Abstract
Purpose
Improvement of left ventricular (LV) diastolic dysfunction (DD) is known to be a good prognostic factor in patients with heart failure with reduced ejection fraction (EF). In the present study, we investigated the predisposing risk factors affecting the reversibility of LV diastolic filling pattern (DFP) in patients with preserved EF.
Materials and Methods
A total of 600 patients with pseudonormal LVDFP and preserved EF who underwent follow-up echocardiography were enrolled between 2011 and 2020. We compared their index and follow-up echocardiography findings and determined the predisposing risk factor affecting the reversibility of LVDFP.
Results
Comparing the index and follow-up echocardiography findings showed that 379 (63%) patients had improved to normal or impaired relaxation LVDFP (improved group) and 221 (37%) patients had maintained or worsened LVDFP (unimproved group). The incidence of paroxysmal atrial fibrillation (PAF) was significantly higher in the unimproved group than in the improved group (4.7% vs. 9.5%, p=0.026). After adjustment for relevant clinical risk factors of diastolic dysfunction, PAF was determined to be an independent predisposing risk factor for the unimproved LVDFP (odds ratio: 2.10, 95% confidence interval: 1.06–4.15, p=0.033). Among the parameters of diastolic dysfunction in follow-up echocardiography, the left atrial volume index, mean E/A ratio, and E/e’ were significantly improved in patients without PAF but remained in patients with PAF.
Conclusion
We identified that PAF was an independent predisposing risk factor of the unimproved LVDFP in patients with pseudonormal LVDFP and preserved EF. Therefore, early detection and management of PAF might be required in patients with LVDD and preserved EF to prevent adverse cardiovascular events.
Keywords: Left ventricle, diastole, atrial fibrillation, heart failure
Graphical Abstract
INTRODUCTION
Left ventricular (LV) diastolic dysfunction (DD) is routinely evaluated during echocardiographic assessment as it is observed in many cardiovascular diseases and is consequently associated with poor prognosis.1 LVDD is mainly explained as an increase in LV filling pressure as a result of abnormalities in LV relaxation, LV stiffness, and LV diastolic recoil. However, LVDD is not only determined by cardiac structures and functions, such as ventricular interactions, left atrial (LA) function, LV systolic and diastolic dyssynchrony, pericardium, and coronary blood flow, but also by various clinical conditions, including aging, hypertension, diabetes mellitus, renal insufficiency, and atrial fibrillation (AF). For example, AF is more likely to occur in patients with LVDD; although structural and functional abnormalities of the LA have been involved in its main mechanism, it has yet to be fully understood.2,3
According to what is known so far, early LVDD may be reversible depending on the state of intravascular volume.4 In clinical practice, it has been reported that symptoms and prognosis improve when DD improves in patients with various cardiovascular diseases. In addition, a previous study showed that patients with heart failure with reduced ejection fraction (HFrEF) had better prognosis when LVDD was improved. However, few data exist regarding factors affecting the reversibility of LVDD in patients with a variety of medical conditions, particularly in patients with preserved EF.5,6 In the present study, we investigated predisposing risk factors that influence the reversibility of LV diastolic filling pattern (DFP) in patients with pseudonormal LVDFP and preserved EF.
MATERIALS AND METHODS
Study population and data collection
This study enrolled 600 patients who met the pre-specified criteria at Inje University Ilsan Paik Hospital between December 2011 and May 2020. The inclusion criteria for this study were as follows: 1) patients with pseudonormal LVDFP (septal e’ <0.08 m/s and 0.8 ≤E/A ratio <2) and preserved EF (>50%) on index echocardiography and 2) patients who had follow-up echocardiography performed at least 1 month later. The exclusion criteria were as follows: patients who 1) had no A-wave velocity of the mitral valve inflow, 2) had regional wall motion abnormality, 3) had acute myocardial infarction (MI) and severe (stages C and D) valvular insufficiency on both the index and follow-up echocardiography, and 4) underwent heart valve surgery (Fig. 1). Past medical history and other demographic information of the patients were obtained from electrical medical records. Paroxysmal atrial fibrillation (PAF) was defined as sinus rhythm on electrocardiography and previous diagnosis of AF. HF was defined as the presence of at least one HF symptom and physical examination finding, or one physical examination finding and B-type natriuretic peptide (BNP) elevation (normal range for N-Terminal-proBNP was lower than 125 pg/mL for adults younger than 75 years and lower than 450 pg/mL for adults 75 years or older), and initiation or intensification of HF treatment.7 The institutional review board of Inje University Ilsan Paik Hospital (IRB nubmer 2021-12-026) approved this study.
Fig. 1. Study design and population. EF, ejection fraction; LVDFP, left ventricular diastolic filling pattern.
