Abstract
Background and Aims
Standardized definitions for outcome measures in randomized clinical trials and observational studies are essential for robust and valid evaluation of medical products, interventions, care, and outcomes. The European Unified Registries for Heart Care Evaluation and Randomised Trials (EuroHeart) project of the European Society of Cardiology aimed to create international data standards for cardiovascular clinical study outcome measures.
Methods
The EuroHeart methods for data standard development were used. From a Global Cardiovascular Outcomes Consortium of 82 experts, five Working Groups were formed to identify and define key outcome measures for: cardiovascular disease (generic outcomes), acute coronary syndrome and percutaneous coronary intervention (ACS/PCI), atrial fibrillation (AF), heart failure (HF) and transcatheter aortic valve implantation (TAVI). A systematic review of the literature informed a modified Delphi method to reach consensus on a final set of variables. For each variable, the Working Group provided a definition and categorized the variable as mandatory (Level 1) or optional (Level 2) based on its clinical importance and feasibility.
Results
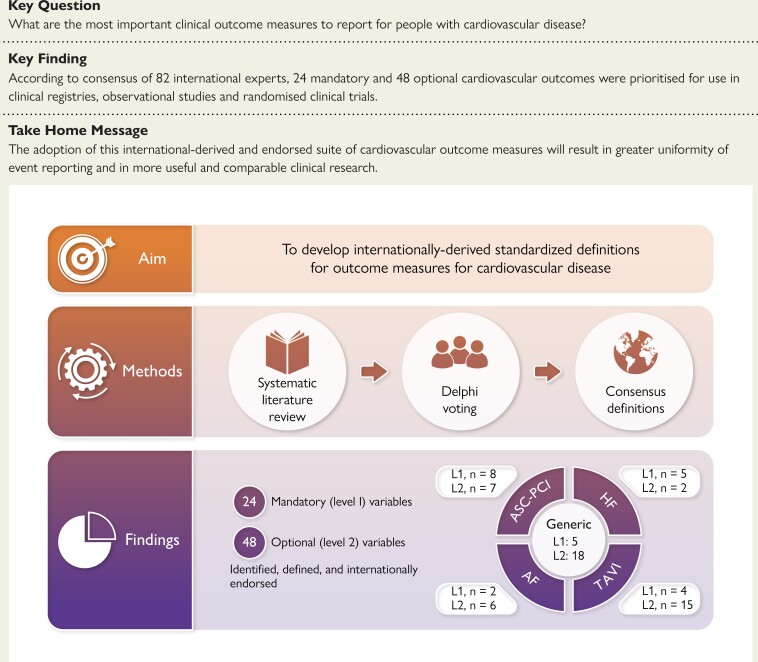
Across the five domains, 24 Level 1 (generic: 5, ACS/PCI: 8, AF: 2; HF: 5, TAVI: 4) and 48 Level 2 (generic: 18, ACS-PCI: 7, AF: 6, HF: 2, TAVI: 15) outcome measures were defined.
Conclusions
Internationally derived and endorsed definitions for outcome measures for a range of common cardiovascular diseases and interventions are presented. These may be used for data alignment to enable high-quality observational and randomized clinical research, audit, and quality improvement for patient benefit.
Keywords: Acute coronary syndrome; Atrial fibrillation; Heart failure; Transcatheter aortic valve intervention; Outcomes; Registry, data, EuroHeart
Structured Graphical Abstract
Structured Graphical Abstract.
Internationally derived, standardized definitions for outcome measures in people with cardiovascular disease. ACS-PCI, acute coronary syndrome-percutaneous coronary intervention; HF, heart failure; AF, atrial fibrillation; TAVI, transcatheter aortic valve intervention
See the editorial comment for this article ‘Data science from EuroHeart: a job in hand’, by A. Timmis, https://doi.org/10.1093/eurheartj/ehae819.
Introduction
Careful selection and use of clinical outcome measures is of paramount importance to enable valid and reliable quantification of the benefits and harms of different management strategies.1,2 Sets of outcome measures have been proposed for cardiovascular disease but these are often limited to single conditions or diseases,3–5 or are specific to individual randomized clinical trials (RCT),6 and lack a structured hierarchy.3–6 At time of development, existing outcome sets have often not included patient stakeholder involvement.7
The definitions of cardiovascular outcomes measures employed in observational research have been inconsistent and heterogeneous,8 yet an increasing proportion of RCTs are using routinely collected healthcare systems and/or clinical registry data for outcome evaluation.9–12 Registry-based RCTs now span multiple geographies.9 It is therefore increasingly important that consistent, internationally endorsed and robustly defined clinical outcome measures are established.13
The European Unified Registries for Heart Care Evaluation and Randomised Trials (EuroHeart) project of the European Society of Cardiology (ESC) has previously published data standards for four common cardiovascular domains: acute coronary syndrome and percutaneous coronary intervention (ACS/PCI), atrial fibrillation (AF), heart failure (HF), and transcatheter aortic valve implantation (TAVI).14–17 These were developed using a standardized, evidence-based method,18 which we now use to select and define cardiovascular outcome measures for assessments in common cardiovascular diseases and interventions. This catalogue of cardiovascular outcome measures will provide a ‘common language’ to facilitate federated, pooled, comparative and meta-analyses of independent yet harmonized data sets, and support the delivery of international registry-based RCTs. It will also improve clinical event reporting—making clinical studies more robust, generalizable, and applicable—and as such it will advance evidence-based assessment of the effectiveness of cardiovascular care.
Methods
We classified cardiovascular outcome measures as either generic or domain-specific. Generic measures were defined as those of potential applicability to all patients with cardiovascular diseases. Domain-specific variables were defined as those applying to patients following diagnosis of ACS, AF, or HF, or after a TAVI procedure. Every participant would be eligible for the collection of generic outcomes in addition to specific domains.
EuroHeart method
We followed the EuroHeart method for cardiovascular data standard development.18 This involved: (i) completion of a systematic review of the literature to synthesize a list of ‘candidate’ variables; (ii) selection and prioritisation of variables by domain experts in the Working Group using a modified Delphi method; and (iii) Working Group feedback.18
Systematic literature review
The protocol was pre-registered.19 We searched Embase and Ovid Medline for studies published in the three medical journals with the highest impact factor: New England Journal of Medicine (NEJM), Lancet or Journal of the American Medical Association (JAMA) on or after 1st January 2000 until 12th October 2021. Studies were included if they reported results from a Phase 3 RCT or a multi-centre observational study, and included adults with coronary artery disease, ACS, PCI, heart rhythm disease, cardiomyopathy, HF, or valve disease, to ensure coverage of all five domains. Conference abstracts or review articles were excluded, as were sub-studies where the main paper was included in the review, and studies in which clinical outcomes were not reported or not defined. The search strategy was developed with a research librarian (see supplementary data online, TablesS1 and S2). Variables that were included in the existing domain registries were reviewed,14–17 and outcome measure variables were included for Delphi voting. These were presented to the Working Group participants, who were able to suggest additional variables based upon clinical expertise.
Working groups
We approached all of the ESC Associations and Working Groups to nominate individuals with relevant clinical expertise to join a Global Cardiovascular Outcomes Consortium, alongside existing members of the EuroHeart team. Additional international clinical experts were approached directly for their specific expertise in clinical trials, registries, and regulators. From this Consortium, five Working Groups were assembled: one for the generic cardiovascular outcome measures, and one for each of the four EuroHeart domains. Some people participated in more than one group, depending on their experience and availability (see Supplementary data).
Variable level
Working group members were asked to take part in a Delphi process to consider three options for each proposed cardiovascular outcome measure variable: include as a mandatory (Level 1) variable; include as an optional (Level 2) variable; or do not include. Voting was conducted in an online poll. Level 1 cardiovascular outcome measure variables are intended for collection in all participants in the registry, whereas Level 2 variables are discretionary and may be useful and available in some (but not all) settings and countries, depending on the purpose of the registry.
Data were collated in MS Excel for calculation of voting proportions. The threshold for inclusion as a Level 1 variable was at least 75% of participants voting for selection of the variable as Level 1.18 The threshold for inclusion as a Level 2 variable was at least 75% of participants selecting for the variable either Levels 1 or 2. Where a cardiovascular outcome measure variable was already included as a Level 1 variable in the generic domain, this was not considered again by the other domain groups (as it would already apply to all registry participants). Where a variable was already included as a Level 2 variable in the generic domain, it could be re-considered by the Working Group for upgrade to Level 1 within that specific domain.
Selection of the final set of variables
The results of the Delphi voting were presented in an online meeting, and the results of each cardiovascular outcome measure variable were discussed. Where a Level 1 confirmatory vote had been made already, these results were presented for information only—because the threshold for inclusion had been made. Where the threshold for Levels 1 or 2 inclusion had not been reached, participants could request for this to be re-phrased based upon their clinical expertise, and this could proceed for a second vote with the same thresholds employed as in the first round.
Definitions
The proposed definitions for each variable were collated from the literature and shared with the invitees from every Working Group for their comment and clarification. These were agreed by consensus. The previously published variables and definitions for each domain included a range of classification systems for bleeding;14–17 for this outcomes domain, however, participants were clear that a single harmonized classification for bleeding outcome measure was essential. Accordingly, an online poll was circulated to all participants to vote on their preference for the Valve Academic Research Consortium (VARC),20 Bleeding Academic Research Consortium (BARC),21 or International Society On Thrombosis and Haemostasis (ISTH)22 classifications of bleeding that would then be employed across all of the cardiovascular outcome domains.
Patient and public involvement
The ESC Patient Forum was invited to contribute to this project from its inception. Their feedback was that the development of data variables and standards for cardiovascular outcomes measures was too technical for their meaningful contribution. Instead, they suggested that the results of the Delphi polls were presented to the Patient Forum for their discussion and comment, which took place in December 2023 prior to the finalisation of the catalogue of cardiovascular outcome measures. Representatives from the ESC Patient Forum are also part of the research team for the development of patient-reported outcome measures.
Results
Systematic review
Of 4728 publications that were screened, 801 (16.9%) were included in the review after full-text evaluation. Of these, 320 (40.0%) were published in the NEJM, 284 (35.5%) in JAMA, and 197 (24.6%) in the Lancet, comprising 620 (77.4%) RCTs and 181 (22.6%) observational studies. The most frequently reported primary outcome measure was a composite (449 studies, 56.1%), followed by all-cause mortality (109 studies, 13.6%). Where a composite was the primary outcome measure, the most frequent components were myocardial infarction (273 studies, 60.8%), all-cause mortality (242 studies, 53.9%), stroke (190 studies, 42.3%), and cardiovascular mortality (178 studies, 39.6%).
