Abstract
Diabetic kidney disease (DKD) is a prevalent and complex disease among patients with diabetes in Korea, requiring comprehensive treatment strategies. Traditional management strategies targeting blood pressure, blood sugar, lipid, and lifestyles are foundational approaches of DKD treatment, each of them still holding importance in current paradigms. The four pillars, renin-angiotensin system(RAS) inhibitors, sodium-glucose cotransporter-2 (SGLT2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and non-steroidal mineralocorticoid receptor antagonists (nsMRA) can enhance DKD treatment. Expanding beyond these pillars with future-oriented pillars including precision medicine, digital health, gut health, anti-inflammatory/fibrotic agents, psychosocial/behavioral health, and regenerative medicine can further advance DKD treatment strategies, offering a more cohesive framework which shifts a disease-centered approach to a patient-centered approach.
Keywords: Diabetic kidney disease, RAS inhibitors, SGLT2 inhibitors, Glucagon-like peptide-1 receptor agonists, Non-steroidal mineralocorticoid receptor antagonists, Precision medicine, Regenerative medicine
INTRODUCTION
Diabetic kidney disease (DKD) has become an increasingly prevalent and significant health burden worldwide, particularly in Korea, where rates of diabetes continue to rise.[1] With Korea’s aging population and evolving lifestyle changes, the incidence of diabetes has surged, directly contributing to an increase in DKD cases1). According to recent epidemiological data, nearly 40% of older patients with diabetes develop kidney complications, placing a strain on both patients and healthcare systems1,2). Additionally, the high prevalence of hypertension, obesity, and metabolic syndrome among Korean adults has escalated the risk of progression from diabetes to DKD1).
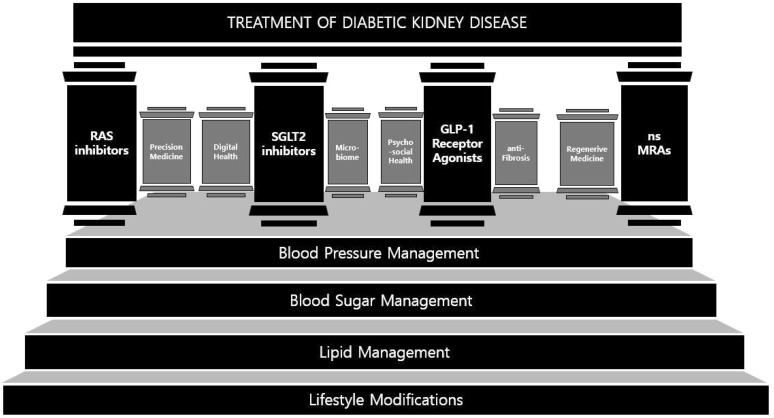
These factors highlight the urgent need for comprehensive and effective management strategies to address the complexities of DKD. Although the traditional foundations of DKD treatment — blood pressure control3), glucose control2), cholesterol control4), and lifestyle modifications5) — provide a strong foundational approach, new pillars are required for better management. Current treatment paradigms integrate traditional foundations with new pillars of DKD treatment, but there are still remaining challenges to overcome2). Expanding beyond this structure by additionally integrating rapidly advancing data science, bioengineering techniques and holistic lifestyle modifications may offer a more personalized and robust defense against the progression of DKD and its complications. The objective of this article is to explore current treatment paradigms and investigate future-oriented innovations to establish a comprehensive DKD management framework (Fig. 1).
Fig. 1. An analogy illustrating the strategies of DKD treatment as pillars in a temple. Traditional managements, which are foundational approaches, are represented as stairs. The pillars include the four pillars and future-oriented pillars suggested in this article.
