Abstract
The Systemic Inflammation Response Index (SIRI), a marker used to assess systemic inflammation, is associated with lower patient survival rates in various cancer types. Factors contributing to the recurrence of colorectal cancer (CRC) have been examined previously using the preoperative SIRI. Herein, we investigated the association between the preoperative SIRI level and both the recurrence-free survival (RFS) and overall survival (OS) in patients diagnosed with CRC. We retrospectively analyzed the case of 406 patients who underwent curative surgery for Stage I-III CRC at a single institution during 2012- 2017. Based on their SIRI levels, we categorized the patients into a low-SIRI group (≤ 1700) and a high-SIRI group (> 1700). Multivariable analyses revealed that a high-SIRI level was an independent risk factor for 5-year RFS (p = 0.045) and OS (p = 0.048) in CRC patients. A Kaplan–Meier analysis demonstrated significantly poorer 5-year RFS and OS outcomes in the high-SIRI group compared to the low-SIRI group (p = 0.0001, p = 0.017 respectively). These findings suggest that the high-SIRI level is significantly associated with a poorer prognosis in patients diagnosed with CRC.
Keywords: Colorectal cancer, SIRI, Prognosis, OS, RFS
Subject terms: Cancer, Biomarkers, Risk factors
Introduction
Colorectal cancer (CRC) is a major health concern globally, contributing to substantial cancer-related morbidity and mortality1. Surgery remains the primary treatment approach, but postoperative complications and the long-term prognosis can vary significantly. Identifying reliable prognostic factors is crucial for guiding treatment decisions and improving patient outcomes. In recent years, the systemic inflammation response index (SIRI) has gained attention as a potential prognostic tool2. The SIRI combines routine hematological parameters to assess the inflammatory response and has shown promise in predicting patient prognoses in various malignancies. Specifically, SIRI can be studied using neutrophil count, monocyte count, and lymphocyte count. In previous studies, patients with high neutrophil and monocyte counts and low lymphocyte counts have been reported to have a poor prognosis2. This article explores the evidence supporting the use of the SIRI as a predictor of the prognosis or postoperative patients with CRC.
Results
Patient characteristics
The study population was 406 patients with median age 66 years (range 24–93 yrs); 239 (58.6%) patients were male and 169 (41.4%) were female. The T-factor (i.e., the depth of tumor invasion) was 133 (32.8%) for T1 / T2 patients, and 273 (67.2%) for the stage T3 or T4 patients. There were 144 (35.5%) cases with lymph node metastasis (N factor +) and 262 (64.5%) patients without lymph node metastasis (N factor -). There were 123 (30.3%) patients with high preoperative CEA levels and 61 (15.2%) cases with high preoperative CA19-9 levels. The optimal cut-off value was 1700 with an area under the curve of 0.600 for RFS. We divided the 406 patients into the low-SIRI group (SIRI ≤ 1700, n = 328, 80.7%) and the high-SIRI group (SIRI > 1700, n = 78, 19.2%). The low-SIRI group was 328 (80.7%) patients, and the high-SIRI group was 78 (19.2%) patients (Table 1). CRC patients, followed for a median period of 1622 days, range of 10–2,759 days), 78 patients (19.2%) experienced disease recurrence. Among the 78 patients with recurrence, the following metastases were observed: 25 (32.1%) had liver metastases, 21 (26.9%) had lung metastases, 9 (11.5%) had peritoneal carcinomatosis, 7 (8.9%) had local recurrence, 7 (8.9%) had para-aortic lymph node involvement, and the remaining 9 (11.5%) had other forms of recurrence.
Table 1.
