Abstract
Purpose
This meta-analysis aimed to determine the efficacy and safety of antioxidant supplementation for treating erectile dysfunction (ED).
Materials and Methods
We systematically searched MEDLINE, Embase, and the Cochrane Library for double-blind, randomized, placebo-controlled trials of oral antioxidant supplementation in men with ED. Erectile function was assessed by the International Index of Erectile Function-Erectile Function domain (IIEF-EF) score. Using random-effects meta-analysis models, antioxidant and placebo groups were compared for erectile function using the mean difference in IIEF-EF score adjusted to a 6–30 scale and for side effects using the log risk ratio.
Results
The review included 23 trials of 1,583 men (median age 51 years) treated with antioxidant supplementation or placebo for a median of 12 weeks (range, 4 weeks to 6 months). Antioxidant supplementation significantly improved erectile function compared to placebo, with a mean difference of 5.5 points (95% confidence interval [CI]: 3.7 to 7.3; p<0.001) on the IIEF-EF. In meta-regression, the treatment benefit was greater in men with more severe ED (p<0.001). Side effects were uncommon, none were serious, and the frequency was comparable between antioxidant (3.8%) and placebo (2.1%) groups (log risk ratio=0.36; 95% CI: -0.24 to 0.97; p=0.24).
Conclusions
Antioxidant supplementation appears safe and significantly improves erectile function in men with ED, particularly those with more severe symptoms. Limitations of this review included unknown long-term efficacy and safety and the inability to make specific product and dosing recommendations due to the variety of antioxidants and regimens studied.
Keywords: Antioxidants, Erectile dysfunction, Meta-analysis, Randomized controlled trial, Systematic review
INTRODUCTION
Erectile dysfunction (ED) is the inability to attain and/or maintain penile erection sufficient for satisfactory sexual performance [1]. ED is highly prevalent globally, with projections estimating a worldwide prevalence of approximately 322 million men by 2025 [2]. The prevalence of ED increases with age, rising from 2%–9% in men aged 40 to 49 years to 20%–40% in men aged 60 to 69 years and 50%–100% in men over 70 years [3,4,5,6]. Lifestyle modifications including regular exercise, dietary modifications, and smoking cessation are often recommended as initial approaches to managing ED. Additional treatment options include oral phosphodiesterase type 5 (PDE5) inhibitors, vacuum erection devices, intracavernosal injections, and penile prostheses [7].
Endothelial dysfunction has been strongly implicated in the pathogenesis of ED [8]. The endothelium regulates erectile function by releasing nitric oxide (NO) and other vasoactive agents, promoting vasodilation, relaxing cavernosal smooth muscle, and facilitating penile erection. Oxidative stress is characterized by excess reactive oxygen species that overwhelm endogenous antioxidant defenses and significantly contribute to endothelial dysfunction in ED [9]. Specifically, oxidative stress disrupts NO synthase enzymes involved in NO production. This disruption of NO signaling leads to reduced NO bioavailability and impaired vasodilation, which interferes with the erectile response [8].
Antioxidants may counteract oxidative stress and improve erectile function through several hypothesized mechanisms. These include neutralizing free radicals, inhibiting pro-inflammatory cytokines and mediators, improving NO bioavailability, and enhancing endothelial-dependent vasodilation [10]. While individual antioxidants have differing molecular structures, their shared mechanism of action in targeting oxidative stress-induced endothelial dysfunction provides a rationale for evaluating them as a therapeutic class [11]. However, interpreting results from previous systematic reviews on the effect of antioxidants on ED has been challenging because of the inclusion of studies utilizing concurrent ED therapies or unblinded control groups [12,13]. This systematic review and meta-analysis aimed to determine the efficacy and safety of antioxidant supplementation in treating ED by evaluating double-blind, randomized, placebo-controlled trials, thus minimizing confounding factors. A secondary aim was to evaluate the association of treatment outcomes with patient characteristics and study design factors.
MATERIALS AND METHODS
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [14].
1. Search strategy
Two reviewers (LM, DF) systematically searched MEDLINE, Embase, and the Cochrane Library from inception to August 2023 using a comprehensive search strategy combining study design-, intervention-, and diagnosis-related search terms (see Table 1 for a complete list of antioxidants included in the review). Supplemental manual searches of the Directory of Open Access Journals, Google Scholar, and reference lists of relevant studies and review articles were also performed. The reviewers independently screened titles, abstracts, and full-text articles, resolving discrepancies through discussion at each stage. Studies were included if they were double-blind, true- or pseudo-randomized trials comparing non-prescription oral antioxidant supplementation to placebo for ED treatment in adult men. Studies were excluded if they were published in non-English journals, lacked International Index of Erectile Function-Erectile Function (IIEF-EF) domain score data, involved recent or concurrent PDE5 inhibitor or extracorporeal shockwave therapy, used hormone-modulating supplements, employed on-demand therapy, were published as abstracts only, or were duplicate publications.