The working group process
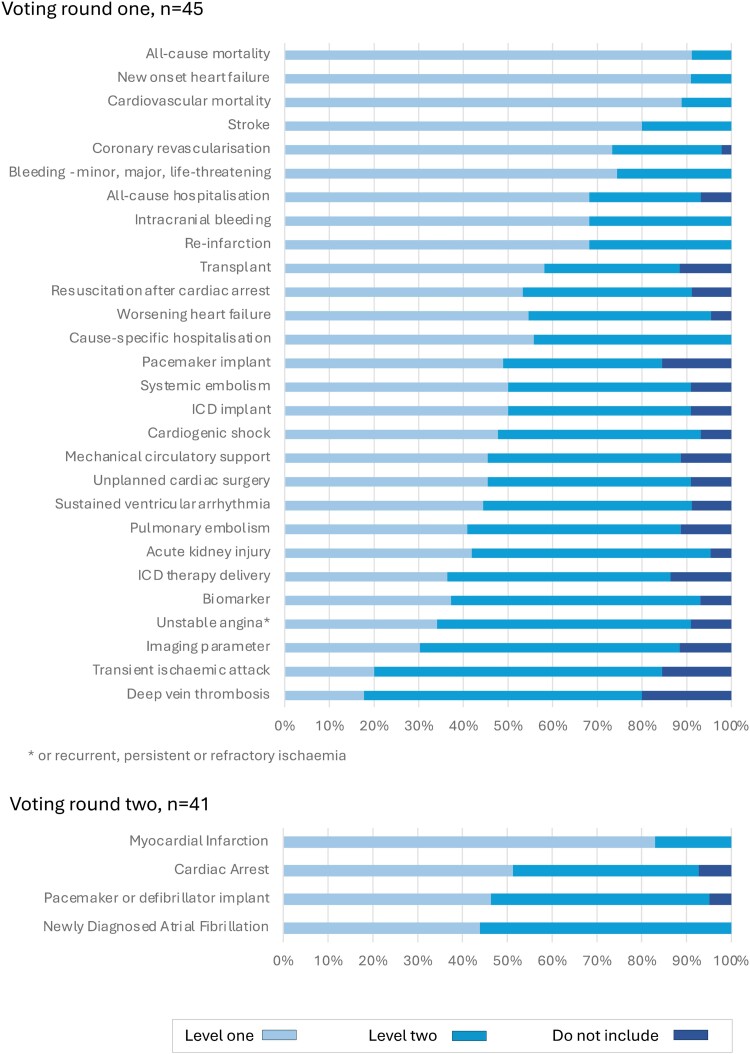
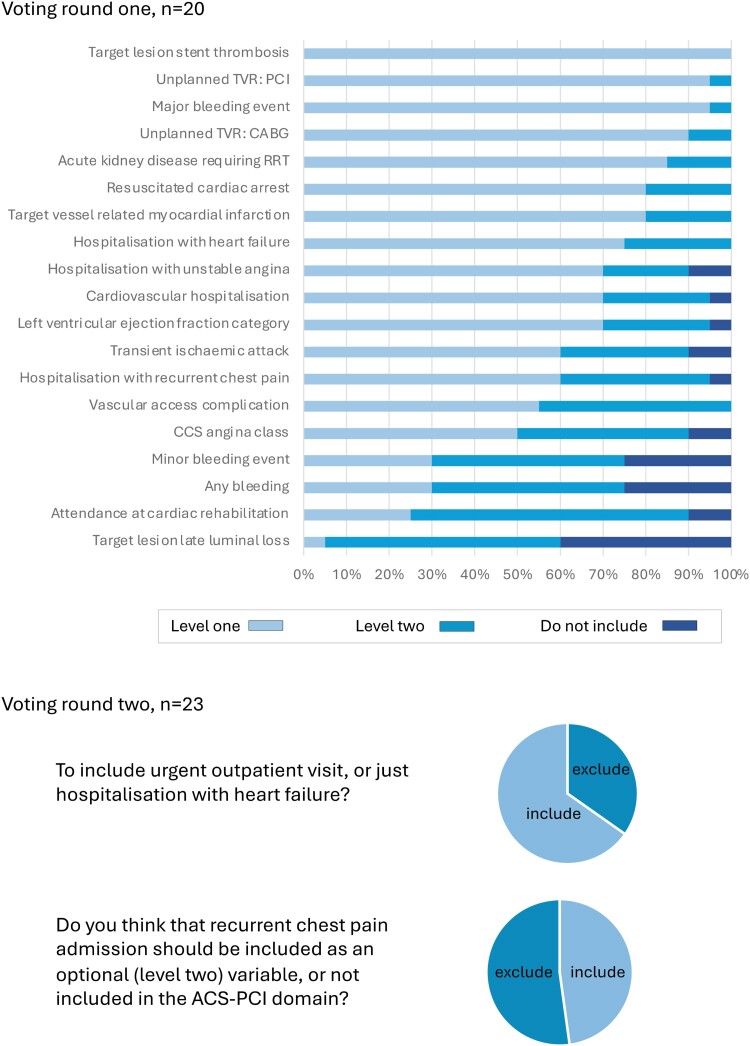
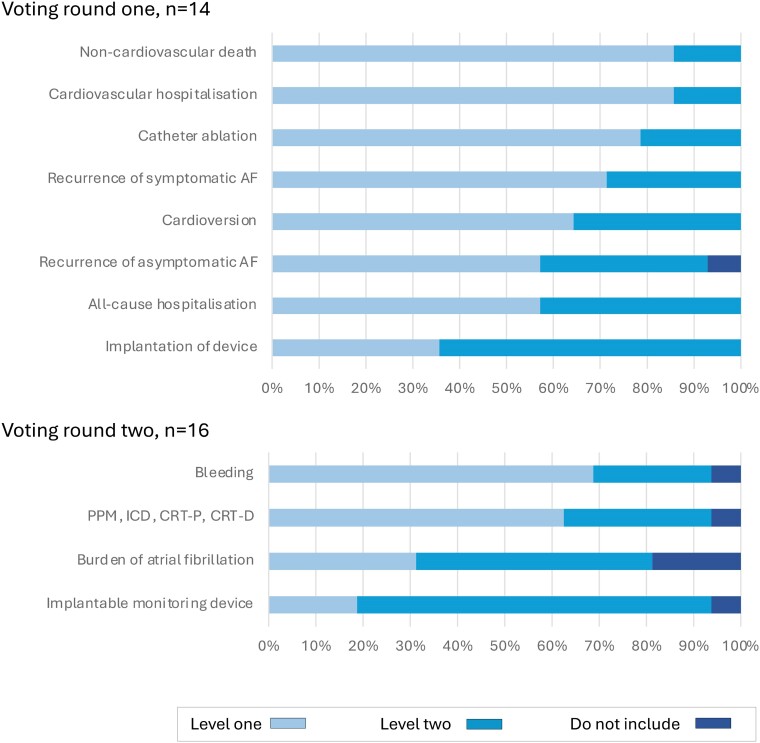
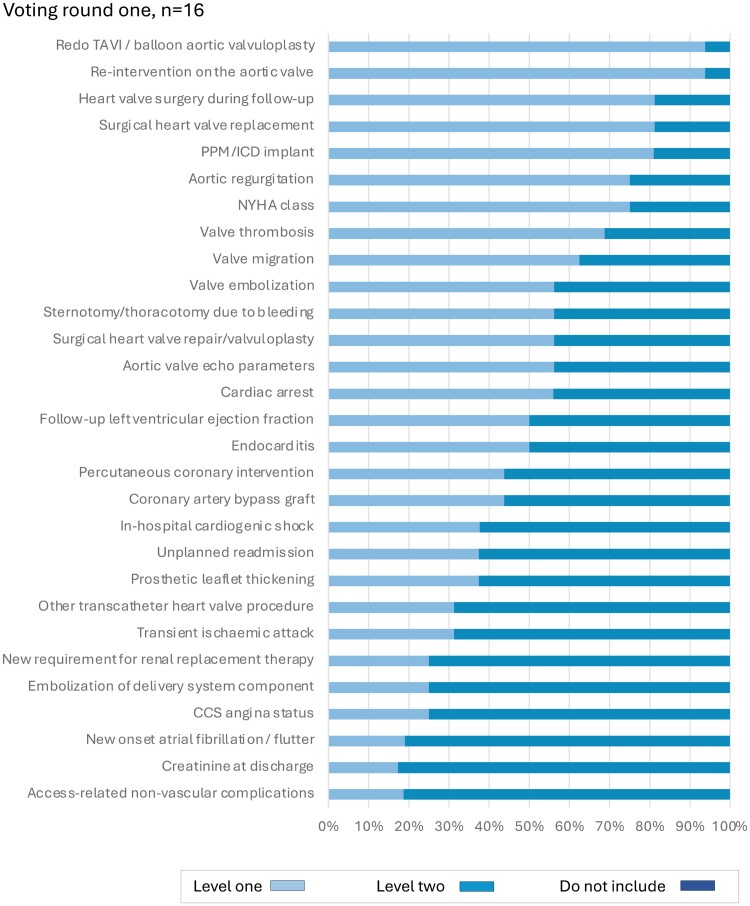
The extracted clinical outcome measures were presented to the five Working Groups for consideration as candidate variables for inclusion. In the first round, 28 candidate variables were considered for the generic cardiovascular outcome measures domain by 45 experts (Figure 1). Following discussion, a further four variables were considered by 41 experts (51 individuals contributed in total). For the ACS/PCI outcome measures domain, 26 experts reviewed 19 candidate variables in two rounds of voting (Figure 2). For the AF outcome measures domain, 18 experts reviewed 12 variables in two rounds of voting (Figure 3). For the HF outcome measures domain, 31 experts reviewed 19 variables in two rounds of voting (Figure 4). In the TAVI outcome measures domain, all 29 variables were already included in the TAVI registry, and so the reference group were asked to consider whether these should be considered as Levels 1 or 2 outcomes, and then the list refined during the meeting, during which 16 experts reviewed 29 variables in one round of voting (Figure 5). The complete list of variables and their definitions was reviewed by all contributors (n = 82).
Figure 1.
Voting results, generic domain. ICD, implantable cardioverter defibrillator. Level 1: mandatory variable; Level 2: optional variable
Figure 2.
Voting results, acute coronary syndrome/percutaneous coronary intervention domain. CABG, coronary artery bypass grafting surgery; CCS, Canadian Cardiovascular Society; ICD, implantable cardioverter defibrillator; PCI, percutaneous coronary intervention; RRT, renal replacement therapy; TVR, target vessel revascularisation. Level 1: mandatory variable; Level 2: optional variable
Figure 3.
Voting results, AF domain. AF, atrial fibrillation/flutter; PPM, permeant pacemaker; ICD, implantable cardioverter defibrillator; CRT-P, cardiac resynchronisation pacemaker; CRT-D, cardiac resynchronisation defibrillator. Level 1: mandatory variable; Level 2: optional variable
Figure 4.
Voting results, heart failure domain. NT-proBNP/BNP, N-Terminal Pro/B-type Natriuretic Peptide; NYHA, New York Heart Association. Level 1: mandatory variable; Level 2: optional variable
Figure 5.
Voting results, transcatheter aortic valve intervention domain. CCS, Canadian Cardiac Society; ICD, implantable cardioverter defibrillator; NYHA, New York Heart Association; PPM, permanent pacemaker; TAVI, transcatheter aortic valve intervention. Level 1: mandatory variable; Level 2: optional variable
Generic domain: Level 1 variables
For the generic cardiovascular outcome measures domain, five Level 1 variables were agreed: all-cause mortality, cardiovascular mortality, myocardial infarction, stroke, and HF (Table 1).
Table 1.
Generic domain—clinical outcomes and their definitions
| Generic domain: Level 1 variables | |
| All-cause mortality | Death from any cause |
| Cardiovascular mortality | Death that is primarily from a cardiovascular cause:
|
| Myocardial infarction | Myocardial infarction, as defined according to the latest universal definition of MI, currently: a rise and/or fall of cardiac troponin with at least one value above the 99th percentile and/or symptoms suggestive of ischaemia, new significant ECG changes, imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischaemic aetiology or identification of a coronary thrombus by angiography/intracoronary imaging or by autopsy. History of myocardial infarction also includes episodes of symptoms suggestive of myocardial ischaemia, which are accompanied by presumed new ischaemic ECG changes or ventricular fibrillation; coronary intervention-related myocardial infarction; and CABG-related myocardial infarction.47 |
| Stroke | An acute episode of focal or global neurological dysfunction (lasting for ≥ 24 h or until death) caused by an infarction or haemorrhage in the brain, spinal cord, or retina resulting in cell damage based on pathological, imaging, or other objective evidence. Stroke does not include non-vascular neurological deficits.
|
| HF | A new clinical diagnosis of HF made by a healthcare professional. HF is a clinical syndrome characterized by typical symptoms (e.g. dyspnoea) and/or signs (e.g. ankle swelling), caused by a structural and/or functional cardiac abnormality (e.g. left ventricular hypertrophy or impairment), and associated with elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion from a cardiogenic origin at rest or with exercise.49 |
| Generic domain: Level 2 variables | |
| Acute kidney injury | Increase in serum creatinine by ≥0.3 mg/dL (≥26.5 µmol/L) within 48 h; or an increase in serum creatinine to ≥1.5 times baseline, which is known or presumed to have occurred within the prior 7 days; or urine volume <0.5 mL/kg/h for 6 h.50 |
| All-cause re-hospitalisation | Unscheduled admission to hospital for any reason, defined as a being admitted for more than 24 h or past a calendar day.6,20 |
| Bleeding events | Type 1: bleeding that is not actionable and does not cause the patient to seek unscheduled performance of studies, hospitalization, or treatment by a healthcare professional. Type 2: any clinically overt sign of haemorrhage that is actionable but does not meet criteria for Type 3, Type 4 (coronary artery bypass graft surgery [CABG]-related), or Type 5 (fatal bleeding) bleeding. The bleeding must require diagnostic studies, hospitalization, or treatment by a healthcare professional. In particular, the bleeding must meet at least one of the following criteria:
Type 3b: overt bleeding plus haemoglobin drop ≥5 g/dL (provided haemoglobin drop is related to bleed); cardiac tamponade; bleeding requiring surgical intervention for control (excluding dental/nasal/skin/haemorrhoid); bleeding requiring intravenous vasoactive drugs. Type 3c: intracranial haemorrhage; sub-categories confirmed by autopsy or imaging, or lumbar puncture; intraocular bleed compromising vision. Type 4: CABG–related bleeding; peri-operative intracranial bleeding within 48 h; re-operation after closure of sternotomy for the purpose of controlling bleeding; transfusion of ≥5 units of whole blood or packed red blood cells within a 48-h period; chest tube output ≥2 L within a 24-h period. Type 5: fatal bleeding.22 |
| Cardiac arrest | Cardiac arrest is defined as a verified sudden cessation of cardiac mechanical activity causing unresponsiveness, absence of normal breathing and no signs of circulation (excluding syncope or profound vagally mediated bradycardia) with ventricular fibrillation, rapid ventricular tachycardia or bradycardia resulting in loss of consciousness, pulseless electrical activity, or asystole as the major causes. Return of spontaneous circulation (ROSC) is defined as the resumption of a sustained heart rhythm that perfuses the body after cardiac arrest. Signs include a palpable pulse, measurable blood pressure and/or respiratory effort.51 |
| Cardiogenic shock | Cardiogenic shock is defined as any one of the following: (1) ‘beginning’ cardiogenic shock or compensated shock where a patient may be volume overloaded, tachycardic, and/or hypotensive but no evidence of hypoperfusion on physical exam or laboratory studies. It also includes patients with a (2) ‘classic’ cardiogenic shock with evidence of hypoperfusion on physical exam and laboratory studies ‘cold and wet.’ Invasive haemodynamics (if available) demonstrate the classic depressed cardiac index associated with cardiogenic shock. Cardiogenic shock also includes patients with (3) ‘deteriorating’ and includes above patients plus failure of initial interventions in restoring adequate perfusion in 30 min and further escalation is required. Cardiogenic shock also includes (4) ‘escalation’ cardiogenic shock which is an increase in the number or intensity of intravenous therapies to address hypoperfusion, or addition of mechanical circulatory support after the initial 30-minute period of observation and treatment. It can also include patients who are highly unstable, often with circulatory collapse and/or refractory cardiac arrest with ongoing CPR. They are being supported by multiple simultaneous acute interventions including ECMO-facilitated CPR (eCPR).52 |
| Cause-specific hospitalisation | Unscheduled hospitalisation due to either cardiovascular or non-cardiovascular causes.