Clinicopathological features of the stage I-III colorectal cancer patients who underwent curative tumor resection.
| Variables | n = 406 |
|---|---|
| Age, yrs; mean | 66.2 |
| Males/females | 239 (58.6%) / 169 (41.4%) |
| BMI, ≤ 22 / ≥ 22 | 189 (46.6%) / 217 (53.4%) |
| Tumor location; right side/left side | 121 (29.8%) / 285 (70.2%) |
| Histology, well or moderate/others | 361 (88.7%) / 45 (11.2%) |
| Depth of tumor invasion, T1–T2/T3–T4 | 133 (32.8%) / 273 (67.2%) |
| Lymph node metastasis, − / + | 262 (64.5%) /144 (35.5%) |
| Lymph invasion, − / + | 224 (60.6%) /182 (39.4%) |
| Venous invasion, − / + | 127 (31.2%) / 279 (68.8%) |
| CEA level, high/normal | 123 (30.3%) / 282 (69.7%) |
| CA19-9 label, high/normal | 61 (15.2%) / 344 (84.8%) |
| Adjuvant chemotherapy, − / + | 298 (73.4%) / 108 (26.6%) |
| SIRI, cut-off (1,700), Low/High | 328 (80.7%) / 78 (19.2%) |
BMI Body mass index, CEA Carcinoembryonic antigen, CA19-9 Carbohydrate antigen 19–9, SIRI Systemic inflammation response index.
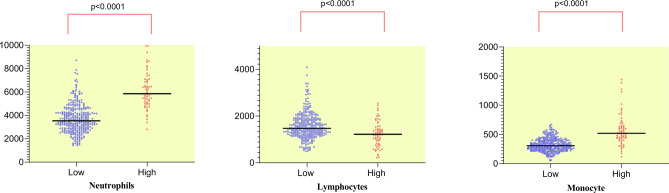
Neutrophils, lymphocytes and monocyte scattergraphs by SIRI levels
The respective mean values of the low-SIRI and high-SIRI groups were as follows. Neutrophil count: 3725 and 4286, lymphocyte count: 1595 and 1235, and monocyte count: 324 and 581(Fig. 1). All three of these variables were significantly different between the two groups (p < 0.0001).
Fig. 1.
Neutrophils, lymphocytes and monocytes scattergraphs by SIRI.
Associations of SIRI quality with clinicopathological factors
Table 2 provides the correlation between the SIRI level and the clinicopathological factors including gender, age, BMI, pT stage, pN stage, lymph / venous invasion, tumor location, pathological type, CEA level and CA19-9 level. The SIRI levels was significantly correlated with pT stage (p < 0.0001), lymph invasion (p = 0.003) and venous invasion (p = 0.0165).
Table 2.
The relationship between SIRI status and clinicopathological factors in the colorectal cancer patients.
| Variables | High-SIRI group (n = 63) | Low-SIRI group (n = 336) | p-value |
|---|---|---|---|
| Age, yrs; ≤ 66, > 66 | 181 (53.8%) / 155 (46.1%) | 42 (66.7%) / 21 (33.3%) | 0.0721 |
| Males/females | 41 (65.1%) / 22 (34.9%) | 196 (58.3%) / 140 (41.7%) | 0.3319 |
| BMI, ≤ 22/ > 22 | 156 (46.7%) / 178 (53.3%) | 28 (44.4%) / 35 (55.6%) | 0.784 |
| Tumor location, right side/left side | 22 (34.9%) /41 (65.1%) | 109 (32.4%) / 227 (67.6%) | 0.7702 |
| Histology, well or moderate/others | 52 (82.4%) /11 (17.5%) | 301 (89.6%) / 35 (10.4%) | 0.1304 |
| Depth of tumor invasion, T1–T2/T3–T4 | 9 (14.3%) / 54 (85.7%) | 125 (37.2%) / 211 (62.8%) | 0.0001 |
| Lymph node metastasis, − / + | 217 (64.6%) /119 (35.4%) | 41 (65.1%) / 22 (34.9%) | 1.0000 |
| Lymph invasion, − / + | 203 (60.6%) /132 (39.4%) | 25 (39.7%) / 38 (60.3%) | 0.0033 |
| Venous invasion, − / + | 110 (32.8%) /225 (67.2%) | 11 (17.5%) / 52 (82.5%) | 0.0165 |
| CEA level, high/normal | 25 (39.7%) / 38 (60.3%) | 98 (29.3%) / 237 (70.7%) | 0.1044 |
| CA19-9 level, high/normal | 48 (14.3%) / 287 (85.7%) | 12 (19.1%) / 51 (80.9%) | 0.3397 |
| Adjuvant chemotherapy, − / + | 251 (75.2%) /83 (24.8%) | 47 (74.6%) / 16 (25.40%) | 1.000 |
SIRI Systemic inflammation response index, High-SIRI group SIRI > 1700, Low SIRI group SIRI ≤ 1700, BMI Body mass index, CEA Carcinoembryonic antigen, CA19-9 Carbohydrate antigen 19-9.
