Abstract
Purpose
The study aimed to comprehensively analyze testosterone and precursor concentrations in the testicular interstitial fluid (TIF) of men with azoospermia, exploring their significance in the testicular microenvironment and their correlation with testicular sperm retrieval outcomes.
Materials and Methods
We analyzed 37 TIF samples, including 5 from men with obstructive azoospermia (OA) and 32 from men with non-obstructive azoospermia (NOA). Liquid chromatography with tandem mass spectrometry quantified testosterone and precursor levels. Comparative assessments of the outcomes of testicular sperm retrieval were performed between the OA and NOA groups as well as among men with NOA.
Results
Men with NOA who had not undergone hormone treatment exhibited significantly higher intratesticular concentrations of testosterone (median 1,528.1 vs. 207.5 ng/mL), androstenedione (median 10.6 vs. 1.9 ng/mL), and 17-OH progesterone (median 13.0 vs. 1.8 ng/mL) than men diagnosed with OA. Notably, in the subgroup of patients with NOA subjected to medical treatment, men with successful sperm retrieval had significantly reduced levels of androstenedione (median androstenedione 5.7 vs. 18.5 ng/mL, p=0.004). Upon a more detailed analysis of these men who underwent hormone manipulation treatment, the testosterone/androstenedione ratio (indicative of HSD17B3 enzyme activity) was markedly increased in men with successful sperm retrieval (median: 365.8 vs. 165.0, p=0.008) compared with individuals with NOA who had unsuccessful sperm recovery. Furthermore, within the subset of men with NOA who did not undergo medical treatment before microdissection testicular sperm extraction but achieved successful sperm retrieval, the ratio of 17-OH progesterone/progesterone (indicative of CYP17A1 activity) was substantially higher.
Conclusions
The study suggests distinct testosterone biosynthesis pathways in men with compromised spermatogenesis and those with normal spermatogenesis. Among NOA men with successful retrieval after hormone optimization therapy, there was decreased androstenedione and increased HSD17B3 enzyme activity. These findings have diagnostic and therapeutic implications for the future.
Keywords: Azoospermia; Extracellular fluid; Infertility, male; Sperm retrieval; Testis; Testosterone
INTRODUCTION
Spermatogenesis is a complex and regulated process that involves the sequential transition of diploid spermatogonia into mature haploid spermatozoa. This process is dependent on a sophisticated interplay of endocrine and paracrine signals, including gonadotropins and androgens, and relies on the proper functioning of various constituents of the hypothalamus-pituitary-gonadal (HPG) axis, Leydig cells, and Sertoli cells [1,2].
Testosterone, the primary androgen synthesized within the testes by Leydig cells in response to luteinizing hormone (LH) stimulation, plays a pivotal role in spermatogenesis [1,2]. Testosterone exerts regulatory control over the expression of androgen-dependent target genes in Sertoli cells, thereby promoting Sertoli cell proliferation and maturation as well as ensuring the structural integrity of the blood-testis barrier, an indispensable element for germ cell development [3,4]. Furthermore, testosterone is essential for the completion of meiosis and differentiation of round spermatids into spermatozoa [5].
Although definitive reference ranges for intratesticular testosterone (ITT) levels that support spermatogenesis remain to be established, studies have revealed that ITT levels are approximately 100 times higher than the corresponding serum testosterone levels observed in men with normal reproductive function [6]. In addition, reducing ITT levels by 50% to 60% does not significantly impede spermatogenesis. However, ITT levels equivalent to serum levels are insufficient to sustain normal sperm production in men [7]. In contrast, men afflicted by non-obstructive azoospermia (NOA) stemming from primary testicular failure may exhibit ITT to serum testosterone ratios surpassing 400-fold [8]. It is plausible that men with NOA manifest modified testicular vascularization, encompassing alterations in the testicular perfusion system and microvascular architecture, compared with men with normal spermatogenesis [8,9]. Testosterone release into the bloodstream is consequently impaired, resulting in diminished negative feedback to the HPG axis and subsequent increased gonadotropin secretion, thereby stimulating testosterone production by Leydig cells within the testes.
While testosterone biosynthesis remains unimpaired in men with NOA (except men with NOA related to hypogonadotropic hypogonadism), as evidenced by Leydig cell hypertrophy and a heightened local testosterone milieu [8,10], alterations occur in the steroidogenesis pathway for these individuals. Unlike men with normal spermatogenesis who predominantly follow the Δ5 pathway, which involves cholesterol conversion to pregnenolone to 17-OH pregnenolone to dehydroepiandrosterone (DHEA) to androstenediol to testosterone, men with defective spermatogenesis exhibit a shift toward the Δ4 pathway due to increased 3β-hydroxysteroid dehydrogenase activity [11,12]. In this alternate pathway, cholesterol is converted to pregnenolone, which is subsequently transformed into progesterone. The pathway then proceeds with the conversion of progesterone to 17-OH progesterone, followed by the conversion of 17-OH progesterone to androstenedione. Finally, androstenedione is converted to testosterone.
In addition to testosterone, the precursors and metabolites of testosterone possess the capacity to induce androgenic effects. Nevertheless, the extent of their contribution to androgenic action in promoting spermatogenesis, particularly in men diagnosed with NOA, remains uncertain. Few current investigations have explored ITT precursors in individuals with impaired spermatogenesis who have undergone surgical testicular sperm extraction. Thus, the primary objective of the present study was to evaluate the concentrations of testosterone and its precursors, namely progesterone, 17-OH progesterone, androstenedione, and DHEA, within the testicular interstitial fluid (TIF) of patients with NOA. Our aim was to assess the potential significance of these precursors within the testicular microenvironment and their association with the outcomes of testicular sperm retrieval.