Echocardiographic evaluation
Two-dimensional echocardiography was performed using five different commercially available equipment (Vivid E95, Vivid E9, Vivid S5, Vivid I, and Vivid 7, GE Vingmed Ultrasound, Horten, Norway). Five cardiac sonographers performed echocardiography under the supervision of five cardiologists. In the present study, LVDFP was defined based on septal e’ and E/A ratio according to the previous guidelines (impaired relaxation: septal e’ <0.08 m/s and E/A ratio <0.8, pseudonormal: septal e’ <0.08 m/s and 0.8≤ E/A ratio <2, restrictive: septal e’ <0.08 m/s and E/A ratio ≥2).1,8 In addition to the above measurements, the following values were also obtained: LA diameter, LA volume index (LAVI), left ventricular ejection fraction (LVEF), LV end-diastolic diameter (LVEDD), LV end-systolic diameter, interventricular septal thickness at end-diastole, LV posterior wall thickness at end-diastole, deceleration time of mitral inflow, E/e’, maximal tricuspid regurgitation velocity (TR Vmax), and right ventricular systolic pressure (RVSP). The improved LVDFP was defined as normal and imparied relaxation LVDFP, and the unimproved LVDFP was defined as pseudonormal and restrictive LVDFP in follow-up echocardiography. Cut-off values of the parameters of LVDD were E/e’ >15, LAVI >34 mL/m2, and TR Vmax >2.8 m/s.
Clinical outcomes
The primary outcome was major adverse cardiac events (MACE), which were composite of all-cause death, non-fatal MI, cardiac hospitalization, or cerebrovascular accident (CVA). All deaths were considered to be of cardiac cause unless a definite non-cardiac cause could be established. MI was defined as elevated cardiac enzyme level greater than the upper limit of the normal range with either ischemic symptoms or electrocardiographic changes implicating ischemia, or MI at readmission that required subsequent hospitalization. Cardiac hospitalization was defined by hospitalization event due to cardiac causes except MI. CVA was defined as evidence of neurological deficit requiring hospitalization with clinically documented lesions on brain imaging.
Statistical analyses
Categorical variables were compared using chi-square tests or Fisher’s exact tests. Continuous variables were compared using Student’s t-test or Wilcoxon signed-rank test after normality test. Paired t-test was used for comparative analysis of parameters of the index and follow-up echocardiography findings. Variables that did not follow normal distribution, such as age and follow-up interval of echocardiography, were described as median with interquartile range, and other variables were described as mean with standard deviation. The multivariate logistic regression analysis was performed after adjusting for relevant clinical risk factors and variables of diastolic dysfunction, including age, sex, body mass index (BMI), PAF, HF, history of MI, chronic kidney disease (CKD), and follow-up interval of echocardiography. Survival rate was compared between groups using Kaplan–Meier curves, and significant differences were calculated using log-rank tests. Statistical significance was set at p<0.05. All statistical analyses were performed using the using the SPSS software, version 23 (IBM Corp., Armonk, NY, USA) and R Statistical Software (version 4.0.5; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Baseline clinical characteristics and laboratory parameters
The study population was divided into two groups, improved (375, 63%) and unimproved (221, 37%) LVDFP, according to changes in follow-up echocardiography findings. Baseline clinical characteristics are summarized in Table 1. There were no statistically significant differences observed in terms of age and sex distribution between the two groups. PAF was more frequently observed in the patients with unimproved LVDFP than in the patients with improved LVDFP (18 vs. 21, p=0.026). In addition, HF was frequently observed in the unimproved group; however, the difference was not statistically significant. Baseline medications were also not different between the two groups. The follow-up interval of echocardiography of the patients with improved LVDFP was significantly longer than that of the patients with unimproved LVDFP (33.3 months vs. 28.2 months, p=0.002). The initial laboratory parameters are listed in Supplementary Table 1 (only online). Protein and triglyceride values were statistically different between the two groups.
Table 1. Baseline Clinical Characteristics According to Changes of LVDFP.