Univariate and multivariate analyses of predictive factors for 5-year RFS and OS
We assessed the relationship between the SIRI level, clinicopathological factors, and the 5-year recurrence-free survival (RFS) rate of the patients. The result of the univariate survival analyses, presented in Table 2, revealed that several factors were significantly associated with a poorer 5-year RFS rate. These factors included the SIRI, histology, lymph invasion, vascular invasion, pT category, pN category, preoperative CEA level, and CA19-9 level. Factors such as age, gender, tumor location, and BMI did not show a significant association with the 5-year RFS rate. To determine the independent prognostic factors for 5-year RFS, we performed a multivariate analysis, and the results identified the following as independent prognostic factors associated with 5-year RFS: the SIRI level, histology, lymph invasion, pT category, pN category, and preoperative CEA level (Table 3).
Table 3.
The univariate and multivariate analysis of prognostic factors for 5-year RFS.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95%CI | p-value | |
| Age, yrs; ≤ 66, > 66 | 1.52 | 0.977–2.366 | 0.063 | |||
| Males/females | 1.08 | 0.701–1.686 | 0.095 | |||
| BMI, ≤ 22/ > 22 | 1.43 | 0.939–2.205 | 0.131 | |||
| Tumor location, right side/left side | 1.16 | 0.741–1.804 | 0.523 | |||
| Histology, well or moderate/others | 2.13 | 1.235–3.667 | 0.0065 | 1.59 | 0.89–2.82 | 0.011 |
| Depth of tumor invasion, T1–T2/T3–T4 | 6.73 | 3.103–14.59 | < 0.0001 | 3.51 | 1.58–7.80 | 0.020 |
| Lymph node metastasis, − / + | 3.17 | 2.051–4.910 | < 0.0001 | 2.24 | 1.38–3.62 | 0.001 |
| Lymph invasion, − / + | 3.31 | 2.099–5.235 | < 0.0001 | 1.89 | 1.15–3.10 | 0.011 |
| Venous invasion, − / + | 2.79 | 1.542–5.027 | 0.0007 | 1.50 | 0.79–2.82 | 0.206 |
| CEA level, normal /high | 2.07 | 1.350–3.183 | 0.0009 | 1.61 | 1.02–2.60 | 0.042 |
| CA19-9 level, normal /high | 3.04 | 1.695–4.367 | < 0.0001 | 1.39 | 0.83–2.33 | 0.209 |
| SIRI | 2.312 | 1.437–3.743 | 0.0006 | 1.60 | 1.04–2.65 | 0.045 |
BMI Body mass index, CEA Carcinoembryonic antigen, CA19-9 Carbohydrate antigen 19–9, SIRI Systemic inflammation response index.
Table 4 provides a summary of the findings from both the univariate and multivariate analyses regarding the patient’s 5-year overall survival (OS) rate. In the univariate analyses, several factors demonstrated a significant association with 5-year OS: histological grade, pT category, pN category, lymph invasion, venous invasion, preoperative CEA level, and SIRI level. In the multivariate analyses focusing on 5-year OS, only the pN category, preoperative CEA level, and the SIRI level were identified as independent predictive factors.
Table 4.