Section 1: The Unwavering Importance of Traditional Risk Factor Management
The current approach to managing DKD has evolved to incorporate a multifaceted framework that integrates both established and emerging therapies. In this approach, traditional foundations such as blood pressure management, blood sugar management, lipid management, and lifestyle modifications remain critical due to their proven roles in slowing the progression of kidney damage in diabetic patients4,5,6,7). These measures, aimed at maintaining cardiovascular and renal stability, emphasize lifestyle adjustments, dietary management, and medication adherence as essential components in preventing complications associated with DKD8). Effective management of blood pressure is particularly important, as hypertension is both a cause and a consequence of DKD3). Proper control of blood pressure reduces mechanical stress on the kidney's vasculature, minimizing glomerular injury and delaying progression to end-stage renal disease (ESRD)3). Similarly, effective blood sugar regulation plays a fundamental role in mitigating hyperglycemia-induced damage and microvascular complications2). Lipid regulation also plays a complementary role by lowering the risk of cardiovascular complications, which are highly prevalent among patients with DKD4).
Blood Pressure Management: Independently, managing hypertension reduces glomerular pressure and protects vascular autoregulation, mitigating renal injury and delaying progression to ESRD3). Maintaining target blood pressure levels has been shown to significantly reduce the risk of kidney failure in diabetic patients6). Cardiovascular events also have been shown to reduce with intensive blood pressure control among diabetic patients in the BPROAD trial9). The SPRINT trial, which tested intensive blood pressure control in patients without diabetes, showed that intensive blood pressure control can lower cardiovascular risk regardless of diabetes status9). Considering that cardiovascular diseases are the most common complications of DKD, blood pressure control is a key factor in DKD patient treatment. Selecting an adequate blood pressure target is important, since there is no RCT specifically determining target levels in DKD patients and excessively low blood pressure can rather cause harm2,3).
Blood Sugar Management: Effective glycemic control minimizes hyperglycemia-induced oxidative stress and renal damage, helping to reduce the incidence of microvascular complications and macrovascular complications, including diabetic nephropathy and cardiovascular events2). The DCCT trial showed that intensive glucose control therapy with the target of 6.05% or less glycosylated hemoglobin, can reduce the risk of nephropathy up to 34% and slow disease progression in diabetic patients7).
Lipid Management: Controlling lipid levels lowers the risk of atherosclerosis and cardiovascular complications, which are major comorbidities in DKD. Statins and other lipid-lowering agents such as ezetimibe, fibrates, bempedoic acid, and PCSK9 inhibitors are required in minimizing cardiovascular risks in these patients4). The CTT meta-analysis showed that statin therapy targeting LDL reduction lowered the risk of cardiovascular disease by 21%4).
Lifestyle Modifications: Lifestyle modifications such as dietary management, exercising, cessation of harmful habits are also important in DKD patients. Dietary management includes sodium restriction, protein control, and phosphorus control. Harmful habits include alcohol consumption, and smoking. These modifications address overall metabolic health, reducing inflammatory and oxidative stressors that contribute to kidney disease progression8,10). A systematic review of behavior change techniques used for lifestyle modifications of chronic kidney disease (CKD) patients identified that education was the most commonly used technique followed by enablement, training, persuading, and environmental restructuring5).
In addition to these traditional measures, recent therapeutic developments focus on novel pharmacologic agents that target mechanisms of renal and cardiovascular disease progression. Key among these are renin-angiotensin system (RAS) inhibitors6,11), sodium-glucose cotransporter-2 (SGLT2) inhibitors12,13,14), Glucagon-like peptide-1 (GLP-1) receptor agonists15,16), and non-steroidal mineralocorticoid receptor antagonists (nsMRAs)17). These developments address that new therapies can lead to a more tailored and advanced approach on DKD patients. However, it is crucial to emphasize that while new therapies can significantly enhance patient outcomes, the foundation of DKD management still relies on traditional risk factor management and that these traditional methods should not be overlooked.
Section 2: The Four Pillars in DKD and their Synergistic Effects with Traditional Management Therapies
The four pillars (RAS inhibitors, SGLT2 inhibitors, GLP-1 receptor agonists, nsMRAs) have been proven as significant DKD treatment choices in large-scale evidences. These pillars complement and enhance the efficacy of established practices, providing a more comprehensive approach to DKD. Each approach offers unique, independent effects in mitigating disease progression, while also demonstrating synergistic benefits when combined with traditional management strategies.
