Abstract
Dengue remains a significant public health concern in Brazil, with all federative units registering occurrences of the disease within their territories despite constant measures to control the Aedes aegypti vector. This study aimed to evaluate the profile of notified dengue cases in the Brazilian Legal Amazon from 2001 to 2021, analyzing National System of Notifiable Diseases (SINAN) data on the disease to assess the risks for its occurrence. Subsequently, statistical analyses were conducted to identify incidence and lethality rates. Over the study period, approximately 1,344,950 cases of the disease were reported, resulting in 863 deaths. The transmission of cases in the Amazonian states was not homogeneous, demonstrating variations and clusters over the years of extreme value for health authorities. Identifying and understanding the spatiotemporal patterns for the disease in the region helps assess the behavior of infections in areas with high susceptibility, promoting targeted interventions and resource allocation for dengue control programs. It is important to encourage future studies that evaluate the disease’s risk based on quantitative variables. Such studies contribute to the formulation of health policies aimed at controlling and preventing dengue, improving public health outcomes.
Keywords: Epidemiology, Dengue, Health Information System, Surveillance
Subject terms: Health policy, Viral infection
Introduction
Unique in the world in terms of complexity and biodiversity, the Amazon biome is related to essential natural processes for life on Earth. Amazonian ecosystems have a fundamental role in the dynamics and control of climate and vector-borne diseases and infections, as it is essential to prevent the collapse of global biodiversity and minimize the negative impacts of climate change worldwide1. Additionally, the largest concentration of isolated indigenous peoples in the world is in the indigenous lands of the Brazilian Amazon2.
Despite its importance, the Brazilian Amazon and its peoples are facing the worst attack in a generation. Indigenous and traditional communities suffer disproportionate violence and repression in defending their rights and forests3. Images of sick and malnourished indigenous peoples (especially children) have become viral on social media and in communication vehicles worldwide, which were important in drawing the federal government’s attention to the problem and initiating more effective actions in the region4. In Brazil, economic and political crises are potential triggers for epidemics of socially determined diseases, such as malaria, dengue, tuberculosis, leishmaniasis, among other neglected tropical diseases that stand out as one of the main risks to the vulnerable population in the Amazon region, as they are associated with socio-environmental vulnerability, including poverty, lack of basic sanitation, and reduced availability of potable water5.
Thus, despite its environmental and cultural richness, the Brazilian Legal Amazon is a vulnerable territory, as are its poorest populations. In this context of socioeconomic and political crisis, Brazil faces the challenge of addressing those problems that have been recently exacerbated in the Brazilian Legal Amazon in recent years. Among these challenges is the increase in socially determined diseases, such as dengue, among the most vulnerable population5. This disease is associated with considerable social and economic losses in the at-risk population, especially those living in precarious conditions regarding health care, housing, and sanitation6. It is considered a serious public health problem worldwide, but especially in developing countries with significant impact on socioeconomic development and quality of life7, being the most widespread arbovirus and causing the highest number of arboviral disease cases in the Region of the Americas8. According to the Pan American Health Organization (PAHO), between 1980 and 1989 there were 1.54 million cases, while between 2010 and 2017, there were 12.68 million cases9.
Endemic in almost 120 countries, dengue is extremely common in the tropical regions, with local variations in cases and severity depending on environmental and social factors10. Asia contributes to 70% of the global dengue burden. Three Asian (Bangladesh, Pakistan, and India) and seven non-Asian countries (Brazil, Peru, Bolivia, Ecuador, Paraguay, Argentina, and Singapore) have reported an increase in the number of dengue cases in recent years11. In the Americas particularly, Brazil is the country with the highest incidence, with 1,450,270 probable cases until the 52nd Epidemiological Week of 2022, representing an incidence rate of 679.9 cases per 100,000 inhabitants12. It is transmitted by Aedes aegypti, a vector that exhibits anthropophilic behavior and is adapted to urban environments, where it finds breeding sites, shelter, and food more easily13. Therefore, the increase in urban conglomerates, waste generation associated with the precariousness of basic sanitation services, is a condition, along with climate change, that favors the rapid proliferation and dissemination of this vector14,15.
In this context, the objective of this study was to analyze the spatiotemporal dynamics of dengue in the Amazon region between the years 2001 and 2021. The contribution of this study lies precisely in the possibility of providing primary care professionals and public managers with a better understanding of dengue in the Brazilian Amazon. It addresses challenges brought up by rapid urbanization, biodiversity loss, a growing and dynamic human population, and significant inequality. Additionally, it considers risks of shocks such as the COVID-19 pandemic and increasingly intense extreme events due to ongoing climate change in the region. Despite those challenges, this epidemiological evaluation has the potential to identify regions at higher risk, therefore more vulnerable to dengue, providing information for surveillance strategies that play an important role in guiding the formulation of disease control plans.
Methodology
Data collection
A study of time series and spatiotemporal analyses was developed, allowing the identification of the disease dynamics in the Brazilian Legal Amazon during the years 2001 to 2021. Data on dengue cases were obtained from individual notification forms made available by the National System of Notifiable Diseases (SINAN) on the TabNet platform on the Ministry of Health/DataSUS website16. The number of cases was downloaded using the municipality as the study area. Population estimates in the municipalities were obtained from the Brazilian Institute of Geography and Statistics (IBGE)17 as well as the geographic informations18, including the shapefile documents used for maps in the figures.
Study location
The term Legal Amazon was established by the Brazilian government with the intention of planning and promoting the socioeconomic development of the states in the Amazon region, which over the years have faced similar economic, political, and social challenges19.
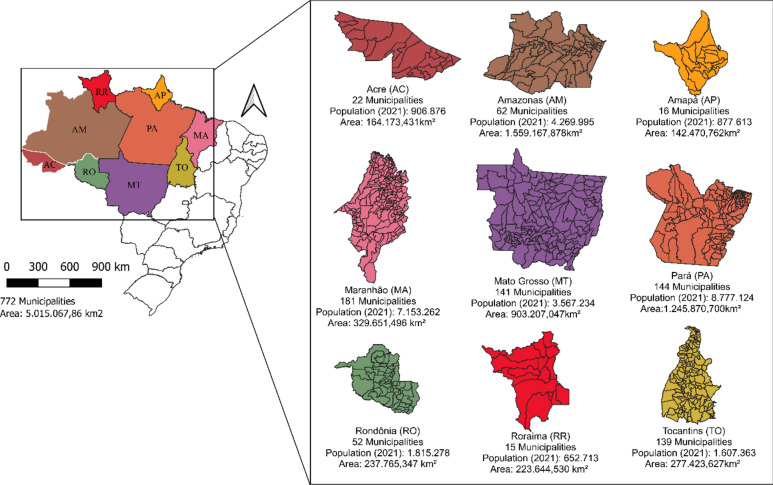
The present study was conducted in the Brazilian Legal Amazon (Fig. 1), a political-administrative region covering 5,015,146.008 km2, corresponding to about 58.93% of the Brazilian territory20,21, divided into 772 municipalities and nine states, namely: Acre (AC), Amapá (AP), Amazonas (AM), Pará (PA), Rondônia (RO), Roraima (RR), Mato Grosso (MT), Tocantins (TO), and part of Maranhão (MA)21. In 1972, the population of this region was 8.2 million inhabitants. By 2020, its population had grown to 28.1 million (5.6 inhabitants per km2), representing 13% of the Brazilian population and having the lowest population density in the country22.
