Abstract
De novo shoot regeneration, characterized by the emergence of adventitious shoots from excised or damaged tissues or organs in vitro, is regulated by the complex interplay between genetic and epigenetic regulatory mechanisms. However, the specific effect of histone deacetylation on shoot regeneration remains poorly understood. This study investigated the effects of trichostatin A (TSA), a histone deacetylase inhibitor, on shoot regeneration in callus derived from root explants. TSA-treated root explants exhibited pronounced callus greening and substantially increasing in multiple shoot formations per callus compared with the control group. Additionally, TSA treatment upregulated shoot apical meristem-specific genes, including WUSCHELL (WUS), RELATED TO AP2.6 L (Rap2.6 L), SHOOT MERISTEMLESS (STM), CUP SHAPED COTYLEDON 2 (CUC2). Notably, TSA treatment enhanced the sensitivity to cytokinins, leading to increase expression of the cytokinin signaling reporter TCS::GFP in the callus. Concomitantly, type-B ARABIDOPSIS RESPONSE REGULATOR (ARR) 10 and 12, which are key regulators of cytokinin signaling, were upregulated in TSA-treated callus, whereas the downstream targets of type-B ARRs, such as ARR5, ARR7, and ARR15, were significantly upregulated during shoot regeneration. Furthermore, mutants deficient in ARR10 and ARR12 showed diminished responsiveness to shoot regenerative capacity, a phenotype that was enhanced by TSA treatment. Our findings underscore the crucial role of histone deacetylation in mediating cytokinin responses and controlling de novo shoot regeneration in plants.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-84860-9.
Keywords: Cytokinin response, Histone deacetylation, Shoot regeneration, Trichostatin A
Subject terms: Plant sciences, Plant development, Plant regeneration
Introduction
Plants possess the plasticity to regenerate new organs and even complete functional organisms after exposure to physical damage. In particular, de novo organogenesis exemplifies the regenerative capacity of plants, in which adventitious shoots or roots originate from differentiated tissues or detached organs. The regenerative capacity is dependent on the proportion of auxin and cytokinin. Callus is a pluripotent cell mass initiated from plant explants on an auxin-rich callus induction medium (CIM) during indirect shoot regeneration. Afterward, the callus is transferred onto cytokinin-rich shoot induction medium (SIM), in which the callus develops de novo shoots. The induction of callus from explants is pivotal for cells to obtain the capacity to regenerate new shoots in the subsequent phase1. Plant regenerative capacity is commonly used for breeding and in vitro micropropagation of horticultural plants.
Shoot regeneration is accomplished by expressing several essential regulatory factors in the callus. The homeodomain transcription factor WUSCHEL (WUS), which is expressed in the organizing center of shoot stem cells, and SHOOT MERISTEMLESS (STM), which encodes a class 1 knotted-like homeodomain transcription factor, are required for the establishment of the shoot apical meristem (SAM) niche2. Loss-of-function mutations in these regulators result in significantly reduced shoot regeneration and even failure to regenerate shoots from the callus in wus mutant3,4. CUP-SHAPED COTYLEDON (CUC1 and CUC2), which are transcriptional activators of the NAC gene family, are involved in callus induction and shoot stem cell initiation5.
Cytokinins are major phytohormones that regulate callus induction and shoot formation6. The cytokinin signaling pathway is mediated by a multi-step phosphorelay based on a two-component system (TCS), in which the phosphoryl group is transferred from a conserved histidine kinase sensor to the conserved aspartate located in the receiver domain and consists of sensor histidine kinases (AHKs) to receive cytokinin signal, histidine phosphotransfer proteins (AHPs) to relay the signal, and response regulators (ARRs) to regulate cytokinin responsive gene expression7. In particular, type-B ARRs activate the transcription of cytokinin-responsive genes and contribute to shoot regeneration in a functionally redundant manner8. Among the seven type-B ARRs, ARR1, ARR10, and ARR12 specify the shoot stem cell niche by positively regulating WUS expression9,10. Double or triple mutants of ARR1, ARR10, and ARR12 are severely defective in sensitivity toward cytokinin and cytokinin-responsive transcriptional regulation and show reduced callus induction and shoot regeneration compared to the wild type. However, the mechanism underlying the role of type-B ARRs in shoot regeneration is not fully understood, and the transcriptional regulation of type-B ARRs during callus induction has not been previously reported.
The epigenetic modifications of DNA and histones are important for callus formation and subsequent shoot regeneration. Among epigenetic processes, histone modifications can alter chromatin conformation and gene expression by altering histone-DNA interactions. Histone deacetylation and acetylation are mediated by histone deacetylases (HDAC) and histone acetyltransferases (HAT), respectively. Histone acetylation refers to an open chromatin state that activates gene transcription by making DNA accessible to the transcriptional machinery, whereas histone deacetylation causes tight interactions between histones and DNA, followed by a closed chromatin state and transcriptional repression. Histone deacetylation inhibitors (HDACi) are chemical compounds that inhibit the function of histone deacetylases, alter the acetylation status of histones, and regulate gene expression11–14. Among HDACi, trichostatin A (TSA) is a well-known pan-inhibitor of the RPD3 and HD2-type subfamilies of HDACs in plants15–18 and is widely used to determine the role of HDAC in plant development, immunity, and stress19–21. TSA treatment induces the de-repression of embryogenesis-related genes and increases the embryogenic potential in Arabidopsis thaliana L., norway spruce (Picea abies L.), pine (Pinus sylvestris L.), wheat (Triticum aestivum L.), and cabbage (Brassica oleracea L.)22–26.
In the present study, we investigated the role of histone deacetylases in de novo shoot regeneration from callus derived from Arabidopsis root explants treated with TSA during callus induction. TSA promoted shoot formation from callus and led to the extensive upregulation of shoot apical meristem-specific genes during shoot regeneration. Furthermore, TSA modulated cytokinin responses in callus by regulating the expression of ARR10 and ARR12, which are positive regulators of the cytokinin response. Based on these results, we propose that TSA promotes de novo shoot regeneration from callus by regulating the cytokinin pathway.