| Variables | Improved LVDFP (n=379) | Unimproved LVDFP (n=221) | p value |
|---|---|---|---|
| Age (yr) | 66.4±11.1 | 66.2±11.8 | 0.84 |
| Female | 155 (40.9) | 101 (45.7) | 0.27 |
| Body mass index (kg/m2) | 25.8±3.8 | 26.1±4.7 | 0.45 |
| Index PCI or CABG | 39 (10.3) | 22 (10.0) | 0.85 |
| Previous PCI | 65 (17.2) | 34 (15.4) | 0.65 |
| Previous CABG | 12 (3.2) | 7 (3.2) | >0.999 |
| Previous MI | 16 (4.2) | 14 (6.3) | 0.25 |
| Paroxysmal AF | 18 (4.7) | 21 (9.5) | 0.026 |
| Heart failure | 29 (7.7) | 27 (12.2) | 0.07 |
| PPM | 5 (1.4) | 1 (0.5) | 0.80 |
| Diabetes mellitus | 131 (34.6) | 85 (38.5) | 0.38 |
| Hypertension | 272 (71.8) | 164 (74.2) | 0.57 |
| Chronic kidney disease | 30 (7.9) | 27 (12.2) | 0.09 |
| Chronic lung disease | 29 (7.7) | 16 (7.7) | 0.47 |
| Cancer | 39 (10.3) | 22 (10.0) | >0.999 |
| Thyroid diseases | 15 (4.0) | 7 (3.2) | 0.92 |
| CVA | 42 (11.1) | 15 (6.8) | 0.11 |
| Alcohol | 80 (21.1) | 50 (22.6) | 0.68 |
| Smoking | 89 (23.5) | 46 (20.8) | 0.56 |
| Renin-angiotensin system blocker | 150 (42.9) | 91 (47.2) | 0.37 |
| Calcium channel blocker | 155 (44.3) | 77 (39.9) | 0.37 |
| Beta blocker | 105 (30.0) | 70 (36.3) | 0.15 |
| Diuretics | 61 (17.4) | 40 (20.7) | 0.36 |
| Follow-up interval of echocardiography (months) | 33.3 (17.6–57.6) | 28.2 (12.6–45.4) | 0.002 |
LVDFP, left ventricular diastolic filling pattern; AF, atrial fibrillation; CABG, coronary artery bypass graft; CVA, cerebrovascular accident; LV, left ventricle; MI, myocardial infarction; PCI, percutaneous coronary intervention; PPM, permanent pacemaker.
Data are presented as mean±standard deviation, n (%), or median (interquartile range).
Echocardiographic parameters
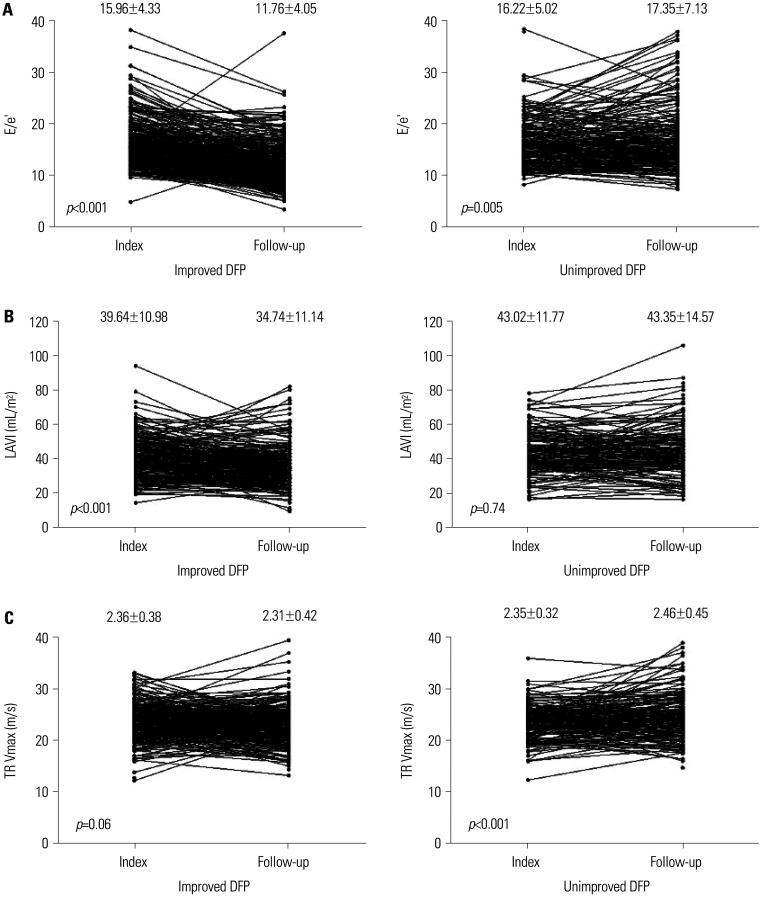
The reasons for the echocardiographic examination were summarized in Supplementary Table 2 (only online). The comparison of changes in echocardiographic parameters between the index and follow-up echocardiography according to the according to improvemnt of LVDFP are summarized in Table 2. In patients with unimproved LVDFP, LVEF was significantly decreased in the follow-up echocardiography compared to the index echocardiography, but it did not change in the patients with improved LVDFP. LVEDD and LA diameter were significantly decreased in the follow-up echocardiography compared to the index echocardiography in the patients with improved LVDFP; however, they did not change in the patients with unimproved LVDFP. The parameters related with LVDD, such as LAVI, E/A ratio, E/e’, TR Vmax, and RVSP, were decreased in the follow-up echocardiography compared to the index echocardiography in the patients with improved LVDFP; however, they did not change or were increased in the patients with unimproved LVDFP (Fig. 2).