Fig. 1.
Location of the Brazilian Legal Amazon with area, population, and number of municipalities per state, 2023.
The climate of the Amazon region is characterized as hot and humid equatorial, with rainfall occurring between December and April23, which contributes to the proliferation of Aedes aegypti (the vector of dengue) every year, and may be related to the probable cases of the disease and variations in incidence rates24.
Statistical analysis
Based on the dengue data, collected over 20 years of observation, incidence rates (IR) (per 100,000 inhabitants) and fatality rates (FR) (%) were calculated, using population projections from the IBGE for each year of interest17. Spatial analyses were conducted to identify geographical and temporal patterns of dengue by municipalities in the nine states of the Brazilian Legal Amazon. Trends in incidence rates were analyzed using Joinpoint models, while the spatiotemporal distribution was analyzed using SaTScan models by state.
Spatial analysis
In this research, different spatial analyses technics were conducted, allowing the identification of patterns of geographical phenomena. In order to verify the existence of clusters and analyze the disease risk between 2001 and 2021, annual thematic maps were constructed regarding the incidence and fatality rates of the disease in the 772 municipalities of the studied region.
SaTScan
SaTScan statistic is a spatial analysis technique that allows defining the scanning window as a time interval, using a circle or cylinder in time and space to record the number of identified and expected cases (within the window) at each screening location25. Thus, each cylinder reflects a cluster of cases. The scanning method sought to identify areas with high (higher risk) and low rates (lower risk), thus creating a scanning radius considering the percentage of the population at risk. This percentage refers to the scanning radius of the scan statistic, which, according to Kulldorff et al.25, is recommended not to exceed 50% of the population, in order to avoid clusters as large as the entire region under study. The scanning radius used in this research was 10%.
The test statistic employs the Monte Carlo method, with  -values less than 0.05 considered significant. SaTScan offers five probabilistic models. For this type of study, the most suitable model is the Poisson model, where the number of events in a geographical area is distributed according to a known underlying risk population25. The Poisson model is given by:
-values less than 0.05 considered significant. SaTScan offers five probabilistic models. For this type of study, the most suitable model is the Poisson model, where the number of events in a geographical area is distributed according to a known underlying risk population25. The Poisson model is given by:
 |
where  is a candidate cluster,
is a candidate cluster,  ) is the probability of the phenomenon under study occurring within the circle, and
) is the probability of the phenomenon under study occurring within the circle, and  is the probability of the phenomenon occurring outside of it. The
is the probability of the phenomenon occurring outside of it. The  is the total number of cases in the entire study region, and
is the total number of cases in the entire study region, and  is the total population. The exponential function is represented by
is the total population. The exponential function is represented by  , where
, where  and
and  are, respectively, the number of cases in circle
are, respectively, the number of cases in circle  and circle
and circle  , and
, and  is the number of individuals at risk in circle
is the number of individuals at risk in circle  26. The relative risk is an indicator used to represent the intensity of the occurrence of a phenomenon in relation to all study regions, thus indicating how many times greater the risk of becoming ill is among the exposed compared to the unexposed. When the relative risk is equal to 1, it indicates that the incidence of the outcome was equal in both compared groups, whereas a relative risk less than 1 indicates that the exposure was a protective factor27. The scan analysis was performed using the open-source software SaTScan28.
26. The relative risk is an indicator used to represent the intensity of the occurrence of a phenomenon in relation to all study regions, thus indicating how many times greater the risk of becoming ill is among the exposed compared to the unexposed. When the relative risk is equal to 1, it indicates that the incidence of the outcome was equal in both compared groups, whereas a relative risk less than 1 indicates that the exposure was a protective factor27. The scan analysis was performed using the open-source software SaTScan28.
Joinpoint
Segmented linear trend analysis for the incidence rate was conducted using the joinpoint regression model. The analysis began with the “0” joinpoint, representing a straight line with no inflection points. We then assessed whether one or more joinpoints (up to three in our analysis) in the model were significant. First introduced by Kim et al.29, joinpoint regression is a widely used method for analyzing trends over time, allowing for the identification of significant points of change and estimating a regression model based on these identified shifts. Joinpoint regression analysis can be a useful tool in epidemiologic frameworks to identify significant changes in temporal trends, such as periods of increased incidence rates, and differentiating trends across various regions, highlighting geographic disparities in disease incidence30.
Using this model, the annual adjusted dengue incidence rates in the Brazilian Legal Amazon were analyzed for segmented temporal trends. The analysis is based on calculating the Annual Percent Changes (APCs) for each segment and the Average Annual Percent Changes (AAPCs) for the entire period, along with their respective 95% confidence intervals (CI. 95%). For this analysis, each joinpoint will indicate a statistically significant change in slope tested using the Monte Carlo permutation test. Trends were considered statistically significant when the APC had a  -value
-value  . Results were interpreted as follows: positive and significant APCs/AAPCs were considered increasing trends, negative and significant APCs/AAPCs were considered decreasing trends; conversely, when there was no significance, the trend was considered stable31–33.
. Results were interpreted as follows: positive and significant APCs/AAPCs were considered increasing trends, negative and significant APCs/AAPCs were considered decreasing trends; conversely, when there was no significance, the trend was considered stable31–33.
Results
Between 2001 and 2021, in the Brazilian Legal Amazon, 1,344,950 cases of dengue were reported, of which 863 cases resulted in death. The average incidence rate during this period was 263.56 cases per 100,000 inhabitants, with a fatality rate of 0.06% (Table 1). From 2001 to 2005, 117 municipalities did not report any cases of dengue. In the periods from 2006 to 2010 and 2011 to 2015, it was observed that the number of municipalities without notifications of the disease decreased to 52 and 34, respectively. In the years 2016 to 2021, 44 municipalities did not report any cases of dengue. Over the 20-year study period, only four of the 772 municipalities in the Brazilian Legal Amazon did not report any cases of dengue, namely: Bacurituba and Marajá do Sena (Maranhão), Pracuúba (Amapá), and Tabocão (Tocantins).
Table 1.