|
| DVT | DVT is the formation of a thrombus (blood clot) in a deep vein, usually in the legs, but may also include the arms, which partially or completely obstructs blood flow.54 |
| Device implantation | Implantation of any of:
|
| Heart transplant | Surgery in which a failing, diseased heart is replaced with a donor heart.57 |
| Hospitalized ventricular tachycardia | The patient was hospitalized with ventricular tachycardia, defined as ≥3 consecutive beats with a rate >100 beats per minute originating from the ventricles, independent from atrial and atrioventricular (AV) nodal conduction.46 |
| ICD therapy delivery | Delivery of either an ICD shock or antitachycardia pacing.55 |
| Mechanical circulatory support | Use of mechanical circulatory support devices, such as left ventricular assist device. |
| AF or AFL | AF is defined as a supraventricular tachyarrhythmia with uncoordinated atrial electrical activation and consequently ineffective atrial contraction. The minimum duration of an ECG tracing of AF required to establish the diagnosis of clinical AF is at least 30 s, or entire 12-lead ECG. AFL is defined as a supraventricular tachyarrhythmia with co-ordinated but overly rapid atrial electrical activation, usually with some degree of AV node conduction block. The minimum duration of an ECG tracing of AF required to establish the diagnosis of clinical AFL is at least 30 s, or entire 12-lead ECG.58 |
| Pulmonary embolism | A condition in which one or more emboli, usually arising from a thrombus formed in the veins, are lodged in and obstruct the pulmonary arterial system.59 |
| Systemic embolism | Systemic embolism is defined as a hospital encounter with a principal diagnosis of an arterial embolism and thrombosis,60 excluding stroke or transient ischaemic attack. |
| Transient ischaemic attack | Transient ischaemic attack is a transient focal neurological signs or symptoms lasting <24 h presumed to be due to focal brain, spinal cord, or retinal ischaemia, but without evidence of acute infarction by neuroimaging or pathology, or with no imaging performed.61 |
| Unplanned cardiac surgery | Unplanned cardiac surgery is defined as an unplanned surgical intervention to the heart and the great vessels.62 |
| Worsening HF | HF is a clinical syndrome characterized by typical symptoms (e.g. dyspnoea) and/or signs (e.g. ankle swelling), caused by a structural and/or functional cardiac abnormality (e.g. left ventricular hypertrophy or impairment), and associated with elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion from a cardiogenic origin at rest or with exercise. Worsening HF is defined as either an unplanned HF hospitalisation or urgent outpatient visit for HF. Unplanned HF hospitalisation is defined as a patient requiring an unscheduled hospital admission for a primary diagnosis of HF with a length of stay that either exceeds 24 h or crosses a calendar day (if hospital admission and discharge times are unavailable). To satisfy the criteria for a worsening HF event, the patient must have an urgent, unscheduled office or emergency visit for HF with signs, symptoms, and diagnostic testing results identical to those already described above. The patient must also require treatment for HF such as significant dose increase of oral diuretics, intravenous diuretics or mechanical or surgical intervention for HF. Importantly, clinic visits for scheduled administration of HF therapies or procedures (e.g. intravenous diuretics, intravenous vasoactive agents, or mechanical fluid removal) do not qualify as non-hospitalized HF events.6,49,63 |
Abbreviations: AF, atrial fibrillation; AFL, atrial flutter; CABG, coronary artery bypass; CPR, cardiopulmonary resuscitation; CRT, cardiac resynchronisation therapy; DVT, deep vein thrombosis; ECG, electrocardiogram; HF, heart failure; ICD, implantable cardioverter defibrillator.
Generic domain: Level 2 variables
For the generic cardiovascular outcome measures domain, 18 Level 2 variables were agreed: acute kidney injury, all-cause re-hospitalisation, bleeding events, cardiac arrest, cardiogenic shock, cause-specific hospitalisation, deep vein thrombosis (DVT), device implantation (including transvenous pacemaker, leadless pacemaker, transvenous implantable cardioverter defibrillator [ICD], subcutaneous ICD, cardiac-resynchronisation therapy pacemaker or defibrillator [CRT-P, CRT-D]), heart transplant, hospitalized ventricular tachycardia, ICD therapy delivery (e.g. cardioversion or antitachycardia therapy), mechanical circulatory support, atrial fibrillation/flutter (AF), pulmonary embolism, systemic embolism, transient ischaemic attack, unplanned cardiac surgery, and worsening HF (Table 1).
Acute coronary syndrome/percutaneous coronary intervention domain: Level 1 variables
For the ACS/PCI outcome measures domain, eight Level 1 variables were agreed: acute kidney injury requiring renal replacement therapy, cardiac arrest, HF hospitalisation, major bleeding event, target lesion stent thrombosis, target vessel related myocardial infarction, unplanned target-vessel coronary artery bypass graft (CABG) surgery, and unplanned target-vessel PCI (Table 2).
Table 2.
Acute coronary syndrome/percutaneous coronary intervention—clinical outcomes and their definitions
| Acute coronary syndrome/PCI: Level 1 variables | |
| Acute kidney injury requiring renal replacement therapy | Renal replacement therapy includes ultrafiltration (haemofiltration), haemodialysis or peritoneal dialysis.50 |
| Cardiac arrest | Cardiac arrest is defined as a verified sudden cessation of cardiac activity causing unresponsiveness, absence of normal breathing and no signs of circulation (excluding syncope or profound vagally mediated bradycardia) with ventricular fibrillation, rapid ventricular tachycardia or bradycardia resulting in loss of consciousness, pulseless electrical activity, or asystole as the major causes. ROSC is defined as the resumption of a sustained heart rhythm that perfuses the body after cardiac arrest. Signs include a palpable pulse, measurable blood pressure and/or respiratory effort.51 |
| Heart failure hospitalisation | Hospital admission primarily due to heart failure. Heart failure is a clinical syndrome characterized by typical symptoms (e.g. dyspnoea) and/or signs (e.g. ankle swelling), caused by a structural and/or functional cardiac abnormality (e.g. left ventricular hypertrophy or impairment), and associated with elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion from a cardiogenic origin at rest or with exercise. Unplanned HF hospitalisation is defined as a patient requiring an unscheduled hospital admission for a primary diagnosis of HF with a length of stay that either exceeds 24 h or crosses a calendar day (if hospital admission and discharge times are unavailable). To satisfy the criteria for a HF hospitalisation, the patient must be admitted primarily for HF with signs, symptoms, and diagnostic testing results identical to those already described above. The patient must also require treatment for HF such as significant augmentation of oral diuretics, intravenous diuretics, or mechanical or surgical intervention for HF.6,49,53,63 Please record worsening heart failure under the Level 2 section of the generic outcomes, if available. |
| Major bleeding event | Type 2: any clinically overt sign of haemorrhage that is actionable but does not meet criteria for Type 3, Type 4 (coronary artery bypass graft surgery [CABG]-related), or Type 5 (fatal bleeding) bleeding. The bleeding must require diagnostic studies, hospitalization, or treatment by a healthcare professional. In particular, the bleeding must meet at least one of the following criteria:
Type 3b: overt bleeding plus haemoglobin drop ≥5 g/dL (provided haemoglobin drop is related to bleed); cardiac tamponade; bleeding requiring surgical intervention for control (excluding dental/nasal/skin/haemorrhoid); bleeding requiring intravenous vasoactive drugs. Type 3c: intracranial haemorrhage; sub-categories confirmed by autopsy or imaging, or lumbar puncture; intraocular bleed compromising vision. Type 4: CABG–related bleeding; peri-operative intracranial bleeding within 48 h; reoperation after closure of sternotomy for the purpose of controlling bleeding; transfusion of ≥5 units of whole blood or packed red blood cells within a 48-h period; chest tube output ≥2 L within a 24-h period. Type 5: fatal bleeding.22 Note: Major bleeding includes fatal bleeding events, symptomatic bleeding in a critical areas or organs (e.g. intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or peri-cardial, or intramuscular with compartment syndrome), and/or fall in haemoglobin level of ≥20 g/L (≥2 g/dL) or transfusion of ≥2 units of blood. In-hospital major bleeding does not include CABG-related bleeding. Fatal bleeding should be selected when bleeding is believed to be the primary cause of death. Intracranial haemorrhage, of any severity, should ideally be confirmed by scanning. Other major bleeding should only be selected if the patient had a major bleeding episode other than those stated above.21 |
| Target lesion stent thrombosis | Target lesion stent thrombosis is defined as occurring when clinical presentation is consistent with acute coronary syndrome of a previously treated lesion. The categories are defined as follows: Definite is defined as angiographic confirmation of stent/scaffold thrombosis, the presence of a thrombus that originates in the stent/scaffold or in the segment 5 mm proximal or distal to the stent/scaffold or in a side branch originating from the stented/scaffolded segment and the presence of at least one of the following criteria:
Silent is defined as the incidental angiographic documentation of stent occlusion in the absence of clinical signs or symptoms is not considered stent thrombosis.64 |
| Target vessel related myocardial infarction | Target vessel myocardial infarction is defined as myocardial necrosis in the vascular territory of a previously treated target vessel. As well as direct evidence of invasive angiography, electrocardiographic or other imaging evidence such as echocardiography (e.g. newly developed regional wall motion abnormality or extension of previous abnormality) can be used to adjudicate the involvement of the target vessel territory. The target vessel was defined as the entire major coronary vessel or bypass graft proximal and distal to the target lesion including upstream and downstream branches and the target lesion itself. The left main coronary artery and any vessel originating from the left main coronary artery, or its major branches is, defined as target vessel.47,64 |
| Unplanned target-vessel coronary artery bypass graft surgery | Repeat revascularisation of the target vessel with unplanned coronary artery bypass grafting (CABG) surgery after the index procedure. Target vessel CABG is defined as any CABG of any segment of the target vessel including the target lesion. The target vessel was defined as the entire major coronary vessel or bypass graft proximal and distal to the target lesion including upstream and downstream branches and the target lesion itself. The left main coronary artery and any vessel originating from the left main coronary artery, or its major branches are, defined as target vessel. CABG is a procedure to bypass diseased segment(s) of the coronary tree using blood vessels derived other parts of the body and connected to the aorta.64,65 |
| Unplanned target-vessel PCI | An unplanned PCI after the index procedure. Repeat target vessel PCI is defined as any repeat PCI of any segment of the target vessel including the target lesion. The target vessel is defined as the entire major coronary vessel or bypass graft proximal and distal to the target lesion including upstream and downstream branches and the target lesion itself. The left main coronary artery and any vessel originating from the left main coronary artery, or its major branches are, defined as target vessel. PCI is defined as the placement of an angioplasty guidewire, balloon, or other device (e.g. stent, atherectomy, brachytherapy, or thrombectomy catheter) into a native coronary artery or a graft for the purpose of mechanical coronary revascularisation. The assessment of coronary lesion severity by fluoroscopy, intracoronary imaging (e.g. intravascular ultrasonography) or physiology (e.g. fractional flow reserve) is not considered a PCI procedure.64,65 |
| Acute coronary syndrome/PCI: Level 2 variables | |
| Attendance at cardiac rehabilitation | Cardiovascular rehabilitation is a multi-factorial and comprehensive intervention in secondary prevention, supervised and carried out by adequately trained health professionals.66 |
| Cardiovascular hospitalisation | Unscheduled hospitalized primarily due to cardiovascular disease. Unscheduled hospitalisation is defined as a being admitted for more than 24 h or past a calendar day due to primarily a cardiovascular condition. Cardiovascular causes include conditions such as heart failure, cardiogenic shock, bioprosthetic or native valve dysfunction, myocardial infarction, stroke, thromboembolism, bleeding, tamponade, vascular complication, arrhythmia or conduction system disturbances, cardiovascular infection (e.g. mediastinitis, endocarditis), or other clear cardiovascular cause.6,53 |
| CCS angina class | CCS Grade I: Ordinary physical activity does not cause angina, such as walking and climbing stairs. Angina with strenuous or rapid or prolonged exertion at work or recreation. CCS Grade II: Slight limitation of ordinary activity. Walking or climbing stairs rapidly, walking uphill, walking or stair climbing after meals, or in cold, or in wind, or under emotional stress, or only during the few hours after awakening. Walking more than two blocks on the level and climbing more than one flight of ordinary stairs at a normal pace and in normal conditions. CCS Grade III: Marked limitation of ordinary physical activity. Walking one or two blocks on the level and climbing one flight of stairs in normal conditions and at normal pace. CCS Grade IV: Inability to carry on any physical activity without discomfort, anginal syndrome may be present at rest.67 |
| Hospitalisation with unstable angina | Unscheduled admission to hospital with unstable angina as the primary cause. Unstable angina is defined as myocardial ischaemia at rest or on minimal exertion in the absence of acute cardiomyocyte injury/necrosis using high-sensitive (hs)-cardiac troponin (cTn). Unscheduled hospitalisation is defined as a being admitted for more than 24 h or past a calendar day due to primarily a cardiovascular condition.6,53,68 |
| Left ventricular ejection fraction | Ejection fraction is ideally measured with echocardiography for consistency. |
| Minor bleeding event | Bleeding that is not actionable and does not cause the patient to seek unscheduled performance of studies, hospitalisation, or treatment by a healthcare professional; may include episodes leading to self-discontinuation of medical therapy by the patient without consulting a healthcare professional (Bleeding Academic Research Consortium Type 1).21 |
| Vascular access complication |
|
Abbreviations: BARC, Bleeding Academic Research Consortium; CABG, coronary artery bypass; CCS, Canadian Cardiovascular Society; PCI, percutaneous coronary intervention.