Table 1. MEDLINE search strategya.
| Design search terms | |
| 1. Control* | |
| 2. Random* | |
| Intervention search terms | |
| 3. Acetylcysteine | |
| 4. Alpha-tocopherol | |
| 5. Alpha-tocotrienol | |
| 6. Antioxidant* | |
| 7. Arginine | |
| 8. Ascorb* | |
| 9. Astaxanthin | |
| 10. Bilberry extract | |
| 11. Carnitine | |
| 12. Carotenoid* | |
| 13. Coenzyme Q10 | |
| 14. Co enzyme Q10 | |
| 15. CoQ 10 | |
| 16. Curcumin | |
| 17. Flavonoid* | |
| 18. Folacin | |
| 19. Folate | |
| 20. Folic acid | |
| 21. Folvite | |
| 22. Ginseng | |
| 23. Glutathione | |
| 24. Green tea | |
| 25. L-acetylcarnitine | |
| 26. L-arginine | |
| 27. L-carnitine | |
| 28. L-citrulline | |
| 29. Levocarnitine | |
| 30. Lutein | |
| 31. Lycopene | |
| 32. Melatonin | |
| 33. N-acetylcysteine | |
| 34. Polyphenol* | |
| 35. Pomegranate | |
| 36. Pycnogenol | |
| 37. Quercetin | |
| 38. Radical scavenger | |
| 39. Resveratrol | |
| 40. Selenium | |
| 41. Ubiquinone | |
| 42. Vitamin C | |
| 43. Vitamin E | |
| 44. Zinc | |
| Diagnosis search terms | |
| 45. Androgen deficien* | |
| 46. ED | |
| 47. Erectile dysfunction | |
| 48. Erectile function | |
| 49. Hypogonadism | |
| 50. IIEF | |
| 51. Impotence | |
| 52. International Index of Erectile Function | |
| 53. Sexual dysfunction | |
| 54. Sexual function | |
| Combination terms | |
| 55. or/1–2 | |
| 56. or/3–44 | |
| 57. or/45–54 | |
| 58. and/55–57 | |
aThe ‘*’ represents a wildcard symbol used in a search query to represent end truncation.
2. Outcomes
The reviewers used piloted data collection forms to independently extract metadata, patient characteristics, study characteristics, treatment regimens, erectile function data, and side effects for each study. Erectile function was measured with the IIEF-EF, a validated questionnaire used to assess erectile function in men, scored from 5 to 25 (IIEF-EF-5) or 6 to 30 (IIEF-EF-6), with higher scores indicating better erectile function [15]. We standardized all IIEF-EF scores to a 6 to 30 scale to ensure consistent results across trials. IIEF-EF scores of 26 to 30 indicated no ED, 22 to 25 indicated mild ED, 17 to 21 indicated mild to moderate ED, 11 to 16 indicated moderate ED, and 6 to 11 indicated severe ED [16]. The frequency of self-reported side effects was compared between antioxidant and placebo groups, and categorized by seriousness. The risk of bias in each study was assessed using the Cochrane Collaboration tool [17].
3. Statistical analysis
We performed a random-effects meta-analysis using restricted maximum likelihood estimation to calculate the mean difference in IIEF-EF scores between the antioxidant and placebo groups. A positive mean difference indicated that the antioxidant group had higher IIEF-EF scores, while a negative value indicated higher scores in the placebo group. The frequency of side effects between groups was compared using the log risk ratio (logRR) statistic. A positive logRR indicated that the antioxidant group had a higher rate of side effects, while a negative value indicated higher rates in the placebo group. For studies that reported the number of individual side effects but not the number of patients experiencing a side effect, this probability was determined using maximum likelihood estimation under a binomial model. We assessed heterogeneity among the studies with the I2 statistic, where a value above 50% indicated substantial/considerable heterogeneity [18]. We performed meta-regression to determine the association of treatment outcomes with patient characteristics and study design factors if significant heterogeneity was identified. The factors examined were age, baseline IIEF-EF value, sample size, treatment duration, and study design. We evaluated publication bias using Egger’s regression test [19] and the trim-and-fill method, which adjusts the meta-analysis estimate based on the estimated number of studies missing due to publication bias [20]. Finally, we performed sensitivity analyses by iteratively removing each study and reestimating the overall outcomes. A two-sided p-value below 0.05 was considered statistically significant. The meta-analysis was performed using Stata v18 (Stata Corp), and the risk of bias was evaluated using Review Manager v5.4 (The Cochrane Collaboration).
4. Ethics statement
This systematic review and meta-analysis did not involve human subjects or animals, so ethical approval was not required. The review protocol was prospectively registered at www.researchregistry.com (reviewregistry1691).