Table 2. Echocardiographic Parameters between Index and Follow-Up Echocardiography According to Changes of LVDFP.
| Parameters | Improved LVDFP (n=379) | p value | Unimproved LVDFP (n=221) | p value | ||
|---|---|---|---|---|---|---|
| Index | Follow-up | Index | Follow-up | |||
| LVEF (%) | 66.83±6.32 | 66.12±6.70 | 0.07 | 66.63±6.41 | 65.24±8.18 | 0.026 |
| LVEDD (mm) | 51.35±4.21 | 49.61±4.76 | <0.001 | 51.39±4.36 | 51.11±4.66 | 0.32 |
| LVESD (mm) | 31.11±4.62 | 30.49±6.82 | 0.10 | 31.32±5.07 | 31.56±5.30 | 0.48 |
| IVSD (mm) | 9.55±1.41 | 9.58±1.74 | 0.67 | 9.61±1.23 | 9.85±1.76 | 0.045 |
| LVPWD (mm) | 9.54±1.19 | 9.44±1.28 | 0.11 | 9.57±1.26 | 9.51±1.25 | 0.55 |
| LA diameter (mm) | 41.43±4.69 | 40.16±4.91 | <0.001 | 42.61±4.25 | 42.71±5.56 | 0.74 |
| LAVI (mL/m2) | 39.67±10.97 | 35.20±12.71 | <0.001 | 42.98±11.76 | 43.52±15.66 | 0.74 |
| E/A ratio | 1.07±0.19 | 0.68±0.22 | <0.001 | 1.16±0.24 | 1.20±0.42 | 0.14 |
| DT (m/s) | 206.50±40.74 | 251.28±64.31 | <0.001 | 196.85±37.96 | 203.83±45.09 | 0.048 |
| e' | 0.06±0.01 | 0.05±0.02 | 0.002 | 0.05±0.01 | 0.05±0.01 | 0.001 |
| E/e' | 15.96±4.33 | 11.74±4.09 | <0.001 | 16.22±5.02 | 17.35±7.15 | 0.005 |
| TR Vmax (m/s) | 2.36±0.38 | 2.31±0.42 | 0.06 | 2.35±0.32 | 2.46±0.45 | <0.001 |
| RVSP (mm Hg) | 32.46±6.98 | 31.35±7.50 | <0.019 | 32.44±6.38 | 35.44±10.61 | <0.001 |
DT, deceleration time; IVSD, interventricular septum diameter; LA, left atrium; LAVI, left atrial volume index; LVDFP, left ventricular diastolic filling pattern; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; LVPWD, left ventricular posterior wall diameter; RVSP, right ventricular systolic pressure; TR Vmax, maximal tricuspid regurgitation velocity.
Data are presented as mean±standard.
Fig. 2. Serial changes of the LVDD parameters between index and follow-up echocardiography according to changes of LVDFP. (A) E/e’. (B) LAVI. (C) TR Vmax. LAVI, left atrial volume index; LVDD, left ventricular diastolic dysfunction; LVDFP, left ventricular diastolic filling pattern; TR Vmax, maximal tricuspid regurgitation velocity.
Predisposing risk factors affecting LVDD
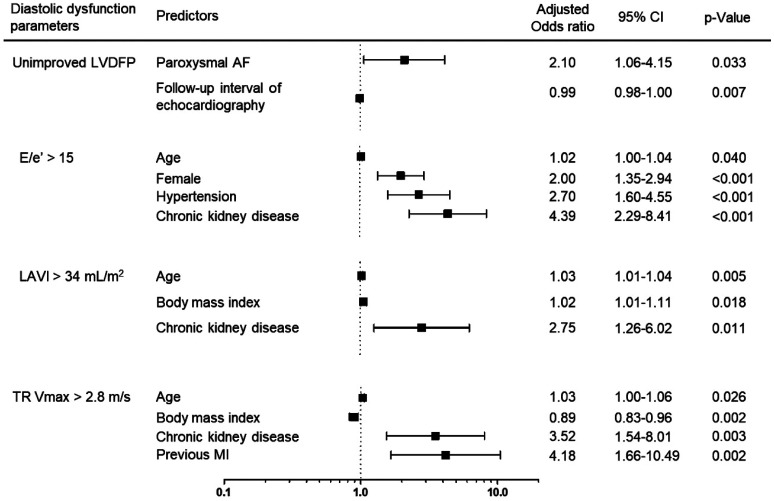
Logistic regression analysis for prognostic factors of unimproved LVDD is summarized in Table 3, Supplementary Tables 3, 4, 5, 6, 7 (only online), and Fig. 3. Multivariate logistic regression analysis revealed that PAF [odds ratio (OR): 2.10, 95% confidence interval (CI): 1.06–4.15, p=0.033] and shorter follow-up interval of echocardiography (OR: 0.99, 95% CI: 0.98–1.00, p=0.007) were independent predisposing risk factors for unimproved LVDFP in the follow-up echocardiography (Table 3). To reduce immortal time bias, we performed a landmark analysis only on the patients whose last clinical follow-up period was within 4.2 years, which was the median follow-up duration. Additionally, we conducted a sensitivity analysis after excluding patients who had follow-up echocardiography within 3 months after the index echocardiography. Consistently, PAF was an independent predisposing risk factor for unimproved LVDFP in the follow-up echocardiography in both analyses (Supplementary Tables 3 and 4, only online). Regarding other parameters of LVDD, age, female sex, hypertension, and CKD were independent predisposing risk factors for E/e’ >15 in the follow-up echocardiography (Supplementary Table 5, only online). Furthermore, age, higher BMI, and CKD were independent predisposing risk factors for LAVI >34 mL/m2 (Supplementary Table 6, only online); and age, lower BMI, previous MI, and CKD were independent predisposing risk factors for TR Vmax >2.8 m/s in the follow-up echocardiography (Supplementary Table 7, only online). PAF significantly increased the risk of E/e’ >15 and LAVI >34 mL/m2 in univariate logistic regression analysis, but not in multivariate logistic regression analysis.