Renin-Angiotensin System(RAS) Inhibitors: RAS inhibitors such as angiotensin-converting enzyme (ACE) inhibitors or angiotensin ii receptor blockers (ARB) directly protect the kidneys by reducing intraglomerular pressure and proteinuria, addressing specific pathways involved in renal injury6,11). The IDNT study demonstrated that irbesartan has renoprotective benefits independent of achieved systolic blood pressure in diabetic patients11). However, combination of ACEi and ARB therapy is not recommended because it can increase the risk of adverse events3,18).
Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors: SGLT2 inhibitors reduce hyperfiltration and improve metabolic control, exerting renoprotective effects through mechanisms beyond glucose reduction12). The CREDENCE trial12) and the CANVAS trial14) showed improved kidney outcomes with canagliflozin in DKD patients. The EMPA-REG OUTCOME trial demonstrated the effect of empagliflozin on improving kidney outcomes in DKD patients13). Through these evidences, SGLT2 inhibitors stand as an important pillar in preventing kidney function decline in DKD patients.
Glucagon-like Peptide-1 (GLP-1) Receptor Agonists: GLP-1 receptor agonists were first recognized to control glucose level, decrease body weight, and reduce cardiovascular risk in diabetic patients16). Recent trials on DKD patients demonstrated that GLP-1 receptor agonists have additional renoprotective benefits along with previously known effects. GLP-1 receptor agonists can improve kidney functions in DKD patients indirectly by preventing hyperglycemia, hypertension, obesity and also directly, by reducing oxidative stress and inflammation19,20). Liraglutide and dulaglutide have been demonstrated to lower albuminuria, slow eGFR decline, and reduce risk of kidney function loss in the LEADER trial15) and the REWIND trial20), respectively. Semaglutide was shown to induce 24% reduction of kidney related outcomes (albuminuria, low eGFR, initiation of RRT, kidney related death) in the FLOW trial16) and the SUSTAIN 6 trial15). Other novel GLP-1 receptor agonists showed similar promising results. Tirzepatide showed renoprotective effects by slowering eGFR decrease and stabilizing urine albumin-creatinine ratio(UACR) in the SURPASS-4 trial21,22). Efpeglenatide also mitigated kidney related outcomes such as albuminuria in the AMPLITUDE-O trial23). These evidences support the position of GLP-1 receptor agonists as a promising new pillar in DKD treatment.
Non-steroidal Mineralocorticoid Receptor Antagonists (nsMRAs): Novel nsMRAs act to reduce inflammation and fibrosis within the kidney, targeting mechanisms that are not adequately addressed by traditional therapies or other agents such as SGLT2 inhibitors and GLP-1 receptor agonists17). By limiting the fibrotic response, these agents help preserve kidney function over time. In the FIDELIO-DKD study and the FIGARO-DKD study, finerenone reduced disease progression and the risk of cardiovascular diseases in DKD patients8,17). The additive effect of finerenone on patients treated with SGLT2 inhibitors and GLP-1 receptor agonists needs further investigation8).
Synergistic Effects between the Four Pillars and Traditional Management Therapies
While each of the four pillars has independent effects, a synergistic benefit arises when traditional risk factor management is combined with the four pillars. For instance, blood pressure control alongside RAS inhibition provides an enhanced protective effect on glomerular pressure. The RENAAL study demonstrated that combining losartan with other BP management therapy can slow ESRD progression6). Similarly, blood sugar regulation such as use of metformin complements the glucose-lowering effect of SGLT2 inhibitors and GLP-1 receptor agonists, together offering greater metabolic control and reduced renal workload2,8,24).
The combination of lipid management with GLP-1 receptor agonists also results in reduced cardiovascular risk, a major cause of morbidity and mortality among patients with DKD8). Lifestyle interventions, such as diet and exercise, have shown synergistic effects with pharmacological therapies, helping to maximize therapeutic outcomes2,10,24). This suggests that significant lifestyle modifications are required to patients undergoing treatment24).
In sum, traditional risk factor management and the four pillars work in concert to provide a multi-layered defense against DKD progression. The independent and synergistic effects underscore the importance of a holistic and comprehensive approach to DKD management, leveraging each component to achieve optimal renal and cardiovascular outcomes for patients.
