypothesis-generating evidence to support the safety and possible benefit of earlier and near-immediate initiation of vasopressin in patients presenting with septic shock. Moreover, the fact that these findings persisted even after accounting for differences in the timing of initiation with entropy-balanced propensity scoring suggests statistical and clinical robustness.
Importantly, three-quarters of the patients in the ‘early’ start group received adjunctive vasopressin within the first 3 h of vasopressor support. Furthermore, it would appear, that, in the Australian context, clinicians are twice as likely to utilise vasopressin early for severe septic shock patients and at a lower noradrenaline threshold than that recommended in the Surviving Sepsis Campaign guidelines.2 This earlier timeframe of initiation, not previously described in the literature, may be used as a key timing threshold for intervention in future prospective studies of adjunctive vasopressin support in septic shock.
4.4. Strengths and limitations
This study had several strengths. Firstly, the cohort was sampled from a large, comprehensive ICU patient database that covers nearly all ICU patient admissions in the third most populous state in Australia. This population is representative of the general Australian population and likely generalisable to other high-income countries. Secondly, our highly granular study data was electronically extracted from a clinical information system in daily clinical use throughout most of the state. All data collected were clinically validated and there were minimal missing data points. Finally, there was a clear separation of more than 12 h between the ‘early’ and ‘late’ start groups with regard to the median time of vasopressin initiation. This is a clinically important time difference and offers practical timing thresholds for prospective randomised studies in this area.
We acknowledge some limitations. First, this is an observational study with the inherent limitations of such studies. In particular, no causal inferences can be drawn from its findings and all associations described are only hypothesis-generating. Second, we do not have data to explain why patients commenced vasopressin early or late. Thus, there may be confounding by indication. However, the APACHE score, the lactate level and the dose of norepinephrine at vasopressin start were higher in the earlier group suggesting greater illness severity in such patients. Thus, greater illness severity may have been a key driver of early vasopressin initiation. If this were the case, our findings of benefit despite greater illness severity would reinforce the argument for an early start. Importantly, our findings remained after entropy balancing suggesting a degree of robustness to selection bias. Finally, we acknowledge patients who received ‘late’ vasopressin may have been a cohort who failed to respond to initial therapy and their admission APACHE score may not be representative of their subsequent clinical trajectory. However, the majority of patients included in this study received vasopressin within the first 24 h of ICU admission and their baseline biological parameters likely still closely reflect their severity of illness at the time of vasopressin initiation.
5. Conclusion
In this large, multicentre, retrospective observational study, we found that earlier timing of adjunctive vasopressin initiation for patients with septic shock was independently associated with decreased hospital mortality. Vasopressin initiation was also associated with decreased noradrenaline dose, reduced tachycardia, less acidemia and falling lactate levels regardless of the timing of initiation. Finally, after propensity adjustment, patients initiated on vasopressin within 6 h of vasopressor infusion start had lower hospital mortality. These findings provide hypothesis-generating evidence to support the safety and possible benefit of earlier initiation of vasopressin in patients presenting with septic shock and the rationale and key information for the design of an interventional trial of early vasopressin therapy in septic shock.
Data availability
Data cannot be shared publicly due to institutional ethics, privacy and confidentiality regulations. Data released for the purposes of research under section 280 of the Public Health Act 2005 requires an application to the Director-General of Queensland Health (PHA@health.qld.gov.au).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
The study conception and design (all authors); data acquisition (KW); analysis (AC, KW); interpretation of data (all authors); article drafting (KW, RCP, AC), article revision for important intellectual content (all authors); final approval of the version submitted for publication (all authors); agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgements
The authors thank the ANZICS CORE management committee and the clinicians, data collectors and researchers at the following contributing sites: Caboolture Hospital, Cairns Hospital, Gold Coast University Hospital, Logan Hospital, Mackay Base Hospital, Princess Alexandra Hospital, Redcliffe Hospital, Rockhampton Hospital, Royal Brisbane and Women's Hospital, Sunshine Coast University Hospital, The Prince Charles Hospital, and The Townsville Hospital.
Collaborators - Queensland Critical Care Research Network Group.