Table 3. Logistic Regression Analysis for Prognostic Factors of Unimproved LVDFP.
| Variables | Univariate analysis | p value | Multivariate analysis | p value | ||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | Odds ratio | 95% CI | |||
| Age | 1.00 | 0.98–1.01 | 0.83 | 0.99 | 0.97–1.01 | 0.20 |
| Female | 1.22 | 0.87–1.70 | 0.25 | 1.26 | 0.89–1.79 | 0.19 |
| Body mass index | 1.02 | 0.98–1.06 | 0.42 | 1.02 | 0.98–1.07 | 0.31 |
| Paroxysmal AF | 2.11 | 1.10–4.05 | 0.025 | 2.10 | 1.06–4.15 | 0.033 |
| Previous MI | 1.53 | 0.73–3.21 | 0.26 | 1.56 | 0.73–3.35 | 0.25 |
| Heart failure | 1.68 | 0.97–2.92 | 0.07 | 1.54 | 0.86–2.73 | 0.15 |
| Diabetes | 1.18 | 0.84–1.67 | 0.34 | 1.13 | 0.77–1.66 | 0.52 |
| Hypertension | 1.13 | 0.78–1.65 | 0.52 | 1.14 | 0.75–1.73 | 0.54 |
| Chronic kidney disease | 1.62 | 0.94–2.80 | 0.09 | 1.19 | 0.65–2.19 | 0.57 |
| Follow-up interval of echocardiography | 0.99 | 0.98–1.00 | 0.003 | 0.99 | 0.98–1.00 | 0.007 |
AF, atrial fibrillation; CI, confidence interval; LVDFP, left ventricular diastolic filling pattern; MI, myocardial infarction.
Fig. 3. Independent predisposing risk factors for unimproved LVDD in the follow-up echocardiography. AF, atrial fibrillation; CI, confidence interval; LAVI, left atrial volume index; LVDD, left ventricular diastolic dysfunction; LVDFP, left ventricular diastolic filling pattern; MI, myocardial infarction; TR Vmax, maximal tricuspid regurgitation velocity.
The comparison of changes in echocardiographic parameters between the index and follow-up echocardiography according to the presence of PAF is shown in Table 4. The E/A ratio, E/e’, and LAVI were significantly decreased in the patients without PAF, but remained unchanged in the patients with PAF in the follow-up echocardiography compared to the index echocardiography.
Table 4. Echocardiographic Parameters in Patients with or without Paroxysmal AF.