Incidence rate (IR) (× 100,000 inhabitants) and fatality rate (FR) (%) of dengue in the Brazilian Legal Amazon states between 2001 and 2021.
| Period | 2001 to 2005 | 2006 to 2010 | 2011 to 2015 | 2016 to 2021 | 2001 to 2021 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| IR average | FR | IR average | FR | IR average | FR | IR average | FR | IR average | FR | |
| Acre | 377.26 | 0.02 | 1,651.23 | 0.03 | 1,539.66 | 0.01 | 912.63 | 0.04 | 1,201.84 | 0.02 |
| Amapá | 532.39 | 0.02 | 374.59 | 0.10 | 336.87 | 0.07 | 85.90 | 0.12 | 312.79 | 0.07 |
| Amazonas | 188.57 | 0.00 | 119.88 | 0.12 | 533.31 | 0.05 | 134.21 | 0.07 | 252.73 | 0.05 |
| Maranhão | 93.00 | 0.07 | 103.81 | 0.25 | 95.20 | 0.26 | 112.96 | 0.06 | 107.77 | 0.15 |
| Mato Grosso | 238.24 | 0.02 | 851.28 | 0.10 | 657.56 | 0.06 | 540.32 | 0.05 | 610.90 | 0.07 |
| Pará | 163.56 | 0.03 | 164.52 | 0.14 | 141.40 | 0.08 | 74.46 | 0.01 | 137.13 | 0.07 |
| Rondônia | 220.88 | 0.02 | 679.74 | 0.10 | 238.76 | 0.07 | 167.08 | 0.04 | 332.77 | 0.07 |
| Roraima | 768.24 | 0.02 | 862.49 | 0.09 | 273.95 | 0.02 | 91.17 | 0.07 | 460.23 | 0.05 |
| Tocantins | 229.18 | 0.04 | 628.34 | 0.03 | 585.96 | 0.04 | 467.72 | 0.04 | 511.82 | 0.04 |
| Legal Amazon | 192.45 | 0.03 | 353.34 | 0.10 | 333.08 | 0.06 | 204.05 | 0.05 | 282.71 | 0.06 |
From 2001 to 2005, the state of Roraima had the highest incidence rate, with 768.24 cases per 100,000 inhabitants, with the capital Boa Vista standing out, reporting 11,902 cases out of 15,549 in the state. In the subsequent periods, Acre had the highest incidence rates compared to other states in the Brazilian Legal Amazon region, with 1651.23 cases per 100,000 inhabitants (2006 to 2010), 1539.66 cases per 100,000 inhabitants (2011 to 2015), and 912.63 cases per 100,000 inhabitants (2016 to 2021), respectively. The state of Maranhão had the lowest incidence rates throughout the study period, with 93.0 cases per 100,000 inhabitants (2001 to 2005), 103.8 cases per 100,000 inhabitants (2006 to 2010), 95.20 cases per 100,000 inhabitants (2011 to 2015), and in the last period analyzed (2016 to 2021), the state of Amapá had 87.0 cases per 100,000 inhabitants (Table 1). Among the municipalities, the capital Rio Branco had the highest dengue incidence rates in the years 2006 to 2010 (331.7 cases per 100,000 inhabitants) and 2016 to 2021 (701.06 cases per 100,000 inhabitants). However, in the period from 2011 to 2015, the municipality of Cruzeiro do Sul had the highest incidence, with 32,344 reported cases (8.1 cases per 100,000 inhabitants).
Regarding lethality, an increase was observed over the evaluated periods, rising from 0.02% between 2001 and 2005 to 0.07% between 2016 and 2021. Maranhão had the highest lethality rates throughout the periods of 2001 to 2005 (0.07%), 2006 to 2010 (0.25%), 2011 to 2015 (0.26%), and 2016 to 2021 (0.06%), with Paço do Lumiar municipality standing out with a lethality rate of 2.7%. Following Maranhão, the states with the highest lethality rates were Tocantins from 2001 to 2005 (0.04%), Pará from 2006 to 2010 (0.03%), and in the last period, the state of Amapá (0.12%) (Table 1).
In most Brazilian Legal Amazon states, the spatial distribution of incidence rates presented a heterogeneous pattern (Figs. 2A). In the region, dengue incidence increased in all states from 2005 onwards, with higher incidences observed between 2006 to 2010 and 2011 to 2015 in the states of Rondônia, Mato Grosso, Tocantins, Amapá, and the southeastern part of Pará. Between 2016 and 2021, higher incidences were mainly observed in the states of Acre, Amazonas, Mato Grosso, and Tocantins. Considering the entire evaluated period (2001 to 2021), Maranhão presented the lowest incidence rates with concentrated cases in the state capital, São Luís. Regarding fatality rates (Figs. 2B), an increase is observed over the years. In the first interval, from 2001 to 2015, higher rates are concentrated in Pará, Maranhão, Mato Grosso, and Tocantins. From 2006 to 2010, it extends to other states such as Rondônia and Amazonas. Between 2011 and 2015, Amapá—which did not appear in previous periods—presented a high fatality rate, as did Maranhão, Pará, and Rondônia, and from 2016 to 2021, the state of Amapá continued with a high fatality rate. When evaluating the entire period (2001 to 2021), higher rates are observed in the North and Southwest of Amazonas; Pará in the southwestern and lower Amazon mesoregions; Rondônia in the Madeira do Guaporé mesoregion; Mato Grosso in the North and Southwest mesoregions; in Eastern Maranhão and Southern Amapá (Fig. 2).
Fig. 2.
Spatial distribution of dengue incidence and fatality rates in municipalities of the Brazilian Legal Amazon, from 2001 to 2021. (IR × 100,000; FR %).
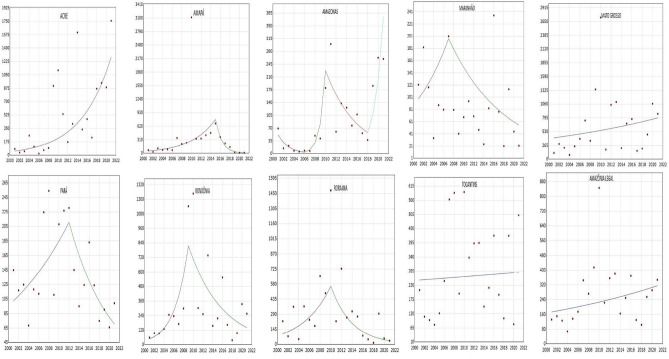
The temporal trends of each state in Brazilian Legal Amazon are presented in Fig. 3, while Table 2 presents the values of APC and AAPC of the trends by state, with their respective confidence intervals and  -values. Considering the entire Brazilian Legal Amazon territory, the dengue incidence rate remained stable throughout the period, as did in the states of Tocantins and Mato Grosso (
-values. Considering the entire Brazilian Legal Amazon territory, the dengue incidence rate remained stable throughout the period, as did in the states of Tocantins and Mato Grosso (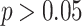 ). On the other hand, an increasing trend in the dengue incidence rate followed by a decrease (
). On the other hand, an increasing trend in the dengue incidence rate followed by a decrease (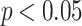 ) in different periods in the last years of the evaluated time series was observed in most states, such as Amapá (2001–2015; APC = 21.7%; 2015–2021; APC = − 54.10%), Maranhão (2001–2007; APC = 12.3%; 2007–2021; APC = − 8.7%), Pará (2001–2012; APC = 6.8; 2012–2021; APC = − 11.4), Rondônia (2001–2009; APC = 43.1; 2009–2021; APC = − 13.6), and Roraima (2001–2010; APC = 20.8; 2010–2021; APC = − 22.4). In the state of Amazonas, decreasing trends were observed throughout most of the time series (
) in different periods in the last years of the evaluated time series was observed in most states, such as Amapá (2001–2015; APC = 21.7%; 2015–2021; APC = − 54.10%), Maranhão (2001–2007; APC = 12.3%; 2007–2021; APC = − 8.7%), Pará (2001–2012; APC = 6.8; 2012–2021; APC = − 11.4), Rondônia (2001–2009; APC = 43.1; 2009–2021; APC = − 13.6), and Roraima (2001–2010; APC = 20.8; 2010–2021; APC = − 22.4). In the state of Amazonas, decreasing trends were observed throughout most of the time series ( ), followed by stability in the last years of the series (2018–2021;
), followed by stability in the last years of the series (2018–2021; 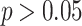 ). Acre was the only state that showed a consistently increasing trend in dengue incidence throughout the entire analyzed period (2001–2021; APC = 17.3%) (Fig. 3; Table 2).