Results
Optimization of TSA concentration and its impact on shoot regeneration
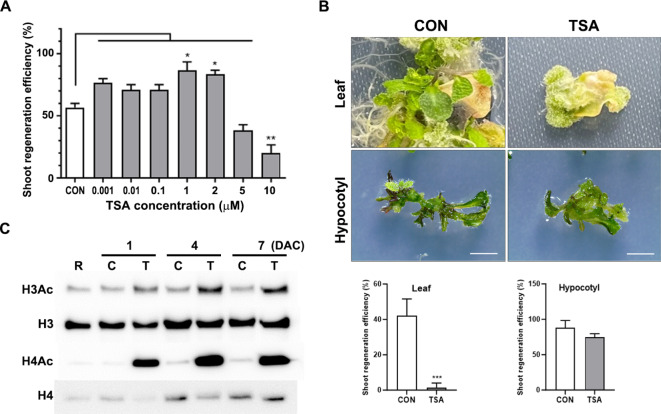
Because the appropriate expression of key regulators during pluripotent callus induction is crucial for complete shoot emergence in indirect shoot regeneration5,27,28, we decided to investigate whether histone deacetylation is implicated in shoot formation from callus. Trichostatin A (TSA) is a well-known pan-HDAC inhibitor that effectively suppresses the activity of class I and II HDACs, leading to histone hyperacetylation, chromatin conformational changes, and upregulation of gene expression11,29. To determine the optimal TSA concentration for promoting shoot regeneration, we evaluated a range of concentrations (0.001 µM, 0.01 µM, 0.1 µM, 1 µM, 2 µM, 5 µM, and 10 µM) using root explants. Root explants excised from 7-day-old seedlings were cultured in CIM supplemented with TSA under continuous dark conditions for 7 days. Subsequently, calli derived from the root explants were transferred to SIM without TSA and incubated under continuous light. The shoot regeneration efficiency increased by approximately 15–20% at low concentrations of TSA (0.001–0.1 µM) compared to the control, with a further increase of approximately 30% observed at 1–2 µM TSA (Fig. 1A). However, the regeneration efficiency decreased at 5 µM and was markedly reduced at 10 µM. Although there was no statistically significant difference in shoot regeneration efficiency between 1 µM and 2 µM TSA, the 2 µM TSA treatment showed a lower standard deviation. Therefore, 2 µM TSA was selected as the optimal concentration for subsequent experiments. We further examined the effect of 2 µM TSA on shoot regeneration in leaf and hypocotyl explants. Unlike root explants, 2 µM TSA treatment inhibited shoot regeneration from leaf explants, with efficiency decreasing markedly from approximately 40% in the control group (Fig. 1B). By contrast, 2 µM TSA treatment in hypocotyl explants showed a regeneration efficiency comparable to that of the control. These findings suggest that the promotive effect of TSA on shoot regeneration is specific to callus derived from root explants.
Fig. 1.
TSA promotes shoot regenerative capacity. (A) Efficiency of shoot regeneration in callus treated with various TSA concentrations. Root explants from 7-day-old seedlings were cultured in CIM with indicated concentration of TSA, then transferred to SIM without TSA and incubated for 18 days under continuous light. Data are expressed as means ± standard deviation of three biological replicates. Significant differences were determined using one-way ANOVA followed by Tukey’s post hoc test, with distinct letters indicating p < 0.05. (B) Upper panels: shoot regeneration of leaf and hypocotyl explants incubated in CIM with or without 2 µM TSA. Hypocotyl explants 7-day-old seedling and leaf explants from 14-day-old seedlings were cultured in CIM, followed by a 4-week incubation in SIM under continuous light. Scale bar = 2 mm. Lower panels: shoot regeneration efficiency of leaf and hypocotyl explants treated with or without 2 µM TSA. Data are expressed as mean ± standard deviation. Statistical significance was assessed using Student’s t-test, with ***p < 0.001. (C) Effect of TSA on histone H3 and H4 acetylation levels during callus induction. Histone proteins were extracted from 7-day-old seedling root explants with callus incubated in CIM with or without TSA after 1, 4, and 7 days. R: Root, C: control callus, T: TSA-treated callus, DAC: days after CIM incubation. Immunoblot images are cropped from supplementary information (Fig. S1).
Furthermore, we measured global histone acetylation during callus induction by western blot analysis to verify whether TSA alters histone H3 and H4 acetylation levels. After 1 day of incubation in CIM supplemented with TSA, the levels of histone H3 and histone H4 acetylation were notably increased compared with those in the control callus (Fig. 1C). Enhanced histone acetylation induced by TSA treatment continued during callus induction, indicating that callus induction from root explants treated with TSA caused prolonged histone H3 and H4 hyperacetylation.
Given the above findings, we focused subsequent experiments on the effect of TSA on root-derived calli. Root explants from 7-day-old seedlings were cultured in CIM with or without 2 µM TSA under continuous dark conditions. After 7 days of callus induction, the calli were transferred to SIM without TSA and incubated under continuous light. TSA-treated calli showed significantly enhanced greening and proliferation compared to controls (Fig. 2A). The shoot regeneration efficiency of TSA-treated calli reached 88% after 2 weeks in SIM, compared to 25% for control calli. After 3 weeks, the maximal shoot regeneration efficiency reached 97% for TSA-treated calli, while control calli showed 74% efficiency (Fig. 2B). Additionally, TSA-treated calli produced twice as many shoots per explant as controls (Fig. 2C). These results indicate that TSA significantly enhances shoot regeneration from calli derived from root explants.
Fig. 2.
Effect of TSA onde novoshoot regeneration from callus derived from root explants. (A) Shoot regeneration of TSA-treated and control calli. Root explants from 7-day-old seedlings were cultured in CIM with or without 2 µM TSA for 7 days in the dark, then transferred to shoot induction medium (SIM) without TSA for 21 days under continuous light. DAS indicates days after SIM incubation. Scale bars = 2 mm. (B) Efficiency of shoot regeneration for TSA-treated and control calli, measured on designated days. (C) Number of regenerated shoots per explant for TSA-treated and control calli. The number of shoots was measured three weeks after transfer to SIM. Data represent means ± standard deviation of three biological replicates. Statistical significance was assessed using Student’s t-test, with significance levels indicated as ***p < 0.001.
Shoot apical meristem-specific genes are upregulated by TSA treatment
The shoot apical meristem-specific WUSCHEL (WUS) and SHOOT MERISTEMLESS (STM) genes are upregulated in the shoot promeristem during shoot induction5. Shoot formation is always preceded by the accumulation of CUP SHAPED COTYLEDON 2 (CUC2)30 and RELATED TO AP2.6 L (RAP2.6 L) in callus31. Appropriate SAM-specific gene expression in callus is a prerequisite for successful regeneration. As the application of TSA to CIM enhanced shoot regenerative capacity in callus, we investigated the effect of TSA on the expression of SAM-specific genes during shoot induction using RT-qPCR. Consistent with the enhanced shoot regeneration efficiency, the expression of WUS, STM, and CUC2 was significantly upregulated in calli cultured with TSA compared to control calli 2 days after transfer into SIM (Fig. 3). The expression of WUS in TSA-treated calli was approximately 50 times higher than that in the control calli after 2 days on SIM, and the transcript level was six times higher than that in the control calli after 4 days on SIM. The levels of STM and CUC2 transcripts increased 7- and 4-fold in response to TSA application on SIM after 2 and 4 days, respectively. Transcript levels of Rap2.6 L were upregulated in calli treated with TSA on SIM after 2 and 4 days. These results indicate that TSA substantially upregulates central shoot apical meristem genes during shoot regeneration.