Acute coronary syndrome/percutaneous coronary intervention domain: Level 2 variables
For the ACS/PCI outcome measures domain, seven Level 2 variables were agreed: attendance at cardiac rehabilitation, cardiovascular hospitalisation, Canadian Cardiovascular Society (CCS) angina class, hospitalisation with unstable angina, left ventricular ejection fraction, minor bleeding event, and vascular access complication (Table 2).
Atrial fibrillation domain: Level 1 variables
For the AF outcome measures domain, two Level 1 variables were agreed: cardiovascular hospitalisation, and catheter ablation (Table 3).
Table 3.
Atrial fibrillation—clinical outcomes and their definitions
| AF: Level 1 variables | |
| Cardiovascular hospitalisation | Admission to hospital primarily due to cardiovascular disease. Unscheduled hospitalisation is defined as a being admitted for more than 24 h or past a calendar day due to primarily a cardiovascular condition. CV causes include conditions such as heart failure, cardiogenic shock, bioprosthetic or native valve dysfunction, myocardial infarction, stroke, thromboembolism, bleeding, tamponade, vascular complication, arrhythmia or conduction system disturbances, cardiovascular infection (e.g. mediastinitis, endocarditis), or other clear cardiovascular cause.6,53 |
| Catheter ablation | Catheter ablation for AF or AFL is defined as a procedure in which catheters are inserted through the veins or arteries to the heart, and energy (e.g. radiofrequency, cryoablation) is delivered to prevent propagation of abnormal AF or AFL. AF is defined as a supraventricular tachyarrhythmia with uncoordinated atrial electrical activation and consequently ineffective atrial contraction. The minimum duration of an ECG tracing of AF required to establish the diagnosis of clinical AF is at least 30 s, or entire 12-lead ECG. AFL is defined as a supraventricular tachyarrhythmia with co-ordinated but overly rapid atrial electrical activation, usually with some degree of AV node conduction block. The minimum duration of an ECG tracing of AFL required to establish the diagnosis of clinical AFL is at least 30 s, or entire 12-lead ECG.17 |
| Atrial fibrillation: Level 2 variables | |
| All-cause hospitalisation | Unscheduled admission to hospital for any reason. Hospitalisation is defined as a being admitted for more than 24 h or past a calendar day.6,53 |
| Burden of atrial fibrillation | Burden is defined as the amount of time spent in atrial fibrillation as a proportion of the total monitoring period. Monitoring can be in the form of invasive and non-invasive monitoring devices. Duration of the device monitoring period is the fixed monitoring period using ambulatory and between downloads of invasive monitoring devices.58 |
| Cardioversion | Electrical cardioversion (external or internal) is defined as a procedure in which direct current is used to restore sinus rhythm. Pharmacologic cardioversion is defined as a procedure in which antiarrhythmic medications are used to restore sinus rhythm.58 |
| Device implantation | Implantation of:
|
| Implantable monitoring device | An implantable device that allows remote rhythm monitoring.55 |
| Recurrence of AF | Recurrence of atrial fibrillation/flutter. AF is defined as a supraventricular tachyarrhythmia with uncoordinated atrial electrical activation and consequently ineffective atrial contraction. The minimum duration of an ECG tracing of AF required to establish the diagnosis of clinical AF is at least 30 s, or entire 12-lead ECG. AFL is defined as a supraventricular tachyarrhythmia with co-ordinated but overly rapid atrial electrical activation, usually with some degree of AV node conduction block. The minimum duration of an ECG tracing of AFL required to establish the diagnosis of clinical AFL is at least 30 s, or entire 12-lead ECG.17 |
Abbreviations: AF, atrial fibrillation; AFL, atrial flutter; CRT, cardiac resynchronisation therapy; ECG, electrocardiogram; ICD, implantable cardioverter defibrillator.
Atrial fibrillation domain: Level 2 variables
For the AF outcome measures domain, six Level 2 variables were agreed: all-cause hospitalisation, burden of AF (time spent in AF out of total monitoring period), cardioversion, device implantation (e.g. pacemaker, CRT, and ICD), implantable monitoring device, and recurrence of AF (Table 3).
Heart failure domain: Level 1 variables
For the HF outcome measures domain, five Level 1 variables were agreed: all-cause hospitalisation, HF re-hospitalisation, heart transplantation, left ventricular ejection fraction, and implant of a left ventricular assist device (Table 4).
Table 4.
Heart failure—clinical outcomes and their definitions
| Heart failure: Level 1 variables | |
| All-cause re-hospitalisation | Unscheduled hospitalisation for any cause, defined as a being admitted for more than 24 h or past a calendar day.6,53 |
| Heart failure re-hospitalisation | Hospital admission primarily related to heart failure. Heart failure is a clinical syndrome characterized by typical symptoms (e.g. dyspnoea) and/or signs (e.g. ankle swelling), caused by a structural and/or functional cardiac abnormality (e.g. left ventricular hypertrophy or impairment), and associated with elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion from a cardiogenic origin at rest or with exercise. Unplanned HF hospitalisation is defined as a patient requiring an unscheduled hospital admission for a primary diagnosis of HF with a length of stay that either exceeds 24 h or crosses a calendar day (if hospital admission and discharge times are unavailable). To satisfy the criteria for a HF hospitalisation, the patient must be admitted primarily for HF with signs, symptoms, and diagnostic testing results identical to those already described above. The patient must also require treatment for HF such as significant augmentation of oral diuretics, intravenous diuretics, or mechanical or surgical intervention for HF.6,49,53,63 |
| Heart transplantation | Receipt of surgery in which a failing, diseased heart is replaced with a healthier donor heart.57 |
| Left ventricular assist device | Implant of a left ventricular assist device. |
| Left ventricular ejection fraction | Ejection fraction, ideally measured with echocardiography. |
| Heart failure domain: Level 2 variables | |
| Device implantation | Implantation of:
|
| Resuscitated ventricular tachyarrhythmia | The patient was successfully resuscitated and had ROSC from a ventricular tachyarrhythmia. |
Abbreviations: CRT, cardiac resynchronisation therapy; HF, heart failure; ICD, implantable cardioverter defibrillator; NYHA, New York Heart Association; ROSC, return of spontaneous circulation.
Heart failure domain: Level 2 variables
For the HF outcome measures domain, two Level 2 variables were agreed: device implant (e.g. pacemaker, CRT, and ICD), and resuscitated ventricular arrhythmia (Table 4). Notably, the HF Working Group advised that additional parameters would be advantageous for monitoring the chronic disease management aspects of HF; these are discussed in detail in a separate publication.23
Transcatheter aortic valve intervention domain: Level 1 variables
For the TAVI outcome measures domain, four Level 1 variables were agreed: aortic regurgitation, device implantation (e.g. pacemaker, CRT, and ICD), New York Heart Association class, and re-intervention on the aortic valve (Table 5).
Table 5.
Transcatheter aortic valve intervention—clinical outcomes and their definitions
| Transcatheter aortic valve intervention—level 1 variables | |
| Aortic regurgitation | Presence of aortic regurgitation, and severity (mild/moderate/severe) as determined by echocardiography based on Doppler parameters according to the criteria of the VARC 3 criteria.53 |
| Device implantation | Implantation of:
|
| NYHA class | NYHA class I: no limitations of physical activity. Ordinary physical activity does not cause undue fatigue, palpitations, or dyspnoea. NYHA class II: slight limitation of physical activity. The patient is comfortable at rest. Ordinary physical activity results in fatigue, palpitations, or dyspnoea. NYHA class III: marked limitation of physical activity. The patient is comfortable at rest. Less than ordinary activity causes fatigue, palpitations, or dyspnoea. NYHA class IV: inability to carry on any physical activity without discomfort. Heart failure symptoms are present even at rest or with minimal exertion.49,63 |
| Re-intervention on the aortic valve: | Re-do TAVI is a different procedure to the index TAVI, and a separate registration form should be completed. Balloon aortic valvuloplasty is a transcatheter balloon dilatation of the implanted aortic valve after the completion of the index procedure. Surgical aortic valve replacement is defined as a deployment of a new (mechanical or bioprosthetic) aortic valve surgically. Other aortic valve surgery is any other surgical intervention on the aortic valve. |
| Transcatheter aortic valve intervention—level 2 variables | |
| Access-related non-vascular complications | Major access-related non-vascular events are defined as one of the following:
|
| All-cause re-hospitalisation | Unscheduled hospital admission for any reason. Unscheduled hospitalisation is defined as a being admitted for more than 24 h or past a calendar day.6,53 |
| Cardiac arrest | Cardiac arrest is defined as a verified sudden cessation of cardiac activity causing unresponsiveness, absence of normal breathing and no signs of circulation (excluding syncope or profound vagally mediated bradycardia) with ventricular fibrillation, rapid ventricular tachycardia, or bradycardia resulting in loss of consciousness, pulseless electrical activity, or asystole as the major causes. ROCS is defined as the resumption of a sustained heart rhythm that perfuses the body after cardiac arrest. Signs include a palpable pulse, measurable blood pressure, and/or respiratory effort.51 |
| Cardiogenic shock | Cardiogenic shock is defined as any one of the following: (1) ‘beginning’ cardiogenic shock or compensated shock where a patient may be volume overloaded, tachycardic, and/or hypotensive but no evidence of hypoperfusion on physical exam or laboratory studies. It also includes patients with a (2) ‘classic’ cardiogenic shock with evidence of hypoperfusion on physical exam and laboratory studies ‘cold and wet.’ Invasive haemodynamics (if available) demonstrate the classic depressed cardiac index associated with cardiogenic shock. Cardiogenic shock also includes patients with (3) ‘deteriorating’ and includes above patients plus failure of initial interventions in restoring adequate perfusion in 30 min and further escalation is required. Cardiogenic shock also includes (4) ‘escalation’ cardiogenic shock in which an increase in the number or intensity of intravenous therapies to address hypoperfusion, or addition of mechanical circulatory support after the initial 30-min period of observation and treatment. It can also include patients who are highly unstable, often with circulatory collapse and/or refractory cardiac arrest with ongoing CPR. They are being supported by multiple simultaneous acute interventions including ECMO-facilitated CPR (eCPR).52 |
| CCS angina status | CCS Grade I: ordinary physical activity does not cause angina, such as walking and climbing stairs. Angina with strenuous or rapid or prolonged exertion at work or recreation. CCS Grade II: slight limitation of ordinary activity. Walking or climbing stairs rapidly, walking uphill, walking or stair climbing after meals, or in cold, or in wind, or under emotional stress, or only during the few hours after awakening. Walking more than two blocks on the level and climbing more than one flight of ordinary stairs at a normal pace and in normal conditions. CCS Grade III: marked limitation of ordinary physical activity. Walking one or two blocks on the level and climbing one flight of stairs in normal conditions and at normal pace. CCS Grade IV: inability to carry on any physical activity without discomfort, anginal syndrome may be present at rest.67 |
| CABG surgery | CABG surgery after the TAVI procedure. CABG is a procedure to bypass diseased segment(s) of the coronary tree using blood vessels derived from other parts of the body and connected to the aorta.62 |
| Creatinine | Serum creatinine assay, in µmol/L. |
| Endocarditis | Infective endocarditis is diagnosed if at least one of the following criteria is met: (1) Fulfilment of the Duke criteria for endocarditis (2) Evidence of abscess, pus, or vegetation confirmed as secondary to infection by histological or microbiological studies during re-operation; and (3) Evidence of abscess, pus, or vegetation confirmed on autopsy.53 |
| Left ventricular ejection fraction | Ejection fraction, ideally measured with echocardiography. |
| AF or AFL | A new diagnosis of AF or AFL. AF is defined as a supraventricular tachyarrhythmia with uncoordinated atrial electrical activation and consequently ineffective atrial contraction. The minimum duration of an ECG tracing of AF required to establish the diagnosis of clinical AF is at least 30 s, or entire 12-lead ECG. AFL is defined as a supraventricular tachyarrhythmia with co-ordinated but overly rapid atrial electrical activation, usually with some degree of atrioventricular node conduction block. The minimum duration of an ECG tracing of AFL required to establish the diagnosis of clinical AFL is at least 30 s, or entire 12-lead ECG.58 |
| Renal replacement therapy | The patient developed a new requirement for renal replacement therapy. Renal replacement therapy includes ultrafiltration (haemofiltration), haemodialysis, or peritoneal dialysis.50 |
| Other transcatheter heart valve procedure | Valve intervention after the index TAVI procedure, excluding repeat aortic valve intervention. |
| PCI | PCI after the TAVI procedure. PCI is the placement of an angioplasty guidewire, balloon, or other device (e.g. stent, atherectomy, brachytherapy, or thrombectomy catheter) into a native coronary artery or a graft for the purpose of mechanical coronary revascularisation. The assessment of the severity of a coronary lesion by fluoroscopy, intracoronary imaging (e.g. intravascular ultrasonography), or intracoronary physiology (e.g. fractional flow reserve) is not considered a PCI procedure.65 |
| Residual aortic stenosis | Stage 1: Evidence of structural valve deterioration, non-structural valve dysfunction (other than paravalvular regurgitation or prosthesis-patient mismatch), thrombosis, or endocarditis without significant haemodynamic changes. Stage 2: Increase in mean trans valvular gradient >10 mm Hg resulting in mean gradient >20 mm Hg with concomitant decrease in effective orifice area (EOA) > 0.3 cm2 or > 25% and/or decrease in Doppler velocity index >0.1 or >20% compared with echocardiographic assessment performed 1–3 months post-procedure. Stage 3: Increase in mean trans valvular gradient >20 mm Hg resulting in mean gradient >30 mm Hg with concomitant decrease in EOA > 0.6 cm2 or > 50% and/or decrease in Doppler velocity index > 0.2 or > 40% compared with echocardiographic assessment performed 1–3 months post-procedure.20 |
| Sternotomy/thoracotomy due to bleeding | The patient had a sternotomy/thoracotomy due to bleeding. |
Abbreviations: AF, atrial fibrillation; AFL, atrial flutter; BARC, Bleeding Academic Research Consortium; CABG, coronary artery bypass; CCS, Canadian Cardiovascular Society; CPR, cardiopulmonary resuscitation; CRT, cardiac resynchronization therapy; ECG, electrocardiogram; ECMO, extracorporeal membrane oxygenation; ICD, implantable cardioverter defibrillator; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; TAVI, transcatheter aortic valve intervention; VARC, Valve Academic Research Consortium.