Section 3: Future-Oriented Expansion of DKD Treatment Pillars
As DKD continues to evolve in both prevalence and complexity, expanding beyond the current paradigm is called for. These future pillars aim to address gaps in treatment and embrace advancing technologies, precision medicine, and comprehensive patient-centered care. The following potential pillars could reshape the DKD treatment landscape:
1. Precision Medicine
Future treatments may incorporate genetic profiling and biomarker-based diagnostics, allowing therapies tailored to each patient’s unique genetic and biological profile25).
In terms of genetic profile, current studies perform genome-wide association studies (GWAS) or investigate experimental models to identify differentially expressed genes (DEG) in DKD groups using bioinformatic technologies26). Therapeutic strategies for disease-mediating genes include small molecules or oligonucleotides such as antisense oligonucleotides, small interfering RNA, and micro RNA27).
In terms of biological profile, kidney biopsy provides histopathological biomarkers, aiding in personalized treatment plans28). Also, assay-based biomarkers of DKD including neutrophil gelatinase-associated lipocalin (NGAL)29), kidney injury molecule-1 (KIM-1)30), and advanced glycation end products (AGEs)2,31) can act as diagnostic tools as well as treatment targets25). Proteomics, metabolomics, and transcriptomics methods can be further used for identifying novel biomarkers for risk stratification. Proteomics has been used to identify proteins such as uromodulin, progranulin, clusterin, α1 acid glycoprotein, and haptoglobin. Metabolomics identified metabolites including octanol, oxalic acid, phosphoric acid, and benzamide. Transcriptomics identified various types of micro RNAs. These identified proteins, metabolites, and micro RNAs have been actively studied for their predictive effects25).
AI-based predictive models can also be used to identify patients at higher risk for DKD progression, facilitating early intervention and prevention strategies32). Based on these comprehensive profiles, a more personalized approach could optimize responses to specific medications and minimize adverse effects, particularly among diverse patient populations25).
2. Digital Health
Continuous Glucose and Blood Pressure Monitoring: Wearable devices and sensors for real-time monitoring could allow patients to manage blood glucose and blood pressure levels with unprecedented accuracy, enhancing adherence and early detection of dysregulation8,33).
Telemedicine and Remote Care Platforms: As digital health solutions evolve, patients could access continuous medical support, enabling timely adjustments to treatment plans based on remote monitoring data, which is particularly beneficial for those in remote or underserved regions.
3. Microbiome and Gut Health
Research on the gut microbiome’s role in kidney health suggests that there is a close interplay between the gut microbiome and DKD. Targeting the microbiome could reduce systemic inflammation and improve metabolic regulation, indirectly benefiting kidney function. Researches suggest elevated levels of gut-microbiota derived metabolites (such as PBUTs) in circulation increase inflammation and exert negative effects including fibrosis34). Specific dietary supplements or probiotic interventions could become standard practices for managing the gut-kidney axis, providing a novel, non-invasive means of supporting renal health34).
4. Anti-Inflammatory and Anti-Fibrotic Therapies
Targeting Inflammatory Pathways: Future therapies are likely to focus on renal inflammation reduction, as inflammation contributes significantly to DKD progression. Upregulation of cytokines such as IL-6, IL-1 are suggested to be associated with decline in kidney function. Anti-inflammatory drugs or biologics targeting key inflammatory mediators could slow kidney damage35).
Targeting Fibrosis Pathways: Tubulointerstitial fibrosis is highly associated with DKD and various fibrotic factors play a key role in the pathogenesis of DKD35). Emerging drugs that specifically inhibit renal fibrosis pathways could directly prevent the scarring that leads to kidney dysfunction, offering an alternative mechanism to traditional therapies. Adding on to nsMRAs, endothelin receptor antagonists are being studied on their effects of reducing fibrosis and kidney inflammation. Atrasentan, avosentan, and zibotentan were studied in the ASCEND trial, the SONAR trial, and the ZENITH-CKD trial, respectively35).