Fig. 3.
Effect of TSA on the expression of shoot meristem-specific genesAtWUS, AtSTM, AtRap2.6 L, andAtCUC2during shoot regeneration. Calli were induced from root explants in CIM with or without 2 µM TSA for 7 days, then transferred to SIM without TSA. Gene expression was normalized to eIF4A. Data represent the means of three biological replicates, with error bars showing standard deviation. Significant differences between TSA concentrations were determined using one-way ANOVA with Tukey’s post hoc test, indicated by distinct letters (p < 0.05).
TSA-promoted shoot regeneration is associated with cytokinin response
The plant hormone cytokinin is highly effective in promoting de novo shoot regeneration. Because TSA treatment during callus induction promotes shoot regeneration from the callus, we hypothesized that TSA might affect the cytokinin response in the callus. To address this, callus derived from root explants incubated in CIM supplemented with or without TSA were transferred onto SIM containing different concentrations of cytokinin (0, 0.05, 0.5, 5, or 50 µM 2-isopentenyladenine; 2-iP) with a fixed auxin concentration (0.9 µM). When calli were exposed to a high cytokinin/auxin ratio SIM (50 µM cytokinin), TSA-treated and control calli showed comparable shoot regenerative capacity (Fig. 4A). However, the low ratio of cytokinin/auxin SIM (0.5 and 5 µM cytokinin) resulted in TSA-treated callus regenerating significantly more shoots compared with control callus. The absence of cytokinin or low-concentration (0.05 µM) of cytokinin caused defective de novo shoot regeneration in the TSA-treated and control calli. TSA-treated calli responded more sensitive to cytokinin than control calli when they were transferred to SIM without TSA. Furthermore, to verify the effect of TSA on the cytokinin response during shoot regeneration, we used a cytokinin-responsive two-component signaling sensor TCS::GFP reporter line, which monitors the transcriptional activation of the type-B response regulators of cytokinin signaling. At SIM 0d, TCS signals were detected at low levels in the TSA-treated calli and the control (Fig. 4B). However, after 24 h when transferred onto SIM, a strong cytokinin response was observed in TSA-treated calli compared to the control calli, and thereafter the TCS::GFP signal vigorously increased and expanded through TSA-treated calli at SIM 48 h, but to a lesser extent in the control calli. These results suggest that the regulation of the cytokinin response in callus may promote TSA-induced shoot regeneration.
Fig. 4.
TSA confers cytokinin hypersensitivity during shoot regeneration. (A) Shoot regeneration efficiency of callus treated with TSA at various 2-iP concentrations. Root explants from 7-day-old seedlings were cultured in CIM with or without 2 µM TSA, then transferred to SIM containing different concentrations of 2-iP under continuous light. Photos were taken two weeks after the transfer. Scale bar = 2 mm. Statistical significance between control and TSA-treated groups at each concentration was determined using Student’s t-test, and the results are presented as mean ± standard deviation (***p < 0.001). (B) Effect of TSA on cytokinin signaling using TCS::GFP reporter. Callus treated with or without TSA was transferred to SIM and imaged by confocal microscopy. Callus was stained with 10 µM propidium iodide to visualize cell outlines; scale bar = 50 μm.
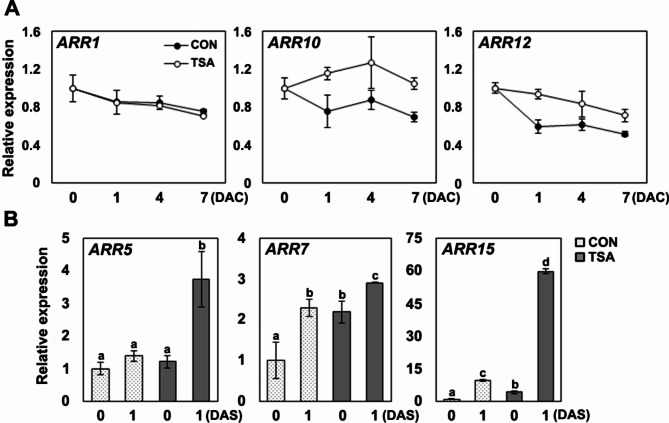
TSA upregulates the ARR10 and ARR12 genes, which are cytokinin signaling regulators
Mutations in cytokinin signaling regulators alter the cytokinin response, and the elevated expression of positive cytokinin regulators leads to cytokinin hypersensitivity during shoot regeneration32–35. To investigate the cytokinin signaling genes that were activated in the callus in response to TSA, we evaluated the expression of three ARABIDOPSIS HISTIDINE KINASEs (AHKs), three ARABIDOPSIS HISTIDINE PHOSPHOTRANSFER PROTEINs (AHPs) – AHP1, AHP2, AHP3 –, and three type-B ARABIDOPSIS RESPONSE REGULATORs (ARRs) – ARR1, ARR10, ARR12 – in callus. The control and TSA-treated calli were harvested seven days after incubation with CIM. RT-qPCR analysis of the three AHK genes revealed that AHK4 expression increased following TSA treatment, AHK3 expression decreased, and AHK2 expression exhibited no statistically significant difference (Fig. 5). Among AHP, sensor histidine kinase, which is activated by AHKs and transfers the phosphoryl group from AHKs to type-A and type-B response regulators, the expression of AHP2 was comparable to that in the control callus, and expression of AHP1 slightly decreased in TSA-treated callus. The transcript levels of AHP3 were slightly increased in the TSA-treated callus. Mediating cytokinin primary transcriptional response, ARR10 and ARR12 exhibited a 1.5-fold and 2-fold increase in expression, respectively, in TSA-treated callus compared to the control, whereas ARR1 transcripts reduced in TSA-treated callus relative to the control. Because ARR1, ARR10, and ARR12 play redundant roles in WUS expression in the shoot apical meristem of Arabidopsis10 and enhanced expression of ARR10 induces hypersensitivity to cytokinin and shoot regeneration in callus induced from hypocotyl explants36, we focused on the gene expression patterns of ARR1, ARR10, and ARR12 in response to TSA treatment during callus induction (Fig. 6A). The transcript levels of ARR1 decreased after inducing callus formation from root explants in both TSA-treated and control calli. In the control group, the expression of ARR10 and ARR12 decreased during callus induction, and minimal expression levels of ARR10 and ARR12 were observed before transfer onto SIM. However, the expression of ARR10 in callus treated with TSA did not decrease compared with that in the root explants (CIM 0 d). Compared with the control callus, the transcript level of ARR10 increased by approximately two-fold at CIM 7 d. The expression pattern of ARR12 decreased during callus induction, but the transcript level was higher than that of the control callus at CIM 7 d. This indicates that TSA affects the transcription patterns of ARR10 and ARR12 during callus induction, which might partially contribute to de novo shoot regeneration. We also examined the expression of three type-A ARRs, cytokinin response genes, and direct target genes of type B ARR34. ARR5, ARR7, and ARR15 were upregulated after incubation with cytokinin-rich SIM; in particular, ARR15 transcripts accumulated more intensively in TSA-treated callus than in the control (Fig. 6B). Collectively, these results suggest that TSA treatment affects the expression of ARR10 and ARR12 throughout the callus induction period, and the enhanced transcript levels of ARR10 and ARR12 stimulate cytokinin hypersensitivity in the callus, leading to improved shoot regeneration after transfer to SIM.