RESULTS
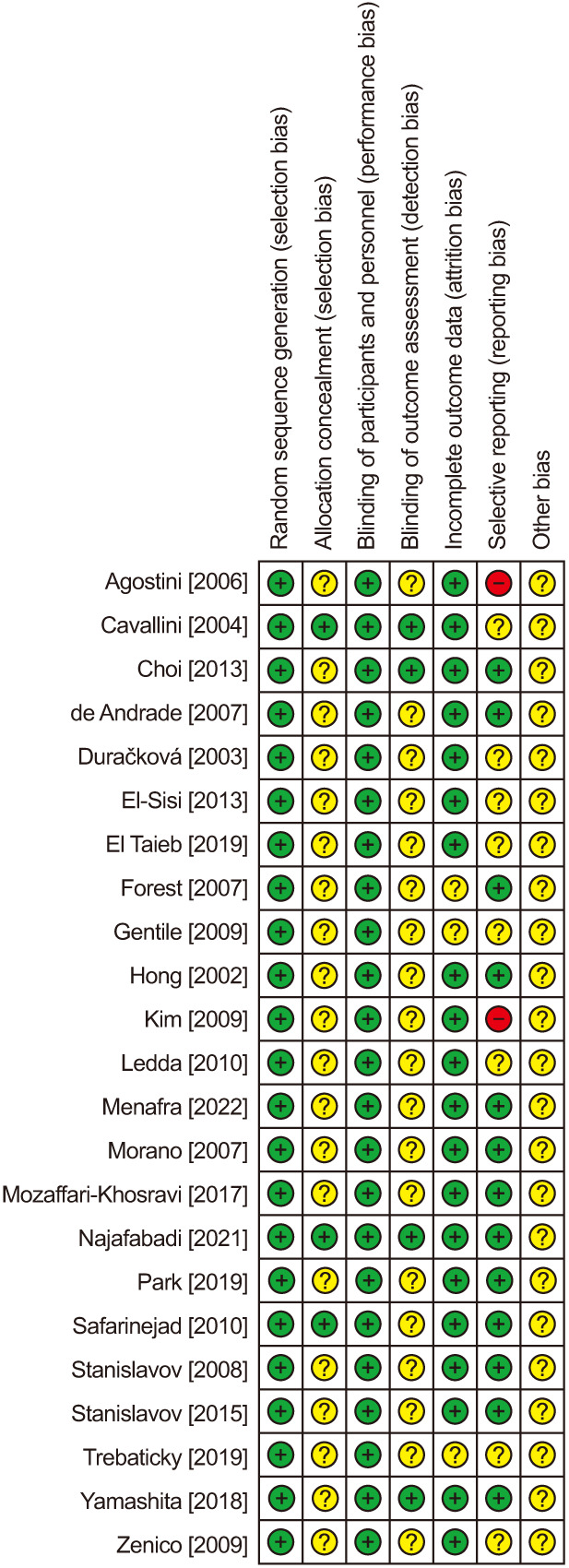
The systematic review identified 23 double-blind, randomized trials [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] of 1,583 men with ED treated with oral antioxidant supplements (n=921) or placebo (n=860) (Fig. 1). The total number of patients differed from the sum of the two groups due to the inclusion of four crossover trials. The mean age of patients ranged from 36 to 65 years (median 51 years). Various antioxidants were studied, most commonly ginseng (7 trials), L-arginine (6 trials), pycnogenol (5 trials), L-carnitine (3 trials), vitamin E (2 trials), and others (1 trial each). Treatment duration ranged from 4 weeks to 6 months (median 12 weeks) (Table 2). The risk of bias summary for each trial is provided in Fig. 2. The most common biases were inadequate descriptions of allocation concealment and unclear outcome assessor blinding.
Fig. 1. PRISMA flow diagram. IIEF-EF: International Index of Erectile Function-Erectile Function.
Table 2. Patient and study characteristics in double-blind, randomized placebo-controlled trials of antioxidant supplementation for erectile dysfunction.
| Study | Design | Numbera | Mean age (range), y | IIEFc | Daily dosage | Duration |
|---|---|---|---|---|---|---|
| Agostini et al (2006) [21] | Parallel | 88/88 | 60 (50–70)b | 15.6 | Myoinositol (4 g); folic acid (0.4 mg) | 12 weeks |
| Cavallini et al (2004) [22] | Parallel | 45/45 | 65 (60–74) | 8.0 | Propionyl-L-carnitine (2 g); acetyl-L-carnitine (2 g) | 6 months |
| Choi et al (2013) [23] | Parallel | 59/59 | 57 (31–69) | 20.8 | Korean ginseng berry extract (1.4 g) | 8 weeks |
| de Andrade et al (2007) [24] | Parallel | 30/30 | 53 (26–70) | 20.0 | Korean red ginseng (3 g) | 12 weeks |
| Duračková et al (2003) [25] | Parallel | 13/8 | 47 (22–69) | 14.5 | Pycnogenol (120 mg) | 3 months |
| El-Sisi et al (2013) [26] | Parallel | 20/20 | 51 (40–60)b | 15.5 | Vitamin E (400 IU) | 6 weeks |
| El Taieb et al (2019) [27] | Parallel | 27/27 | 43 (-) | 14.1 | L-arginine (5 g) | 8 weeks |
| Forest et al (2007) [28] | Crossover | 53 | 46 (21–70) | 20.8 | Pomegranate juice (1.5 mm total polyphenol) | 4 weeks |
| Gentile et al (2009) [29] | Parallel | 10/10 | 55 (50–60)b | 15.6 | Propionyl-L-carnitine (250 mg); L-arginine (2.5 g); nicotinic acid (20 mg) | 12 weeks |
| Hong et al (2002) [30] | Crossover | 45 | 54 (-) | 12.7 | Korean red ginseng (2.7 g) | 8 weeks |
| Kim et al (2009) [31] | Parallel | 65/21 | 58 (33–79) | 14.1 | Panax ginseng (2 g) | 8 weeks |
| Ledda et al (2010) [32] | Parallel | 54/57 | 44 (30–50) | 15.2 | Pycnogenol (80 mg); L-arginine aspartate (2.8 g) | 6 months |
| Menafra et al (2022) [33] | Parallel | 51/47 | 51 (20–73) | 20.0 | L-arginine (6 g) | 3 months |
| Morano et al (2007) [34] | Parallel | 8/8 | 56 (45–70) | 19.0 | Propionyl L-carnitine (2 g) | 12 weeks |
| Mozaffari-Khosravi et al (2017) [35] | Parallel | 34/35 | 51 (25–55) | 16.2 | L-arginine (5 g) | 4 weeks |
| Najafabadi et al (2021) [36] | Parallel | 26/26 | 41 (32–57) | 18.4 | Vitamin E (100 IU); Korean ginseng (67 mg); Siberian ginseng (40 mg) | 6 weeks |
| Park et al (2019) [37] | Parallel | 21/20 | 57 (35–73) | 15.7 | Ginseng Radix Rubra, Cornus officinalis Sieb. et Zucc., Lycium chinense Mill, Daphnandra tenuipes, and Curcuma longa Linn (dose n/a) | 8 weeks |
| Safarinejad (2010) [38] | Parallel | 93/93 | 52 (35–60) | 9.8 | Coenzyme Q10 (300 mg) | 24 weeks |
| Stanislavov et al (2008) [39] | Crossover | 50 | 37 (30–50) | 14.0 | L-arginine aspartate (3 g); pycnogenol (80 mg) | 1 month |