The univariate and multivariate analysis of prognostic factors for 5-year OS.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-value | HR | 95%CI | p-value | |
| Age, yrs; ≤ 66, > 66 | 1.52 | 0.677–2.366 | 0.098 | |||
| Males/females | 1.26 | 0.701–2.340 | 0.461 | |||
| BMI, ≤ 22/ > 22 | 0.98 | 0.548–1.778 | 0.965 | |||
| Tumor location, right side/left side | 1.54 | 0.354–1.181 | 0.156 | |||
| Histology, well or moderate/others | 2.12 | 1.023–4.415 | 0.043 | 1.59 | 0.89–2.82 | 0.120 |
| Depth of tumor invasion, T1–T2/T3–T4 | 3.05 | 1.360–6.823 | 0.002 | 3.51 | 0.69–3.80 | 0.244 |
| Lymph node metastasis, − / + | 2.42 | 1.344–4.357 | 0.003 | 2.24 | 1.28–2.96 | 0.041 |
| Lymph invasion, − / + | 2.55 | 1.385–4.700 | 0.002 | 1.89 | 0.76–2.97 | 0.236 |
| Venous invasion, − / + | 2.32 | 1.078–4.972 | 0.019 | 1.50 | 0.57–2.87 | 0.230 |
| CEA level, normal /high | 2.92 | 1.625–5.241 | 0.0004 | 1.61 | 1.40–4.66 | 0.002 |
| CA19-9 level, normal /high | 2.36 | 1.217–4.570 | 0.021 | 1.39 | 0.83–2.33 | 0.209 |
| SIRI | 2.11 | 1.122–3.965 | 0.018 | 1.60 | 1.03–2.43 | 0.048 |
BMI Body mass index, CEA Carcinoembryonic antigen, CA19-9 Carbohydrate antigen 19–9, SIRI Systemic inflammation response index.
TNM stage in the low-SIRI group and-high-SIRI groups
Among the total patient series, 106 patients (26.1%) had been diagnosed with stage I cancer, 156 (38.4%) with stage II, and 144 (36.5%) with stage III. In the high-SIRI group, there were 8 patients with stage I cancer (7.5%), 38 with stage II (24.4%), and 21 with stage III (14.6%). We observed a significant trend in which the number of patients with a low-SIRI value increased as the stage progressed (RFS: p = 0.002) (Table 5).
Table 5.
Correlation between colorectal cancer stage and SIRI status.
| Low SIRI | High SIRI | p-value | |
|---|---|---|---|
| Stage I: n = 106 (26.1%) | 98 (92.4%) | 8 (7.6%) | 0.002 |
| Stage II: n = 156 (38.4%) | 118 (75.6%) | 38 (24.4%) | |
| Stage III: n = 144 (36.5%) | 112 (77.8%) | 32 (22.2%) |
SIRI Systemic inflammation response index, High-SIRI group: SIRI > 1700, Low SIRI group: SIRI ≤ 1700.
Kaplan–Meier curve of SIRI in CRC patients
We conducted survival analyses comparing the low-SIRI group and high-SIRI group based on the defined cutoff value for the SIRI. The Kaplan–Meier curves demonstrated significant differences between the two groups in terms of both the 5-year RFS and OS rate (p = 0.0001, p = 0.017, respectively), indicating that the SIRI may have prognostic value. As depicted in Fig. 2, the high-SIRI and low-SIRI groups’ 5-year RFS rates were 65.9 and 81.9%, and their 5-year OS rates were 79.4 and 90.1% respectively.
Fig. 2.
Kaplan–Meier analysis for the RFS of colorectal cancer patients in all stages according to SIRI (A) and OS (B).
Our analysis of the association between the SIRI and TNM staging, revealed that the high-SIRI group was significantly associated with poorer prognosis in stages I and III of CRC (p = 0.002, p = 0.033, respectively; Fig. 3A,C). In stage II CRC, although there was a tendency towards poorer prognosis in the high-SIRI group, the difference was not significant (P = 0.09, Fig. 3B).
Fig. 3.
Kaplan–Meier analysis for the RFS of colorectal cancer patients in stratification analysis based on TNM stage: stage I (A), stage II (B) and stage III (C).
The prognostic value of the SIRI in CRC
The analysis of the ROC curve confirmed the prognostic value of the SIRI for patients with CRC. The values used for comparison were systemic immune-inflammation index (SII), neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR). SII is a prognostic indicator using inflammatory markers, which is calculated with the formula SII = (platelet count × neutrophil count)/lymphocyte count3. We observed that the area under the curve (AUC) for the SIRI at the 5-year mark, i.e., 0.60 was larger than the areas for SII (0.59), NLR (0.57) PLR (0.54), and LMR (0.55), as depicted in Fig. 4.