Mahesh Ramanan, Prashanti Marella, Patrick Young, Pip McIlroy, Ben Nash, James McCullough, Kerina J Denny, Mandy Tallott, Andrea Marshall, David Moore, Hayden White, Sunil Sane, Aashish Kumar, Lynette Morrison, Pam Dipplesman, Jennifer Taylor, Stephen Luke, Anni Paasilahti, Ray Asimus, Kyle White, Jason Meyer, Rod Hurford, Meg Harward, James Walsham, Neeraj Bhadange, Wayne Stevens, Kevin Plumpton, Sainath Raman, Andrew Barlow, Alexis Tabah, Hamish Pollock, Stuart Baker, Kylie Jacobs, Antony G. Attokaran, David Austin, Jacobus Poggenpoel, Josephine Reoch, Kevin B. Laupland, Felicity Edwards, Tess Evans, Jayesh Dhanani, Marianne Kirrane, Pierre Clement, Nermin Karamujic, Paula Lister, Vikram Masurkar, Peter Garrett, Lauren Murray, Jane Brailsford, Todd Erbacher, Kiran Shekar, Jayshree Lavana, George Cornmell, Siva Senthuran, Stephen Whebell, Michelle Gatton, Robert Andrews, Sam Keogh.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ccrj.2024.09.002.
Contributor Information
Kyle C. White, Email: kyle.white@health.qld.gov.au.
Queensland Critical Care Research Network (QCCRN):
Mahesh Ramanan, Prashanti Marella, Patrick Young, Pip McIlroy, Ben Nash, James McCullough, Kerina J. Denny, Mandy Tallott, Andrea Marshall, David Moore, Hayden White, Sunil Sane, Aashish Kumar, Lynette Morrison, Pam Dipplesman, Jennifer Taylor, Stephen Luke, Anni Paasilahti, Ray Asimus, Kyle White, Jason Meyer, Rod Hurford, Meg Harward, James Walsham, Neeraj Bhadange, Wayne Stevens, Kevin Plumpton, Sainath Raman, Andrew Barlow, Alexis Tabah, Hamish Pollock, Stuart Baker, Kylie Jacobs, Antony G. Attokaran, David Austin, Jacobus Poggenpoel, Josephine Reoch, Kevin B. Laupland, Felicity Edwards, Tess Evans, Jayesh Dhanani, Marianne Kirrane, Pierre Clement, Nermin Karamujic, Paula Lister, Vikram Masurkar, Peter Garrett, Lauren Murray, Jane Brailsford, Todd Erbacher, Kiran Shekar, Jayshree Lavana, George Cornmell, Siva Senthuran, Stephen Whebell, Michelle Gatton, Robert Andrews, and Sam Keogh
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Russell J.A., Gordon A.C., Williams M.D., Boyd J.H., Walley K.R., Kissoon N. Vasopressor therapy in the intensive care unit. Semin Respir Crit Care Med. 2021;42(1):59–77. doi: 10.1055/s-0040-1710320. [DOI] [PubMed] [Google Scholar]
- 2.Evans L., Rhodes A., Alhazzani W., Antonelli M., Coopersmith C.M., French C., et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–1247. doi: 10.1007/s00134-021-06506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheeren T.W.L., Bakker J., De Backer D., Annane D., Asfar P., Boerma E.C., et al. Current use of vasopressors in septic shock. Ann Intensive Care. 2019;9(1):20. doi: 10.1186/s13613-019-0498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vail E.A., Gershengorn H.B., Hua M., Walkey A.J., Wunsch H. Epidemiology of vasopressin use for adults with septic shock. Ann Am Thorac Soc. 2016;13(10):1760–1767. doi: 10.1513/AnnalsATS.201604-259OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell J.A., Walley K.R., Singer J., Gordon A.C., Hébert P.C., Cooper D.J., et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358(9):877–887. doi: 10.1056/NEJMoa067373. [DOI] [PubMed] [Google Scholar]
- 6.Gordon A.C., Mason A.J., Thirunavukkarasu N., Perkins G.D., Cecconi M., Cepkova M., et al. Effect of early vasopressin vs norepinephrine on kidney failure in patients with septic shock: the VANISH randomized clinical trial. JAMA. 2016;316(5):509–518. doi: 10.1001/jama.2016.10485. [DOI] [PubMed] [Google Scholar]
- 7.Gordon A.C., Russell J.A., Walley K.R., Singer J., Ayers D., Storms M.M., et al. The effects of vasopressin on acute kidney injury in septic shock. Intensive Care Med. 2010;36(1):83–91. doi: 10.1007/s00134-009-1687-x. [DOI] [PubMed] [Google Scholar]
- 8.Nagendran M., Russell J.A., Walley K.R., Brett S.J., Perkins G.D., Hajjar L., et al. Vasopressin in septic shock: an individual patient data meta-analysis of randomised controlled trials. Intensive Care Med. 2019;45(6):844–855. doi: 10.1007/s00134-019-05620-2. [DOI] [PubMed] [Google Scholar]