Fig. 5.
Effect of TSA on expression of cytokinin signaling during callus induction. Root explants taken from seedling of 7 days old were cultured in CIM treated with or without 2 µM TSA. After 7 days incubation, the calli were harvested to extract total RNA for quantitative RT-PCR analysis. Gene expression was adjusted to be comparable to that of the eIF4A gene. Each value indicates means of three biological replicates and error bars show standard deviation. Statistical significance was determined using student t-test (*p < 0.05, **p < 0.01 and ***p < 0.001).
Fig. 6.
Effect of TSA on the expression of cytokinin signaling regulators during shoot regeneration. (A) Expression profiles of ARR1, ARR10, and ARR12 during callus induction with TSA treatment. Root explants from 7-day-old seedlings were cultured in CIM with or without TSA, and callus was harvested at indicated days for RNA extraction. (B) Relative expression levels of ARR5, ARR7, and ARR15 during shoot regeneration. Callus was induced from root explants in CIM with or without TSA for 7 days, then transferred to SIM. RNA was extracted from callus incubated for 7 days in CIM (0 DAS) and 7 days in CIM plus 1 day in SIM (1 DAS). Transcript levels were normalized to eIF4A. Data represent means of three biological replicates, with error bars showing standard deviation. Significant differences between treatments were determined using one-way ANOVA with Tukey’s post hoc test, indicated by distinct letters (p < 0.05).
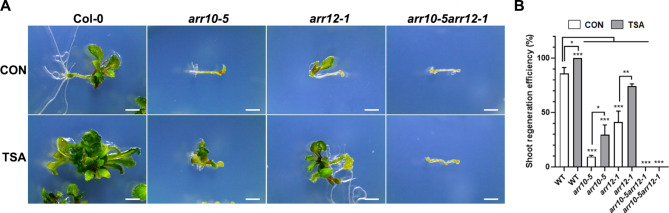
Enhanced shoot regeneration conferred by TSA treatment is contributed by regulation of ARR10 and ARR12 expression
To confirm that the increased transcription of ARR10 and ARR12 conferred by TSA treatment during callus induction is important for shoot regeneration, we examined the shoot-regenerative capacities of ARR10 and ARR12 mutants in response to TSA. Root explants of arr10-5, arr12-1, and arr10-5arr12-1 mutants were cultured on CIM supplemented with TSA for 7 days and then transferred into SIM without TSA. The arr10-5 and arr12-1 mutants exhibited fewer shoots on root explant than the Col-0 wild type (Fig. 7A). However, when arr10-5 and arr12-1 calli were induced on CIM supplemented with TSA, their shoot regenerative capacity was enhanced compared to non-treated calli of the each mutants. As shown in Fig. 7B, the arr10-5 and arr12-1 single mutants showed reduced shoot regeneration rates by approximately 75% and 45%, respectively, compared to the wild-type. Under TSA treatment, the arr10-5 mutant increased by approximately 20% relative to the control, while the arr12-1 mutant increased by approximately 33%. However, the arr10-5 arr12-1 double mutant was completely defective in shoot formation compared to the single mutants, even with TSA treatment. These results indicate that TSA might regulate the transcription of ARR10 and ARR12 during callus induction, and the increased transcripts of ARR10 and ARR12 might change the cytokinin response and induce subsequent shoot formation from the callus during shoot regeneration.
Fig. 7.
Effect of TSA on shoot regeneration ofarr10-5, arr12-1, andarr10-5arr12-1mutants. (A) Root explants from 7-day-old seedlings were incubated on CIM with 2 µM TSA for 7 days then transferred to SIM without TSA. Photos were taken 3 weeks after transfer. The scale bar = 2 mm. (B) Shoot regeneration efficiency of arr10-5, arr12-1, and arr10-5arr12-1 mutants treated with or without 2 µM TSA. Data are presented as mean ± standard deviation (n = 3 samples) and are representative of three independent experiments. Statistical significance was assessed using Student’s t-test, with *p < 0.05, **p < 0.01, and ***p < 0.001.
Discussion
Histone acetylation is generally considered to involve transcriptional activation. Histone deacetylases catalyze the elimination of acetyl groups from histone proteins, which causes transcriptional repression. Several studies have revealed that treatment with the broad-spectrum histone deacetylase inhibitor TSA can block histone deacetylase activity following histone hyperacetylation16,18,37. We found that TSA treatment increased global acetylation levels of histone H3 and H4 during callus induction. TSA treatment causes changes in plant regeneration due to histone hyperacetylation. Treatment of Arabidopsis cotyledons excised from immature zygotes with TSA promotes somatic embryo induction without exogenous treatment with phytohormones26. TSA leads to massive embryogenic cell proliferation and triggers a transition from pollen to embryogenic cell proliferation, promoting totipotency in the male gametophyte Brassica napus23.