Fig. 4.
Predictive ability of the SIRI in colorectal cancer was compared with SII, NLR, PLR and MLR by ROC curves in 5-years.
Discussion
We assessed of the prognostic implications of the SIRI in a cohort of 406 patients diagnosed with CRC. Our comprehensive analyses demonstrated that the SIRI exhibited independent prognostic significance for both recurrence-free survival (RFS) and overall survival (OS) outcomes. Notably, the SIRI emerged as a valuable prognostic indicator that offers distinct advantages in terms of cost-effectiveness and ease of measurement compared to conventional approaches such as TNM classification and tumor marker assessment.
Multiple clinical studies have indicated that chronic inflammation plays a significant role in the development of cancer, and that persistent inflammation acts as a driving force in the progression of cancer4. Several markers have been identified as independent indicators of the prognosis of patients with CRC, including the NLR, PLR, LMR, and systemic inflammation scores5–7. Numerous research studies have consistently highlighted the significant influence of nutrition-related factors and patients’ immune system status on the outlook and progression of cancer patients8–10. In addition, prognostic prediction tools using nutritional indicators, inflammatory indicators, and gene mutations have been developed11.
The SIRI emerged relatively recently as a novel of marker inflammation called. The SIRI is defined based on the levels of neutrophils, monocytes, and lymphocytes in the peripheral blood. Initially, the SIRI was observed to be an independent risk factor for survival in patients with pancreatic cancer12. We conducted the present study to assess the predictive ability of the SIRI and that of clinical factors on post-operative survival in patients with CRC. The prognostic scores prognostic nutritional index and modified Glasgow Prognostic Score, which use inflammatory and nutritional indices, have cut-off values that have been determined13. In comparison, the cut-off value of SIRI is more variable due to the small number of cases. In the present study, we calculated the cut-off for SIRI using receiver operating characteristic curve analysis for RFS. A multivariate analysis showed that the independent prognostic factors for CRC patients in terms of RFS were the SIRI, histology, T factor, N factor, lymph invasion, and CEA level. For OS, the N factor, the CEA level and the SIRI were identified as independent prognostic factor. This independence highlights the potential of the SIRI as a standalone prognostic tool that can aid in risk stratification and treatment decision-making. In addition, the ROC curve analysis demonstrated that among these markers, the SIRI exhibited greater validity and accuracy in predicting patient prognoses. The predictive value of the SIRI can be attributed to its ability to reflect the host-tumor interaction, immune competence, and systemic inflammatory status.
The systemic inflammatory response plays a crucial role in cancer progression and affects the tumor microenvironment, promoting tumor growth, invasion, and metastasis. The SIRI, calculated based on the ratio of neutrophils, monocytes, and lymphocytes, represents a comprehensive assessment of the systemic inflammatory response14,15. Elevated SIRI levels indicate an increase in neutrophils and/or monocytes, along with a decrease in lymphocytes, reflecting an imbalance between pro-inflammatory and anti-inflammatory components and thus indicating a dysregulated immune response16. Neutrophils have been shown to promote cancer progression by releasing cytokines such as tumor necrosis factor alpha (TNFα), interleukin (IL-1), IL-6, and vascular endothelial growth factor (VEGF)17–20. They also suppress the host’s T-cell immunity. Monocytes, in contrast, are considered pro-tumor cells. They interact with tumor cells and are recruited to the tumor tissue as tumor-associated macrophages (TAMs), thereby suppressing the antitumor immune response and promoting the migration and metastasis of tumor cells21,22. Particularly TAMs derived from circulating monocytes, significantly impact the tumor microenvironment by promoting tumor progression and metastasis. Additionally, research has shown that altering factors secreted by neutrophils and TAMs can influence the stem cell-like properties of tumor cells, thereby affecting their responsiveness to chemotherapy23. Conversely, lymphocytes, particularly CD3 + T-cells and natural killer (NK) cells, play a role in inhibiting tumor growth and/or metastasis through their inherent anti-cancer immune activity24,25. In addition, lymphocytes are crucial in tumor immune surveillance and defense, inducing cytotoxic cell death26. The high SIRI value thus indicates pro-tumor activity and a reduction in anti-cancer immunity.