5. Comprehensive Psychosocial and Behavioral Health Support
Psychosocial Health Support: Mental health support may become an integral part of DKD management. Patients with diabetes and CKD have higher risk of depression, and as a combination of these two diseases, DKD is expected to increase risk of depression36). It is important to acknowledge stress and depression in diabetic patients due to its association with treatment adherence37). One way to improve mental health status in DKD patients is changing weight to optimal level through lifestyle modifications36). Additional holistic approaches for depression are required to enhance patient engagement and treatment adherence.
Patient Education and Self-Management Tools: Digital applications for patient education, tailored lifestyle coaching, and enhanced self-management would empower patients to actively engage in their health, addressing lifestyle factors crucial to DKD management8).
6. Regenerative Medicine
Stem Cell Therapy: Stem cell-based therapies, aimed at repairing damaged kidney tissue or regenerating functional kidney cells, hold future promise for halting or even reversing DKD progression. Strategies for regenerating kidney cells include decellularized scaffold technology, 3D bioprinting, and kidney organoid fabrication. Strategies for repairing kidney cells include injection of stem cells or secretome such as growth factors38,39).
Bioengineered Renal Tissue: Advances in microphysiological systems and bioengineered kidney tissues could provide functional alternatives or support to damaged kidneys, delaying or potentially eliminating the need for dialysis40).
Looking forward, the integration of these future pillars could revolutionize DKD management by expanding from a disease-centered approach to a comprehensive, patient-centered approach. Precision medicine, digital health tools, advanced anti-inflammatory and anti-fibrotic therapies, comprehensive psychosocial support, and regenerative treatments represent forward-oriented paths that hold the potential to mitigate DKD progression and improve quality of life. Together, these future-oriented approaches form a cohesive framework that supports early intervention, personalized treatment, and holistic patient care.
CONCLUSION
In summary, continued research and collaboration among healthcare providers will be crucial to successfully integrate these treatment pillars into everyday clinical practice. A multi-dimensional approach that integrates traditional risk factor management, the four pillars, and emerging future pillars is needed for effective DKD management. Traditional foundations —control of blood pressure, blood sugar, and cholesterol along with lifestyle modifications—remain foundational to slowing DKD progression. The four pillars—RAS inhibitors, SGLT2 inhibitors, GLP-1 receptor agonists, and nsMRAs—have added crucial, targeted therapies that address both renal and cardiovascular risks.
Future-oriented pillars such as precision medicine, digital health innovations, microbiome modulation, advanced anti-inflammatory, anti-fibrotic therapies, behavioral and psychosocial support, and regenerative medicine offer promising new avenues to further enhance DKD management. Combining these pillars creates a comprehensive framework that addresses the complex and evolving needs of DKD patients. Developing these pillars and discussing ways to effectively and safely integrate them will be essential.
Footnotes
Acknowledgement: This research was supported by a grant of Patient-Centered Clinical Research Coordinating Center (PACEN) funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI19C0481, HC20C0054).
References