). Acre was the only state that showed a consistently increasing trend in dengue incidence throughout the entire analyzed period (2001–2021; APC = 17.3%) (Fig. 3; Table 2).
Fig. 3.
Joinpoint Regression of dengue incidence rates (per 100,000 inhabitants) in the Brazilian Amazon between 2001 and 2021.Y-axis = incidence; X-axis = years.
Table 2.
Joinpoint Regression of segmented linear trend of dengue incidence rates in the Brazilian Legal Amazon between 2001 and 2021.
| Area | Period | APC (IC 95%) |
 -values -values |
Trend | AAPC (IC 95%) | Trend |
|---|---|---|---|---|---|---|
| Legal Amazon | 2001–2021 | 3.0 (− 0.9, − 7.1) | 0.121 | Stable | 3.0 (− 0.9, 7.1) | Stable |
| Acre | 2001–2021 | 17.3 (10.6, − 24.4) | 0.001 | Increasing | 17.3 (10.6, − 24.4) | Increasing |
| Amapá | 2001–2015 | 21.7 (11.3, − 33.1) | < 0.001 | Increasing | − 9.1 (− 17.2, − 0.4) | Decreasing |
| 2015–2021 | − 54.1 (− 64.6, − 40.5) | 0.001 | Decreasing | |||
| Amazonas | 2001–2006 | − 40.7 (− 58.4, − 15.4) | 0.008 | Decreasing | 10.5 (− 10.5, 36.4) | Stable |
| 2006–2010 | 179.0 (18.5, 556.9) | 0.023 | Increasing | |||
| 2010–2018 | − 15.8 (− 27.9, − 1.7) | 0.033 | Decreasing | |||
| 2018–2021 | 86.7 (− 20.5, 338.5) | 0.135 | Stable | |||
| Maranhão | 2001–2007 | 12.3 (1.0, − 24.8) | 0.035 | Increasing | − 2.8 (− 7.3, 1.9) | Stable |
| 2007–2021 | − 8.7 (− 13.7, − 3.3) | 0.004 | Decreasing | |||
| Mato Grosso | 2001–2021 | 3.3 (− 2.9, − 10) | 0.284 | Stable | 3.3 (− 2.9–10) | Stable |
| Pará | 2001–2012 | 6.8 (0.5, 13.5) | 0.036 | Increasing | − 1.8 (− 7.0− 3.6) | Stable |
| 2012–2021 | − 11.4 (− 20.2, − 1.6) | 0.026 | Decreasing | |||
| Roraima | 2001–2010 | 20.8 (− 7.3, 7.40) | 0.149 | Stable | − 5.3 (− 16.6, 7.6) | Stable |
| 2010–2021 | − 22.4 (− 31.6, − 11.9) | 0.001 | Decreasing | |||
| Rondônia | 2001–2009 | 43.1 (3.9, 97.0) | 0.030 | Increasing | 5.7 (7.6,21.0) | Stable |
| 2009–2021 | − 13.6 (− 231, − 2.9) | 0.018 | Decreasing | |||
| Tocantins | 2001–2021 | 0.6 (− 4.8, 6.3) | 0.830 | Stable | 0.6 (− 4.8 6.3) | Stable |
APC: annual percentage change (%); AAPC = average annual percentage change (%).
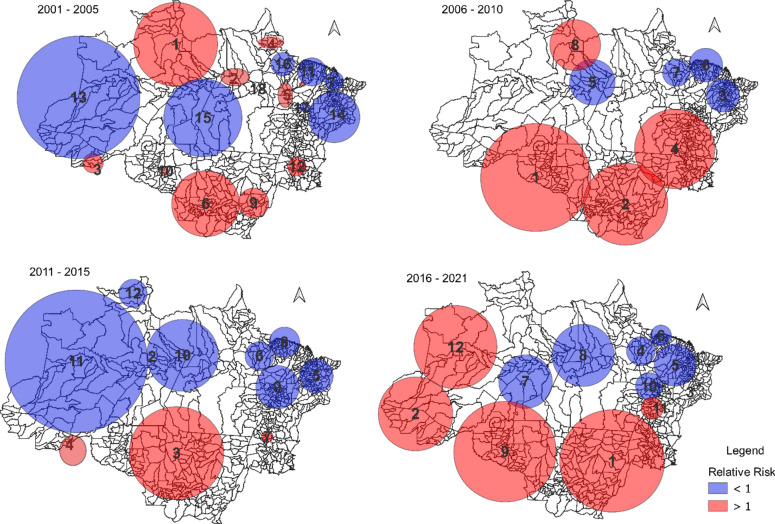
In addition to these results, through spatiotemporal analyses, 40 significant clusters ( ) were identified in the Brazilian Legal Amazon between 2001 and 2021, of which 26 were high-risk clusters for dengue (Fig. 4; supplementary material 1). The clusters were distributed heterogeneously over the period because, except for the state of Maranhão, high-risk clusters were observed in all states of the Brazilian Legal Amazon between 2001 and 2021, distributed over different time frames. From 2001 to 2005, high-risk clusters were observed mainly in the states of Roraima (1; pop = 1.9 million), northern Amazonas (1; pop = 1.9 million), and Mato Grosso (6; pop = 1.7 million), while between 2006 and 2010, in the states of Mato Grosso (1 and 2; pop = 4.5 million), Rondônia (1; pop = 2.2 million), Tocantins (4; pop = 2.0 million), and Roraima (8; pop = 0.3 million). Between 2011 and 2015, high-risk clusters were identified mainly in the state of Mato Grosso (3; pop = 2.2 million), while between 2011 and 2021, an increase in the number of municipalities in the Brazilian Legal Amazon region presenting high risk for dengue was observed, located mainly in the states of Tocantins (1; pop = 2.4 million), Mato Grosso (1 and 9; pop = 4.5 million), Amazonas (2 and 12; pop = 1.3 million), Acre (2; pop = 0.9 million), and Rondônia (9; pop = 2.1 million).