MATERIALS AND METHODS
1. Ethics statement
The study protocol conformed to the ethical guidelines of the current Declaration of Helsinki and was approved by local ethics committees (IRB: 2022-01-036AC). Written informed consent was obtained from all participants.
2. Study population
Following approval from the institutional review board, we conducted a prospective study from January 2021 to December 2021 to acquire TIF from two distinct categories. The first category included men diagnosed with NOA who underwent microdissection testicular sperm extraction (mTESE) as their initial surgical sperm retrieval. The second category consisted of men with obstructive azoospermia (OA) resulting from prior vasectomy procedures who underwent vasectomy reversal in conjunction with concurrent testicular sperm extraction for sperm preservation. Individuals who had received invasive procedures to differentiate NOA from OA as well as those with hypogonadotropic hypogonadism, were excluded. To ensure a comprehensive evaluation, all participants underwent a complete assessment to ascertain the underlying etiology of azoospermia. This evaluation encompassed an in-depth analysis of medical history, physical examination, and measurement of serum levels of follicle-stimulating hormone (FSH), LH, testosterone, prolactin, and estradiol. Baseline endocrine levels were measured in all patients at the time of azoospermia diagnosis. In addition, men diagnosed with NOA underwent further cytogenetic and Y-chromosome microdeletion testing to identify potential genetic abnormalities.
3. Hormone optimization therapy prior to mTESE
The pre-mTESE therapeutic protocol for NOA individuals with hypogonadal status (total testosterone <300 ng/dL) encompasses the administration of clomiphene citrate, aromatase inhibitors, or human chorionic gonadotropin (hCG). For patients exhibiting LH levels within the normal range (1.24–7.8 IU/L), a daily dosage of clomiphene citrate 50 mg was employed. Alternatively, in cases where the testosterone: estradiol ratios were below 10, anastrozole at 1 mg was administered. Men with elevated LH levels (>7.8 IU/L) typically received hCG at 5,000 U twice weekly. This therapeutic regimen was sustained for a minimum duration of 8 weeks preceding the mTESE procedure.
4. mTESE and TIF collection
After sterilization and draping of the scrotal skin, a 3-cm midline incision was made to gain access to the testis by dissecting through the layers of the dartos muscle and tunica vaginalis. Using an operating microscope, a singular incision in the tunica albuginea was made, and intratesticular fluid was aspirated before the testicular parenchyma was examined to identify the dilated and opaque seminiferous tubules that were more likely to contain viable spermatozoa [13,14]. To obtain micro-biopsies of the tubules, tissue micro-forceps were employed. In cases of initial samples that did not yield spermatozoa, additional samples were collected from both the same testis and the contralateral testis, if necessary. The collected specimens were transferred to a sterile tube containing 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid-buffered sperm medium, where they were subjected to further examination by an embryologist. Furthermore, a small portion of the testicular tissue was sent for histological evaluation. The histological diagnosis depended on the degree of spermatogenesis and included a Sertoli cell only pattern (characterized by seminiferous tubules devoid of germ cells), maturation arrest (impaired maturation up to the primary spermatocyte level or arrested at the spermatid stage), and hypospermatogenesis (reduced number of spermatozoa but all stages of sperm cell development present). In cases of mixed patterns, the most advanced histopathological diagnosis from all specimens was considered.
5. Intratesticular fluid analysis: quantification of testosterone levels and its precursors
The intratesticular fluid was kept at a temperature of -80 ℃ until analysis. To extract the analytes, 10 µL of intratesticular fluid was mixed with 90 µL of acetonitrile (ACN) and vortexed for 30 seconds to precipitate the protein. The sample was then centrifuged at 16,000×g for 10 minutes, and the supernatant was collected. The supernatant was then reconstituted using a 100 µL solution containing 0.1% formic acid in water and 0.1% formic acid in ACN (80:10, v/v). Liquid chromatography with tandem mass spectrometry (LC-MS/MS) was used to measure the levels of testosterone and its precursors, including progesterone, 17-OH progesterone, androstenedione, and DHEA. The internal standard used was D3-testosterone. The analysis was performed using SCIEX Triple Quad 5500 in electrospray ionization positive ion mode. The quantification of the analytes was determined using multiple reaction monitoring of the protonated precursor ions (testosterone, m/z 289.2; D3-testosterone, m/z 292.2) and the related product ions (m/z 109 and m/z 97). The data were processed using MultiQuant™ (version 3.0; Sciex). To assess enzymatic activity (CYP17A1, HSD17B3, and HSD3B1/2), ratios of 17-OH progesterone/progesterone (indicative of CYP17A1 activity), androstenedione/17-OH progesterone (indicative of CYP17A1 activity), testosterone/androstenedione (indicative of HSD17B3 enzyme activity), and androstenedione/DHEA (indicative of HSD3B1/2 activity) within TIF samples were analyzed.
6. Statistical analysis
Categorical and nominal variables are summarized as absolute numbers along with corresponding percentages, and their significance was evaluated using Fisher's exact test. Continuous variables that did not follow a normal distribution, as determined by the Shapiro–Wilk test, were compared using the Mann–Whitney U test for two groups or the Kruskal–Wallis test for three or more groups. The results for continuous variables are reported as medians with interquartile ranges (IQRs), while categorical variables are reported as frequencies with proportions. The primary outcome measure of interest was the rate of successful sperm retrieval. Statistical significance was defined as a two-tailed p-value of less than 0.05. All statistical analyses were performed using IBM SPSS software, version 24.0 (IBM Corp.). Graphical illustrations were generated using GraphPad Prism, version 9.5.1 (GraphPad Software).