| Parameters | No paroxysmal AF (n=561) | p value | Paroxysmal AF (n=39) | p value | ||
|---|---|---|---|---|---|---|
| Index | Follow-up | Index | Follow-up | |||
| LVEF (%) | 66.91±6.26 | 65.89±7.11 | 0.003 | 64.62±7.35 | 64.54±9.36 | 0.96 |
| LVEDD (mm) | 51.36±4.25 | 50.16±4.78 | <0.001 | 51.44±4.45 | 50.33±4.80 | 0.09 |
| LVESD (mm) | 31.11±4.76 | 30.82±6.34 | 0.31 | 32.26±5.08 | 31.85±5.88 | 0.65 |
| IVSD (mm) | 9.58±1.36 | 9.66±1.77 | 0.27 | 9.46±1.16 | 10.04±1.42 | 0.037 |
| LVPWD (mm) | 9.56±1.21 | 9.45±1.28 | 0.024 | 9.41±1.25 | 9.80±1.14 | 0.11 |
| LA diameter (mm) | 41.74±4.55 | 41.00±5.24 | <0.001 | 43.62±4.41 | 42.46±5.95 | 0.15 |
| LAVI (mL/m2) | 40.27±11.12 | 37.34±12.87 | <0.001 | 48.39±12.04 | 44.70±14.93 | 0.10 |
| E/A ratio | 1.10±0.21 | 0.85±0.36 | <0.001 | 1.17±0.28 | 1.14±0.72 | 0.75 |
| DT (m/s) | 203.23±39.77 | 234.09±62.41 | <0.001 | 198.87±43.13 | 229.54±60.99 | 0.007 |
| e' | 0.06±0.01 | 0.05±0.01 | <0.001 | 0.05±0.01 | 0.05±0.02 | 0.047 |
| E/e' | 16.00±4.59 | 13.65±5.89 | <0.001 | 16.87±4.64 | 16.32±7.36 | 0.61 |
| TR Vmax (m/s) | 2.35±0.37 | 2.36±0.43 | 0.78 | 2.43±0.30 | 2.51±0.47 | 0.24 |
| RVSP (mm Hg) | 32.35±6.78 | 32.67±8.82 | 0.46 | 33.80±6.35 | 36.23±11.18 | 0.14 |
AF, atrial fibrillation; DT, deceleration time; IVSD, interventricular septum diameter; LA, left atrium; LAVI, left atrial volume index; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; LVPWD, left ventricular posterior wall diameter; RVSP, right ventricular systolic pressure; TR Vmax, maximal tricuspid regurgitation velocity.
Data are presented as mean±standard.
Clinical outcomes according to LVDD
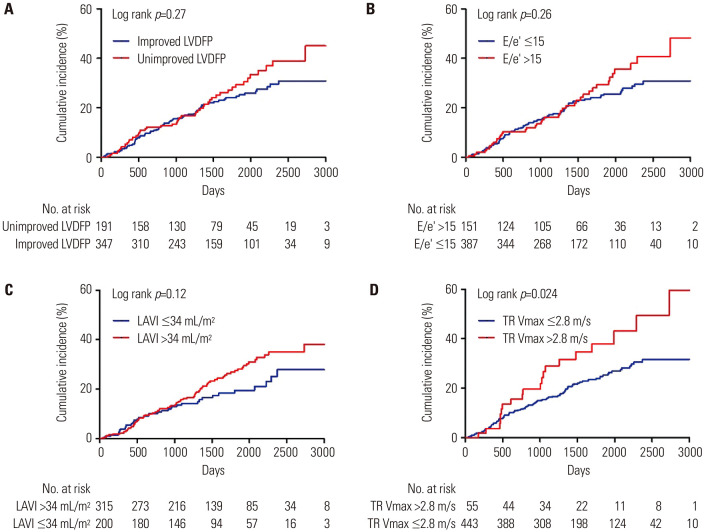
The median follow-up duration was 45.9 months (interquartile range: 27.3 to 68.5 months). Compared to the patients in the improved LVDFP group, those in the unimproved LVDFP group had a higher cumulative incidence of MACE (31.3% vs. 45.4%), but this difference was not statistically significant (p=0.27) (Fig. 4A). Similarly, patients with E/e’ >15 or LAVI >34 mL/m2 had a higher cumulative incidence of MACE compared to those with E/e’ ≤15 or LAVI ≤34 mL/m2, but these differences were also not statistically significant (Fig. 4B and C). However, patients with TR Vmax >2.8 m/s had a significantly higher cumulative incidence of MACE compared to those with TR Vmax ≤2.8 m/s (22.5% vs. 35.7%, p=0.024) (Fig. 4D).
Fig. 4. Kaplan–Meier curves for MACE according to LVDD. (A) LVDFP, (B) E/e’, (C) LAVI, (D) TR Vmax. LAVI, left atrial volume index; LVDD, left ventricular diastolic dysfunction; LVDFP, left ventricular diastolic filling pattern; TR Vmax, maximal tricuspid regurgitation velocity.
DISCUSSION
In the present study, more than half of the patients with pseudonormal LVDFP and preserved EF had improved LVDFP upon follow-up echocardiography. More importantly, we identified that PAF was an independent predisposing risk factor of the unimproved LVDFP in patients with preserved EF. Considering this, the possibility of LVDFP recovery might be decreased in patients with pseudonormal LVDFP and preserved EF who have PAF. Indeed, parameters reflecting LVDD, such as E/A ratio, E/e’, and LAVI, did not improve upon follow-up echocardiography in patients with PAF compared to patients without PAF. Regarding other parameters of LVDD, age and CKD were common independent predisposing risk factors for E/e’ >15, LAVI >34 mL/m2, and TR Vmax >2.8 m/s. PAF also increased the risk of E/e’ >15 and LAVI >34 mL/m2 in univariate logistic regression analysis, but not in multivariate logistic regression analysis.