Transcatheter aortic valve intervention domain: Level 2 variables
For the TAVI outcome measures domain, 15 Level 2 variables were agreed: access-related non-vascular complications, all-cause re-hospitalisation, cardiac arrest, cardiogenic shock, CCS angina status, CABG surgery, creatinine, endocarditis, left ventricular ejection fraction, AF/flutter, renal replacement therapy, other transcatheter heart valve procedure, PCI, residual aortic stenosis, and sternotomy or thoracotomy due to bleeding (Table 5).
Bleeding
Various classification systems for bleeding are used across the EuroHeart data standards,14–17 reflecting the relative strengths of each in specific in-patient settings. Of 59 respondents, the preferred bleeding classification for the outcome domain was BARC as selected by 25 (42%), with 18 (31%) voting for VARC, and 16 (27%) voting for ISTH as their first choice.
Medical devices
Whenever a medical device featured within the outcomes domain is implanted or used, the associated unique device identification code will be recorded as a Level 1 variable to enable longitudinal device surveillance.
Discussion
Following the ESC methodology for data standards development, we have derived and defined a suite of internationally agreed cardiovascular outcome measures. This catalogue spans ACS/PCI, AF, HF, and TAVI, and includes generic cardiovascular outcome measures that are applicable across a range of cardiovascular diseases. The cardiovascular outcome measures have been evaluated by international experts, and classified hierarchically as Level 1, meaning that collection of the variable is mandatory; or Level 2, where inclusion is optional and is based upon specific study goals. In total, we present 24 Level 1 and 48 Level 2 cardiovascular outcome measures.
The concept of standardized cardiovascular endpoints is not new. Similar work has been published, but is limited to specific cardiovascular diseases,3–5 or RCTs.6 Unlike previous frameworks, our cardiovascular outcome measure catalogue is purposefully designed to span many research study designs, as well as for the design of and utilisation in structured electronic health records. Differentiating between Level 1 and 2 variables means that centres may participate in data collection of only the ‘essentials’, and select additional optional variables to suit their specific needs. This will maximize the potential for implementation across heterogeneous healthcare environments with differing availability of electronic health records and central infrastructure to support data collection.
The EuroHeart initiative offers a toolset that is likely to enhance the quality and impact of cardiovascular healthcare research and patient care globally.24 Seemingly small differences in definitions may alter study conclusions, may potentially be misleading, and make comparisons between studies challenging.25–27 Indeed, regulatory authorities and most major clinical journals prefer prospective identification of a primary outcome measure with a robust statistical approach to multiplicity in outcomes.28,29 The alignment of these measures is necessary: the number of RCTs published is increasing year on year, and now exceeds 35 000 annually.30 In addition, researchers are employing novel methods by which to conduct RCTs and observational studies,31 including the use of routinely collected healthcare systems data and registry-based RCTs.32 Such use of structured data, not purposely designed for the research at hand, compels the use of clinical outcome measures selected from a finite range of variables,6,33 although we recognize that difficulties in determining whether the definition was met in individual cases, particularly where clinical data are incomplete.
Historically, outcomes such as myocardial infarction have limited specificity and sensitivity in administrative data.34 The ADAPTABLE trial [NCT02697916] used a common data model as the primary source of endpoint ascertainment without adjudication, and found that the positive predictive values for hospitalisation for myocardial infarction, stroke, and major bleeding, compared with adjudication were 90%, 72%, and 93%, respectively.35 Adjudication of outcome measures has traditionally been considered important to minimize noise and mitigate bias,36 however, recent evidence indicates that it may be supplemented or replaced by the use of routinely collected healthcare systems data for clinical outcome measure assessments.36–39 Similarly, HF re-hospitalisation rates within 30 days vary substantially depending on how they are measured—between 6.5% and 15% at 30 days according to a recent registry study.40 There is therefore a need to standardize the cardinal clinical outcome measures and their definitions to maximize the opportunities that are afforded by these developments in methodology to be fully realized.13 Additionally, in light of the increased use of composite outcomes in clinical trials,41 a consistent and universally agreed selection of the variables to be included in the design phase is important to minimize the risk of bias in these circumstances.42
Strengths and limitations
EuroHeart provides an opportunity for co-ordinated collection and analysis of cardiovascular data across Europe and beyond. Within the first year of data collection, data were collected using internationally endorsed data standards for over 40 000 patients with ACS across seven countries.14–17,43 The present work extends and complements this, using an established and robust methodology,18 and harnessing the expertise of a wide range of international experts from diverse healthcare settings. However, we recognize the limitations of this work. In particular, the reliance on a select group of leading experts to define and classify outcome measures can introduce selection bias. The perspectives and experiences of these experts might not fully represent the diverse patient demographics or the full spectrum of clinical realities in different settings. This might limit the universality of the adopted measures. Although the importance of patient-reported outcomes measures (PROMs) and experiences (PREMs) is increasingly recognized,44,45 our remit for this project was limited to clinical outcomes.
This suite of outcomes may be used in clinical studies and for data alignment, and will be integrated into the EuroHeart IT platform to enable standardized measurement and federated research.
Conclusion
We present a suite of internationally developed and prioritized outcome measures and their associated definitions for four common cardiovascular conditions, derived through an expert-led consensus process. These will be implemented within EuroHeart. Their consistent use is encouraged in other registries, healthcare systems data, RCTs, and observational research.
Supplementary Material
Acknowledgements
Many thanks to the national cardiac societies of Denmark, Estonia, Hungary, Iceland, Italy, Lithuania, Portugal, Republic of Ireland, Romania, Singapore and Sweden for their support and commitment to EuroHeart. We are grateful to the staff and board of the ESC, and the ESC Working Groups and Associations including: the ESC Patient forum, Association of Cardiovascular Nursing and Allied Professions (ACNAP), Association for Acute CardioVascular Care (ACVC), European Association of Cardiovascular Imaging (EACVI), European Association of Percutaneous Cardiovascular Interventions (EAPCI), European Association of Preventive Cardiology (EAPC), European Heart Rhythm Association (EHRA), Heart Failure Association (HFA), EURObservational Research Programme (EORP) committee, WG Cardiovascular Regenerative and Reparative Medicine, WG on Adult Congenital Heart Disease, WG on Aorta and Peripheral Vascular Diseases, WG on Atherosclerosis and Vascular Biology, WG on Cardiac Cellular Electrophysiology, WG on Cardiovascular Pharmacotherapy, WG on Cardiovascular Surgery, WG on Cellular Biology of the Heart, WG on Coronary Pathophysiology and Microcirculation, WG on Development Anatomy and Pathology, WG on e-Cardiology, WG on Myocardial and Pericardial Diseases, WG on Myocardial Function, WG on Pulmonary Circulation and Right Ventricular Function, Working Group on Thrombosis, and the Committee for Young Cardiovascular Professionals. We are indebted to Mrs Catherine Reynolds (project manager) in the Data Science Group, University of Leeds, for her role in co-ordinating the Working Groups and managing and analysing the survey responses. Many thanks to Deidre Andre, research librarian at the University of Leeds for her assistance with the search strategy. We would like to post-humously acknowledge Professor Jean-Philippe Collet for his invaluable advice and support in the earlier stages of this research.
Contributor Information
Chris Wilkinson, Hull York Medical School, University of York, YO10 5DD York, UK; Academic Cardiovascular Unit, South Tees NHS Foundation Trust, James Cook University Hospital, Middlesbrough, UK.
Asad Bhatty, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, UK; Leeds Institute for Data Analytics, University of Leeds, Leeds, UK; Department of Cardiology, Leeds Teaching Hospitals NHS Trust, Leeds, UK.
Gorav Batra, Department of Medical Sciences, Cardiology and Uppsala Clinical Research Centre, Uppsala University, Uppsala, Sweden.
Suleman Aktaa, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, UK; Leeds Institute for Data Analytics, University of Leeds, Leeds, UK; Department of Cardiology, Leeds Teaching Hospitals NHS Trust, Leeds, UK.
Adam B Smith, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, UK; Leeds Institute for Data Analytics, University of Leeds, Leeds, UK.
Jeremy Dwight, European Society of Cardiology Patient Forum.
Marcin Ruciński, European Society of Cardiology Patient Forum.
Sam Chappell, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, UK; Leeds Institute for Data Analytics, University of Leeds, Leeds, UK.
Joakim Alfredsson, Department of Cardiology, Linköping University Hospital, Linköping, Sweden.
David Erlinge, Department of Clinical Sciences, Lund University, Lund, Sweden.
Jorge Ferreira, Department of Cardiology, Hospital de Santa Cruz, Centro Hospitalar de Lisboa Ocidental, Carnaxide, Portugal.
Ingibjörg J Guðmundsdóttir, Department of Cardiology, Landspitali University Hospital, Reykjavik, Iceland.
Þórdís Jóna Hrafnkelsdóttir, Department of Cardiology, Landspitali University Hospital, Reykjavik, Iceland.
Inga Jóna Ingimarsdóttir, Department of Cardiology, Landspitali University Hospital, Reykjavik, Iceland; Department of Health Sciences, Faculty of Medicine, University of Iceland, Reykjavik, Iceland.
Alar Irs, Heart Clinic, Tartu University Hospital, Tartu, Estonia.
András Jánosi, György Gottsegen National Cardiovascular Institute, Budapest, Hungary.
Zoltán Járai, Department of Cardiology, South Buda Center Hospital, Szent Imre Teaching Hospital, Budapest, Hungary.
Manuel Oliveira-Santos, Cardiology Department, Unidade Local de Saúde de Coimbra, Coimbra, Portugal.
Bogdan A Popescu, Cardiology Clinic, University of Medicine and Pharmacy Carol Davila, Emergency Institute for Cardiovascular Diseases Prof Dr C C Iliescu, Bucharest, Romania.
Peter Vasko, Department of Cardiology, Linköping University Hospital, Linköping, Sweden.
Dragos Vinereanu, Cardiology Department, Unidade Local de Saúde de Coimbra, Coimbra, Portugal; Cardiology and Cardiovascular Surgery, University and Emergency Hospital, Bucharest, Romania.
Jonathan Yap, Department of Cardiology, National Heart Centre Singapore, Singapore.
Raffaele Bugiardini, Department of Experimental, Diagnostic and Specialty Medicine, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy.
Edina Cenko, Department of Experimental, Diagnostic and Specialty Medicine, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy.
Ramesh Nadarajah, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, UK; Leeds Institute for Data Analytics, University of Leeds, Leeds, UK; Department of Cardiology, Leeds Teaching Hospitals NHS Trust, Leeds, UK.
Matthew R Sydes, BHF Data Science Centre, HDR UK, London, UK; MRC Clinical Trials Unit at UCL, Institute of Clinical Trials and Methodology, UCL, London, UK.
Stefan James, Department of Medical Sciences, Cardiology and Uppsala Clinical Research Centre, Uppsala University, Uppsala, Sweden.
Aldo P Maggioni, ANMCO Research Centre, Heart Care Foundation, 50121 Florence, Italy.
Lars Wallentin, Department of Medical Sciences, Cardiology and Uppsala Clinical Research Centre, Uppsala University, Uppsala, Sweden.
Barbara Casadei, Division of Cardiovascular Medicine, NIHR Oxford Biomedical Research Centre, University of Oxford, Oxford, UK.
Chris P Gale, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, UK; Leeds Institute for Data Analytics, University of Leeds, Leeds, UK; Department of Cardiology, Leeds Teaching Hospitals NHS Trust, Leeds, UK.
