Abstract
Background and Aims
Overtesting of low-risk patients with suspect chronic coronary syndrome (CCS) is widespread. The acoustic-based coronary artery disease (CAD)-score has superior rule-out capabilities when added to pre-test probability (PTP). FILTER-SCAD tested whether providing a CAD-score and PTP to cardiologists was superior to PTP alone in limiting testing.
Methods
At six Danish and Swedish outpatient clinics, patients with suspected new-onset CCS were randomized to either standard diagnostic examination (SDE) with PTP, or SDE plus CAD-score, and cardiologists provided with corresponding recommended diagnostic flowcharts. The primary endpoint was cumulative number of diagnostic tests at one year and key safety endpoint major adverse cardiac events (MACE).
Results
In total, 2008 patients (46% male, median age 63 years) were randomized from October 2019 to September 2022. When randomized to CAD-score (n = 1002), it was successfully measured in 94.5%. Overall, 13.5% had PTP ≤ 5%, and 39.5% had CAD-score ≤ 20. Testing was deferred in 22% with no differences in diagnostic tests between groups (P for superiority = .56). In the PTP ≤ 5% subgroup, the proportion with deferred testing increased from 28% to 52% (P < .001). Overall MACE was 2.4 per 100 person-years. Non-inferiority regarding safety was established, absolute risk difference 0.49% (95% confidence interval −1.96–0.97) (P for non-inferiority = .003). No differences were seen in angina-related health status or quality of life.
Conclusions
The implementation strategy of providing cardiologists with a CAD-score alongside SDE did not reduce testing overall but indicated a possible role in patients with low CCS likelihood. Further strategies are warranted to address resistance to modifying diagnostic pathways in this patient population.
Keywords: Chronic coronary syndrome, Coronary artery disease, Heart sound, Pre-test probability, Risk stratification, Deferred testing, Diagnostic strategy, Implementation strategy
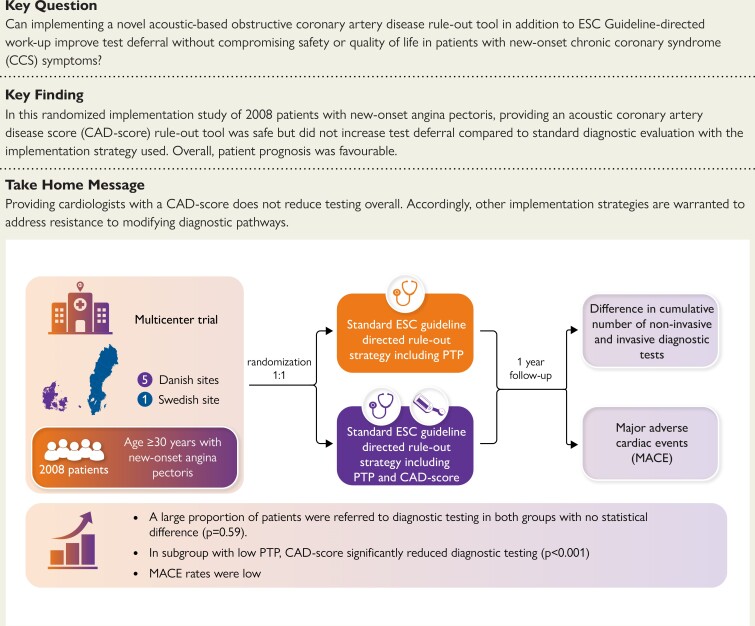
Structured Graphical Abstract
Graphical Abstract.
Summary of the aim, methods and main findings of the FILTER-SCAD trial.
See the editorial comment for this article ‘The Ulysses syndrome of the patient with chronic coronary syndrome', by E. Picano, https://doi.org/10.1093/eurheartj/ehae796.
Introduction
Stable chest pain is a common complaint, affecting ∼6.7% of the adult population.1 Consequently, diagnosing its cause is a significant aspect of cardiology practice. Current guidelines from the European Society of Cardiology (ESC) suggest initial risk stratification using a pre-test probability (PTP) model. For patients with a low likelihood of obstructive coronary artery disease (CAD), further diagnostic testing may be deferred.2 However, despite a declining incidence of CAD, there has been a rise in referrals for functional stress tests and anatomical imaging.3,4 Recent studies have shown that much of this testing yields low diagnostic value, with <10% of patients ultimately requiring coronary revascularization, and favourable cardiovascular (CV) outcomes over five years.5–7 Given this evidence, refining deferred testing strategies to reduce costs, resource consumption, and patient concerns is crucial in this large patient population.
Non-invasive, radiation-free technologies based on acoustic detection of coronary artery stenosis have shown promise in early patient stratification.8 The CADScor® System analyses sounds from blood flow turbulence and myocardial motion to generate a CAD-score incorporating the patient characteristics age, sex, and hypertension status.9–11 This system effectively rules out obstructive CAD in patients with intermediate likelihood, identifying additional candidates suitable for a deferred testing strategy.11–13
Despite awareness among cardiologists of the low diagnostic yield of current testing strategies, there is significant resistance to reducing unnecessary testing for patients with a low likelihood of CAD. This inertia is influenced by factors such as familiarity with existing methods and specific expectations from assessments as well as patient expectations. Therefore, before implementing the CADScor®System in routine clinical practice, we conducted the FILTER-SCAD trial to assess its acceptability among clinicians and patients and determine whether its use could reduce testing for low-risk patients without compromising safety, patient symptom burden, or quality of life.14 This trial aims to promote more appropriate and efficient diagnostic work-up strategies by combining guideline-directed risk assessment with advanced acoustic technology.