| Stanislavov and Rohdewald (2015) [40] | Crossover | 50 | 37 (30–50) | 16.8 | L-arginine (1.92 g); pycnogenol (80 mg); citrulline (1.2 g); roburins (40 mg) | 4 weeks |
| Trebaticky et al (2019) [41] | Parallel | 32/21 | 49 (-) | 12.4 | Pycnogenol (120 mg) | 3 months |
| Yamashita (2018) [42] | Parallel | 22/22 | 48 (-) | 12.0 | Sanchi ginseng extract (62.5 mg total ginsenosides) | 12 weeks |
| Zenico et al (2009) [43] | Parallel | 25/25 | 36 (-) | 19.0 | Maca (2.4 g) | 12 weeks |
IIEF-EF, International Index of Erectile Function-Erectile Function.
aAntioxidant group/placebo group. bEstimated mean values. cIIEF-EF reported on a 6 to 30 scale.
Fig. 2. Risk of bias summary. Review authors’ judgments about each risk of bias item for each included study.
The baseline IIEF-EF score across all studies was 15.7 (95% confidence interval [CI]: 14.6–16.7), indicating moderate ED overall. Antioxidant supplementation statistically improved IIEF-EF compared to placebo, with a mean difference between groups of 5.5 points (95% CI: 3.7–7.3; p<0.001). High heterogeneity (I2=98%) was observed among the trials (Fig. 3). In the metaregression analysis, the effect of antioxidants on erectile function was greater in men with lower baseline IIEF-EF scores (p<0.001) and over longer treatment durations (p=0.01). In multivariable meta-regression, a lower baseline IIEF-EF score was the only factor associated with a greater treatment effect of antioxidants, with no independent association observed with treatment duration, study design, study sample size, or patient age (Table 3, Fig. 4). In an exploratory subgroup analysis of individual antioxidants, pycnogenol and L-arginine were associated with statistically significant benefits that also exceeded the minimal clinically important difference (MCID) of 6 points for men with moderate ED (Table 4) [44].
Fig. 3. Effect of antioxidant supplementation on erectile function. Values reported as the difference in IIEF-EF on a 6 to 30 scale between antioxidant and placebo groups. The mean difference and 95% CI are plotted for each study. The pooled mean difference (diamond apex) and 95% CI (diamond width) are calculated using a random effects model. Mean difference=5.5; 95% CI: 3.4–7.6; p<0.001; I2=98%). IIEF-EF: International Index of Erectile Function-Erectile Function, CI: confidence interval.
Table 3. Association of patient- and study-factors on the mean difference in IIEF-EF comparing antioxidant to placebo groups.
| Variable | Z-scorea | p-value | |
|---|---|---|---|
| Univariable | Multivariable | ||
| Lower baseline IIEF-EF score | 3.73 | <0.001 | <0.001 |
| Longer treatment duration | 2.51 | 0.01 | |
| Larger sample size | 1.85 | 0.07 | |
| Crossover vs. parallel | 0.80 | 0.43 | |
| Older age | 0.32 | 0.75 | |
IIEF-EF: International Index of Erectile Function-Erectile Function.
aPositive z-value indicates the variable improved the overall benefit of antioxidant supplementation.
Fig. 4. Bubble plot of the association between the treatment benefit of antioxidants in IIEF-EF and baseline IIEF-EF score. Blue circles represent values of individual studies where the circle size is proportional to the study weight in the random-effects model. The red line represents the regression line of best fit. The regression equation for the IIEF-EF difference with antioxidant supplementation=18.2–(0.8×Baseline IIEF-EF); p<0.001, where IIEF-EF scores were converted to a 6–30 scale. IIEF-EF: International Index of Erectile Function-Erectile Function.