LVDD is an independent predictor of poor prognosis in various cardiovascular diseases.4 Previous studies have shown that HF patients with restrictive filling patterns have better prognosis after improvement of LVDD through appropriate treatment. 6,9 These results are based on the reversibility of LVDD, which implies that some patients with restrictive LVDD patterns may have improved or worsened DD under certain circumstances. 10 However, patients with LVDD do not always have improvement in both LVDD and clinical outcomes due to the heterogeneous etiologies of LVDD. Notably, our study showed that LVDD parameters were not improved in patients with PAF who had pseudonormal LVDFP and preserved EF.
AF could be a cause and result of LVDD. LVDD increases LV filling pressure and is accompanied by LA remodeling and dysfunction, causing AF. While AF itself can worsen LA remodeling and LVDD, LA size and annular dimension are restored through rhythm control of AF.11 A previous study reported that LV mass and LA enlargement, rather than LVDD, were associated with PAF, irrespective of BMI, blood pressure, and renal function. These findings suggest that cardiac remodeling may occur very early in the natural history of AF. In our study, patients with PAF already had LVDD, and therefore, might already have had advanced and irreversible cardiac remodeling. Indeed, our results showed that the patients with unimproved LVDFP had more enlarged LA diameter (41.43±4.69 vs. 42.61±4.25, p=0.002) and increased LAVI (39.67±10.97 vs. 42.98±11.76, p=0.003) compared to those with improved LVDFP in the index echocardiography. Moreover, in our study, patients were well-controlled in terms of heart rate and rhythm by taking medications (Supplementary Table 8, only online), but most of these patients did not have improved or worsened LVDFP. It might be possible that the reversibility of LVDFP may be reduced despite the intake of these medications, as it is already accompanied by some remodeling in its early stages.
Until now, there remains to be no effective medications except for sodium glucose co-transporter two inhibitors in HFpEF patients that improve clinical outcomes.12 Therefore, early detection of the etiology and risk factors for progression of LVDD and HFpEF is crucial for improving the prognosis.13 From this point of view, the reversibility of LVDFP pointed out in our study provides an important insight into the treatment strategy of HFpEF. In the recently developed scoring system for the diagnosis of HFpEF, AF is the highest risk factor compared to all other risk factors. Furthermore, a recent study reported that increased LV filling pressure in patients with PAF is associated with LA dysfunction and more frequent strokes or transient ischemic attacks.14 Our study also showed that the patients with PAF had higher risk of unimproved LVDFP, and those with unimproved LVDFP had a higher tendency for MACE among patients who had pseudonormal LVDFP and preserved EF. Therefore, it is important to detect and manage AF early to improve LVDD and prevent HF and stroke.
Our study has several inherent limitations. First, the retrospective analysis of echocardiography registry data could have resulted in variations in the baseline clinical characteristics contributing to LVDD. Specifically, data regarding reversible factors of LVDD, such as volume status and medication during follow-up, were lacking. Additionally, we were unable to control for the reasons and intervals of echocardiography follow-up. To address these limitations, we categorized the reasons for echocardiographic examinations and conducted multivariate logistic regression analysis, adjusting for the follow-up interval of echocardiography, and additionally performed the landmark and sensitivity analyses. Nonetheless, these limitations should be addressed through a prospective study in the future.
In conclusion, we identified that PAF was an independent predisposing risk factor for the unimproved LVDFP in patients with pseudonormal LVDFP and preserved EF. Therefore, early detection and management of PAF might be necessary in patients with LVDD and preserved EF to prevent adverse cardiovascular events.
ACKNOWLEDGEMENTS
This research was funded by the Basic Science Research Program through the National Research Foundation (NRF) of Korea as funded by the Ministry of Science and ICT (MSIT) (2020R1C1C1015104).
We would like to thank the staff of the echocardiography room at Inje University Ilsan Paik Hospital who established the echocardiographic registry data and continuously filled data. We would also like to thank Editage (www.editage.co.kr) for the English language editing.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Sung Woo Cho.
- Data curation: Dong-Gil Kim, Seongjin Park, and Gi Rim Kim.
- Formal analysis: Sung Woo Cho and Dong-Gil Kim.
- Funding acquisition: Sung Woo Cho.
- Investigation: Sung Woo Cho and Sungsoo Cho.
- Methodology: Kyu-Yong Ko and Ji-won Hwang.
- Project administration: Dong-Gil Kim.
- Resources: Joon-Hyung Doh, Sung Uk Kwon, Jae-Jin Kwak, and June Namgung.
- Software: Sung Woo Cho, Kyu-Yong Ko, and Ji-won Hwang.
- Supervision: Sung Woo Cho, Sungsoo Cho, Kyu-Yong Ko, and Sung Eun Kim.
- Validation: Sung Woo Cho and Kyu-Yong Ko.
- Visualization: Sung Woo Cho and Dong-Gil Kim.
- Writing—original draft: Sung Woo Cho and Dong-Gil Kim.
- Writing—review & editing: Sung Woo Cho and Sungsoo Cho.
- Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIALS
Baseline Laboratory Parameters According to Changes of LVDFP
Reasons for Echocardiographic Examination
Logistic Regression Analysis for Prognostic Factors of Unimproved LVDFP on Patients Whose Last Clinical Follow-Up Period was Within 4.2 Years (n=274)
Logistic Regression Analysis for Prognostic Factors of Unimproved LVDFP after Excluding Patients Who Had Follow-Up Echocardiography Within 3 Months after the Index Echocardiography (n=572)
Logistic Regression Analysis for Prognostic Factors of E/e’ >15
Logistic Regression Analysis for Prognostic Factors of LAVI >34 mL/m2
Logistic Regression Analysis for Prognostic Factors of TR Vmax >2.8 m/s
Diagnosis Time, Antiarrhythmic Drugs, Anticoagulants, and RFCA Status in Patients with Paroxysmal Atrial Fibrillation
References
- 1.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg MA, Manning WJ. Diastolic dysfunction and risk of atrial fibrillation: a mechanistic appraisal. Circulation. 2012;126:2353–2362. doi: 10.1161/CIRCULATIONAHA.112.113233. [DOI] [PubMed] [Google Scholar]
- 3.Tsang TS, Gersh BJ, Appleton CP, Tajik AJ, Barnes ME, Bailey KR, et al. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol. 2002;40:1636–1644. doi: 10.1016/s0735-1097(02)02373-2. [DOI] [PubMed] [Google Scholar]
- 4.Nagueh SF. Left ventricular diastolic function: understanding pathophysiology, diagnosis, and prognosis with echocardiography. JACC Cardiovasc Imaging. 2020;13(1 Pt 2):228–244. doi: 10.1016/j.jcmg.2018.10.038. [DOI] [PubMed] [Google Scholar]
- 5.Beladan CC, Botezatu S, Popescu BA. Reversible left ventricular diastolic dysfunction-overview and clinical implications. Echocardiography. 2020;37:1957–1966. doi: 10.1111/echo.14838. [DOI] [PubMed] [Google Scholar]
- 6.Temporelli PL, Corrà U, Imparato A, Bosimini E, Scapellato F, Giannuzzi P. Reversible restrictive left ventricular diastolic filling with optimized oral therapy predicts a more favorable prognosis in patients with chronic heart failure. J Am Coll Cardiol. 1998;31:1591–1597. doi: 10.1016/s0735-1097(98)00165-x. [DOI] [PubMed] [Google Scholar]
- 7.Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on clinical data standards (writing committee to develop cardiovascular endpoints data standards) J Am Coll Cardiol. 2015;66:403–469. doi: 10.1016/j.jacc.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 8.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Pozzoli M, Traversi E, Cioffi G, Stenner R, Sanarico M, Tavazzi L. Loading manipulations improve the prognostic value of Doppler evaluation of mitral flow in patients with chronic heart failure. Circulation. 1997;95:1222–1230. doi: 10.1161/01.cir.95.5.1222. [DOI] [PubMed] [Google Scholar]
- 10.Sohn DW. Heart failure due to abnormal filling function of the heart. J Cardiol. 2011;57:148–159. doi: 10.1016/j.jjcc.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015;8:295–303. doi: 10.1161/CIRCHEARTFAILURE.114.001667. [DOI] [PubMed] [Google Scholar]
- 12.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 13.Obokata M, Reddy YNV, Borlaug BA. Diastolic dysfunction and heart failure with preserved ejection fraction: understanding mechanisms by using noninvasive methods. JACC Cardiovasc Imaging. 2020;13(1 Pt 2):245–257. doi: 10.1016/j.jcmg.2018.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim TH, Shim CY, Park JH, Nam CM, Uhm JS, Joung B, et al. Left ventricular diastolic dysfunction is associated with atrial remodeling and risk or presence of stroke in patients with paroxysmal atrial fibrillation. J Cardiol. 2016;68:104–109. doi: 10.1016/j.jjcc.2015.10.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline Laboratory Parameters According to Changes of LVDFP
Reasons for Echocardiographic Examination
Logistic Regression Analysis for Prognostic Factors of Unimproved LVDFP on Patients Whose Last Clinical Follow-Up Period was Within 4.2 Years (n=274)
Logistic Regression Analysis for Prognostic Factors of Unimproved LVDFP after Excluding Patients Who Had Follow-Up Echocardiography Within 3 Months after the Index Echocardiography (n=572)
Logistic Regression Analysis for Prognostic Factors of E/e’ >15
Logistic Regression Analysis for Prognostic Factors of LAVI >34 mL/m2
Logistic Regression Analysis for Prognostic Factors of TR Vmax >2.8 m/s
Diagnosis Time, Antiarrhythmic Drugs, Anticoagulants, and RFCA Status in Patients with Paroxysmal Atrial Fibrillation