RESULTS
1. Patients
A total of 37 patients were prospectively enrolled in the study. Five patients who had previously undergone vasectomy and sought vasectomy reversal were identified as having OA with normal spermatogenesis. These patients who had not undergone testosterone replacement therapy served as the control group. The remaining 32 patients were diagnosed with NOA. Karyotype analysis and Y-chromosome microdeletion analysis using the polymerase chain reaction method revealed that one patient had Klinefelter syndrome, while three patients had Y-chromosome AZFc microdeletion. All 32 patients with NOA underwent mTESE. Histopathological examination of testicular tissue samples from these patients revealed that 16 had hypospermatogenesis, 7 had maturation arrest, and 9 had Sertoli cell-only histology. The detailed clinical characteristics and demographic information of the study participants are presented in Table 1.
Table 1. Clinical characteristics, serum hormone levels, and intratesticular concentrations of testosterone and its precursors among different testicular histopathological groups.
| Characteristic | SCO (n=9) | MA (n=7) | HS (n=16) | NR (n=5) | p-valuea | p-valueb | |
|---|---|---|---|---|---|---|---|
| Age (y) | 37 (35.0–38.5) | 34.0 (31.0–38.0) | 35.0 (32.0–40.5) | 33.0 (29.5–47.0) | 0.489 | 0.055 | |
| Right testis size (mL) | 9.0 (6.5–15.5) | 13 (7–20) | 7.0 (4.5–10.5) | 22.0 (16.5–24.0) | 0.108 | 0.003* | |
| Left testis size (mL) | 10.0 (7.5–15.0) | 16 (13–20) | 8 (5–14) | 21.0 (17.0–23.5) | 0.120 | 0.002* | |
| Baseline serum endocrine levels | |||||||
| FSH (IU/L) | 25.6 (14.4–32.6) | 12.8 (10.5–15.2) | 24.4 (15.7–33.0) | 3.6 (2.3–4.3) | 0.620 | <0.001* | |
| LH (IU/L) | 7.7 (5.8–28.5) | 5.8 (5.4–7.2) | 12.5 (8.1–15.8) | 6.0 (4.0–6.7) | 0.373 | 0.014* | |
| Testosterone (ng/mL) | 3.7 (2.1–6.5) | 2.4 (2.1–4.8) | 3.7 (2.9–4.6) | 3.9 (3.1–4.8) | 0.477 | 0.647 | |
| Prolactin (ng/mL) | 11.3 (8.7–12.3) | 11.5 (7.5–18.7) | 11.5 (8.3–15.5) | 8.9 (6.6–16.5) | 0.785 | 0.646 | |
| Estradiol (pg/mL) | 22.4 (16.1–31.1) | 24.3 (14.0–31.5) | 23.9 (15.6–34.0) | 26.9 (22.3–31.3) | 0.982 | 0.890 | |
| Intratesticular endocrine levels | |||||||
| Testosterone (ng/mL) | 1,812.5 (1,330.8–2,655.1) | 2,112.6 (1,115.9–3,178.8) | 2,395.7 (1,495.1–3,060.1) | 207.5 (151.6–307.8) | 0.665 | 0.002* | |
| Androstenedione (ng/mL) | 7.6 (4.1–10.1) | 7.5 (1.8–22.8) | 6.8 (4.2–10.8) | 1.9 (1.4–2.5) | 0.932 | 0.065 | |
| 17-OHP (ng/mL) | 15.1 (9.4–23.7) | 8.8 (6.8–15.2) | 20.4 (12.5–28.4) | 1.8 (1.0–2.4) | 0.079 | <0.001* | |
| Progesterone (ng/mL) | 1.3 (0.2–2.2) | 2.1 (0.3–2.8) | 1.2 (0.8–2.1) | 0.7 (0.6–3.4) | 0.731 | 0.914 | |
| DHEA (ng/mL) | 3.4 (1.1–6.7) | 10.1 (4.1–13.5) | 4.6 (2.0–11.4) | 2.0 (2.0–2.2) | 0.218 | 0.156 | |
Values are presented as median (interquartile range).
NR: normal spermatogenesis, HS: hypospermatogenesis, MA: maturation arrest, SCO: Sertoli cell only, FSH: follicle-stimulating hormone, LH: luteinizing hormone, 17-OHP: 17 hydroxyprogesterone, DHEA: dehydroepiandrosterone.
Statistically significant (*p<0.05).
aSCO vs. MA vs. HS; bSCO vs. MA vs. HS vs. NR.
The median baseline serum endocrine levels for men with OA were as follows: FSH, 3.6 IU/L; LH, 6.0 IU/L; testosterone, 3.9 ng/mL; prolactin, 8.9 ng/mL; and estradiol, 26.9 pg/mL. Conversely, for men with NOA, the median baseline serum endocrine levels were FSH, 20.8 IU/L; LH, 8.1 IU/L; testosterone, 3.6 ng/mL; prolactin, 11.4 ng/mL; and estradiol, 24 pg/mL (Table 1, 2).