To investigate whether histone deacetylation is involved in callus-to-shoot transition in de novo shoot regeneration, we treated TSA on CIM to induce callus derived from Arabidopsis root explants and transferred the callus into SIM without TSA. We found that the callus treated with TSA regenerated de novo shoots earlier than the control callus and formed multiple shoot clusters. Bie et al.38 demonstrated that TSA could improve the frequency of shoot regeneration derived from mature embryos compared to the control group in a common wheat cultivar. Similar to TSA, 4-phenylbutyric acid (4PBA), an HDAC inhibitor, enhances callus formation and subsequent shoot formation39, whereas SAHA, an HDAC inhibitor, stimulates microspore embryogenesis and direct plant regeneration in Pak choi40. Our results showed that TSA-mediated broad HDAC inhibition promoted shoot regeneration; however, each HDAC may play a different role in shoot regeneration. Temman et al.41 revealed that the loss of histone deacetylase19 (HDA19) reduced shoot regenerative ability by regulating the expression of ENHANCER OF SHOOT REGENERATION 1 (ESR1) and CUC2, which are responsible for SAM formation during shoot induction in A. thaliana. We hypothesized that TSA might block the action of several HDACs, thereby regulating important genes involved in shoot formation, although it is necessary to study the role of specific HDACs in shoot regeneration. In addition, we found that the response of shoot regeneration varies according to the explant source. TSA only promoted shoot regeneration in callus derived from root explants, whereas reduced shoot regeneration was observed in callus derived from leaf explants. Similarly, TSA-treated leaf explants exhibited reduced callus formation, whereas TSA-treated hypocotyl explants exhibited accelerated callus formation42,43. The process of callus induction from explants is similar to the mechanism of lateral root formation, and key regulatory genes expressed in the root apical meristem play a crucial role in callus induction44,45. In contrast to root explants, the expression of genes related to callus induction occurs after the global suppression of leaf regulatory gene expression in leaf explants during the leaf-to-callus transition. He et al.46 reported that the expression of leaf regulatory genes is silenced with the increase of the PRC2-mediated H3K27me3 mark, and that the leaf-to-callus transition is defective in PRC2 mutants, demonstrating that the disruption of key regulators in the leaf-to-callus transition significantly reduces callus formation, highlighting their critical role in cellular reprogramming. Similarly, our results showed that TSA treatment on CIM markedly inhibits shoot regeneration from leaf explants, suggesting that deacetylation may play crucial roles suppression of leaf regulatory gene expression and the activation of genes crucial for callus formation during shoot regeneration. The shoot apical meristem is initiated and maintained by the action of several transcription factors that function in stem cell niches and upregulate genes to stimulate shoot regeneration. Ectopic activation of AtWUS is sufficient to develop leaf-like organs with guard cells and trichomes in Arabidopsis47 and to form multiple shoots on callus in cotton48. In this study, TSA significantly upregulated shoot apical meristem-specific genes, including WUS, STM, CUC2, and Rap2.6 L, during shoot regeneration. Co-treatment with TSA and BAP led to increase histone H3 and H4 acetylation in the WUS genomic region and the rapid activation of WUS expression in the shoot apex tissue of A. thaliana49. Overexpression of CUC2, which is expressed throughout the proliferating callus and marks the promeristems during shoot regeneration, promotes successful shoot regeneration in the compromised shoot regenerative capacities of plt3, plt5-3, and plt727. Furthermore, increases in H3K9ac have been observed in the promoter and coding sequences of CUC2, which are associated with the activation of CUC2 expression during shoot regeneration50. Rap2.6 L also functions in both callus induction and shoot initiation, and its promoter region is regulated by acetylation-mediated transcriptional modifications31,51.
The TSA-treated callus was sensitive to cytokinin and could quickly and successfully regenerate shoots at low concentrations of cytokinin, which prevented the callus from struggling to form new shoots. Similarly, Furuta et al.42 reported that TSA promoted cytokinin hypersensitivity for the growth and greening of callus derived from hypocotyl explants. Overexpression of the cytokinin signaling regulator type-B ARR causes cytokinin hypersensitivity and enhances shoot regeneration35,36,52,53. Considering that TSA treatment has a distinct effect on gene expression in plants26,54,55, TSA appears to regulate the transcription of cytokinin signaling regulators in callus. In support of this, we found that the expression of type-B ARR, ARR10 and ARR12, which act as positive regulators in the cytokinin signaling pathway9,56, were selectively increased in callus-treated with TSA in CIM for 7 days, and their expression did not decrease, unlike that in the control callus during callus induction from root explants. ARR10 and ARR12 contribute to SAM establishment by colocalizing with the expression of WUS, which marks the future SAM initiation region during shoot regeneration10. In particular, ARR10 and ARR12 directly bind to the WUS promoter, and cytokinin treatment leads to an overall increase in ARR10 binding to the target gene35. Based on these results, we might suggest that the increased expression of ARR10 and ARR12 induced by TSA during the callus induction caused hypersensitivity to cytokinin response when transferred to SIM containing cytokinin. This likely enhanced the expression of meristem-related factors such as WUS, promoting shoot regeneration even under low cytokinin conditions. To clarify this hypothesis, it is necessary to examine the increase in the histone acetylation status of the promoter regions of ARR10 and ARR12 in the callus following TSA treatment, as well as their increased binding to the WUS promoter region after being transferred to SIM. In addition, we observed the upregulation of type-A ARR, ARR5, ARR7, and ARR15 in TSA-treated calli following transfer into cytokinin-rich SIM, which is a cytokinin-mediated target of type-B ARR and is induced by type-B ARR overexpression36,53,57. Considering that type-B ARR promotes cytokinin-induced target gene expression, we assumed that TSA treatment induces de-repression of ARR10 and ARR12 in the callus, leading to hypersensitivity to cytokinins after the callus is transferred into cytokinin-rich SIM and subsequently enhances shoot regenerative capacity during shoot regeneration. Consistent with this assumption, Hill36 showed that transgenic expression of ARR10 increased cell proliferation of explants, callus greening, and shoot regeneration in arr1 and arr12 mutants, even when compared with the wild type. Furthermore, single mutants arr10-5 and arr12-1 had reduced shoot regeneration capacity compared to wild-type Col-0, which was consistent with previous observations8, but TSA enhanced the shoot regenerative capacity of arr10-5 and arr12-1. However, the double mutant arr10-5arr12-1 showed defective shoot regeneration, even with TSA treatment. Because of the functional redundancy of ARR10 and ARR12, single mutants might be responsive to TSA treatment following improved shoot regenerative capacity compared to single mutants. However, double mutants could not respond to TSA and exhibited defective shoot formation. Our results suggest that TSA-induced shoot regeneration is associated with ARR10- and ARR12-mediated cytokinin responses, triggering enhanced regenerative capacity.