) were identified in the Brazilian Legal Amazon between 2001 and 2021, of which 26 were high-risk clusters for dengue (Fig. 4; supplementary material 1). The clusters were distributed heterogeneously over the period because, except for the state of Maranhão, high-risk clusters were observed in all states of the Brazilian Legal Amazon between 2001 and 2021, distributed over different time frames. From 2001 to 2005, high-risk clusters were observed mainly in the states of Roraima (1; pop = 1.9 million), northern Amazonas (1; pop = 1.9 million), and Mato Grosso (6; pop = 1.7 million), while between 2006 and 2010, in the states of Mato Grosso (1 and 2; pop = 4.5 million), Rondônia (1; pop = 2.2 million), Tocantins (4; pop = 2.0 million), and Roraima (8; pop = 0.3 million). Between 2011 and 2015, high-risk clusters were identified mainly in the state of Mato Grosso (3; pop = 2.2 million), while between 2011 and 2021, an increase in the number of municipalities in the Brazilian Legal Amazon region presenting high risk for dengue was observed, located mainly in the states of Tocantins (1; pop = 2.4 million), Mato Grosso (1 and 9; pop = 4.5 million), Amazonas (2 and 12; pop = 1.3 million), Acre (2; pop = 0.9 million), and Rondônia (9; pop = 2.1 million).
Fig. 4.
SaTScan Space–time analysis of dengue in the Brazilian Amazon 2001–2021. The blue color represents low-risk clusters (Relative Risk—RR < 1) and the red color represents high-risk clusters (RR > 1) for dengue.
Among the capitals, Palmas, the capital of Tocantins, stands out, where a high risk was observed in all periods of the analyzed time series, and Rio Branco, the capital of the state of Acre, where high-risk clusters were observed between 2001 and 2005 (RR = 2.56), 2006 and 2010 (RR = 5.72), and 2011 and 2015 (RR = 4.4), thus in three of the four periods analyzed. Manaus presented high-risk clusters in two periods, namely 2001 to 2005 (RR = 7.13) and 2011 to 2015 (RR = 10.5), as well as Porto Velho in 2006 to 2010 (RR 8.26) and 2016 to 2021 (RR 2.71). Boa Vista, the capital of Roraima, presented high-risk clusters only between 2006 and 2010 (RR = 3.95). Macapá and Belém were the capitals that showed low-risk clusters (RR < 1) throughout the entire studied time series (Fig. 4).
Discussion
Our study demonstrated the evolution of the geographic distribution and the complexity of dengue occurrence in a region with unique environmental characteristics in the world during the first 21 years of the twenty-first century. In addition, the study provides new insights into the occurrence of dengue, which are essential to guide surveillance and control actions, focusing on the most vulnerable areas. The results also highlighted the difficulties in controlling this disease, considering the increase in the number of dengue cases in different time frames between 2001 and 2021, and showed the areas to be prioritized for disease control and prevention strategies. Growth trends in incidence rates were observed in all states, especially in the early years of the time series, followed by a decrease in recent years in some states, such as Amapá, Maranhão, Pará, Rondônia, and Roraima. In the year 2020, a decrease in the number of dengue notifications occurred in all states.
According to Leandro et al.34, there was a decrease in the number of cases, not representing a decrease in incidence, but rather underreporting motivated by the consequences of the COVID-19 pandemic. Cardona-Ospina et al.35 also found similar results in a study in Colombia regarding the overlap of dengue and COVID-19, where a coincidental seasonal reduction in dengue notifications was observed with the increase in coronavirus infection notifications. According to the Ministry of Health in Brazil, the number of reported cases up to epidemiological week 17 of 2020 surpassed the number of cases during epidemiological weeks 07 and 11 of 2019. However, from epidemiological week 10 onwards, when the country’s health measures were intensified to combat COVID-19, a decline in the number of dengue notifications was observed, suggesting a possible underreporting from this period, which is seasonally expected to see an increase in dengue cases in the country36.
The present study identified significant high incidence rates of the disease in the Brazilian Legal Amazon region throughout the observation period. Specifically, regarding Acre, between 2009 and 2019, there were 1,502.06 cases per 100,000 inhabitants, values above those observed in other Brazilian states such as Goiás (988.21 cases per 100,000 inhabitants), Mato Grosso do Sul (856.83 cases per 100,000 inhabitants), Minas Gerais (769.39 cases per 100,000 inhabitants), Espírito Santo (755.85 cases per 100,000 inhabitants), and Mato Grosso (621.46 cases per 100,000 inhabitants)1. Additionally, an increase in dengue lethality was observed in the region, rising from 0.02% between 2001 and 2005 to 0.07% between 2016 and 2021.
When analyzing Brazil for 2022 up to Epidemiological Week 52, the lethality rate was at 0.07%, considering 1,016 deaths for 1,450,270 cases10. In a spatiotemporal analysis of dengue cases conducted in 2020 in the Brazilian Northeast, it was possible to observe an incidence rate related to the population size per municipality over the four years of the time series (2014 to 2017), being 1.92% higher in locations with more than 100,000 inhabitants compared to municipalities with 50,000 inhabitants37. In a longer temporal series, between 1990 and 2017, across Brazil, conducted by Andrioli et al.38, an incidence rate of 448.0 cases/100,000 inhabitants was found in the Midwest region, followed by the Southeast region with 242 cases/100,000 inhabitants and the Northeast region with 198 cases/100,000 inhabitants, with lethality rates ranging from 0.00 to 0.09% in these regions. In the state of Amazonas, in the Amazon region, from 2018 to 2022, an incidence rate of 754.8 cases per 100,000 inhabitants were observed39.
An increase in the number of cases was observed in some states of the BLA starting in 2010, such as Acre, Amazonas, Pará, Rondônia, and Roraima. The increase in these areas might be due to the extremely high burden of dengue circulating throughout the region in 2010, which likely affected areas where the dengue virus was not previously circulating. In 2010, severe epidemics were reported in several countries in the Americas, including Brazil, Colombia, Ecuador, Mexico, Venezuela, and Peru40–42. Soares et al.43 discuss that Boa Vista, the capital of Roraima, also experienced an epidemic in 2010, notably marked by the reintroduction of the DENV-4 serotype, highlighting the region’s ongoing vulnerability to the reintroduction of different viral serotypes. This could explain the increase in incidence observed in our study, particularly in states such as Acre, Amazonas, Pará, Rondônia, and Roraima. Notably, except for Pará, these states share borders with the countries affected by the dengue epidemic in 2010.
Several factors influence the transmission of diseases in border regions, such as the legal and illegal movement of people and goods, border conflicts, cultural differences, and differences in national regulations on public health44. Strategies for controlling these diseases in border areas involve coordinated work between both countries. Sharing epidemiological information systematically can be the first step to initiate a coordinated work process45. In a case study conducted by Krisher et al.44, it is emphasized the critical importance of supporting local public health leaders who understand the context and nuances of their local population and invest in long-term collaborations.