- 9.Goradia S., Sardaneh A.A., Narayan S.W., Penm J., Patanwala A.E. Vasopressor dose equivalence: a scoping review and suggested formula. J Crit Care. 2021;61:233–240. doi: 10.1016/j.jcrc.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 10.See E.J., Chaba A., Spano S., Maeda A., Clapham C., Liu J., et al. Exploring the norepinephrine to angiotensin II conversion ratio in patients with vasodilatory hypotension: a post-hoc analysis of the ARAMIS trial. J Crit Care. 2024;79 doi: 10.1016/j.jcrc.2023.154453. [DOI] [PubMed] [Google Scholar]
- 11.Vincent J.L., de Mendonça A., Cantraine F., Moreno R., Takala J., Suter P.M., et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Knaus W.A., Wagner D.P., Draper E.A., Zimmerman J.E., Bergner M., Bastos P.G., et al. The Apache III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 13.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacha G.L., Lam S.W., Wang L., Duggal A., Reddy A.J., Bauer S.R. Association of catecholamine dose, lactate, and shock duration at vasopressin initiation with mortality in patients with septic shock. Crit Care Med. 2022;50(4):614–623. doi: 10.1097/CCM.0000000000005317. [DOI] [PubMed] [Google Scholar]
- 15.Sacha G.L., Bauer S.R. Optimizing vasopressin use and initiation timing in septic shock: a narrative review. Chest. 2023;164(5):1216–1227. doi: 10.1016/j.chest.2023.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brask A.L., Shemanski S.M., Barnes T.E., Holmes A.K. Timing of vasopressin addition to norepinephrine and efficacy outcomes in patients with septic shock. Ann Pharmacother. 2023;57(5):521–526. doi: 10.1177/10600280221118903. [DOI] [PubMed] [Google Scholar]
- 17.Jakowenko N.D., Murata J., Kopp B.J., Erstad B.L. Influence of timing and catecholamine requirements on vasopressin responsiveness in critically ill patients with septic shock. J Intensive Care Med. 2022;37(11):1512–1519. doi: 10.1177/08850666221081836. [DOI] [PubMed] [Google Scholar]
- 18.Rydz A.C., Elefritz J.L., Conroy M., Disney K.A., Miller C.J., Porter K., et al. Early initiation of vasopressin reduces organ failure and mortality in septic shock. Shock. 2022;58(4):269–274. doi: 10.1097/SHK.0000000000001978. [DOI] [PubMed] [Google Scholar]
- 19.Patel B.M., Chittock D.R., Russell J.A., Walley K.R. Beneficial effects of short-term vasopressin infusion during severe septic shock. Anesthesiology. 2002;96(3):576–582. doi: 10.1097/00000542-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Dünser M.W., Mayr A.J., Ulmer H., Knotzer H., Sumann G., Pajk W., et al. Arginine vasopressin in advanced vasodilatory shock: a prospective, randomized, controlled study. Circulation. 2003;107(18):2313–2319. doi: 10.1161/01.CIR.0000066692.71008.BB. [DOI] [PubMed] [Google Scholar]
- 21.Torgersen C., Dünser M.W., Wenzel V., Jochberger S., Mayr V., Schmittinger C.A., et al. Comparing two different arginine vasopressin doses in advanced vasodilatory shock: a randomized, controlled, open-label trial. Intensive Care Med. 2010;36(1):57–65. doi: 10.1007/s00134-009-1630-1. [DOI] [PubMed] [Google Scholar]
- 22.Barzegar E., Ahmadi A., Mousavi S., Nouri M., Mojtahedzadeh M. The therapeutic role of vasopressin on improving lactate clearance during and after vasogenic shock: microcirculation, is it the black box? Acta Med Iran. 2016;54(1):15–23. [PubMed] [Google Scholar]
- 23.White K.C., Serpa-Neto A., Hurford R., Clement P., Laupland K.B., See E., et al. Sepsis-associated acute kidney injury in the intensive care unit: incidence, patient characteristics, timing, trajectory, treatment, and associated outcomes. A multicenter, observational study. Intensive Care Med. 2023;49(9):1079–1089. doi: 10.1007/s00134-023-07138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mochizuki K., Fujii T., Paul E., Anstey M., Pilcher D.V., Bellomo R. Early metabolic acidosis in critically ill patients: a binational multicentre study. Crit Care Resusc. 2021;23(1):67–75. doi: 10.51893/2021.1.OA6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data cannot be shared publicly due to institutional ethics, privacy and confidentiality regulations. Data released for the purposes of research under section 280 of the Public Health Act 2005 requires an application to the Director-General of Queensland Health (PHA@health.qld.gov.au).