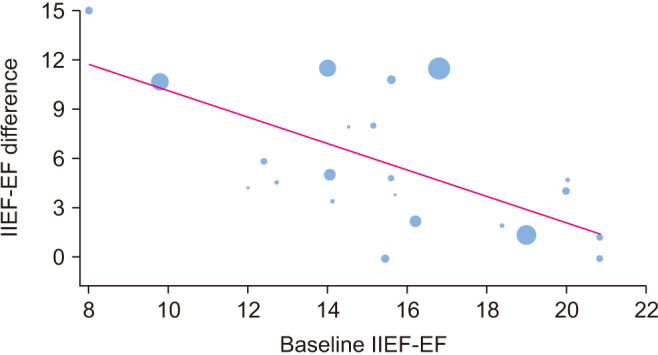
Table 4. Treatment effect of individual antioxidants on erectile function among included studies.
| Antioxidant | Studies | IIEF-EF | 95% CI | p-value |
|---|---|---|---|---|
| Mean difference | ||||
| Pycnogenol | 5 | 9.2 | 6.9 to 11.5 | <0.001 |
| L-carnitine | 3 | 7.4 | -0.8 to 15.7 | 0.08 |
| L-arginine | 7 | 6.7 | 3.9 to 9.5 | <0.001 |
| Other antioxidants | 4 | 5.7 | 0.0 to 11.5 | 0.05 |
| Ginseng | 7 | 2.9 | 1.5 to 4.4 | <0.001 |
| Vitamin E | 2 | 0.4 | -1.3 to 2.0 | 0.67 |
IIEF-EF: International Index of Erectile Function-Erectile Function, CI: confidence interval.
Side effects were uncommon overall, occurring in 3.8% of patients in the antioxidant group and 2.1% in the placebo group. There was no statistically significant difference in the frequency of side effects between groups (logRR=0.36; 95% CI: -0.24–0.97; p=0.24), and heterogeneity was negligible (I2=0%) (Fig. 5). The most common side effects in the antioxidant group were headache (n=10), gastrointestinal disturbances (n=10), itching (n=4), upper respiratory infection (n=4), insomnia (n=3), dry mouth (n=2), dizziness (n=2), hypertension (n=1), and an unspecified side effect (n=1). None of the side effects were classified as serious or severe.
Fig. 5. Safety profile of antioxidant supplementation for treatment of erectile dysfunction. Values reported as the log risk ratio (logRR) between antioxidant and placebo groups. The logRR and 95% confidence interval (CI) are plotted for each study. The pooled logRR (diamond apex) and 95% CI (diamond width) are calculated using a random effects model. The percentage of patients reporting a side effect was 3.7% with antioxidants and 2.0% with placebo (logRR=0.36; 95% CI: -0.24 to 0.97; p=0.24). Heterogeneity was negligible (I2=0%).
The conclusions from the primary analyses were corroborated in various sensitivity analyses. Compared to the 5.5-point mean difference in IIEF-EF from the primary analysis, iterative removal of one study at a time resulted in mean difference values ranging from 5.1 to 5.8 (all p<0.001) for IIEF-EF, and the publication-bias adjusted trim-and-fill IIEF-EF value was 6.2 (Egger p=0.14). Compared to the nonsignificant logRR of 0.36 for side effects from the primary analysis, iterative removal of one study at a time resulted in logRR values ranging from 0.29 to 0.42, with all p-values ≥0.18. Furthermore, the publication-bias adjusted trim-and-fill logRR value for side effects remained unchanged at 0.36 (Egger p=0.91).
DISCUSSION
Endothelial dysfunction and impaired NO signaling are common contributors to ED [8]. Interventions that target oxidative stress may help treat ED resulting from endothelial dysfunction. This systematic review and meta-analysis of 23 double-blind, randomized, placebo-controlled trials aimed to evaluate the potential therapeutic role of oral antioxidant supplementation for treating ED. The results demonstrated that antioxidant supplementation led to statistically significant and clinically important improvements in erectile function compared to placebo. Furthermore, the beneficial effect of antioxidants on erectile function was greater in men with more severe ED. Additionally, side effects were uncommon, non-serious, and the risk did not significantly differ between antioxidant and placebo groups. However, the supplementation protocols were generally short-term, with a median duration of 12 weeks. Overall, antioxidant supplementation appears to be a low-risk, non-pharmacologic intervention with demonstrated short-term efficacy for improving erectile function in men with ED.
The magnitude of improvement in erectile function with antioxidants was statistically significant and clinically meaningful in this meta-analysis. On a 6 to 30-point IIEF-EF scale, the MCID is 2.4 points for mild ED, 6.0 points for moderate ED, and 8.4 points for severe ED [44]. The IIEF-EF improvement in this study was 5.5 points and favorably compared to 2 points for testosterone replacement, 4 points for shockwave therapy, and 4 to 8 points for PDE5 inhibitors [45]. In addition, the safety profile of antioxidant supplementation was favorable with minor side effects occurring in only 3.8% of patients, a rate comparable to placebo. This compares favorably to the safety profile of PDE5 inhibitors where side effects such as headache and flushing occur in over 25% of patients, a rate 2 to 3 times higher than with placebo [46,47].
The results of this meta-analysis suggest that antioxidant supplementation may offer a low-risk treatment option for individuals across the spectrum of ED severities. Of significance is that patients with ED could consider antioxidant supplementation as an alternative to PDE5 inhibitors, potentially delaying or avoiding the need for drug therapy. The 5.5-point improvement in IIEF-EF with antioxidants is comparable to PDE5 inhibitors [45], thus warranting consideration for inclusion in ED treatment algorithms. Some have postulated that the treatment benefit of antioxidants may depend on ED etiology. Aldemir et al [48] demonstrated that total antioxidant capacity was lower and oxidative stress markers were higher in men with versus without ED. Furthermore, these differences were more pronounced in men with arteriogenic ED, suggesting that antioxidants might hold greater promise in treating ED of arteriogenic etiology. Additional research is needed to confirm whether antioxidant supplementation provides differing therapeutic benefits based on ED etiology.