- 1.Kim NH, Seo MH, Jung JH, Han KD, Kim MK, Kim NH, Grp DKDR, Assoc KD. 2023 Diabetic Kidney Disease Fact Sheet in Korea. Diabetes Metab J. 2024;48:463–472. doi: 10.4093/dmj.2023.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tong LL, Adler SG. Diabetic kidney disease treatment: new perspectives. Kidney Res Clin Pract. 2022;41:S63–S73. doi: 10.23876/j.krcp.21.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim Y, Kim W, Kim JK, et al. Blood Pressure Control in Patients with Diabetic Kidney Disease. Electrolyte Blood Press. 2022;20:39–48. doi: 10.5049/EBP.2022.20.2.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zac-Varghese S, Mark P, Bain S, et al. Clinical practice guideline for the management of lipids in adults with diabetic kidney disease: abbreviated summary of the Joint Association of British Clinical Diabetologists and UK Kidney Association (ABCD-UKKA) Guideline 2024. BMC Nephrol. 2024;25:216. doi: 10.1186/s12882-024-03664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evangelidis N, Craig J, Bauman A, Manera K, Saglimbene V, Tong A. Lifestyle behaviour change for preventing the progression of chronic kidney disease: a systematic review. BMJ Open. 2019;9:e031625. doi: 10.1136/bmjopen-2019-031625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakris GL, Weir MR, Shanifar S, et al. Effects of blood pressure level on progression of diabetic nephropathy - Results from the RENAAL study. Arch Intern Med. 2003;163:1555–1565. doi: 10.1001/archinte.163.13.1555. [DOI] [PubMed] [Google Scholar]
- 7.Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 8.de Boer IH, Khunti K, Sadusky T, et al. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2022;102:974–989. doi: 10.1016/j.kint.2022.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Bi Y, Li M, Liu Y, et al. Intensive Blood-Pressure Control in Patients with Type 2 Diabetes. N Engl J Med. 2024 doi: 10.1056/NEJMoa2412006. [DOI] [PubMed] [Google Scholar]
- 10.Jo SM. Understanding and Treatment Strategies of Hypertension and Hyperkalemia in Chronic Kidney Disease. Electrolyte Blood Press. 2023;21:24–33. doi: 10.5049/EBP.2023.21.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pohl MA, Blumenthal S, Cordonnier DJ, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: clinical implications and limitations. J Am Soc Nephrol. 2005;16:3027–3037. doi: 10.1681/ASN.2004110919. [DOI] [PubMed] [Google Scholar]
- 12.Wada T, Mori-Anai K, Kawaguchi Y, et al. Renal, cardiovascular and safety outcomes of canagliflozin in patients with type 2 diabetes and nephropathy in East and South-East Asian countries: Results from the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation Trial. J Diabetes Invest. 2022;13:54–64. doi: 10.1111/jdi.13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wanner C, Inzucchi SE, Zinman B, et al. Consistent effects of empagliflozin on cardiovascular and kidney outcomes irrespective of diabetic kidney disease categories: Insights from the EMPA-REG OUTCOME trial. Diabetes Obes Metab. 2020;22:2335–2347. doi: 10.1111/dom.14158. [DOI] [PubMed] [Google Scholar]
- 14.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 15.Shaman AM, Bain SC, Bakris GL, et al. Effect of the Glucagon-Like Peptide-1 Receptor Agonists Semaglutide and Liraglutide on Kidney Outcomes in Patients With Type 2 Diabetes: Pooled Analysis of SUSTAIN 6 and LEADER. Circulation. 2022;145:575–585. doi: 10.1161/CIRCULATIONAHA.121.055459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perkovic V, Tuttle KR, Rossing P, et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N Engl J Med. 2024;391:109–121. doi: 10.1056/NEJMoa2403347. [DOI] [PubMed] [Google Scholar]
- 17.Humle K, Klanger B, Kolkhof P, et al. Summary of Research: Cardiovascular and Kidney Outcomes with Finerenone in Patients with Type 2 Diabetes and Chronic Kidney Disease-The FIDELITY Pooled Analysis. Diabetes Ther. 2024;15:1861–1864. doi: 10.1007/s13300-024-01591-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung HH. Hypertension Management in Patients with Chronic Kidney Disease in the Post-SPRINT Era. Electrolyte Blood Press. 2021;19:19–28. doi: 10.5049/EBP.2021.19.2.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu JH, Park SY, Lee DY, Kim NH, Seo JA. GLP-1 receptor agonists in diabetic kidney disease: current evidence and future directions. Kidney Res Clin Pract. 2022;41:136–149. doi: 10.23876/j.krcp.22.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394:131–138. doi: 10.1016/S0140-6736(19)31150-X. [DOI] [PubMed] [Google Scholar]
- 21.Del Prato S, Kahn SE, Pavo I, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021;398:1811–1824. doi: 10.1016/S0140-6736(21)02188-7. [DOI] [PubMed] [Google Scholar]
- 22.Heerspink HJL, Sattar N, Pavo I, et al. Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: post-hoc analysis of an open-label, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2022;10:774–785. doi: 10.1016/S2213-8587(22)00243-1. [DOI] [PubMed] [Google Scholar]