Global Cardiovascular Outcomes Consortium and in collaboration with ACNAP, ACVC, EACVI, EAPC, EAPCI, EHRA, ESC Committee for Young CV Professionals, ESC Registry Committee, HFA, ESC Patient Forum and these Working Groups: aorta and peripheral vascular diseases, atherosclerosis and vascular biology, cardiac cellular electrophysiology, cardiovascular pharmacotherapy, cardiovascular regenerative and restorative medicine, cardiovascular surgery, cellular biology of the heart, e-cardiology, myocardial function, pulmonary circulation and right ventricular function and thrombosis:
Victor Aboyans, Ana G Almeida, Dan Atar, Gorav Batra, Antoni Bayés-Genís, Asad Bhatty, Giuseppe Biondi-Zoccai, Marc P Bonaca, Nikolaos Bonaros, Bianca J J M Brundel, Raffaele Bugiardini, Gianluca Campo, Barbara Casadei, Ruben Casado-Arroyo, Claudio Ceconi, Edina Cenko, Ovidiu Chioncel, Michele Ciccarelli, Louise Coats, Jean-Philippe Collet, Gheorghe-Andrei Dan, Victoria Delgado, Polychronis Dilaveris, Dobromir Dobrev, Erwan Donal, David Duncker, Sarah Moharem Elgamal, Justin A Ezekowitz, Gregg Fonarow, Alan G Fraser, Chris P Gale, George Giannakoulas, Bruna Gigante, Massimiliano Gnecchi, Can Gollmann-Tepeköylü, Stephen J Greene, Jordi Heijman, Jonathan Howes, Bernard Iung, Stefan James, Magnus T Jensen, Vijay Kunadian, Malgorzata Lelonek, Sergio Leonardi, Erik Lerkevang Grove, Luca Liberale, Riccardo Liga, A Michael Lincoff, Roberto Lorusso, Aldo P Maggioni, Mamas Mamas, Olivia Manfrini, Fabio Mangiacapra, Nina Ajmone Marsan, María Martín-Fernandez, Jose L Merino, Marco Metra, Alessandro Parolari, Cinzia Perrino, Lorenz Räber, Benyamin Rahmani, Peter P Rainer, Giuseppe M C Rosano, Alexia Rossi, Andrea Rubboli, Tanja Rudolph, Sigrid Sandner, Gianluigi Savarese, Jolanta Siller-Matula, Samuel Sossalla, Cristiano Spadaccio, Eugenio Stabile, David Tanne, Jurrien ten Berg, Matthias Thielmann, Roderick W Treskes, Izabella Uchmanowicz, Jacob A Udell, Roland R J van Kimmenade, Lars Wallentin, Chris Wilkinson, and Marija Zdravkovic
Notes
Membership of the Global Cardiovascular Outcomes Consortium: Victor Aboyans, Ana G. Almeida, Dan Atar, Gorav Batra, Antoni Bayés-Genís, Asad Bhatty, Giuseppe Biondi-Zoccai, Marc P. Bonaca, Nikolaos Bonaros, Bianca J.J.M. Brundel, Raffaele Bugiardini, Gianluca Campo, Barbara Casadei, Ruben Casado-Arroyo, Claudio Ceconi, Edina Cenko, Ovidiu Chioncel, Michele Ciccarelli, Louise Coats, Jean-Philippe Collet, Gheorghe-Andrei Dan, Victoria Delgado, Polychronis Dilaveris, Dobromir Dobrev, Erwan Donal, David Duncker, Sarah Moharem Elgamal, Justin A. Ezekowitz, Gregg Fonarow, Alan G. Fraser, Chris P. Gale, George Giannakoulas, Bruna Gigante, Massimiliano Gnecchi, Can Gollmann-Tepeköylü, Stephen J. Greene, Jordi Heijman, Jonathan Howes, Bernard Iung, Stefan James, Magnus T. Jensen, Vijay Kunadian, Malgorzata Lelonek, Sergio Leonardi, Erik Lerkevang Grove, Luca Liberale, Riccardo Liga, A. Michael Lincoff, Roberto Lorusso, Aldo P. Maggioni, Mamas Mamas, Olivia Manfrini, Fabio Mangiacapra, Nina Ajmone Marsan, María Martín-Fernandez, Jose L. Merino, Marco Metra, Alessandro Parolari, Cinzia Perrino, Lorenz Räber, Benyamin Rahmani, Peter P. Rainer, Giuseppe M.C. Rosano, Alexia Rossi, Andrea Rubboli, Tanja Rudolph, Sigrid Sandner, Gianluigi Savarese, Jolanta Siller-Matula, Samuel Sossalla, Cristiano Spadaccio, Eugenio Stabile, David Tanne, Jurrien ten Berg, Matthias Thielmann, Roderick W. Treskes, Izabella Uchmanowicz, Jacob A. Udell, Roland R.J. van Kimmenade, Lars Wallentin, Chris Wilkinson, and Marija Zdravkovic.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
There are no declarations with respect to this manuscript. Outside this work: A.M.L. reports institutional research grants from AbbVie, CSL Behring, Esperion, Novartis, Eli Lilly and AstraZeneca; consulting fees from Novo Nordisk, Eli Lilly, Akebia, Alnylam, Becton-Dickson, Brainstorm Cell, Cadrenal, Endologix, FibroGen, GlaxoSmithKline, Intarcia, Medtronic, Neovasc, Provention Bio and Recor; support for attending meetings and/or travel from Novo Nordisk, Eli Lilly and Esperion. A.G.F. reports grants or contracts from European Union Horizon 2020; support for attending meetings and/or travel from European Society of Cardiology and Biomedical Alliance in Europe; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from the Regulatory Affairs Committee and Biomedical Alliance in Europe. A.I. reports grants or contracts from Novartis, Abbott and Medtronic; support for attending meetings and/or travel from Abbott and Medtronic; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid as board member and Secretary for the Estonian Society of Cardiology; other financial or non-financial interests as a part-time employee of the Estonian Agency of Medicines. A.P.M. reports fees for participation on a data safety monitoring board or advisory board from Bayer; paid or unpaid leadership or fiduciary role in other board, society, committee, or advocacy group from AstraZeneca, Novartis and Sanofi. A. Rossi has been an employee of Astrazeneca Italia with the role of Medical Advisor in Vaccine and ImmunoTherapies since 15/09/2023. A. Rubboli reports support for attending meetings and/or travel from Daiichi Sankyo. A.B.G. reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Abbott, AstraZeneca, Bayer, Boehringer, Ingelheim, Medtronic, Novartis, Novo Nordisk, Roche Diagnostics and Vifor. A.B. reports grants or contracts from the European Society of Cardiology. B.c. reports other financial or non-financial interests as the Co-chair of the Executive Committee of EuroHeart (not remunerated). B.R. reports support for attending meetings and/or travel from British Standards Institution; patents planned, issued or pending from University College London. B.J.J.M.B. reports institutional research grants from the Dutch Heart Foundation and NWA-ORC project CIRCULAR NOW; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid as Chairman of AFIP Foundation. B.A.P. reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from GE Healthcare and Hitachi-Aloka; receipt of equipment, materials, drugs, medical writing, gifts or other services from GE Healthcare and Hitachi-Aloka. C.P.G. reports grants or contracts from the Alan Turing Institute, British Heart Foundation, National Institute for Health Research, Horizon 2020, Abbott Diabetes, Bristol Myers Squibb and European Society of Cardiology; consulting fees from AI Nexus, AstraZeneca, Amgen, Bayer, Bristol Myers Squibb, Boehringer-Ingelheim, Cardiomatics, Chiesi, Daiichi Sankyo, GPRI Research B.V., Menarini, Novartis, iRhythm, Organon, The Phoenix Group; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, Boston Scientific, Menarini, Novartis, Raisio Group, Wondr Medical, Zydus; support for attending meetings and/or travel from AstraZeneca; participation on a data safety monitoring board or advisory board from DANBLOCK trial, TARGET CTCA trial; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from EHJ Quality of Care and Clinical Outcomes (Deputy Editor), NICE Indicator Advisory Committee, European Society of Cardiology Quality Indicator Committee (Chair); stock or stock options from Cardiomatics; receipt of equipment, materials, drugs, medical writing, gifts or other services from EchoNous. CW reports institutional grants from the British Heart Foundation and National Institute for Health Research, and unpaid roles with EHJ Quality of Care and Clinical Outcomes (Associate Editor), and NICE Indicator Advisory Committee. D.A. reports institutional research grants from BMS/Pfizer, Medtronic, Bayer and Roche-Diagnostics; speaker fees from Amgen, Amarin, AstraZeneca, Bayer, Boehringer-Ingelheim, BMS, MSD, Novartis, Novo Nordisk, Pfizer, Pharmacosmos, Philips, Roche-Diagnostics, Sanofi, Takeda and Vifor. D. Duncker reports research funding from Roche, consulting fees from Sanofi, lecture honoraria from Abbott, AstraZeneca, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, CVRx, Metronic, Pfizer, and Zoll; participation on advisory boards for Cardiomatics, Edwards and Medtronic; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid as Chair of the EHRA e-communication committee. D. Dobrev reports consulting fees from AbbVie Deutschland GmBH & Co. KG; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Daiichi-Sankyo. D.V. reports research grants from Boehringer Ingelheim and CeleCor and consulting fees from Boehringer Ingelheim and Bayer. E.C. reports leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid as Chairperson-Elect of the European Society of Cardiology Working Group on Coronary Pathophysiology and Microcirculation. E.L.G. reports research grants from Boehringer Ingelheim, AstraZeneca, Idorsia and Bayer; consulting fees from Bayer and Bristol Myers Squibb; lecture fees from AstraZeneca, Bayer, Pfizer and Bristol Myers Squibb; support for attending meetings and/or travel from AstraZeneca and Bayer; participation on a data safety monitoring board or advisory board for Lundbeck Pharma, AstraZeneca, Bristol Myers Squibb, Bayer, Novo Nordisk and Pfizer; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid as Chair of the Danish Society of Thrombosis & Haemostasis, nucleus member of Working Group on Cardiovascular Pharmacotherapy (European Society of Thrombosis and Haemostasis), past nucleus member of the European Society of Cardiology Working Group on Thrombosis. G.G. reports institutional research grants from Win Medica, Galenica, Pfizer, AstraZeneca, Boehringer Ingelheim, Menarini and Roche Diagnostics; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educations events from Pfizer, Roche Diagnostics, Bayer, MSD, AstraZeneca, Boehringer Ingelheim, Janssen, Bausch Health, Novartis, Menarini, Genesis Pharma, Sanofi, ELPEN Pharmaceuticals, Amgen, Gilead Sciences, VIANEX, Servier, Ferrer and Gossamer Bio; support for attending meetings and/or travel from Galenica, Sanofi, Menarini, Pfizer, Bayer and Amgen; participation on a data safety monitoring board or advisory board for Boehringer Ingelheim, Janssen, MSD, AstraZeneca, GlaxoSmithKline, Sanofi, Amgen, Roche Diagnostics, Bayer, Novartis and Bausch Health. G.A.d. reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis, Berlin Chemie, KRKA, Sanofi and AOP; support for attending meetings and/or travel from Berlin Chemie. G.C. reports research grants from SMT, Siemens Healthcare and Medis. G.S. reports grants or contracts from Vifor Pharma, Boehringer Ingelheim, AstraZeneca, Merck, Cytokinetics, Novartis, Boston Scientific, Pharmacosmos, Bayer and Horizon 2022 funding; consulting fees from TEVA, Medical Education Global Solutions, Genesis, Agence Recherche, Translational Medicine Academy Foundation, Servier, Ministero dell'istruzione, dell'università e della ricerca, Atheneum, CSL Vifor, AstraZeneca; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis, Cytokinetics, Medtronic, Dynamicom Education, Vifor Pharma, INTAS, Roche, Translational Medicine Academy Foundation, Medical Education Global Solutions, AstraZeneca, Abbott and Pharmacosmos; support for attending meetings and/or travel from Boehringer Ingelheim; participation on a data safety monitoring board or advisory board for AstraZeneca, Uppsala Clinical Research Centre, Servier, Edwards Lifesciences and CSL Vifor. G.B.Z. reports consulting fees from Amarin, Balmed, Cardionovum, CrannMed, EndoCore Lab, Eukon, Guidotti, InnovHeart, Meditrial, MicroPort, OpSens Medical, Terumo and Translumina. G.B. reports institutional research grants from the Swedish Heart Lung Foundation, P.O. Zetterlings Foundation, Jeanssons Foundation, AstraZeneca and Pfizer; consulting fees from Bayer; honoraria for lectures from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Novo Nordisk, Pfizer and Sanofi. G.F. reports institutional research grants from the National Institutes of Health; consulting fees from Medtronic, Novartis, Janssen, Amgen, Pfizer, Merck, Cytokinetics, AstraZeneca and Abbott; payment or honoraria for lectures presentations, speakers bureaus, manuscripts, writing or educational events from Novartis; participation on a data safety monitoring board or advisory board for Pfizer. I.J.I. reports support for attending meetings and/or travel from Novartis. J.A.U. reports institutional research grants from Bayer, Boehringer Ingelheim, Janssen and Sanofi; consulting fees from Boehringer Ingelheim, Novartis, Novavax, Novo Nordisk and Sanofi; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline and Sanofi. J.D. reports royalties or licenses from Oxford University Press; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events for PassPACES; support for attending meetings and/or travel from the European Society of Cardiology Patient Forum. J.A. reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Boehringer Ingelheim, AstraZeneca, MSD, Bayer and Novartis; participation on a data safety monitoring board or advisory board from AstraZeneca and Novartis. J.S.M. reports grants or contracts from Novartis and Chiesi; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Daiichi Sankyo, Cordis and