The TNM stage is widely used as a postoperative staging evaluation system for various cancers worldwide, including CRC. It serves as a crucial guide for postoperative follow-up and treatment in CRC patients27,28. However, it is frequently observed that CRC patients with the same TNM stage exhibit significant survival heterogeneity, indicating that the TNM stage alone is insufficient for individual prognosis prediction6,29. One possible explanation for this discrepancy is that the TNM stage primarily classifies patients based on postoperative pathological findings and does not consider the patient’s inflammation status. In recent years, there has been a growing focus on the tumor environment, particularly the patient’s nutritional and inflammatory status30,31. By incorporating the SIRI and classifying CRC patients according to both the cancer stage and the SIRI, the ability to predict prognoses has been enhanced. We therefore believe that (i) determining patients’ SIRI values can effectively complement the use of their TNM stage and, (ii) the SIRI can play a vital role in evaluating the individual prognosis of CRC patients.
This study has some limitations. It was retrospective in design and included patients from a single institution, prospective data from a multicenter cohort is needed to validate their findings. Overcoming potential biases in observational studies requires controlled randomized controlled trials comparing each SIRI risk group. Second, the patients in this study had undergone a variety of surgical procedures for CRC, and we did not take into account differences among surgical procedures. Third, there is currently no consensus regarding the SIRI cut-off value, and this makes it difficult to use the SIRI in clinical settings. We selected the SIRI herein by performing a ROC analysis. The SIRI is a non-specific marker of inflammation, and this implies that another systemic disease can affect the SIRI. Our present findings need further review and validation in greater numbers of CRC patients.
Conclusions
Our findings provide presented compelling evidence supporting the clinical significance and practical applicability of the SIRI as a prognostic biomarker in CRC. The evaluation of our newly developed SIRI demonstrated its potential to identify CRC patients with an unfavorable prognosis.
Patients and methods
Patient selection
We diagnosed with stage I–III colorectal cancer (CRC) according to the 8th edition of the United States Joint Commission on Cancer (AJCC)32 staging system. These patients underwent elective curative resection at Teikyo University Hospital in Japan between 2013 and 2017. Patients’ written informed consent for their data to be used was obtained, and enrolled 406 patients. This study has been approved by Teikyo University Ethics (Registration Number; 19–127). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and its later amendments.
SIRI calculation method
The SIRI was calculated as: = neutrophil count × monocyte count / lymphocyte count, in reference to Huang et al.14. According to the SIRI items, data on the neutrophil count, monocyte count and lymphocyte count of patients with CRC were collected within 1 week before the surgical procedure.
Survival follow-up
The surgical resection was considered curative when there were no signs of tumor recurrence and complete histological and macroscopic removal of distant metastases was confirmed. After the surgery, the patients were regularly followed up according to a specific schedule. During the first 3 years, follow-up visits occurred every 3 months, followed by visits every 6 months for the next 2 years. At each follow-up, a physical examination was conducted, and the levels of serum tumor markers, such as carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9), were measured. A colonoscopy examination was performed 1–2 years after surgery (or annually in the case of rectal cancer). Thoraco-abdominal computed tomography scans were typically conducted every 6 months.
The criteria for CRC recurrence included radiological, clinical, and/or pathological evidence of cancer cells appearing either locally or in distant locations from their original site. This comprehensive follow-up approach aimed to promptly detect any signs of tumor recurrence or metastasis and initiate appropriate treatment as necessary.
Determination of cut-off values
The cut off value of SIRI is controversial due to the small number of cases. In the present study, we sought to obtain better results. We determined that cutoff value for the SIRI levels in this study by performing a receiver-operating characteristic (ROC) curve analysis, using Youden’s index to assess survival. For patients with a body mass index (BMI) ≥ 22, the upper limits of the normal ranges of the serum tumor markers at our hospital are set at 5 ng/mL for CEA and 37 U/mL for CA19-9.