The Brazilian Legal Amazon is predominantly composed of the Amazon biome, but also includes the Cerrado and Pantanal biomes. Regarding incidence rates specifically for the Amazon biome, results indicated increases from 2005 onwards. From 2001 to 2005, the state of Roraima had the highest incidence rate, with particular emphasis on the capital Boa Vista. Higher incidences were observed between 2006 to 2010 and 2011 to 2015 in the states of Rondônia, Amapá, and the southeastern part of Pará. Between 2016 and 2021, higher incidences were observed mainly in the states of Acre and Amazonas. Among municipalities, Rio Branco (in southern Acre) had the highest incidence rate (between 2006–2010 and 2016–2021), and in the subsequent periods (2011–2015), Cruzeiro do Sul (in northern Acre) presented the highest incidence. These results corroborate those of Lee et al.46, highlighting that new areas of the Amazon, which were previously isolated from infected hosts and vectors, also began experiencing outbreaks associated with increased connectivity between rural areas and larger cities. Lee et al.46 emphasize that the introduction of dengue in the state of Acre has been associated with increased connectivity of the state after the construction of a highway between the two largest cities, Rio Branco and Cruzeiro do Sul. The authors highlight this relationship, as they identified that the outbreak jumped from Rio Branco to Cruzeiro do Sul in 2014, instead of spreading to neighboring regions, and conclude that the expansion of the dengue transmission zone in the Amazon continued in the twenty-first century, driven by a network of highly interconnected cities and high levels of urbanization.
In the Brazilian Legal Amazon, the distribution of cases was heterogeneous among municipalities. The SaTScan analysis showed the spatiotemporal dynamics of risk areas during the study period. Parts of the states of Amazonas and Mato Grosso appeared as high-risk areas for dengue in all periods analyzed between 2001 and 2021. In the last years of the time series, high-risk areas were also identified, mainly in Amazonas, Tocantins, Mato Grosso, Rondônia, and Acre. This expansion of dengue cases from the southern to the western part of the Brazilian Amazon corroborates with what was observed by Alves et al.47 who, when studying the state of Mato Grosso, found epidemic peaks of the disease from 2008 to 2020. Furthermore, the growth in dengue incidence observed in all states in different periods, but with emphasis on what was observed in the state of Acre, where there was growth throughout the entire time series.
The SaTScan analysis also identified a change in the configuration of high-risk clusters for dengue among the studied biomes over the analyzed period. That is, the results indicated high-risk clusters between 2001 and 2005 mainly for the Amazon biome (in Roraima and northern Amazonas) and the Cerrado biome (central portion of Mato Grosso), namely, the northern and southern portions of the Legal Amazon. Regarding incidence rates specifically for the Cerrado biome, the results indicate that Mato Grosso, which encompasses three Brazilian biomes (the Amazon occupies the northern portion, the Cerrado in the central portion of the state, and the Pantanal in the southern part of the state), was among the states where dengue incidence increased from 2005 onwards, with higher incidences observed between 2006 to 2010 and 2011 to 2015. The states within the Cerrado biome (Mato Grosso and Tocantins) were among those with the highest incidences between 2016 and 2021. These results are similar to those identified by Codeço et al.48, who found that municipalities with dominant trajectories TT7 (in the northern and southern portions of the Brazilian Amazon) contributed the most to deforestation between 2006 and 2017, followed by municipalities with TT4 (characterized by productive systems that converge almost exclusively to livestock—beef production—in the southern and eastern portions of Brazilian Amazon and that are expanding into areas that still have extensive forest cover), which in this study correspond to the portions of high-risk clusters identified in southwestern Pará, Mato Grosso, Rondônia, and Tocantins.
In the last period of analysis (2011 to 2021), a similar pattern to the 2006–2010 period was observed. However, in this last period of analysis, high-risk clusters were added for the states of Amazonas (northwestern portion of the state) and Acre, while the cluster for Roraima no longer appears as a high-risk cluster. The results found in this last period, as argued earlier, reinforce the evidence pointed out by Lee et al.46 that recent epidemiological bulletins show the expansion of dengue in the most recent years to areas previously unaffected.
The cluster analysis in this study highlights that each studied biome has its own particularities, with the Federal Government being responsible for instituting environmental and health policies tailored to each of these biomes. This study, similar to Lee et al.46, underscores a new configuration of dengue expansion in the Legal Amazon, where new areas, previously considered relatively protected from outbreaks (such as rural parts of the Amazon), which were previously isolated from infected hosts and vectors, are also experiencing outbreaks. These results reinforce the argument of Pereira da Silva et al.49 regarding the need for region-specific health and environmental policies for each studied biome.
Due to its importance in terms of public health, dengue is a disease subject to immediate compulsory notification50, but despite its high incidence, it is still considered a neglected and underreported disease51. Underreporting occurs due to a lack of seeking medical care by the population or a lack of correct diagnosis, which can lead to an increase in the disease’s lethality rate52. Incomplete reporting is also a problem, as the lack of information hinders data evaluation, making it difficult to correctly identify affected individuals, as well as other important information for case outcomes, and prevents the assembly of an epidemiological profile of the disease in populations.
The use of secondary data from surveillance systems implies working with numerous limitations53. We utilized secondary data, where cases were reported by healthcare professionals, healthcare services, and by the public. Study limitations included, among others, difficulties in providing information on variables in the database due to lack of uniformity in data collection. However, even with possible notification biases, the analysis of this data is extremely valuable for health authorities, as it provides a way to analyze the behavior of various health problems and, thus, direct efforts and resources to make surveillance and control more effective, as well as anticipate risk situations and plan actions aimed at population health54,55. In other words, identifying areas at higher risk for dengue allows for highlighting these areas of higher vulnerability to dengue and other diseases that share the same causal determinants. Essentially, this contributes to promoting equity by enabling more resources and attention, at national and international levels, to be allocated to these territories and vulnerable populations. Therefore, the results of this study have the potential to support better targeting of dengue control strategies for each affected area. In the case of the Legal Amazon, with its vast dimension, this complexity increases due to various determinants, including biological, social, environmental, climatological, infrastructure, hydrological, presence of vulnerable groups such as rural populations, riverside dwellers, quilombola communities and indigenous peoples.
This study analyzed the spatiotemporal dynamics of dengue occurrence in the Brazilian Legal Amazon between the years 2001 and 2021. For future research, we suggest to evaluate economic activities and other environmental variables, with emphasis on those related to environmental and social vulnerability, climate change, and the study of specific states and smaller areas, especially those located in border regions.
Overall, this study revealed heterogeneous patterns in the spatial and temporal distribution of dengue in the Brazilian Legal Amazon between 2001 and 2021, highlighting important regional dynamics. While the average incidence rate remained stable over the period, states like Acre exhibited a consistently increasing trend, whereas others, such as Pará, Amapá, Rondônia, Maranhão, and Roraima, showed an initial increase followed by a decline. Fatality rates also showed an upward trend, with Maranhão and specific municipalities, such as Paço do Lumiar, standing out due to concerning rates.
The identification of 26 significant high-risk clusters for dengue reinforces the complexity of the region’s epidemiological dynamics. These clusters, widely distributed across nearly all states, highlight Palmas, Rio Branco, and Manaus as capitals with persistent high risk during different periods. In contrast, capitals such as Macapá and Belém consistently exhibited low risk.