Chiesi. J.Y. reports grants or contracts from Singhealth Cardiovascular ACP; speakers honoraria from Abbott, Biosensors, Biotronik, Boston Scientific, Edwards, GE Healthcare, J&J, Kaneka, Medtronic and Terumo; patents planned, issued or pending for Ecg and Pcg Monitoring System for Detection of Heart Anomaly; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid as Treasurer of Singapore Cardiac Society. J.H. reports leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid as the Chairperson of the European Society of Cardiology Working Group on Cardiac Cellular Electrophysiology 2022–24. J.F. reports consulting fees from Boehringer Ingelheim; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Amgen and Bayer; support for attending meetings and/or travel from Amgen; participation on a data safety monitoring board or advisory board for Boehringer Ingelheim, Merck, Sharpe & Dohme and Novartis; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid through Steering Committee positions for VESALIUS-CV, OCEAN(a) Outcomes, VICTORION-2-Prevent, ESSENCE CS9 and LIBREXIA-AF. J.L.M. reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Abbott, Milestone Pharmaceuticals, Biotronik, AstraZeneca, MicroPort and ZOLL; support for attending meetings and/or travel from Bayer and ZOLL; participation on a data safety monitoring board or advisory board for Medtronic and Sanofi. J.E. declares grants and personal fees from Amgen, Bayer, American Regent, Merck, Otsuka, Novo Nordisk, Applied Therapeutics, Cardurion, CSL Vifor, AstraZeneca and BI-Lilly. L.R. reports consulting fees from Abbott, Medtronic, Novo Nordisk and Sanofi; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Abbott, Amgen, Occlutech and Novo Nordisk. L.C. reports leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid as a British Congenital Cardiac Council Member; Other financial or non-financial interests including research funding from National Institute for Health Research and British Heart Foundation and research collaboration with CardiacSense. L.L. reports grants or contracts from the Swiss Heart Foundation; royalties or licences from XBiotech; support for attending meetings and/or travel from the European Society for Clinical Investigation and EU-COST action; patents planned, issued or pending as the Co-Inventor on the international patent WO/2020/226993; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid as Counsellor of the European Society for Clinical Investigation and nucleus member of the European Society of Cardiology Working Group in Atherosclerosis and Vascular Biology. M.T.J. reports support for attending meetings and/or travel from the Novo Nordisk Foundation; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid as CEO of Steno Diabetes Centre Copenhagen and Chair-Elect of the European Society of Cardiology Working Group on eCardiology; stock or stock options with various index funds, not related to the manuscript. M.L. reports grants or contracts from Novartis, Novo Nordisk, Bayer, AstraZeneca, Boehringer Ingelheim and Servier; consulting fees from Novo Nordisk, Novartis, Bayer, AstraZeneca, Boehringer Ingelheim and Servier; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novo Nordisk, Novartis, Bayer, AstraZeneca, Boehringer Ingelheim and Servier; support for attending meetings and/or travel from AstraZeneca, Novo Nordisk and Boehringer Ingelheim. M. Metra reports consulting fees from Abbott Structural Heart, AstraZeneca, Bayer, Boreringher Ingelheim, Edwards LifeSciences and Riche Diagnostics. M. Mamas reports grants or contracts from Terumo and Abbott; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Terumo, Biosensors and Boehringer Ingelheim; participation on a data safety monitoring board or advisory board for Boehringer Ingelheim; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid as a Senior Clinical Editor for TCTMD. M.O.S. reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Edwards, Boston, Daiichi Sankyo, Biotronik, Abbott and Bayer; support for attending meetings and/or travel from Daiichi Sankyo, Medinfar and Medtronic. M.P.B. is the Executive Director of CPC, a non-profit academic research organization affiliated with the University of Colorado, that receives or has received research grant/consulting funding between August 2021 and present from Abbott Laboratories, Agios Pharmaceuticals, Inc., Alexion Pharma, Alnylam Pharmaceuticals, Inc., Amgen Inc., Angionetics, Inc., Anthos Therapeutics, ARCA Biopharma, Inc., Array BioPharma, Inc., AstraZeneca and Affiliates, Atentiv LLC, Audentes Therapeutics, Inc., Bayer and Affiliates, Beth Israel Deaconess Medical Center, Better Therapeutics, Inc., Boston Clinical Research Institute, Bristol-Meyers Squibb Company, Cambrian Biopharma, Inc., Cardiol Therapeutics Inc., CellResearch Corp., Cleerly Inc., Cook Regentec LLC, CSL Behring LLC, Eidos Therapeutics, Inc., EP Trading Co. Ltd., EPG Communication Holdings Ltd., Epizon Pharma, Inc., Esperion Therapeutics, Inc., Everly Well, Inc., Exicon Consulting Pvt. Ltd., Faraday Pharmaceuticals, Inc., Foresee Pharmaceuticals Co. Ltd., Fortress Biotech, Inc., HDL Therapeutics Inc., HeartFlow Inc., Hummingbird Bioscience, Insmed Inc., Ionis Pharmaceuticals, IQVIA Inc., Janssen and Affiliates, Kowa Research Institute, Inc., Kyushu University, Lexicon Pharmaceuticals, Inc., Medimmune Ltd., Medpace, Merck & Affiliates, Nectero Medical Inc., Novartis Pharmaceuticals Corp., Novo Nordisk, Inc., Osiris Therapeutics Inc., Pfizer Inc., PhaseBio Pharmaceuticals, Inc., PPD Development, LP, Prairie Education and Research Cooperative, Prothena Biosciences Limited, Regeneron Pharmaceuticals, Inc., Regio Biosciences, Inc., Saint Luke’s Hospital of Kansas City, Sanifit Therapeutics S.A., Sanofi-aventis Groupe, Silence Therapeutics PLC, Smith & Nephew plc, Stanford Center for Clinical Research, Stealth BioTherapeutics Inc., State of Colorado CCPD Grant, The Brigham & Women's Hospital, Inc., The Feinstein Institutes for Medical Research, Thrombosis Research Institute, University of Colorado, University of Pittsburgh, VarmX, Virta Health Corporation, Worldwide Clinical Trials Inc., WraSer, LLC, and Yale Cardiovascular Research Group. M.G. reports support for attending meetings and/or travel for the Annual Meeting of the International Society of Cell and Gene Therapy; leadership or fiduciary role in other board, society, committee or advocacy group as an unpaid Board Member of the International Society of Cell and Gene Therapy 2021–23. N.B. reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Edwards Lifesciences and Medtronic. N.A.M. reports institutional research grants from Alnylam, Pfizer and Pie Medical; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or education events from GE Healthcare, Philips Ultrasound, Pfizer, Abbott Vascular and Omron. O.C. reports support for attending meetings and/or travel from Servier. P.P.R. reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis, Boehringer Ingelheim, Pfizer, AstraZeneca, Vifor and Zoll; support for attending meetings and/or travel from Novartis and Boehringer Ingelheim; participation on a data safety monitoring board or advisory board for Novartis and Boehringer Ingelheim. P.D. reports honoraria for lectures from Medtronic and Abbott; support for attending meetings and/or travel from Biotronik, Philips and Abbott. R.B. reports participation on a data safety monitoring board or advisory board for DMC NCT05758896 Phase 2 Trial Aptabio Therapeutics. R.N. reports grants or contracts from British Heart Foundation, Leeds Hospital Charity, Daiichi Sankyo, British Heart Foundation Clinical Research Collaborative, Health Data Research UK, National Institute of Health and Care Research; consulting fees from Vitacam. R. Lorusso reports institutional research grants from Medtronic and Livanova; speaker fees from Medtronic and Xenios; participation on a data safety monitoring board or advisory board for Eurosets and Xenios. R.W.T. reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Boston Scientific and Pfizer; support for attending meetings and/or travel from Boston Scientific; leadership or fiduciary role in other board, society, committee or advocacy group as a nucleus member of the European Society of Cardiology Working Group on eCardiology and as a member of the Dutch Society of Cardiology Working Group on eHealth & AI. R.R.J.v.K. reports grants or contracts from the Dutch Heart Foundation and Hartekind; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis and Bayer; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid with the European Society of Cardiology Working Group on Aorta & Peripheral Artery Disease. R.C.A. reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Abbott and Boston Scientific. S. Sossalla reports research grants from DFG, German Research Foundation; consulting fees from AstraZeneca, Novartis, Bristol Myers Squibb, Boehringer Ingelheim and Lilly; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, Novartis, Berlin-Chemie, Bristol Myers Squibb, Boehringer Ingelheim and Lilly; support for attending meetings and/or travel from Boehringer Ingelheim; participation on a data safety monitoring board or advisory board for CASTLE-HTX-Trial. S.M.E. reports leadership or fiduciary role in other board, society, committee or advocacy group through unpaid positions as a European Association of Cardiovascular Imaging Board Member, European Association of Cardiovascular Imaging Chair of Web and Communication and European Society of Cardiology Communication Committee Member. S.L. reports grants or contracts from AstraZeneca and ICON; consulting fees from Bayer, Bristol Myers Squibb, Novo Nordisk, AstraZeneca and Johnson & Johnson. S.J. reports grants or contracts from AstraZeneca, Amgen and Elixir; consulting fees from Novartis, Novo Nordisk, MSD and Medtronic; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Pfizer and Boehringer Ingelheim; participation on a Data Safety Monitoring Board or Advisory Board from New Amsterdam. S.J.G. reports grants or contracts from Novartis, Sanofi, Pfizer, AstraZeneca, Cytokinetics, Merck and Boehringer Ingelheim; consulting fees from Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Cytokinetics, Boehringer Ingelheim, PharmaIN, Urovant Pharmaceuticals, Eli Lilly, Merck, Sanofi, Tricog Health, Corteria Therapeutics, and Roche Diagnostics; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Boehringer Ingelheim, Cytokinetics, Lexicon, Bayer and Roche Diagnostics. S.A. reports support for attending meetings and/or travelling from the European Society of Cardiology; participation on a data safety monitoring board or advisory board for the British Cardiovascular Intervention Society; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid for the European Society of Cardiology quality of care programme. T.R. reports consulting fees from Medtronic and JenaValve; speaker’s honoraria from Medtronic, Edwards Lifesciences, Boston and JenaValve; participation on an advisory board for Medtronic. V.A. reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Boehringer Ingelheim, Organon and Novo Nordisk; other financial or non-financial interests as Editor-in-Chief of the European Journal of Preventive Cardiology and as a board member of the European Association of Preventive Cardiology and European Society of Cardiology. V.D. reports consulting fees from Edwards Lifesciences, MSD and Novo Nordisk; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Edwards Lifesciences, GE Healthcare, JenaValve, Novartis and Philips. Z.J. reports consulting fees from AstraZeneca, Novo Nordisk and Gedeon Richter; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, Bayer, Berlin Chemie, Boehringer, Egis, KRKA, Merck, Novartis, Novo Nordisk, Pfizer, Gedeon Richter, Sandoz and TEVA; support for attending meetings and/or travel from AstraZeneca, Egis, Pfizer and Sandoz; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid as President of the Hungarian Society of Hypertension, Secretary of the Hungarian Society of Cardiology and Council Member of the European Society of Hypertension; A.B.S.; A.P.; A.G.A.; A.J.; B.I.; B.G.; C.G.T.; C.P.; C.C.; C.S.; D.E.; E.D.; E.S.; F.M.; G.M.C.R.; I.J.G.; I.U.; J. Howes; J.t.B.; L.W.; M.R.; M.M.F.; M.Z.; M.T.; M.C.; O.M.; P.V.; R. Liga; S.C.; S. Sandner; Þ.J.H. and V.K. have nothing to declare.
Data Availability
The data underlying this article are available in the article and in its online supplementary material.
Funding
Pilot phase (up to June 2022) industry partners: Astra Zeneca AB, Daiichi Sankyo Europe GmbH, Amgen (Europe) GmbH, Novartis Pharma AG, Edwards Lifesciences, Boehringer Ingelheim, Swedish Heart Lung Foundation, Janssen Global Services LLC, Bayer AG and Medtronic International Trading SARL. Consolidation phase (since October 2022) industry partners: Astra Zeneca AB, Boehringer Ingelheim, Novartis Pharma AG, Roche, Swedish Heart Lung Foundation, Bayer AG.
Ethical Approval
Ethical approval was not required.
Pre-registered Clinical Trial Number
None: this was not a clinical trial.