Conclusion
This study investigated the effects of trichostatin A (TSA), a histone deacetylase inhibitor, on de novo shoot regeneration in root explant-derived callus. TSA-treated root explants exhibited pronounced callus greening and a substantial increase in the formation of multiple shoots per callus compared with the control. Furthermore, the TSA treatment resulted in the upregulation of shoot apical meristem-specific genes such as WUS, Rap2.6 L, STM, and CUC2 during shoot regeneration. Notably, the TSA treatment increased sensitivity to cytokinin, leading to enhanced cytokinin signaling, as evidenced by increased TCS::GFP expression in the callus. This was accompanied by the upregulation of ARR10 and 12, which are key regulators of cytokinin signaling. Additionally, the downstream targets of type-B ARRs, including ARR5, ARR7, and ARR15, were significantly upregulated during shoot regeneration. This study further demonstrates that TSA treatment enhances the regenerative capacity of ARR10 and ARR12 deficient mutants. Overall, our findings highlight the pivotal role of histone deacetylation in mediating cytokinin responses and controlling de novo shoot regeneration in plants.
Materials and methods
Plant materials and growth conditions
Wild-type and mutant lines of Arabidopsis thaliana were of the Columbia ecotype background. The arr10-5 (CS39989), arr12-1 (CS6978), and arr10-5arr12-1 (CS39991) mutants were obtained from the Arabidopsis Biological Resource Center (ABRC). Seeds were surface-sterilized for 3 min in 1% NaOCl, rinsed several times with sterile distilled water, and the sterilized seeds were sown on a half-strength Murashige and Skoog medium58 (Duchefa Biochemie, Haarlem, Netherlands) supplemented with 1% (w/v) sucrose, 0.5 g/L 2-morpholinoethanesulfonic acid (MES), and 0.8% (w/v) phytagel (pH 5.7). Seedlings were grown vertically at 22 °C with 16 h light and 8 h dark cycles for 7 days.
Callus induction and shoot regeneration
For callus induction, root and hypocotyl explants were cut into 5–10 mm parts from 7-day-old seedlings and incubated on callus induction medium (CIM) containing Gamborg B5 medium59, 0.5 mg/L 2,4-dichlorophenoxylacetic acid, 0.05 mg/L kinetin, 0.5 g MES, 20 g glucose, and 0.8% phytoagar (pH 5.7) for 7 days at 22 °C in the dark. Leaf explants were cut from 2-week-old sterile seedlings and incubated on CIM at 22 °C in the dark for 2 weeks. To induce shoot regeneration, root, hypocotyl, or leaf derived explants were transferred to SIM containing Gamborg B5 medium, 0.9 µM 3-indoleacetic acid, 2.5 µM 2-iP, 20 g glucose, 0.5 g/L MES, and 0.8% phytoagar (pH 5.7) or low-cytokinin SIM supplemented with 0.5 µM 2-isopentenyladenine and 0.9 µM 3-indoleacetic acid, then incubated at 22 °C under continuous light conditions. Trichostatin A (Sigma Aldrich, #T1952) was added to the CIM at concentrations of 0.001, 0.01, 0.1, 1, 2, 5, and 10 µM. As dimethyl sulfoxide (DMSO) was used as a solvent for TSA, and the control group was treated with the same volume of DMSO as the TSA-treated group.
Immunoblot analysis
The callus was harvested, powdered in liquid nitrogen, suspended in a Triton Extraction Buffer (TEB: PBS containing 0.5% Triton X-100 and 2 mM phenylmethylsulfonyl fluoride (PMSF), and then left on ice for 10 min while being gently stirred. The lysed cells were centrifuged at 6500 × g for 10 min at 4 °C, and the supernatant was removed. The lysed cells were resuspended in half the volume of TEB and centrifuged at 6500 × g for 10 min at 4 °C to separate the supernatants. Histone proteins were extracted using 0.2 N HCl overnight at 4 °C, and the sample was centrifuged at 6500 × g for 10 min at 4 °C, and the supernatant was neutralized with 1/10 volume of 2 N NaOH. A 15% SDS-PAGE gel was used to analyze the histone extracts, which were suspended in an SDS polyacrylamide gel electrophoresis (SDS-PAGE) sample loading buffer. Histones were detected using antibodies against histone H3 (Merck Millipore, 06-599), H4 (Merck Millipore, 05-858), acetylated histone H3 (Abcam, ab1791), and acetylated histone H4 (Abcam, ab177790)60.
RNA extraction and quantitative RT-PCR analysis
The TRIzol reagent (Invitrogen) was used to extract total RNA according to the manufacturer’s instructions. Total RNA concentration was determined using a UV-Vis spectrophotometer. cDNA was synthesized using the SuperScript IV First-Strand Synthesis System Kit (Invitrogen). The RT-qPCR was performed using a CFX96 real-time PCR detection system (Bio-Rad, CFX96). The following thermal parameters were used: (1) 15 min at 95 °C, (2) 45 cycles of 20 s at 95 °C, and 40 s at 60 °C. After normalization to the internal control, EUKARYOTIC TRANSLATION INITIATION FACTOR 4A1 (eIF4A) gene (At3g13920) transcript levels were calculated. Relative expression levels of the examined genes were analyzed using the comparative Ct method. For each biological sample, RT-qPCR was conducted three times for independent biological replicates and three times for technical replicates.
Statistical analysis
Statistical analyses were conducted using Student’s t-test and one-way analysis of variance (ANOVA) to evaluate and interpret the results presented in the manuscript. GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA) was used for statistical analysis. Significant differences were determined using one-way ANOVA with Tukey’s post-hoc test (p < 0.05).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by grants from the KRIBB Initiative Program [grants No. KGM5382414 and KGM5282432], and a National Research Council of Science & Technology (NST) grant from the Korean government (MSIT) [grant number CAP23051-200], and the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) [grant number NRF-2020M3A9I4038354]. We thank to Dr. Bruno Muller at Massachusetts General Hospital for the development of transgenic TCS::GFP-expressing plant. The material was provided to us through Dr. Youngsook Lee at Pohang University of Science and Technology.
Author contributions
Su Hyun Park: Investigation, validation, and writing-original draft. Yu Jeong Jeong: Investigation and validation. Soyoung Kim: Investigation. Jiyoung Lee: Validation and writing-review & editing. Cha Young Kim: Conceptualization and validation. Jae Cheol Jeong: Project administration, validation, conceptualization and writing-review & editing.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
Experimental research and field studies on plants, including the collection of plant material, complying with relevant institutional, national, and international guidelines and legislation.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cha Young Kim, Email: kimcy@kribb.re.kr.
Jae Cheol Jeong, Email: jcjeong@kribb.re.kr.