This meta-analysis included trials that utilized a variety of antioxidants, including amino acids, vitamins, enzymes, and plant extracts. While these compounds differ in their molecular structure, they share common mechanisms of action in combating oxidative stress, which are particularly relevant to ED where oxidative stress significantly impairs endothelial cell function and NO signaling [8]. By counteracting oxidative stress, antioxidants may facilitate improved vasodilation and smooth muscle relaxation critical to the erectile response [11]. Furthermore, individual or combined antioxidants can modulate endothelial NO synthase uncoupling by scavenging free radicals and impairing radical formation pathways [49]. Thus, analyzing antioxidants as a unified class for meta-analytic purposes is justified. However, analyzing the treatment effect of individual antioxidants was complicated by several factors such as differences in daily dosages, treatment durations, and their inclusion in combination products where the contribution from individual compounds could not be quantified. Although the results support a beneficial effect for antioxidants overall and the subgroup analysis suggested the potential for pycnogenol and L-arginine to improve erectile function, additional research is needed to evaluate and isolate the effect of specific antioxidant products.
The strengths of this review include the generation of Level 1 evidence and identification of factors influencing erectile function with antioxidant supplementation. However, several limitations of the review warrant discussion. First, factors such as comorbidities, diet, and lifestyle were not routinely reported in the trials and may have influenced the treatment response. Second, the antioxidant supplementation regimens varied among studies. Although we identified the potential for certain antioxidants such as pycnogenol and L-arginine, they were often components of combination products such that determining the treatment effect of individual components was not possible. Consequently, determining the optimal duration, dosage, frequency, and antioxidant type required to produce significant improvements in erectile function warrants additional investigation where the treatment effects of individual compounds can be determined. Further, the meta-regression and subgroup analysis results should be interpreted cautiously and considered hypothesis-generating only. Third, the mechanism by which erectile function improved with antioxidant supplementation was unreported in most studies, suggesting the need for more studies linking treatment outcomes with mechanistic changes. Finally, the supplementation protocols were generally short-term; thus, the long-term efficacy and safety of antioxidant supplementation for the treatment of ED remains to be determined and warrants additional investigation.
CONCLUSIONS
Antioxidant supplementation appears safe and significantly improves erectile function in men with ED, particularly those with more severe symptoms. Limitations of this review included unknown long-term efficacy and safety and the inability to make specific product and dosing recommendations due to the variety of antioxidants and regimens studied.
Acknowledgements
We thank David Fay, PhD (Miller Scientific) for assistance with the literature review and data extraction.
Footnotes
Conflict of Interest: RR reports consultancy with Boston Scientific.
SB reports employment with Boston Scientific.
TPK has nothing to disclose.
LEM reports consultancy with Boston Scientific.
Funding: This study was supported by Boston Scientific.
- Conceptualization: SB, LEM.
- Data curation: LEM.
- Formal analysis: LEM.
- Funding acquisition: SB.
- Investigation: SB, LEM.
- Methodology: SB, LEM.
- Project administration: SB, LEM.
- Resources: SB, LEM.
- Software: LEM.
- Supervision: SB, LEM.
- Validation: RR, SB, TPK.
- Visualization: LEM.
- Writing – original draft: LEM.
- Writing – review & editing: RR, SB, TPK.
References
- 1.National Institutes of Health (NIH) Impotence. NIH Consensus Statement. 1992;10:1–33. [PubMed] [Google Scholar]
- 2.Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84:50–56. doi: 10.1046/j.1464-410x.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- 3.Braun M, Wassmer G, Klotz T, Reifenrath B, Mathers M, Engelmann U. Epidemiology of erectile dysfunction: results of the 'Cologne Male Survey'. Int J Impot Res. 2000;12:305–311. doi: 10.1038/sj.ijir.3900622. [DOI] [PubMed] [Google Scholar]
- 4.Pinnock CB, Stapleton AM, Marshall VR. Erectile dysfunction in the community: a prevalence study. Med J Aust. 1999;171:353–357. doi: 10.5694/j.1326-5377.1999.tb123691.x. [DOI] [PubMed] [Google Scholar]
- 5.Nicolosi A, Glasser DB, Kim SC, Marumo K, Laumann EO GSSAB Investigators' Group. Sexual behaviour and dysfunction and help-seeking patterns in adults aged 40-80 years in the urban population of Asian countries. BJU Int. 2005;95:609–614. doi: 10.1111/j.1464-410X.2005.05348.x. [DOI] [PubMed] [Google Scholar]