Table 2. Characteristics of OA men and NOA men with successful/unsuccessful sperm retrieval.
| Characteristic | OA men (n=5) | NOA men (n=32) | p-value | NOA men with successful sperm retrieval (n=16) | NOA men with unsuccessful sperm retrieval (n=16) | p-value | |
|---|---|---|---|---|---|---|---|
| Age (y) | 33.0 (29.5–47.0) | 36.0 (33.0–38.5) | 0.190 | 35.0 (32.5–40.5) | 36.0 (33.5–38.0) | 0.881 | |
| Right testis size (mL) | 22.0 (16.5–24.0) | 8.0 (5.5–15.0) | 0.003* | 7.0 (4.5–10.5) | 13.0 (7.0–16.5) | 0.054 | |
| Left testis size (mL) | 21.0 (17.0 –23.5) | 13.0 (7.0–15.0) | 0.002* | 8.0 (5.0–14.0) | 13.5 (9.0 –16.0) | 0.124 | |
| Medication prior to mTESE (%) | 0 (0.0) | 15 (46.9) | 0.067 | 9 (56.3) | 6 (37.5) | 0.480 | |
| Etiology (%) | |||||||
| Idiopathic | 0 (0.0) | 23 (71.9) | 0.005* | 9 (56.3) | 14 (87.5) | 0.113 | |
| Cryptorchidism | 0 (0.0) | 2 (6.3) | 1.000 | 1 (6.2) | 1 (6.2) | 1.000 | |
| Previous testicular cancer | 0 (0.0) | 2 (6.3) | 1.000 | 2 (12.5) | 0 (0.0) | 0.484 | |
| Previous mumps and orchitis | 0 (0.0) | 1 (3.1) | 1.000 | 1 (6.2) | 0 (0.0) | 1.000 | |
| Klinefelter, 47 XXY | 0 (0.0) | 1 (3.1) | 1.000 | 0 (0.0) | 1 (6.2) | 1.000 | |
| AZFc microdeletion | 0 (0.0) | 3 (9.3) | 1.000 | 3 (2.8) | 0 (0.0) | 0.226 | |
| Baseline serum endocrine levels | |||||||
| FSH (IU/L) | 3.6 (2.3–4.3) | 20.8 (12.9–28.8) | <0.001* | 24.4 (17.4) | 15.8 (12.5–27.4) | 0.147 | |
| LH (IU/L) | 6.0 (4.0–6.7) | 8.1 (5.8–15.2) | 0.038* | 12.5 (7.7) | 7.0 (5.6–8.1) | 0.080 | |
| Testosterone (ng/mL) | 3.9 (3.1–4.8) | 3.6 (2.7–4.8) | 0.638 | 3.7 (1.7) | 3.2 (2.1–5.5) | 0.298 | |
| Prolactin (ng/mL) | 8.9 (6.6–16.5) | 11.4 (8.3–15.2) | 0.258 | 11.5 (7.1) | 11.4 (8.7–14.0) | 0.952 | |
| Estradiol (pg/mL) | 26.9 (22.3–31.3) | 24 (14.2–33.1) | 0.465 | 23.9 (18.4) | 24.3 (14–31.5) | 0.920 | |
| Intratesticular endocrine levels | |||||||
| Testosterone (ng/mL) | 207.5 (151.6–307.8) | 1,871.9 (1,182.6–2,721.3) | <0.001* | 2,395.7 (1,495.1–3,060.1) | 1,876.0 (1,330.8–2,752.9) | 0.119 | |
| Androstenedione (ng/mL) | 1.9 (1.4–2.5) | 7.5 (3.6–10.8) | 0.008* | 6.8 (4.2–10.8) | 7.9 (2.6–13.1) | 0.912 | |
| 17-OHP (ng/mL) | 1.8 (1.0–2.4) | 15.1 (8.8–23.7) | <0.001* | 20.4 (12.5–28.4) | 10.8 (8.3–19.1) | 0.067 | |
| Progesterone (ng/mL) | 0.7 (0.6–3.4) | 1.3 (0.5–2.1) | 0.873 | 1.2 (0.8–2.1) | 1.5 (0.3–2.2) | 0.865 | |
| DHEA (ng/mL) | 2.0 (2.0–2.2) | 4.3 (1.8–10.6) | 0.162 | 4.6 (2.0–11.4) | 4.3 (1.5–10.6) | 0.779 | |
| CYP17A1 activity | |||||||
| 17-OHP/progesterone | 2.6 (0.4–4.0) | 16.1 (7.9–29.3) | 0.001* | 16.8 (11.6–26.5) | 10.8 (5.4–42.1) | 0.535 | |
| Androstenedione/17-OHP | 1.1 (1.0–2.0) | 0.4 (0.3–0.8) | 0.008* | 0.4 (0.3–0.7) | 0.5 (0.3–0.8) | 0.535 | |
| HSD17B3 activity | |||||||
| Testosterone/androstenedione | 114.0 (60.4–242.4) | 270.1 (179.1–395.6) | 0.035* | 311.7 (217.6–389.4) | 217.5 (165.0–415.9) | 0.373 | |
| HSD3B1/2 activity | |||||||
| Androstenedione/DHEA | 0.9 (0.6–1.2) | 1.5 (0.5–5.2) | 0.190 | 1.6 (0.5–4.2) | 1.4 (1.7–6.2) | 0.960 | |
Values are presented as median (interquartile range) and proportion (%).
OA: obstructive azoospermia, NOA: non-obstructive azoospermia, mTESE: microdissection testicular sperm extraction, FSH: follicle-stimulating hormone, LH: luteinizing hormone, 17-OHP: 17 hydroxyprogesterone, DHEA: dehydroepiandrosterone.
Statistically significant (*p<0.05).
Among the 32 patients with NOA, 15 received hormone optimization treatment including clomiphene citrate, anastrozole, or hCG for a period of 2 to 3 months (Table 2). For those who received hormone treatment to optimize testosterone levels and those who did not, the sperm retrieval rate was 60.0% (9/15) and 41.2% (7/17), respectively, but the difference in successful retrieval rates did not reach statistical significance (p=0.480).