These findings provide clear evidence that targeted interventions, tailored to local characteristics, are crucial for addressing the challenges posed by dengue. The integration of spatiotemporal analyses and regional trends should guide more effective and sustainable public policies, considering both environmental and social determinants of health in the Brazilian Legal Amazon.
Supplementary Information
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) - Finance Code 001 and by the Departamento de Ciência e Tecnologia da Secretaria de Ciência, Tecnologia, Inovação e Complexo da Saúde do Ministério da Saúde and by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (FRPB; Grant number 316426/2021-0 and 444913/2023-8); (NCPB; Grant number 420718/2023-0).
Author contributions
The following is a summary of each author’s contributions to this manuscript: R.H.: Contributed to the study, data analysis, interpretation of results, and reviewed the manuscript. B.B.: Contributed to the study, prepared the figures, contributed to data interpretation, and reviewed the manuscript. C.C.: Contributed to the study, edited the manuscript to conform with the guidelines, and reviewed the manuscript. S.S., A.S., J.L.: Contributed to the study, contributed to data interpretation, and reviewed the manuscript. N.B. and F.B.: Advisors, conceptualization; project administration, supervision, writing – review & editing; formal Analysis. All authors reviewed and approved the final manuscript.
Data availability
All data generated or analyzed during this study are public and included in this published article.
Declarations
Ethics section
According to the Ethics Committee of Universidade Federal de Pelotas (UFPel) approval (CAAE 46019321.6.0000.5317), the consent statement from all subjects and/or their legal guardian(s) was not necessary for this study since it is a retrospective study. As a retrospective study, using secondary data, the Ethics Committee approved this study and guarantees it follows all ethical principles of the current legislation involving human subjects. Confidentiality of information is ensured, and its use is solely for research purposes.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-84119-3.
References
- 1.Silva, J. M. C. D., Barbosa, L. C. F., Topf, J., Vieira, I. C. G. & Scarano, F. R. Minimum costs to conserve 80% of the Brazilian Amazon. Perspect. Ecol. Conserv.20, 216–222 (2022). [Google Scholar]
- 2.Villén-Pérez, S., Anaya-Valenzuela, L., Conrado Da Cruz, D. & Fearnside, P. M. Mining threatens isolated indigenous peoples in the Brazilian Amazon. Glob. Environ. Change72, 102398 (2022). [Google Scholar]
- 3.Ribeiro, F., Aparicio, M. & Matos, B. D. A. Isolamento Como Declaração de Recusa: Políticas Indígenas Contra A Violência do Estado Brasileiro. Tipití J. Soc. Anthropol. Lowl. S. Am.18, 148–152 (2022). [Google Scholar]
- 4.Lobo, M. S. D. C. & Cardoso, M. L. D. M. Lições de tempos urgentes: a experiência da atenção à saúde Yanomami ontem e hoje. Cad. Saúde Pública39, e00065623 (2023). [DOI] [PubMed] [Google Scholar]
- 5.Rorato, A. C. et al. Trajetorias: a dataset of environmental, epidemiological, and economic indicators for the Brazilian Amazon. Sci. Data10, 65 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alves, M. D. S., Araújo, W. C. D. & Silva, F. A. M. D. Uma revisão sistemática da literatura: análise sobre desigualdade estrutural em decorrência de casos de dengue e sua influência no cenário brasileiro. Res. Soc. Dev.10, e71101119355 (2021). [Google Scholar]
- 7.Santos, C. S. et al. Social representations of health professionals on negligenced diseases. Esc. Anna Nery Rev. Enferm.10.5935/1414-8145.20170016 (2017). [Google Scholar]
- 8.WHO. Dengue - Global situation. https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON498 (World Health Organization (WHO), 2023).
- 9.PAHO. Number of Reported Cases of Dengue and Severe Dengue in the Americas, by Country - September 22 2017 (EW 36). https://www.paho.org/en/documents/2017-number-reported-cases-dengue-and-severe-dengue-americas-country-september-22-2017-ew (2017).
- 10.Alla, D. et al. Dengue & COVID-19: A comparison and the challenges at hand. Cureus (2022) [DOI] [PMC free article] [PubMed]
- 11.Khan, S. et al. Dengue infections during COVID-19 period: Reflection of reality or elusive data due to effect of pandemic. IJERPH19, 10768 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brasil. Monitoramento dos casos de arboviroses até a semana epidemiológica 52 de 2022. Boletim Epidemiológico. v.54, no1. (2023).
- 13.Donalisio, M. R., Freitas, A. R. R. & Zuben, A. P. B. V. Arboviruses emerging in Brazil: challenges for clinic and implications for public health. Rev. Saúde Pública51, 30 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rey, J. R. & Lounibos, P. Ecología de Aedes aegypti y Aedes albopictus en América y la transmisión de enfermedades. Biomédica10.7705/biomedica.v35i2.2514 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Faria, M. T. D. S., Ribeiro, N. R. D. S., Dias, A. P., Gomes, U. A. F. & Moura, P. M. Saúde e saneamento: uma avaliação das políticas públicas de prevenção, controle e contingência das arboviroses no Brasil. Ciênc. Saúde Coletiva28, 1767–1776 (2023). [DOI] [PubMed] [Google Scholar]
- 16.Brasil. Ministério da Saúde. Informações de Saúde (TABNET) – DATASUS. https://datasus.saude.gov.br/informacoes-de-saude-tabnet/ (2024).
- 17.IBGE. Sistema IBGE de Recuperação Automática - SIDRA. https://sidra.ibge.gov.br/pesquisa/pnadca/tabelas (2024).
- 18.IBGE. Mapas regionais. https://www.ibge.gov.br/geociencias/cartas-e-mapas/mapas-regionais.html (2024).
- 19.Campos, N. B. D. et al. Twenty-Two years of dengue fever (1996–2017): an epidemiological study in a Brazilian city. Int. J. Environ. Health Res.31, 315–324 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Rorato, A. C., Escada, M. I. S., Camara, G., Picoli, M. C. A. & Verstegen, J. A. Environmental vulnerability assessment of Brazilian Amazon Indigenous Lands. Environ. Sci. Policy129, 19–36 (2022). [Google Scholar]
- 21.IBGE. Amazônia Legal | IBGE. https://www.ibge.gov.br/geociencias/cartas-e-mapas/mapas-regionais/15819-amazonia-legal.html?=&t=o-que-e (2022).
- 22.Santos, D., Salomão, R. & Veríssimo, A. Fatos da Amazônia 2021. https://amazonia2030.org.br/wp-content/uploads/2021/04/AMZ2030-Fatos-da-Amazonia-2021-3.pdf (2021) 10.59346/report.amazonia2030.202103.ed3.