- 6.Nicolosi A, Moreira ED, Jr, Shirai M, Bin Mohd Tambi MI, Glasser DB. Epidemiology of erectile dysfunction in four countries: cross-national study of the prevalence and correlates of erectile dysfunction. Urology. 2003;61:201–206. doi: 10.1016/s0090-4295(02)02102-7. [DOI] [PubMed] [Google Scholar]
- 7.Burnett AL, Nehra A, Breau RH, Culkin DJ, Faraday MM, Hakim LS, et al. Erectile dysfunction: AUA guideline. J Urol. 2018;200:633–641. doi: 10.1016/j.juro.2018.05.004. Erratum in: J Urol 2022;207:743. [DOI] [PubMed] [Google Scholar]
- 8.Blick C, Ritchie RW, Sullivan ME. Is erectile dysfunction an example of abnormal endothelial function? Curr Vasc Pharmacol. 2016;14:163–167. doi: 10.2174/1570161114666151202205950. [DOI] [PubMed] [Google Scholar]
- 9.Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 10.Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 11.Azadzoi KM, Schulman RN, Aviram M, Siroky MB. Oxidative stress in arteriogenic erectile dysfunction: prophylactic role of antioxidants. J Urol. 2005;174:386–393. doi: 10.1097/01.ju.0000161209.39959.67. [DOI] [PubMed] [Google Scholar]
- 12.Su L, Yang ZT, Qu H, Luo CL, Yuan GX, Wu J, et al. Effect of antioxidants supplementation on erectile dysfunction: a systematic review and meta-analysis of randomized controlled trials. Sex Med Rev. 2022;10:754–763. doi: 10.1016/j.sxmr.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Leisegang K, Finelli R. Alternative medicine and herbal remedies in the treatment of erectile dysfunction: a systematic review. Arab J Urol. 2021;19:323–339. doi: 10.1080/2090598X.2021.1926753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 15.Cappelleri JC, Rosen RC, Smith MD, Mishra A, Osterloh IH. Diagnostic evaluation of the erectile function domain of the International Index of Erectile Function. Urology. 1999;54:346–351. doi: 10.1016/s0090-4295(99)00099-0. [DOI] [PubMed] [Google Scholar]
- 16.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 21.Agostini R, Rossi F, Pajalich R. Myoinositol/folic acid combination for the treatment of erectile dysfunction in type 2 diabetes men: a double-blind, randomized, placebo-controlled study. Eur Rev Med Pharmacol Sci. 2006;10:247–250. [PubMed] [Google Scholar]
- 22.Cavallini G, Caracciolo S, Vitali G, Modenini F, Biagiotti G. Carnitine versus androgen administration in the treatment of sexual dysfunction, depressed mood, and fatigue associated with male aging. Urology. 2004;63:641–646. doi: 10.1016/j.urology.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Choi YD, Park CW, Jang J, Kim SH, Jeon HY, Kim WG, et al. Effects of Korean ginseng berry extract on sexual function in men with erectile dysfunction: a multicenter, placebo-controlled, double-blind clinical study. Int J Impot Res. 2013;25:45–50. doi: 10.1038/ijir.2012.45. [DOI] [PubMed] [Google Scholar]
- 24.de Andrade E, de Mesquita AA, Claro Jde A, de Andrade PM, Ortiz V, Paranhos M, et al. Study of the efficacy of Korean red ginseng in the treatment of erectile dysfunction. Asian J Androl. 2007;9:241–244. doi: 10.1111/j.1745-7262.2007.00210.x. [DOI] [PubMed] [Google Scholar]
- 25.Duračková Z, Trebatický B, Novotný V, Žitňanová I, Breza J. Lipid metabolism and erectile function improvement by pycnogenol®, extract from the bark of pinus pinaster in patients suffering from erectile dysfunction-a pilot study. Nutr Res. 2003;23:1189–1198. [Google Scholar]
- 26.El-Sisi AA, Hegazy SK, Salem KA, AbdElkawy KS. Atorvastatin improves erectile dysfunction in patients initially irresponsive to Sildenafil by the activation of endothelial nitric oxide synthase. Int J Impot Res. 2013;25:143–148. doi: 10.1038/ijir.2012.46. [DOI] [PubMed] [Google Scholar]
- 27.El Taieb M, Hegazy E, Ibrahim A. Daily oral l-arginine plus tadalafil in diabetic patients with erectile dysfunction: a double-blinded, randomized, controlled clinical trial. J Sex Med. 2019;16:1390–1397. doi: 10.1016/j.jsxm.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Forest CP, Padma-Nathan H, Liker HR. Efficacy and safety of pomegranate juice on improvement of erectile dysfunction in male patients with mild to moderate erectile dysfunction: a randomized, placebo-controlled, double-blind, crossover study. Int J Impot Res. 2007;19:564–567. doi: 10.1038/sj.ijir.3901570. [DOI] [PubMed] [Google Scholar]
- 29.Gentile V, Antonini G, Antonella Bertozzi M, Dinelli N, Rizzo C, Ashraf Virmani M, et al. Effect of propionyl-L-carnitine, L-arginine and nicotinic acid on the efficacy of vardenafil in the treatment of erectile dysfunction in diabetes. Curr Med Res Opin. 2009;25:2223–2228. doi: 10.1185/03007990903138416. [DOI] [PubMed] [Google Scholar]
- 30.Hong B, Ji YH, Hong JH, Nam KY, Ahn TY. A double-blind crossover study evaluating the efficacy of Korean red ginseng in patients with erectile dysfunction: a preliminary report. J Urol. 2002;168:2070–2073. doi: 10.1016/S0022-5347(05)64298-X. [DOI] [PubMed] [Google Scholar]