Statistical analyses
Differences in categorical variables were examined using the χ2 test or Fisher’s exact test. Relapse-free survival (RFS) was calculated from the date of the patient’s surgery to that of recurrence or death. We used the Kaplan–Meier method to calculate the overall survival (OS) from the date of the patient’s surgery to that of death. Univariate and multivariate analyses were performed using a Cox proportional hazards regression model for RFS and OS. Multivariate analyses were performed using the factors that were significant in the univariate analyses. The clinical variables that we considered for the univariate and multivariate analyses, in addition to the target SIRI value, were previously identified confounding factors with an impact on the prognosis of CRC: sex, age at the diagnosis, histology, pathological T stage (T1/2 or T3/4), lymph invasion, venous invasion, lymph-node metastasis (present or absent), BMI (≥ 22 or < 22), CEA level (≥ 5.0 ng/mL or < 5.9 ng/mL), and CA-19–9 level (≥ 37 ng/mL or < 37 U/mL). To better assess the predictive value of the SIRI, four indicators of inflammation, namely the SII, NLR, PLR and MLR, were introduced for comparison. AUC was used as the index for comparison. Probability (p)-values ≤ 0.05 were considered significant. All statistical analyses were performed using JMP 15 software (https://www.jmp.com/ja_jp/home.html. SAS, Cary, NC, USA).
Abbreviations
- AUC
Area under the curve
- CEA
Carcinoembryonic antigen
- CA19-9
Carbohydrate antigen 19-9
- CRC
Colorectal cancer
- IL
Interleukin
- LMR
Lymphocyte-to-monocyte ratio
- NK
Natural killer
- NLR
Neutrophil-to-lymphocyte ratio
- OS
Overall survival
- PLR
Platelet-to-lymphocyte ratio
- RFS
Relapse-free survival
- ROC
Receiver-operating characteristic
- SII
Systemic immune-inflammation index
- SIRI
Systemic inflammation response index
- TNF
Tumor necrosis factor
- TAM
Tumor-associated macrophage
- VEGF
Vascular endothelial growth factor
Author contributions
T.H were involved in study concept and design, analysis and interpretation of data and drafting of the manuscript. T.O was involved in analysis and interpretation of data. H.O was involved in acquisition of data. T.F, S.F and T.M were involved in critical revision of the manuscript for important intellectual content and material support. K.N, Y.F, K.K, T.M and K.A were involved in study concept and study supervision.
Funding
This study was supported by Grant-in-Aid for Scientists from Japan Society for Promotion of Science (JSPS KAKENHI grants JP22K08784).
Data availability
The datasets collected in this study can be obtained from the corresponding author upon a reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics
This study was approved by the Teikyo University Ethics Committee (No. 19-127).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Morgan, E. et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut72(2), 338–344 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Wei, L., Xie, H. & Yan, P. Prognostic value of the systemic inflammation response index in human malignancy: A meta-analysis. Medicine (Baltimore)99(50), e23486 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, J. H. et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J. Gastroenterol.23(34), 6261–6272 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kundu, J. K. & Surh, Y. J. Inflammation: gearing the journey to cancer. Mutat. Res.659(1–2), 15–30 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Hayama, T. et al. Significance of the 7th postoperative day neutrophil-to-lymphocyte ratio in colorectal cancer. Int. J. Colorectal Dis.35(1), 119–124 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Hayama, T. et al. Impact of colon cancer location on the prognostic significance of nutritional indexes and inflammatory markers. Vivo35(2), 1261–1269 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozawa, T. et al. Impact of a lymphocyte to monocyte ratio in stage IV colorectal cancer. J. Surg. Res.199(2), 386–392 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Hayama, T. et al. The pretreatment controlling nutritional status (CONUT) score is an independent prognostic factor in patients undergoing resection for colorectal cancer. Sci. Rep.10(1), 13239 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayama, T. et al. The preoperative geriatric nutritional risk index (GNRI) is an independent prognostic factor in elderly patients underwent curative resection for colorectal cancer. Sci. Rep.12(1), 3682 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayama, T. et al. Predicting overall survival using