2. Quantification of ITT and its precursor levels among men with OA and NOA
The median (IQR) ITT, androstenedione, 17-OH progesterone, progesterone, and DHEA levels were 207.5 ng/mL (151.6–307.8 ng/mL), 1.9 ng/mL (1.4–2.5 ng/mL), 1.8 ng/mL (1.0–2.4 ng/mL), 0.7 ng/mL (0.6–3.4 ng/mL), and 2.0 ng/mL (2.0–2.2 ng/mL), respectively, in men with OA. Men with NOA exhibited significantly higher ITT, androstenedione, and 17-OH progesterone levels than men with OA. However, the levels of progesterone and DHEA were comparable between the two groups (Table 2). Moreover, among men with NOA who had not undergone hormone treatment, there were significantly elevated levels of ITT (median 1,528.1 vs. 207.5 ng/mL), androstenedione (median 10.6 vs. 1.9 ng/mL), and 17-OH progesterone (median 13.0 vs. 1.8 ng/mL) compared to men with OA. Notably, there were no significant differences in the levels of progesterone and DHEA when comparing men with NOA without prior hormone treatment to those with OA. Regarding ITT and its precursors, no significant differences in levels were observed between individuals who underwent medical treatment and those who did not.
3. Quantification of ITT and its precursor levels and their association with the outcome of sperm retrieval
When comparing the intratesticular levels between men with NOA with and without successful sperm retrieval, no significant differences were observed in the ITT, androstenedione, 17-OH progesterone, progesterone, and DHEA levels (Table 2). However, in the subgroup of patients who did not receive medical treatment, men with successful sperm retrieval had significantly higher levels of androstenedione and testosterone (median [IQR] androstenedione: 10.6 ng/mL [5.0–19.2] vs. 4.1 ng/mL [1.8–7.6], p=0.036; testosterone: 2,441.5 ng/mL [1,821.0–2,982.3] vs. 1,330.8 ng/mL [764.3–1,528.1], p=0.046). Conversely, in the subgroup of patients who received medical treatment, men with successful sperm retrieval had significantly lower levels of androstenedione (median [IQR] androstenedione: 5.7 ng/mL [2.8–9.9] vs. 18.5 ng/mL [12.0–29.1], p=0.004) (Fig. 1).
Fig. 1. Intratesticular testosterone and its precursor levels and their association with the outcome of sperm retrieval in men with NOA. 17-OHP: 17 hydroxyprogesterone, DHEA: dehydroepiandrosterone, NOA: non-obstructive azoospermia. Statistically significant (*p<0.05, **p<0.01).
The ITT levels were approximately 445.3 times higher than the simultaneously collected serum testosterone levels in men with NOA who had unsuccessful surgical sperm retrieval. Although numerically lower, this increase in ITT levels compared with serum testosterone levels is similar to the approximately 623-fold increase observed in men with NOA who had successful surgical sperm retrieval (p=0.254). The ITT/serum testosterone ratio was also comparable in men with NOA whose testicular tissue showed different phenotypes (~479 in Sertoli cell only syndrome vs. 401 in maturation arrest vs. ~623 in hypospermatogenesis, p=0.463). Furthermore, this ratio showed no significant difference between men with NOA who received hormone therapy prior to surgical sperm retrieval and those who did not (~492 vs. ~583, p=0.254).
4. Comparison of 17α-hydroxylase/17,20-lyase (CYP17A1), 3β-hydroxysteroid dehydrogenase (HSD17B3), and 3β-hydroxysteroid dehydrogenase (HSD3B1/2) activity in men with OA and NOA
The results revealed higher 17-OH progesterone/progesterone and testosterone/androstenedione ratios in men with NOA than in those diagnosed with OA. However, a lower androstenedione/17-OH progesterone ratio was observed in men with NOA (Table 2).
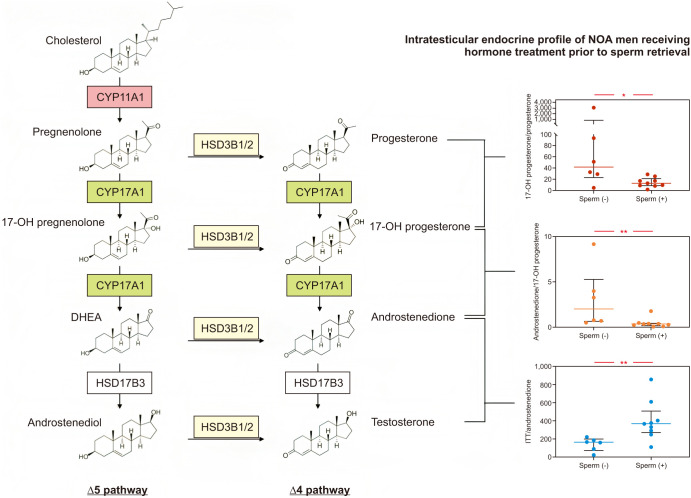
Furthermore, the outcomes indicated no significant differences in these ratios among men with NOA with varying sperm retrieval outcomes. However, upon further examination of a subset of men who underwent hormone manipulation treatment to optimize testosterone levels, discernible disparities emerged in the ratios of testosterone/androstenedione, androstenedione/17-OH progesterone, and 17-OH progesterone/progesterone between individuals who experienced successful sperm recovery and those who did not. Specifically, the ratios of 17-OH progesterone/progesterone and androstenedione/17-OH progesterone were significantly lower (median [IQR]: 13.2 [8.9–21.6] vs. 42.1 [29.4–93.8], p=0.029; and 0.4 [0.2–0.5] vs. 2.0 [0.7–4.0], p=0.006), whereas the testosterone/androstenedione ratio was notably higher in men with successful sperm retrieval (median [IQR]: 365.8 [271.9–505.3] vs. 165.0 [92.6–189.9], p=0.008) than in individuals with NOA who yielded no sperm during mTESE (Fig. 2). Furthermore, within the subset of men with NOA who did not undergo medical treatment before mTESE but achieved successful sperm retrieval, the ratio of 17-OH progesterone/progesterone was substantially higher (median [IQR]: 17.3 [15.9–60.1] vs. 6.6 [4.4–12.7], p=0.017) than in those without successful sperm retrieval (Fig. 3).