- 23.De Almeida, R. B. & Aleixo, N. C. R. Análise socioambiental da morbidade da malária em Manaus, Amazonas, Brasil. Rev. Bras. Climatol.30, 845–866 (2022). [Google Scholar]
- 24.Moreira, L. S. D. B. et al. Perfil clínico e epidemiológico da dengue no estado de Minas Gerais. Braz. J. Health Rev.5, 373–387 (2022). [Google Scholar]
- 25.Kulldorff, M. et al. Multivariate scan statistics for disease surveillance. Stat. Med.26, 1824–1833 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Kulldorff, M. A spatial scan statistic. Commun. Stat. - Theory Methods26, 1481–1496 (1997). [Google Scholar]
- 27.Kirkwood, B. R. & Sterne, J. A. C. Essential Medical Statistics (Blackwell Science, 2003). [Google Scholar]
- 28.Kulldorff, M. SaTScanTM v8.0: Software for the Spatial and Space-Time Scanstatistics (Information Management Services, Inc., 2009). [Google Scholar]
- 29.Kim, H.-J., Fay, M. P., Feuer, E. J. & Midthune, D. N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med.19, 335–351 (2000). [DOI] [PubMed] [Google Scholar]
- 30.Santos, V. S. D. O. et al. Tendência temporal dos casos de dengue no Brasil e suas regiões no período de 2001 a 2020. Res. Soc. Dev.11, e53011831403 (2022). [Google Scholar]
- 31.Martins-Melo, F. R., Ramos, A. N., Alencar, C. H. & Heukelbach, J. Trends and spatial patterns of mortality related to neglected tropical diseases in Brazil. Parasite Epidemiol. Control1, 56–65 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costa De Albuquerque, M. A., Dias, D. M., Vieira, L. T., Lima, C. A. & Da Silva, A. M. Mortality trends for neglected tropical diseases in the State of Sergipe, Brazil, 1980–2013. Infect. Dis. Poverty6, 20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ribeiro, C. J. N. et al. Space-time risk cluster of visceral leishmaniasis in Brazilian endemic region with high social vulnerability: An ecological time series study. PLoS Negl. Trop. Dis.15, e0009006 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leandro, C. D. S., Barros, F. B. D., Cândido, E. L. & Azevedo, F. R. D. Redução da incidência de dengue no Brasil em 2020: controle ou subnotificação de casos por COVID-19?. Res. Soc. Dev.9, e76891110442 (2020). [Google Scholar]
- 35.Cardona-Ospina, J. A. et al. Dengue and COVID-19, overlapping epidemics? An analysis from Colombia. J. Med. Virol.93, 522–527 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ministério da Saúde. Monitoramento dos casos de arboviroses urbanas transmitidas pelo Aedes aegypti (dengue, chikungunya e zika), Semanas Epidemiológicas 1 a 17, 2020. Bol Epidemiol 51(18) https://www.saude.gov.br/images/pdf/2020/May/04/Boletim-epidemiologico-SVS-18.pdf (2020).
- 37.Do Carmo, R. F., Silva Júnior, J. V. J., Pastor, A. F. & De Souza, C. D. F. Spatiotemporal dynamics, risk areas and social determinants of dengue in Northeastern Brazil, 2014–2017: an ecological study. Infect. Dis. Poverty9, 153 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andrioli, D. C., Busato, M. A. & Lutinski, J. A. Spatial and temporal distribution of dengue in Brazil, 1990–2017. PLoS ONE15, e0228346 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paixão, F. A. W. & Oliveira, M. A. D. Casos de dengue no Amazonas nos anos de 2018 a 2022. Res. Soc. Dev.11, e30111932053 (2022). [Google Scholar]
- 40.Padilha, J. C., Rojas, D. P. & Sáenz-Gómez, R. Dengue en Colombia: epidemiología de la reemergencia a la hiperendemia. Rev. Sal. Bosq5, 81 (2015). [Google Scholar]
- 41.Forshey, B. M. et al. incomplete protection against dengue virus type 2 re-infection in Peru. PLoS Negl. Trop. Dis.10, e0004398 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartinger, S. M. et al. The 2022 South America report of The Lancet Countdown on health and climate change: trust the science. Now that we know, we must act. Lancet Reg. Health Am.20, 100470 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soares, S. R. et al. Prevalência das arboviroses urbanas em Roraima. Rev. DELOS17, e2529 (2024). [Google Scholar]
- 44.Krisher, L. K. et al. Successful malaria elimination in the Ecuador-Peru border region: epidemiology and lessons learned. Malar. J.15, 573 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saldanha, R. et al. Contributing to elimination of cross-border malaria through a standardized solution for case surveillance, data sharing, and data interpretation: development of a cross-border monitoring system. JMIR Public Health Surveill.6, e15409 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee, S. A., Economou, T., De Castro Catão, R., Barcellos, C. & Lowe, R. The impact of climate suitability, urbanisation, and connectivity on the expansion of dengue in 21st century Brazil. PLoS Negl. Trop. Dis.15, e0009773 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alves, L. D., Lana, R. M. & Coelho, F. C. A framework for weather-driven dengue virus transmission dynamics in different Brazilian regions. Int. J. Environ. Res. Public. Health18, 9493 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Codeço, C. T. et al. Epidemiology, biodiversity, and technological trajectories in the Brazilian Amazon: from malaria to COVID-19. Front. Public Health9, 647754 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pereira Da Silva, A. A. et al. The fewer, the better fare: Can the loss of vegetation in the Cerrado drive the increase in dengue fever cases infection?. PLoS ONE17, e0262473 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brasil. Lista Nacional de Notificação Compulsória de Doenças, Agravos e Eventos de Saúde Pública. Ministério da Saúdehttps://www.gov.br/saude/pt-br/composicao/svsa/notificacao-compulsoria/lista-nacional-de-notificacao-compulsoria-de-doencas-agravos-e-eventos-de-saude-publica (2024).
- 51.Cardoso Lucena, L., Souto, A. A., Lucena, L. C. & Marques, T. N. Avaliação do perfil epidemiológico dos casos de dengue no município de Porto Nacional, Tocantins. Rev. Patol. Tocantins6, 18–23 (2019). [Google Scholar]
- 52.De Mattos Almeida, M. C., Caiaffa, W. T., Assunção, R. M. & Proietti, F. A. Spatial vulnerability to dengue in a Brazilian urban area during a 7-year surveillance. J. Urban Health84, 334–345 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goto, D. Y. N., Larocca, L. M., Felix, J. V. C., Kobayashi, V. L. & Chaves, M. M. N. Avaliação da oportunidade de notificação da dengue no Estado do Paraná. Acta Paul. Enferm.29, 355–362 (2016). [Google Scholar]
- 54.Ferreira, J. S. A., Vilela, M. B. R., Aragão, P. S., Oliveira, R. A. D. & Tiné, R. F. Avaliação da qualidade da informação: linkage entre SIM e SINASC em Jaboatão dos Guararapes (PE). Ciênc. Saúde Coletiva16, 1241–1246 (2011). [DOI] [PubMed] [Google Scholar]
- 55.Pereira, C. M. Medidas de Educação e Saúde na Escola: Prevenção contínua contra o vírus da Dengue (Universidade Federal do Paraná, 2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are public and included in this published article.