preoperative nutritional and inflammation status for colorectal cancer. Vivo36(1), 450–457 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyata, T. et al. Predicting prognosis in colorectal cancer patients with curative resection using albumin, lymphocyte count and RAS mutations. Sci. Rep.14(1), 14428 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi, Q. et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer122(14), 2158–2167 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Ishizuka, M., Nagata, H., Takagi, K., Horie, T. & Kubota, K. Inflammation-based prognostic score is a novel predictor of postoperative outcome in patients with colorectal cancer. Ann. Surg.246(6), 1047–1051 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Huang, H. et al. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci. Rep.9(1), 3284 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valero, C. et al. Prognostic capacity of systemic inflammation response index (SIRI) in patients with head and neck squamous cell carcinoma. Head Neck42(2), 336–343 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Xia, Y. et al. Systemic immune inflammation index (SII), system inflammation response index (SIRI) and risk of all-cause mortality and cardiovascular mortality: A 20-year follow-up cohort study of 42,875 US adults. J. Clin. Med.12(3), 1128 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Street, M. E. et al. Interleukin-1beta (IL-1beta) and IL-6 modulate insulin-like growth factor-binding protein (IGFBP) secretion in colon cancer epithelial (Caco-2) cells. J. Endocrinol.179(3), 405–415 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Schietroma, M. et al. Systemic inflammation response index (SIRI) as predictor of anastomotic leakage after total gastrectomy for gastric cancer. Surg. Oncol.43, 101791 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Asako, K. et al. Prognostic value of KRAS exon-specific mutations in patients with colorectal cancer. Anticancer Res.43(4), 1563–1568 (2023). [DOI] [PubMed] [Google Scholar]
- 20.Hayama, T. et al. Ceramide synthase CERS4 gene downregulation is associated with KRAS mutation in colorectal cancer. Sci. Rep.13(1), 16249 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen, S. R. & Schmid, M. C. Macrophages as key drivers of cancer progression and metastasis. Mediat. Inflamm.2017, 9624760 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong, L. et al. The loss of RNA N(6)-adenosine methyltransferase Mettl14 in tumor-associated macrophages promotes CD8(+) T cell dysfunction and tumor growth. Cancer Cell39(7), 945–57.e10 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Yoon, C. et al. CD44 expression denotes a subpopulation of gastric cancer cells in which Hedgehog signaling promotes chemotherapy resistance. Clin. Cancer Res.20(15), 3974–3988 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Schmohl, J. U., Gleason, M. K., Dougherty, P. R., Miller, J. S. & Vallera, D. A. Heterodimeric bispecific single chain variable fragments (scFv) killer engagers (BiKEs) enhance NK-cell activity against CD133+ colorectal cancer cells. Target Oncol.11(3), 353–361 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeuchi, Y. & Nishikawa, H. Roles of regulatory T cells in cancer immunity. Int. Immunol.28(8), 401–409 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature454(7203), 436–444 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Sano, T. et al. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer20(2), 217–225 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cserni, G., Chmielik, E., Cserni, B. & Tot, T. The new TNM-based staging of breast cancer. Virchows Arch.472(5), 697–703 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Li, J. et al. TNM staging of colorectal cancer should be reconsidered by T stage weighting. World J. Gastroenterol.20(17), 5104–5112 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Funamizu, N., Nakabayashi, Y., Iida, T. & Kurihara, K. Geriatric nutritional risk index predicts surgical site infection after pancreaticoduodenectomy. Mol. Clin. Oncol.9(3), 274–278 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okugawa, Y. et al. Lymphocyte-C-reactive protein ratio as promising new marker for predicting surgical and oncological outcomes in colorectal cancer. Ann. Surg.272(2), 342–351 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Weiser, M. R. AJCC 8th edition: Colorectal cancer. Ann. Surg. Oncol.25(6), 1454–1455 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets collected in this study can be obtained from the corresponding author upon a reasonable request.