Fig. 2. CYP17A1 and HSD17B3 enzymes activity of NOA men receiving hormone treatment prior to sperm retrieval. 17-OHP: 17 hydroxyprogesterone, DHEA: dehydroepiandrosterone, NOA: non-obstructive azoospermia. Statistically significant (*p<0.05, **p<0.01).
Fig. 3. CYP17A1 and HSD17B3 enzymes activity of NOA men “not” receiving hormone treatment prior to sperm retrieval. 17-OHP: 17 hydroxyprogesterone, DHEA: dehydroepiandrosterone, NOA: non-obstructive azoospermia. Statistically significant (*p<0.05).
DISCUSSION
In this study, we investigated the altered Leydig cell steroidogenic function in men with primary testicular failure. A key finding of this study is that men with NOA who achieved successful sperm retrieval and underwent medical treatment prior to the procedure exhibited lower androstenedione levels, decreased activity of the CYP17A1 enzyme, and higher HSD17B3 activity in the intratesticular environment compared to those with unsuccessful sperm retrieval despite medical treatment. To the best of our knowledge, this study is the first to explore the relationship between ITT precursors and the outcome of surgical testicular sperm extraction.
The pathways of androgen biosynthesis in men with defective spermatogenesis have also been extensively investigated and documented in the scientific literature. In individuals presenting with oligozoospermia and severe Sertoli cell dysfunction, characterized by elevated FSH levels, the administration of hCG elicits an atypical response marked by excessive secretion or accumulation of 17-OH progesterone. This abnormal response is accompanied by an elevated ratio of 17-OH progesterone to testosterone, indicating a dysregulation in the androgen biosynthesis pathways within these individuals [15]. A recent study by Belli et al [16]. demonstrated that the administration of a relatively high dose of hCG (5,000 IU) to patients with Klinefelter syndrome resulted in an increased serum ratio of 17-OH progesterone to progesterone; however, this ratio remained significantly lower than the levels observed in the control group. Furthermore, these patients exhibited a reduced ability to produce testosterone in response to gonadotropin stimulation compared with the control group. These findings suggest a diminished stimulatory effect of hCG on the conversion rate of progesterone or 17-OH progesterone in men with defective spermatogenesis.
The enzymatic activity of HSD17B3 is reliant on the utilization of the nicotinamide cofactors NADPH/NADP+ and NADH/NAD+ [17]. The availability and abundance of these cofactors play crucial roles in determining the magnitude of the steroidogenic hydroxysteroid dehydrogenase reactions mediated by HSD17B3. The intracellular redox state directly impacts the abundance of nicotinamide cofactors [17]. Thus, the metabolic state of the cell, as reflected by its intracellular redox state, can exert a significant influence on the activity of HSD17B3 involved in steroid metabolism. In our study, we observed enhanced HSD17B3 enzyme activity, as inferred by the testosterone/androstenedione ratio, in men with NOA who presented focal areas of sperm production within their testes and achieved successful sperm recovery after undergoing medical hormonal treatment. It is plausible that in these individuals, hormone manipulation collaboratively regulates cellular redox states under both normal and pathological conditions, leading to augmented HSD17B3 activity. However, to provide robust evidence supporting the potential benefits of pharmacological interventions or bioactive NAD+ precursors in improving sperm development through increasing HSD17B3 enzyme activity, further measurements of the redox status and HSD17B3 activity must be validated before and after medical treatment.
An earlier in vitro investigation illustrated that oxidative stress increased the enzyme activity of CYP17A1 through the phosphorylation of CYP17A1 via mitogenactivated protein kinase 14 [18]. Consistent with our study's results, the identified reduction in CYP17A1 activity among men with NOA who successfully underwent sperm retrieval after prior medical treatment suggests a potentially diminished level of oxidative stress within the testicular microenvironment.
According to the findings of the present study, NOA men who achieved successful sperm retrieval following hormone optimization therapy exhibited a decrease in androstenedione and an increase in HSD17B3 enzyme activity. These observed endocrine alterations subsequent to hormone manipulation are theoretically detectable in the bloodstream. Therefore, our subsequent investigative step will involve examining the correlation between testosterone precursors in serum and those in the TIF. If this correlation is substantiated, men with NOA undergoing mTESE could potentially undergo hormone treatment, followed by monitoring serum testosterone precursor levels to estimate their likelihood of successful sperm retrieval.
In contrast to men with NOA attributed to secondary hypogonadism who exhibit low ITT levels leading to inhibited spermatogenesis, men with NOA resulting from primary gonadal failure have been reported to have elevated ITT levels [8]. These elevated ITT levels can exceed serum levels by more than 400 times, which is compared with the approximately 100 times in men with normal spermatogenesis [6,8]. Consequently, the rationale for employing hormone stimulation in men with NOA undergoing surgical sperm retrieval to further increase ITT levels remains a topic of debate [19]. Nevertheless, there are reports in the literature demonstrating that hormone stimulation improves sperm retrieval rates in men with NOA undergoing initial or rescue mTESE procedures [20,21]. In accordance with the findings of a previous study by Shinjo et al [21], our results showed that the ITT levels were comparable between men with NOA with and without successful sperm retrieval, and they had no predictive value for sperm recovery. Moreover, in accordance with the findings of Tuttelmann et al [8], the ITT/testosterone ratio in men with NOA was significantly higher than that of fertile controls. These findings confirm the idea that testosterone is not the sole factor that impacts spermatogenesis. In addition, the high ITT/testosterone ratio in men with NOA may be related to changes in the testicular vasculature, where disturbed testicular circulation hinders testosterone release into the bloodstream, leading to the sequestration of ITT. Furthermore, a reduced vascular bed could contribute to diminished release of testosterone into the bloodstream [8]. However, our study identified a higher ITT concentration than the previous study conducted by Shiraishi et al [22]. This discrepancy is likely attributable to differences in the methods used to collect TIF samples. In our approach a 20-gauge Jelco peripheral IV catheter–attached syringe was employed to primarily collect interstitial fluid, thereby minimizing the collection of intra-seminiferous fluid [14]. In contrast, Shiraishi et al [22] utilized a butterfly needle, which might inherently collect fluid from both within the seminiferous tubules and interstitial fluid. A previous study has confirmed significantly higher ITT concentrations in interstitial fluid compared to the seminiferous tubule compartment [6], potentially explaining the disparity in ITT levels between our study and that of Shiraishi et al [22].