- 31.Kim TH, Jeon SH, Hahn EJ, Paek KY, Park JK, Youn NY, et al. Effects of tissue-cultured mountain ginseng (Panax ginseng CA Meyer) extract on male patients with erectile dysfunction. Asian J Androl. 2009;11:356–361. doi: 10.1038/aja.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ledda A, Belcaro G, Cesarone MR, Dugall M, Schönlau F. Investigation of a complex plant extract for mild to moderate erectile dysfunction in a randomized, double-blind, placebo-controlled, parallel-arm study. BJU Int. 2010;106:1030–1033. doi: 10.1111/j.1464-410X.2010.09213.x. [DOI] [PubMed] [Google Scholar]
- 33.Menafra D, de Angelis C, Garifalos F, Mazzella M, Galdiero G, Piscopo M, et al. Long-term high-dose L-arginine supplementation in patients with vasculogenic erectile dysfunction: a multicentre, double-blind, randomized, placebo-controlled clinical trial. J Endocrinol Invest. 2022;45:941–961. doi: 10.1007/s40618-021-01704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morano S, Mandosi E, Fallarino M, Gatti A, Tiberti C, Sensi M, et al. Antioxidant treatment associated with sildenafil reduces monocyte activation and markers of endothelial damage in patients with diabetic erectile dysfunction: a double-blind, placebo-controlled study. Eur Urol. 2007;52:1768–1774. doi: 10.1016/j.eururo.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 35.Mozaffari-Khosravi H, Fallahi M, Afkhami-Ardekani M. Effect of oral supplementation of L-arginine on sexual function in men with type 2 diabetes: a double-blind clinical trial. J Nutr Food Secur. 2017;2:165–172. [Google Scholar]
- 36.Najafabadi BT, Jafarinia M, Ghamari K, Shokraee K, Tadayyon F, Akhondzadeh S. Vitamin E and ginseng combined supplement for treatment of male erectile dysfunction: a double-blind, placebo-controlled, randomized, clinical trial. Adv Integr Med. 2021;8:44–49. [Google Scholar]
- 37.Park NC, Kim SW, Hwang SY, Park HJ. Efficacy and safety of an herbal formula (KBMSI-2) in the treatment of erectile dysfunction: a preliminary clinical study. Investig Clin Urol. 2019;60:275–284. doi: 10.4111/icu.2019.60.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Safarinejad MR. Safety and efficacy of coenzyme Q10 supplementation in early chronic Peyronie's disease: a double-blind, placebo-controlled randomized study. Int J Impot Res. 2010;22:298–309. doi: 10.1038/ijir.2010.20. [DOI] [PubMed] [Google Scholar]
- 39.Stanislavov R, Nikolova V, Rohdewald P. Improvement of erectile function with Prelox: a randomized, double-blind, placebo-controlled, crossover trial. Int J Impot Res. 2008;20:173–180. doi: 10.1038/sj.ijir.3901597. [DOI] [PubMed] [Google Scholar]
- 40.Stanislavov R, Rohdewald P. Improvement of erectile function by a combination of French maritime pine bark and roburins with aminoacids. Minerva Urol Nefrol. 2015;67:27–32. [PubMed] [Google Scholar]
- 41.Trebaticky B, Muchova J, Ziaran S, Bujdak P, Breza J, Durackova Z. Natural polyphenols improve erectile function and lipid profile in patients suffering from erectile dysfunction. Bratisl Lek Listy. 2019;120:941–944. doi: 10.4149/BLL_2019_158. [DOI] [PubMed] [Google Scholar]
- 42.Yamashita S. Effects of Sanchi ginseng extract on the sexual function in Japanese men—a randomized, double-blind, placebo-controlled, parallel-group trial—. Jpn Pharmacol Ther. 2018;46:561–580. [Google Scholar]
- 43.Zenico T, Cicero AF, Valmorri L, Mercuriali M, Bercovich E. Subjective effects of Lepidium meyenii (Maca) extract on well-being and sexual performances in patients with mild erectile dysfunction: a randomised, double-blind clinical trial. Andrologia. 2009;41:95–99. doi: 10.1111/j.1439-0272.2008.00892.x. [DOI] [PubMed] [Google Scholar]
- 44.Rosen RC, Allen KR, Ni X, Araujo AB. Minimal clinically important differences in the erectile function domain of the International Index of Erectile Function scale. Eur Urol. 2011;60:1010–1016. doi: 10.1016/j.eururo.2011.07.053. [DOI] [PubMed] [Google Scholar]
- 45.Ciocanel O, Power K, Eriksen A. Interventions to treat erectile dysfunction and premature ejaculation: an overview of systematic reviews. Sex Med. 2019;7:251–269. doi: 10.1016/j.esxm.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lui JL, Shaw NM, Abbasi B, Hakam N, Breyer BN. Adverse reactions of PDE5 inhibitors: an analysis of the World Health Organization pharmacovigilance database. Andrology. 2023;11:1408–1417. doi: 10.1111/andr.13430. [DOI] [PubMed] [Google Scholar]
- 47.Madeira CR, Tonin FS, Fachi MM, Borba HH, Ferreira VL, Leonart LP, et al. Efficacy and safety of oral phosphodiesterase 5 inhibitors for erectile dysfunction: a network meta-analysis and multicriteria decision analysis. World J Urol. 2021;39:953–962. doi: 10.1007/s00345-020-03233-9. [DOI] [PubMed] [Google Scholar]
- 48.Aldemir M, Okulu E, Neşelioğlu S, Erel O, Ener K, Kayıgil Ö. Evaluation of serum oxidative and antioxidative status in patients with erectile dysfunction. Andrologia. 2012;44 Suppl 1:266–271. doi: 10.1111/j.1439-0272.2011.01174.x. [DOI] [PubMed] [Google Scholar]
- 49.Varadharaj S, Kelly OJ, Khayat RN, Kumar PS, Ahmed N, Zweier JL. Role of dietary antioxidants in the preservation of vascular function and the modulation of health and disease. Front Cardiovasc Med. 2017;4:64. doi: 10.3389/fcvm.2017.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]