References
- 1.Christianson, M. L. & Warnick, D. A. Competence and determination in the process of in vitro shoot organogenesis. Dev. Biol.95, 288–293. 10.1016/0012-1606(83)90029-5 (1983). [DOI] [PubMed] [Google Scholar]
- 2.Mayer, K. et al. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell95, 805–815. 10.1016/S0092-8674(00)81703-1 (1999). [DOI] [PubMed] [Google Scholar]
- 3.Barton, M. & Poethig, R. S. Formation of the shoot apical meristem in Arabidopsis thaliana—An analysis of development in wild type and in the SHOOT MERISTEMLESS mutant. Development119 (1993).
- 4.Chatfield, S. P. et al. Incipient stem cell niche conversion in tissue culture: Using a systems approach to probe early events in WUSCHEL-dependent conversion of lateral root primordia into shoot meristems. Plant. J.73, 798–813. 10.1111/tpj.12085 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Gordon, S. P. et al. Pattern formation during de novo assembly of the Arabidopsis shoot meristem. Development134, 3539–3548. 10.1242/dev.010298 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Valvekens, D., Van Montagu, M. & Van Lijsebettens, M. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc. Natl. Acad. Sci. U S A. 85, 5536–5540. 10.1073/pnas.85.15.5536 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko, D. et al. Arabidopsis ABCG14 is essential for the root-to-shoot translocation of cytokinin. Proc. Natl. Acad. Sci. U S A. 111, 7150–7155. 10.1073/pnas.1321519111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishida, K., Yamashino, T., Yokoyama, A. & Mizuno, T. Three type-B response regulators, ARR1, ARR10 and ARR12, play essential but redundant roles in cytokinin signal transduction throughout the life cycle of Arabidopsis thaliana. Plant. Cell. Physiol.49, 47–57. 10.1093/pcp/pcm165 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Mason, M. G. et al. Multiple type-B response regulators mediate cytokinin signal transduction in Arabidopsis. Plant. Cell.17, 3007–3018. 10.1105/tpc.105.035451 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng, W. J. et al. Type-B ARABIDOPSIS RESPONSE REGULATORs specify the shoot stem cell niche by dual regulation of WUSCHEL. Plant. Cell.29, 1357–1372. 10.1105/tpc.16.00640 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorisch, S. M., Wachsmuth, M., Toth, K. F., Lichter, P. & Rippe, K. Histone acetylation increases chromatin accessibility. J. Cell. Sci.118, 5825–5834. 10.1242/jcs.02689 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Martinez, O., Arjones, V., Gonzalez, M. V. & Rey, M. Histone deacetylase inhibitors increase the embryogenic potential and alter the expression of embryogenesis-related and HDAC-encoding genes in Grapevine (Vitis vinifera L., Cv. Mencia). Plants (Basel). 10, 1164. 10.3390/plants10061164 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mengel, A. et al. Nitric oxide modulates histone acetylation at stress genes by inhibition of histone deacetylases. Plant. Physiol.173, 1434–1452. 10.1104/pp.16.01734 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venturelli, S. et al. Plants release precursors of histone deacetylase inhibitors to suppress growth of competitors. Plant. Cell.27, 3175–3189. 10.1105/tpc.15.00585 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brosch, G., Goralik-Schramel, M. & Loidl, P. Purification of histone deacetylase HD1-A of germinating maize embryos. FEBS Lett.393, 287–291. 10.1016/0014-5793(96)00909-x (1996). [DOI] [PubMed] [Google Scholar]
- 16.Earley, K. et al. Erasure of histone acetylation by Arabidopsis HDA6 mediates large-scale gene silencing in nucleolar dominance. Genes Dev.20, 1283–1293. 10.1101/gad.1417706 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu, C. et al. HDA18 affects cell fate in Arabidopsis root epidermis via histone acetylation at four kinase genes. Plant. Cell.25, 257–269. 10.1105/tpc.112.107045 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo, M. et al. Regulation of flowering time by the histone deacetylase HDA5 in Arabidopsis. Plant. J.82, 925–936. 10.1111/tpj.12868 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Niu, Y. et al. HISTONE DEACETYLASE 9 transduces heat signal in plant cells. Proc. Natl. Acad. Sci. U S A. 119, e2206846119. 10.1073/pnas.2206846119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu, C. R. et al. Histone acetylation affects expression of cellular patterning genes in the Arabidopsis root epidermis. Proc. Natl. Acad. Sci. USA. 102, 14469–14474. 10.1073/pnas.0503143102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu, Y. et al. A histone deacetylase inhibitor enhances rice immunity by derepressing the expression of defense-related genes. Front. Plant. Sci.13, 1041095. 10.3389/fpls.2022.1041095 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang, F. et al. Trichostatin A increases embryo and green plant regeneration in wheat. Plant. Cell. Rep.36, 1701–1706. 10.1007/s00299-017-2183-3 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Li, H. et al. The histone deacetylase inhibitor trichostatin a promotes totipotency in the male gametophyte. Plant. Cell.26, 195–209. 10.1105/tpc.113.116491 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka, M., Kikuchi, A. & Kamada, H. The Arabidopsis histone deacetylases HDA6 and HDA19 contribute to the repression of embryonic properties after germination. Plant. Physiol.146, 149–161. 10.1104/pp.107.111674 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uddenberg, D. et al. Embryogenic potential and expression of embryogenesis-related genes in conifers are affected by treatment with a histone deacetylase inhibitor. Planta234, 527–539. 10.1007/s00425-011-1418-8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wojcikowska, B. et al. Trichostatin A triggers an embryogenic transition in Arabidopsis explants via an auxin-related pathway. Front. Plant. Sci.9, 1353. 10.3389/fpls.2018.01353 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kareem, A. et al. PLETHORA genes control regeneration by a two-step mechanism. Curr. Biol.25, 1017–1030. 10.1016/j.cub.2015.02.022 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim, J. Y. et al. Epigenetic reprogramming by histone acetyltransferase HAG1/AtGCN5 is required for pluripotency acquisition in Arabidopsis. EMBO J.3710.15252/embj.201798726 (2018). [DOI] [PMC free article] [PubMed]