Our comparative analysis of ITT levels between men with NOA and OA provides insights into the distinctive hormonal profiles in the testis microenvironment in men with defective spermatogenesis. Intriguingly, men with NOA exhibited significantly elevated ITT levels, androstenedione, and 17-OH progesterone, compared with their OA counterparts. These findings underscore the complex endocrine disruptions characteristic of NOA, suggesting potential dysregulation of the HPG axis and Leydig cell function. The observed shift in steroidogenesis pathways, particularly towards the Δ4 pathway, implies a compensatory mechanism in response to impaired spermatogenesis.
This study represents the first known investigation of ITT levels and its precursors using TIF in relation to the surgical sperm retrieval outcome in men with NOA. While we observed a higher testosterone/androstenedione ratio in men with NOA who underwent successful sperm retrieval with prior hormone treatment, it is important to consider several potential limitations and strengths when interpreting these findings. One notable strength of this study is the use of TIF, which provides a more specific representation of testicular steroidogenesis than serum measurements, as the evaluated hormones are exclusively secreted by Leydig cells. Unlike serum measurements, which include a mixture of testicular and adrenal hormone sources, individual steroid concentrations and their relative ratios in TIF can provide insights into Leydig cell steroidogenic enzyme activity. Another strength is the utilization of LC-MS/MS for precise identification and quantification of endogenous steroid profiles, requiring only minute sample volumes in a single measurement. However, this study has certain limitations that warrant thorough discussion. First, it was a single-center study with a limited sample size, which may restrict the generalizability of the findings. This underscores the necessity for larger, multicenter studies to validate and extend our findings. Second, most of the patients with NOA in this study had an idiopathic etiology, indicating the need for a larger sample size that includes men with NOA of various etiologies to determine the general applicability of these results. In addition, the assessment of enzyme activity was inferred from the ratios of individual steroid concentrations in TIF and was not validated using techniques such as immunohistochemistry or enzymatic assays. Therefore, further research incorporating immunohistochemistry or other techniques to assess enzyme expression directly within Leydig cells is necessary to acknowledge enzyme activity and contribute valuable insights into the expression levels of CYP17A1, HSD17B3, and HSD3B1/2 enzymes in men with azoospermia.
CONCLUSIONS
In summary, our study contributes to a deeper understanding of the complex hormonal environment within the testis, with significant implications for spermatogenesis in men with impaired sperm production. We found that men diagnosed with NOA and primary testicular failure who underwent hormonal optimization therapy and achieved successful sperm retrieval exhibited reduced intratesticular androstenedione levels, diminished CYP17A1 enzyme activity and increased HSD17B3 enzyme activity. Additionally, when compared to men with OA, men with NOA exhibited significantly higher ITT, androstenedione, and 17-OH-progesterone levels.
Acknowledgements
The authors thank Howard En-Hao Tien and Irene Yea-Lan Chang for their valuable statistical assistance.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: This work was supported by the Taipei Veterans General Hospital (VGH109-C-132 to William J. Huang and V112B-034 to I-Shen Huang) and Urological Surgical Medical Research and Development Foundation.
- Conceptualization: ISH, WJH.
- Data curation: ISH, WJC, WJH.
- Formal analysis: all authors.
- Funding acquisition: ISH, WJH.
- Investigation: ISH, LHL, WJH.
- Methodology: ISH, LHL, WJH.
- Project administration: ISH, CCJ, WJH.
- Resources: WJH.
- Software: ISH.
- Supervision: CCJ, WJH.
- Validation: ISH, WJH.
- Visualization: ISH.
- Writing – original draft: all authors.
- Writing – review & editing: all authors.
Data Sharing Statement
The data analyzed for this study have been deposited in HARVARD Dataverse and are available at https://doi.org/10.7910/DVN/PWYDIP.