References
- 1. Evidence-Based Medicine Working Group . Evidence-based medicine. A new approach to teaching the practice of medicine. JAMA 1992;268:2420–5. 10.1001/jama.1992.03490170092032 [DOI] [PubMed] [Google Scholar]
- 2. Williamson PR, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E, et al. Developing core outcome sets for clinical trials: issues to consider. Trials 2012;13:132. 10.1186/1745-6215-13-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McNamara RL, Spatz ES, Kelley TA, Stowell CJ, Beltrame J, Heidenreich P, et al. Standardized outcome measurement for patients with coronary artery disease: consensus from the international consortium for health outcomes measurement (ICHOM). J Am Heart Assoc 2015;4:e001767. 10.1161/JAHA.115.001767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seligman WH, Das-Gupta Z, Jobi-Odeneye AO, Arbelo E, Banerjee A, Bollmann A, et al. Development of an international standard set of outcome measures for patients with atrial fibrillation: a report of the International Consortium for Health Outcomes Measurement (ICHOM) atrial fibrillation working group. Eur Heart J 2020;41:1132–40. 10.1093/eurheartj/ehz871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burns DJP, Arora J, Okunade O, Beltrame JF, Bernardez-Pereira S, Crespo-Leiro MG, et al. International consortium for health outcomes measurement (ICHOM): standardized patient-centered outcomes measurement set for heart failure patients. JACC Heart Fail 2020;8:212–22. 10.1016/j.jchf.2019.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hicks KA, Mahaffey KW, Mehran R, Nissen SE, Wiviott SD, Dunn B, et al. 2017 cardiovascular and stroke endpoint definitions for clinical trials. Circulation 2018;137:961–72. 10.1161/CIRCULATIONAHA.117.033502 [DOI] [PubMed] [Google Scholar]
- 7. Kearney A, Gargon E, Mitchell JW, Callaghan S, Yameen F, Williamson PR, et al. A systematic review of studies reporting the development of core outcome sets for use in routine care. J Clin Epidemiol 2023;158:34–43. 10.1016/j.jclinepi.2023.03.011 [DOI] [PubMed] [Google Scholar]
- 8. Bosco E, Hsueh L, McConeghy KW, Gravenstein S, Saade E. Major adverse cardiovascular event definitions used in observational analysis of administrative databases: a systematic review. BMC Med Res Methodol 2021;21:241. 10.1186/s12874-021-01440-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. James S, Erlinge D, Storey RF, McGuire DK, de Belder M, Eriksson N, et al. Dapagliflozin in myocardial infarction without diabetes or heart failure. NEJM Evid 2024;3:EVIDoa2300286. 10.1056/EVIDoa2300286 [DOI] [PubMed] [Google Scholar]
- 10. Gale CP, Stocken DD, Aktaa S, Reynolds C, Gilberts R, Brieger D, et al. Effectiveness of GRACE risk score in patients admitted to hospital with non-ST elevation acute coronary syndrome (UKGRIS): parallel group cluster randomised controlled trial. BMJ 2023;381:e073843. 10.1136/bmj-2022-073843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hofmann R, James SK, Jernberg T, Lindahl B, Erlinge D, Witt N, et al. Oxygen therapy in suspected acute myocardial infarction. N Engl J Med 2017;377:1240–9. 10.1056/NEJMoa1706222 [DOI] [PubMed] [Google Scholar]
- 12. Frobert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T, Maeng M, et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med 2013;369:1587–97. 10.1056/NEJMoa1308789 [DOI] [PubMed] [Google Scholar]
- 13. Bhatty A, Wilkinson C, Sydes M, Gale CP. Defining the need for cardiovascular event definitions. Eur Heart J Qual Care Clin Outcomes 2024;10:105–7. 10.1093/ehjqcco/qcae008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Batra G, Aktaa S, Wallentin L, Maggioni AP, Ludman P, Erlinge D, et al. Data standards for acute coronary syndrome and percutaneous coronary intervention: the European unified registries for heart care evaluation and randomised trials (EuroHeart). Eur Heart J 2022;43:2269–85. 10.1093/eurheartj/ehac133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Batra G, Aktaa S, Camm AJ, Costa F, Di Biase L, Duncker D, et al. Data standards for atrial fibrillation/flutter and catheter ablation: the European unified registries for heart care evaluation and randomized trials (EuroHeart). Eur Heart J Qual Care Clin Outcomes 2023;9:609–20. 10.1093/ehjqcco/qcac068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aktaa S, Batra G, Cleland JGF, Coats A, Lund LH, McDonagh T, et al. Data standards for heart failure: the European unified registries for heart care evaluation and randomized trials (EuroHeart). Eur Heart J 2022;43:2185–95. 10.1093/eurheartj/ehac151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aktaa S, Batra G, James SK, Blackman DJ, Ludman PF, Mamas MA, et al. Data standards for transcatheter aortic valve implantation: the European unified registries for heart care evaluation and randomised trials (EuroHeart). Eur Heart J Qual Care Clin Outcomes 2023;9:529–36. 10.1093/ehjqcco/qcac063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Batra G, Aktaa S, Wallentin L, Maggioni AP, Wilkinson C, Casadei B, et al. Methodology for the development of international clinical data standards for common cardiovascular conditions: European unified registries for heart care evaluation and randomised trials (EuroHeart). Eur Heart J Qual Care Clin Outcomes 2023;9:161–8. 10.1093/ehjqcco/qcab052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilkinson C, Aktaa S, Batra G, Beska B, Wu J, Gale CP.. Data Standards for Clinical Endpoints in Cardiovascular Disease: A Literature Review Protocol. 10.25405/data.ncl.19264346 [DOI] [Google Scholar]
- 20. Varc-3 Writing COMMITTEE, Genereux P, Piazza N, Alu MC, Nazif T, Hahn RT, et al. Valve academic research consortium 3: updated endpoint definitions for aortic valve clinical research. J Am Coll Cardiol 2021;77:2717–46. 10.1016/j.jacc.2021.02.038 [DOI] [PubMed] [Google Scholar]
- 21. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011;123:2736–47. 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 22. Schulman S, Kearon C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692–4. 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 23. Bhatty A, Wilkinson C, Batra G, Aktaa S, Smith AB, Wahab A, et al. Standardised and hierarchically classified heart failure and complementary disease monitoring outcome measures: european Unified Registries for heart Care evaluation and randomised trials (EuroHeart). Eur Heart J Qual Care Clin Outcomes 2024:qcae086. 10.1093/ehjqcco/qcae086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cordoba G, Schwartz L, Woloshin S, Bae H, Gotzsche PC. Definition, reporting, and interpretation of composite outcomes in clinical trials: systematic review. BMJ 2010;341:c3920. 10.1136/bmj.c3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hara H, Serruys PW, Takahashi K, Kawashima H, Ono M, Gao C, et al. Impact of peri-procedural myocardial infarction on outcomes after revascularization. J Am Coll Cardiol 2020;76:1622–39. 10.1016/j.jacc.2020.08.009 [DOI] [PubMed] [Google Scholar]
- 26. Stone GW, Kappetein AP, Sabik JF, Pocock SJ, Morice MC, Puskas J, et al. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med 2019;381:1820–30. 10.1056/NEJMoa1909406 [DOI] [PubMed] [Google Scholar]
- 27. Cohen D, Brown E. New England Journal of Medicine reviews controversial stent study. BMJ 2020;368:m878. 10.1136/bmj.m878 [DOI] [PubMed] [Google Scholar]
- 28. U.S. Department of Health and Human Services Food and Drug Administration . Multiple Endpoints in Clinical Trials: Guidance for Industry. FDA, 2022. https://www.fda.gov/media/162416/download [Google Scholar]
- 29. Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. 10.1136/bmj.c869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. US National Library of Medicine . ClinicalTrials.gov. www.clinicaltrials.gov (24 October 2024, date last accessed).
- 31. Noor NM, Love SB, Isaacs T, Kaplan R, Parmar MKB, Sydes MR. Uptake of the multi-arm multi-stage (MAMS) adaptive platform approach: a trial-registry review of late-phase randomised clinical trials. BMJ Open 2022;12:e055615. 10.1136/bmjopen-2021-055615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sydes MR, Barbachano Y, Bowman L, Denwood T, Farmer A, Garfield-Birkbeck S, et al. Realising the full potential of data-enabled trials in the UK: a call for action. BMJ Open 2021;11:e043906. 10.1136/bmjopen-2020-043906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Karanatsios B, Prang KH, Verbunt E, Yeung JM, Kelaher M, Gibbs P. Defining key design elements of registry-based randomised controlled trials: a scoping review. Trials 2020;21:552. 10.1186/s13063-020-04459-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of myocardial infarction diagnoses in administrative databases: a systematic review. PLoS One 2014;9:e92286. 10.1371/journal.pone.0092286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marquis-Gravel G, Hammill BG, Mulder H, Roe MT, Robertson HR, Wruck LM, et al. Validation of cardiovascular end points ascertainment leveraging multisource electronic health records harmonized into a common data model in the ADAPTABLE randomized clinical trial. Circ Cardiovasc Qual Outcomes 2021;14:e008190. 10.1161/CIRCOUTCOMES.121.008190 [DOI] [PubMed] [Google Scholar]
- 36. Meah MN, Denvir MA, Mills NL, Norrie J, Newby DE. Clinical endpoint adjudication. Lancet 2020;395:1878–82. 10.1016/S0140-6736(20)30635-8 [DOI] [PubMed] [Google Scholar]
- 37. GBD 2015 Mortality and Causes of Death Collaborators . Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1459–544. 10.1016/S0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harper C, Mafham M, Herrington W, Staplin N, Stevens W, Wallendszus K, et al. Comparison of the accuracy and completeness of records of serious vascular events in routinely collected data vs clinical trial-adjudicated direct follow-up data in the UK: secondary analysis of the ASCEND randomized clinical trial. JAMA Netw Open 2021;4:e2139748. 10.1001/jamanetworkopen.2021.39748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harper C, Mafham M, Herrington W, Staplin N, Stevens W, Wallendszus K, et al. Reliability of major bleeding events in UK routine data versus clinical trial adjudicated follow-up data. Heart 2023;109:1467–72. 10.1136/heartjnl-2023-322616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Salimian S, Virani SA, Roston TM, Yao RJR, Turgeon RD, Ezekowitz J, et al. Impact of the method of calculating 30-day readmission rate after hospitalization for heart failure. Data from the VancOuver CoastAL Acute Heart Failure (VOCAL-AHF) registry. Eur Heart J Qual Care Clin Outcomes 2024;10:523–30. 10.1093/ehjqcco/qcae026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shi L, Lindsell CJ, Liu D. Trends in use of composite endpoints in clinical trials: a comparison between acute heart failure trials and COVID-19 trials. J Clin Transl Sci 2024;8:e55. 10.1017/cts.2024.492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Diaz-Quijano FA. Estimating and testing an index of bias attributable to composite outcomes in comparative studies. J Clin Epidemiol 2021;132:1–9. 10.1016/j.jclinepi.2020.12.003 [DOI] [PubMed] [Google Scholar]
- 43. Bhatty A, Wilkinson C, Batra G, Alfredsson J, Erlinge D, Ferreira J, et al. Cohort profile: the European unified registries on heart care evaluation and randomised trials (EuroHeart)—acute coronary syndrome and percutaneous coronary intervention. Eur Heart J Qual Care Clin Outcomes 2024;10:386–90. 10.1093/ehjqcco/qcae025 [DOI] [PubMed] [Google Scholar]
- 44. Moons P, Norekval TM, Arbelo E, Borregaard B, Casadei B, Cosyns B, et al. Placing patient-reported outcomes at the centre of cardiovascular clinical practice: implications for quality of care and management. Eur Heart J 2023;44:3405–22. 10.1093/eurheartj/ehad514 [DOI] [PubMed] [Google Scholar]
- 45. Wilkinson C, Bhatty A, Smith A, Dwight J, Sanders J, Gale C. Embracing the promise of patient reported outcome measures in cardiology. Eur Heart J Qual Care Clin Outcomes 2024:qcae073. in press. 10.1093/ehjqcco/qcae073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2022;43:3997–4126. 10.1093/eurheartj/ehac262 [DOI] [PubMed] [Google Scholar]
- 47. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J 2019;40:237–69. 10.1093/eurheartj/ehy462 [DOI] [PubMed] [Google Scholar]
- 48. Sacco RL, Kasner SE, Broderick JP, Caplan, LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:2064–89. 10.1161/STR.0b013e318296aeca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bozkurt B, Coats AJS, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail 2021;23:352–80. 10.1002/ejhf.2115 [DOI] [PubMed] [Google Scholar]
- 50. Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013;158:825–30. 10.7326/0003-4819-158-11-201306040-00007 [DOI] [PubMed] [Google Scholar]
- 51. Grasner JT, Herlitz J, Tjelmeland IBM, Wnent J, Masterson S, Lilja G, et al. European resuscitation council guidelines 2021: epidemiology of cardiac arrest in Europe. Resuscitation 2021;161:61–79. 10.1016/j.resuscitation.2021.02.007 [DOI] [PubMed] [Google Scholar]
- 52. Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv 2019;94:29–37. 10.1002/ccd.28329 [DOI] [PubMed] [Google Scholar]
- 53. Varc-3 Writing Committee, Genereux P, Piazza N, Alu MC, Nazif T, Hahn RT, et al. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. Eur Heart J 2021;42:1825–57. 10.1093/eurheartj/ehaa799 [DOI] [PubMed] [Google Scholar]
- 54. Di Nisio M, van Es N, Buller HR. Deep vein thrombosis and pulmonary embolism. Lancet 2016;388:3060–73. 10.1016/S0140-6736(16)30514-1 [DOI] [PubMed] [Google Scholar]
- 55. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2021;42:3427–520. 10.1093/eurheartj/ehab364 [DOI] [PubMed] [Google Scholar]
- 56. Friedman P, Murgatroyd F, Boersma LVA, Manlucu J, O'Donnell D, Knight BP, et al. Efficacy and safety of an extravascular implantable cardioverter-defibrillator. N Engl J Med 2022;387:1292–302. 10.1056/NEJMoa2206485 [DOI] [PubMed] [Google Scholar]
- 57. Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant 2016;35:1–23. 10.1016/j.healun.2015.10.023 [DOI] [PubMed] [Google Scholar]
- 58. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C,, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 59. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing G-J, Harjola V-P, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2020;41:543–603. 10.1093/eurheartj/ehz405 [DOI] [PubMed] [Google Scholar]
- 60. Hsieh MT, Hsieh CY, Tsai TT, Wang YC, Sung SF. Performance of ICD-10-CM diagnosis codes for identifying acute ischemic stroke in a national health insurance claims database. Clin Epidemiol 2020;12:1007–13. 10.2147/CLEP.S273853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fonseca AC, Merwick A, Dennis M, Ferrari J, Ferro JM, Kelly P, et al. European Stroke Organisation (ESO) guidelines on management of transient ischaemic attack. Eur Stroke J 2021;6:CLXIII–CLXXXVI. 10.1177/2396987321992905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Authors/Task Force M, Kunst G, Milojevic M, Boer C, De Somer FMJJ, Gudbjartsson T, et al. 2019 EACTS/EACTA/EBCP guidelines on cardiopulmonary bypass in adult cardiac surgery. Br J Anaesth 2019;123:713–57. 10.1016/j.bja.2019.09.012 [DOI] [PubMed] [Google Scholar]
- 63. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–726. 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 64. Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, et al. Standardized end point definitions for coronary intervention trials: the academic research consortium-2 consensus document. Circulation 2018;137:2635–50. 10.1161/CIRCULATIONAHA.117.029289 [DOI] [PubMed] [Google Scholar]
- 65. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. 10.1093/eurheartj/ehy394 [DOI] [PubMed] [Google Scholar]
- 66. Ambrosetti M, Abreu A, Corra U, Davos CH, Hansen D, Frederix I, et al. Secondary prevention through comprehensive cardiovascular rehabilitation: from knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur J Prev Cardiol 2021;28:460–95. 10.1177/2047487320913379 [DOI] [PubMed] [Google Scholar]
- 67. Campeau L. The Canadian Cardiovascular Society grading of angina pectoris revisited 30 years later. Can J Cardiol 2002;18:371–9. [PubMed] [Google Scholar]
- 68. Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J 2023;44:3720–826. 10.1093/eurheartj/ehad191 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.