- 29.Martin, B. J. E. et al. Transcription shapes genome-wide histone acetylation patterns. Nat. Commun.12, 210. 10.1038/s41467-020-20543-z (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motte, H., Verstraeten, I., Werbrouck, S. & Geelen, D. CUC2 as an early marker for regeneration competence in Arabidopsis root explants. J. Plant. Physiol.168, 1598–1601. 10.1016/j.jplph.2011.02.014 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Che, P., Lall, S., Nettleton, D. & Howell, S. H. Gene expression programs during shoot, root, and callus development in Arabidopsis tissue culture. Plant. Physiol.141, 620–637. 10.1104/pp.106.081240 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higuchi, M. et al. In planta functions of the Arabidopsis cytokinin receptor family. Proc. Natl. Acad. Sci. USA. 101, 8821–8826. 10.1073/pnas.0402887101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoue, T. et al. Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature409, 1060–1063. 10.1038/35059117 (2001). [DOI] [PubMed] [Google Scholar]
- 34.To, J. P. et al. Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. Plant. Cell.16, 658–671. 10.1105/tpc.018978 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zubo, Y. O. et al. Cytokinin induces genome-wide binding of the type-B response regulator ARR10 to regulate growth and development in Arabidopsis. Proc. Natl. Acad. Sci. USA. 114, E5995–E6004. 10.1073/pnas.1620749114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill, K. et al. Functional characterization of type-B response regulators in the Arabidopsis cytokinin response. Plant. Physiol.162, 212–224. 10.1104/pp.112.208736 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cusack, M. et al. Distinct contributions of DNA methylation and histone acetylation to the genomic occupancy of transcription factors. Genome Res.30, 1393–1406. 10.1101/gr.257576.119 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bie, X. M. et al. Trichostatin A and sodium butyrate promotes plant regeneration in common wheat. Plant. Signal. Behav.15, 1820681. 10.1080/15592324.2020.1820681 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwase, A. et al. 4-Phenylbutyric acid promotes plant regeneration as an auxin by being converted to phenylacetic acid via an IBR3-independent pathway. Plant. Biotechnol. (Tokyo). 39, 51–58. 10.5511/plantbiotechnology.21.1224b (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang, L. et al. Effects of histone deacetylase inhibitors on microspore embryogenesis and plant regeneration in Pakchoi (Brassica rapa ssp. chinensis L). Sci. Hort.209, 61–66. 10.1016/j.scienta.2016.05.001 (2016). [Google Scholar]
- 41.Temman, H. et al. Histone deacetylation regulates de novo shoot regeneration. PNAS Nexus. 2, pgad002. 10.1093/pnasnexus/pgad002 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furuta, K. et al. The CKH2/PKL chromatin remodeling factor negatively regulates cytokinin responses in Arabidopsis Calli. Plant. Cell. Physiol.52, 618–628. 10.1093/pcp/pcr022 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Lee, K., Park, O. S., Jung, S. J. & Seo, P. J. Histone deacetylation-mediated cellular dedifferentiation in Arabidopsis. J. Plant. Physiol.191, 95–100. 10.1016/j.jplph.2015.12.006 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Sugimoto, K., Jiao, Y. & Meyerowitz, E. M. Arabidopsis regeneration from multiple tissues occurs via a root development pathway. Dev. Cell.18, 463–471. 10.1016/j.devcel.2010.02.004 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Atta, R. et al. Pluripotency of Arabidopsis xylem pericycle underlies shoot regeneration from root and hypocotyl explants grown in vitro. Plant. J.57, 626–644. 10.1111/j.1365-313X.2008.03715.x (2009). [DOI] [PubMed] [Google Scholar]
- 46.He, C., Chen, X., Huang, H. & Xu, L. Reprogramming of H3K27me3 is critical for acquisition of pluripotency from cultured Arabidopsis tissues. PLoS Genet.8, e1002911. 10.1371/journal.pgen.1002911 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gallois, J. L., Nora, F. R., Mizukami, Y. & Sablowski, R. WUSCHEL induces shoot stem cell activity and developmental plasticity in the root meristem. Genes Dev.18, 375–380. 10.1101/gad.291204 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bouchabke-Coussa, O. et al. Wuschel overexpression promotes somatic embryogenesis and induces organogenesis in cotton (Gossypium hirsutum L.) tissues cultured in vitro. Plant. Cell. Rep.32, 675–686. 10.1007/s00299-013-1402-9 (2013). [DOI] [PubMed] [Google Scholar]
- 49.Wang, J. et al. Cytokinin Signaling activates WUSCHEL expression during Axillary Meristem initiation. Plant. Cell.29, 1373–1387. 10.1105/tpc.16.00579 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song, Y. G., Liu, Y. L., Qiu, N. W. & Dong, W. Involvement of histone modification in regulating CUP-SHAPED COTYLEDON genes during shoot regeneration in Arabidopsis. Biol. Plant.61, 197–200. 10.1007/s10535-016-0661-z (2017). [Google Scholar]
- 51.Rymen, B. et al. Histone acetylation orchestrates wound-induced transcriptional activation and cellular reprogramming in Arabidopsis. Commun. Biol.2, 404. 10.1038/s42003-019-0646-5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Imamura, A., Kiba, T., Tajima, Y., Yamashino, T. & Mizuno, T. In vivo and in vitro characterization of the ARR11 response regulator implicated in the his-to-asp phosphorelay signal transduction in Arabidopsis thaliana. Plant. Cell. Physiol.44, 122–131. 10.1093/pcp/pcg014 (2003). [DOI] [PubMed] [Google Scholar]
- 53.Sakai, H. et al. ARR1, a transcription factor for genes immediately responsive to cytokinins. Science294, 1519–1521. 10.1126/science.1065201 (2001). [DOI] [PubMed] [Google Scholar]
- 54.Choi, S. H. et al. TSA promotes CRISPR/Cas9 editing efficiency and expression of cell division-related genes from plant protoplasts. Int. J. Mol. Sci.22, 7817. 10.3390/ijms22157817 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu, Y. et al. Trichostatin A selectively suppresses the cold-induced transcription of the ZmDREB1 gene in maize. PLoS One. 6, e22132. 10.1371/journal.pone.0022132 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Argyros, R. D. et al. Type B response regulators of Arabidopsis play key roles in cytokinin signaling and plant development. Plant. Cell.20, 2102–2116. 10.1105/tpc.108.059584 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tajima, Y. et al. Comparative studies on the type-B response regulators revealing their distinctive properties in the his-to-asp phosphorelay signal transduction of Arabidopsis thaliana. Plant. Cell. Physiol.45, 28–39. 10.1093/pcp/pcg154 (2004). [DOI] [PubMed] [Google Scholar]
- 58.Murashige, T. & Skoog, F. A. Revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant.15, 473–497. 10.1111/j.1399-3054.1962.tb08052.x (2006). [Google Scholar]
- 59.Gamborg, O. L., Miller, R. A. & Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell. Res.50, 151–158. 10.1016/0014-4827(68)90403-5 (1968). [DOI] [PubMed] [Google Scholar]
- 60.Lee, M. H. et al. The effect of sodium butyrate on adventitious shoot formation varies among the plant species and the explant types. Int. J. Mol. Sci.21, 8451. 10.3390/ijms21228451 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.