References
- 1.Roberts KP, Zirkin BR. Androgen regulation of spermatogenesis in the rat. Ann N Y Acad Sci. 1991;637:90–106. doi: 10.1111/j.1749-6632.1991.tb27303.x. [DOI] [PubMed] [Google Scholar]
- 2.Smith LB, Walker WH. The regulation of spermatogenesis by androgens. Semin Cell Dev Biol. 2014;30:2–13. doi: 10.1016/j.semcdb.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker WH, Cheng J. FSH and testosterone signaling in Sertoli cells. Reproduction. 2005;130:15–28. doi: 10.1530/rep.1.00358. [DOI] [PubMed] [Google Scholar]
- 4.Mruk DD, Cheng CY. The mammalian blood-testis barrier: its biology and regulation. Endocr Rev. 2015;36:564–591. doi: 10.1210/er.2014-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai MY, Yeh SD, Wang RS, Yeh S, Zhang C, Lin HY, et al. Differential effects of spermatogenesis and fertility in mice lacking androgen receptor in individual testis cells. Proc Natl Acad Sci U S A. 2006;103:18975–18980. doi: 10.1073/pnas.0608565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarow JP, Chen H, Rosner TW, Trentacoste S, Zirkin BR. Assessment of the androgen environment within the human testis: minimally invasive method to obtain intratesticular fluid. J Androl. 2001;22:640–645. [PubMed] [Google Scholar]
- 7.Coviello AD, Bremner WJ, Matsumoto AM, Herbst KL, Amory JK, Anawalt BD, et al. Intratesticular testosterone concentrations comparable with serum levels are not sufficient to maintain normal sperm production in men receiving a hormonal contraceptive regimen. J Androl. 2004;25:931–938. doi: 10.1002/j.1939-4640.2004.tb03164.x. [DOI] [PubMed] [Google Scholar]
- 8.Tuttelmann F, Damm OS, Luetjens CM, Baldi M, Zitzmann M, Kliesch S, et al. Intratesticular testosterone is increased in men with Klinefelter syndrome and may not be released into the bloodstream owing to altered testicular vascularization– a preliminary report. Andrology. 2014;2:275–281. doi: 10.1111/j.2047-2927.2014.00190.x. [DOI] [PubMed] [Google Scholar]
- 9.Xue H, Wang SY, Cui LG, Hong K. Can contrast-enhanced ultrasound increase or predict the success rate of testicular sperm aspiration in patients with azoospermia? AJR Am J Roentgenol. 2019;212:1054–1059. doi: 10.2214/AJR.18.20436. [DOI] [PubMed] [Google Scholar]
- 10.Tash JA, McCallum S, Hardy MP, Knudsen B, Schlegel PN. Men with nonobstructive azoospermia have Leydig cell hypertrophy but not hyperplasia. J Urol. 2002;168:1068–1070. doi: 10.1016/S0022-5347(05)64576-4. [DOI] [PubMed] [Google Scholar]
- 11.Yanaihara T, Troen P. Studies of the human testis. I. Biosynthetic pathways for androgen formation in human testicular tissue in vitro. J Clin Endocrinol Metab. 1972;34:783–792. doi: 10.1210/jcem-34-5-783. [DOI] [PubMed] [Google Scholar]
- 12.Sato Y, Asahina K, Yoshiike M, Nozawa S, Otoi T, Iwamoto T. A change in the steroid metabolic pathway in human testes showing deteriorated spermatogenesis. Reprod Biol. 2020;20:210–219. doi: 10.1016/j.repbio.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod. 1999;14:131–135. doi: 10.1093/humrep/14.1.131. [DOI] [PubMed] [Google Scholar]
- 14.Huang IS, Li LH, Chen WJ, Huang EY, Juan CC, Huang WJ. Proteomic analysis of testicular interstitial fluid in men with azoospermia. Eur Urol Open Sci. 2023;54:88–96. doi: 10.1016/j.euros.2023.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anapliotou ML, Liparaki M, Americanos N, Goulandris N, Papaioannou D. Increased 17-OH-progesterone levels following hCG stimulation in men with idiopathic oligozoospermia and raised FSH levels. Int J Androl. 1994;17:192–198. doi: 10.1111/j.1365-2605.1994.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 16.Belli S, Santi D, Leoni E, Dall'Olio E, Fanelli F, Mezzullo M, et al. Human chorionic gonadotropin stimulation gives evidence of differences in testicular steroidogenesis in Klinefelter syndrome, as assessed by liquid chromatography-tandem mass spectrometry. Eur J Endocrinol. 2016;174:801–811. doi: 10.1530/EJE-15-1224. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal AK, Auchus RJ. Minireview: cellular redox state regulates hydroxysteroid dehydrogenase activity and intracellular hormone potency. Endocrinology. 2005;146:2531–2538. doi: 10.1210/en.2005-0061. [DOI] [PubMed] [Google Scholar]
- 18.Zhu W, Han B, Fan M, Wang N, Wang H, Zhu H, et al. Oxidative stress increases the 17,20-lyase-catalyzing activity of adrenal P450c17 through p38α in the development of hyperandrogenism. Mol Cell Endocrinol. 2019;484:25–33. doi: 10.1016/j.mce.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Minhas S, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. European Association of Urology guidelines on male sexual and reproductive health: 2021 update on male infertility. Eur Urol. 2021;80:603–620. doi: 10.1016/j.eururo.2021.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Hussein A, Ozgok Y, Ross L, Rao P, Niederberger C. Optimization of spermatogenesis-regulating hormones in patients with non-obstructive azoospermia and its impact on sperm retrieval: a multicentre study. BJU Int. 2013;111(3 Pt B):E110–E114. doi: 10.1111/j.1464-410X.2012.11485.x. [DOI] [PubMed] [Google Scholar]
- 21.Shinjo E, Shiraishi K, Matsuyama H. The effect of human chorionic gonadotropin-based hormonal therapy on intratesticular testosterone levels and spermatogonial DNA synthesis in men with non-obstructive azoospermia. Andrology. 2013;1:929–935. doi: 10.1111/j.2047-2927.2013.00141.x. [DOI] [PubMed] [Google Scholar]
- 22.Shiraishi K, Oka S, Matsuyama H. Testicular testosterone and estradiol concentrations and aromatase expression in men with nonobstructive azoospermia. J Clin Endocrinol Metab. 2021;106:e1803–e1815. doi: 10.1210/clinem/dgaa860. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed for this study have been deposited in HARVARD Dataverse and are available at https://doi.org/10.7910/DVN/PWYDIP.