- 23.Gerstein HC, Sattar N, Rosenstock J, et al. Cardiovascular and Renal Outcomes with Efpeglenatide in Type 2 Diabetes. N Engl J Med. 2021;385:896–907. doi: 10.1056/NEJMoa2108269. [DOI] [PubMed] [Google Scholar]
- 24.Morales J, Dagogo-Jack S, Fonseca V, Neumiller JJ, Rosas SE. Perspectives on Chronic Kidney Disease With Type 2 Diabetes and Risk Management: Practical Viewpoints and a Paradigm Shift Using a Pillar Approach. Clin Diabetes. 2023;41:553–566. doi: 10.2337/cd22-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung CY, Yoo TH. Novel biomarkers for diabetic kidney disease. Kidney Res Clin Pract. 2022;41:S46–S62. doi: 10.23876/j.krcp.22.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Y, Li L, Tang P, et al. Identifying key genes for diabetic kidney disease by bioinformatics analysis. BMC Nephrol. 2023;24:305. doi: 10.1186/s12882-023-03362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasquez-Rios G, De Cos M, Campbell KN. Novel Therapies in APOL1-Mediated Kidney Disease: From Molecular Pathways to Therapeutic Options. Kidney Int Rep. 2023;8:2226–2234. doi: 10.1016/j.ekir.2023.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim WJ, Oh T, Heo NH, et al. Kidney biopsy can help to predict renal outcomes of patients with type 2 diabetes mellitus. Kidney Res Clin Pract. 2024 doi: 10.23876/j.krcp.23.059. [DOI] [PubMed] [Google Scholar]
- 29.Tang XY, Zhou JB, Luo FQ, et al. Urine NGAL as an early biomarker for diabetic kidney disease: accumulated evidence from observational studies. Ren Fail. 2019;41:446–454. doi: 10.1080/0886022X.2019.1617736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gohda T, Kamei N, Koshida T, et al. Circulating kidney injury molecule-1 as a biomarker of renal parameters in diabetic kidney disease. J Diabetes Investig. 2020;11:435–440. doi: 10.1111/jdi.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koska J, Gerstein HC, Beisswenger PJ, Reaven PD. Advanced Glycation End Products Predict Loss of Renal Function and High-Risk Chronic Kidney Disease in Type 2 Diabetes. Diabetes Care. 2022;45:684–691. doi: 10.2337/dc21-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makino M, Yoshimoto R, Ono M, et al. Artificial intelligence predicts the progression of diabetic kidney disease using big data machine learning. Sci Rep. 2019;9:11862. doi: 10.1038/s41598-019-48263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jancev M, Vissers T, Visseren FLJ, et al. Continuous glucose monitoring in adults with type 2 diabetes: a systematic review and meta-analysis. Diabetologia. 2024;67:798–810. doi: 10.1007/s00125-024-06107-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang Z, Yu S, Pan Y. The gut microbiome tango in the progression of chronic kidney disease and potential therapeutic strategies. J Transl Med. 2023;21:689. doi: 10.1186/s12967-023-04455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazzieri A, Porcellati F, Timio F, Reboldi G. Molecular Targets of Novel Therapeutics for Diabetic Kidney Disease: A New Era of Nephroprotection. Int J Mol Sci 25. 2024 doi: 10.3390/ijms25073969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi HS, Kim B, Han KD, et al. Weight change and risk of depression in patients with diabetic kidney disease: a nationwide population-based study. Kidney Res Clin Pract. 2023;42:86–97. doi: 10.23876/j.krcp.21.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez JS, Peyrot M, McCarl LA, et al. Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care. 2008;31:2398–2403. doi: 10.2337/dc08-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuji K, Kitamura S, Wada J. Potential Strategies for Kidney Regeneration With Stem Cells: An Overview. Front Cell Dev Biol. 2022;10:892356. doi: 10.3389/fcell.2022.892356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu D, Cheng F, Pan S, Liu Z. Stem cells: a potential treatment option for kidney diseases. Stem Cell Res Ther. 2020;11:249. doi: 10.1186/s13287-020-01751-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawlor KT, Vanslambrouck JM, Higgins JW, et al. Cellular extrusion bioprinting improves kidney organoid reproducibility and conformation. Nat Mater. 2021;20:260–271. doi: 10.1038/s41563-020-00853-9. [DOI] [PMC free article] [PubMed] [Google Scholar]