Methods
Study design and participants
FILTER-SCAD was a pragmatic, unblinded, multicentre, randomized, controlled trial done at six study sites: Five outpatient clinics in northeastern Denmark and one in southern Sweden.14 The study protocol was approved by the Danish Medical Agency (2019024326), the Danish National Committee on Health Research Ethics (H-19012579), and the Swedish Ethical Review Authority (Dnr 2019-04252). ClinicalTrial.gov ID is NCT04121949. The study protocol was designed in accordance with the 2013 ESC guidelines on the management of stable coronary artery disease15 applicable at the time of study initiation and updated in 2019 when the current 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes2 were published.
Patients ≥ 30 years without known CAD who signed informed consent form and were able and willing to comply with the investigation plan were consecutively included when referred for assessment due to symptoms suggestive of stable CAD. Exclusion criteria were diagnostic testing for suspected CAD within 6 months prior to randomization, implanted donor heart, mechanical heart, mechanical heart pump or electronic equipment in the area around the heart, same-day treatment with nitroclycerine, pregnancy, or if a CAD-score measurement was deemed impossible (e.g. due to pacemaker or compromised skin). A full list of in- and exclusion criteria has been reported previously and is available in Supplementary data online, Table S1.14
Study procedures
After providing written informed consent, patients were randomly assigned in a 1:1 ratio to receive either a standard diagnostic examination (SDE) including the 2019 ESC PTP-guided strategy for deferred diagnostic testing, or a strategy guided by SDE plus a CAD-score measurement.
The CAD-score is a risk stratification score for obstructive CAD based on acoustic detection of CAD-related soundwaves combined with the patient’s sex, age, and hypertension status. A sound recording and a CAD-score measurement are automatically done over a total of 10 min by the non-invasive device CADScor®System (Acarix A/S) attached to the supine patient’s chest.10 Heart sound recordings are obtained transcutaneously over 3 min using a microphone positioned at the left fourth intercostal space. Immediately after the recording, a CAD-score ranging from 0 to 99 is estimated by a fully automated algorithm relying on eight acoustic features combined with a clinical risk based on sex, age, and known arterial hypertension.9,10,16 With a sensitivity of 88.7%, specificity of 41.5%, a negative predictive value of 97.2%, and a positive predictive value of 13% in a population with a CAD prevalence of 9.4% (n = 2245), a CAD-score ≤ 20 indicates a low likelihood of obstructive CAD on invasive coronary angiography (ICA).11 The high sensitivity and ease of use make the CADScor®System a suitable first-line rule-out tool in populations with suspected CAD and a low prevalence of disease.
At enrolment, patients completed the Seattle Angina Questionnaire (SAQ) and the 5-level version of the EuroQol 5 dimensions (EQ-5D-5L) questionnaire. A trained study nurse recorded anginal symptoms, heart rate, blood pressure, and PTP on a clinical decision sheet. The PTP was estimated for each patient using age, sex, and type of angina symptoms according to current 2019 ESC guidelines.2 The SDE followed ESC guidelines and included standard blood tests, echocardiography, and evaluation of medical history and CV risk factors and was conducted by the treating physician. The further diagnostic strategy followed the 2019 ESC guidelines: patients with a PTP ≤ 5% (low) should be ruled out without further diagnostic testing, in patients with PTP 6%–15% (intermediate) diagnostic test may be performed depending on overall clinical likelihood, and patients with PTP > 15% (high) should be referred for a non-invasive test (NIT).2 In the SDE plus CAD-score group, a CAD-score measurement was added to the SDE including the PTP estimation, and should guide the further diagnostic strategy by ruling out patients with low CAD-score ≤ 20. A recommendation on further testing was indicated on the clinical decision sheet, which was then handed over to the treating physician (clinical decision sheet available in Supplementary data online, Figure S1). Any deviations from a recommended rule-out strategy needed to be justified and recorded by the treating physician in the clinical decision sheet. Adherence to the rule-out strategy was thus strongly encouraged as described but was not mandatory, as adherence to the protocol was part of the primary outcome.
After enrolment, patients were contacted by study personnel at 3 and 12 months for a re-evaluation of symptoms using the SAQ and EQ-5D-5L questionnaire.
All treating physicians responsible for diagnostic decisions received a standardized training programme, including both written and verbal information on study tasks, an introduction to the decision sheet, and evidence base for the CAD-score. A 3-month run-in period was undertaken at each study site to ensure proper training and experience with using the CADScor®System and the decision sheet.
Randomization and blinding
A permuted block randomization with a block size of eight was generated with stratification on enrolling sites and on PTP (≤5% vs. >5%). The sequence was generated by an independent statistician using a computerized system. The allocation sequence was blinded for all, but patients and treating physicians were not masked to treatment allocation. All elements of the composite key secondary endpoint were adjudicated by a clinical events committee masked to treatment allocation.
Outcomes
The primary outcome was cumulative number of diagnostic tests, including any NIT and invasive coronary angiographies at one year after randomization. Non-invasive tests included cardiac computed tomography angiography (CCTA), myocardial perfusion imaging, exercise ECG, stress echocardiography, or cardiac magnetic resonance imaging.
The key secondary outcome was major adverse cardiac events (MACE) — a composite of death, myocardial infarction (MI), hospitalization for unstable angina pectoris (UAP), heart failure (HF), or ischaemic stroke, and major complications from cardiovascular procedures up to one year after randomization. Definitions of all-cause mortality, MI, UAP, HF, and ischaemic stroke follow the American College of Cardiology/American Heart Association description of key data elements and definitions for cardiovascular endpoint events in clinical trials.17 Major complication from CV procedures or diagnostic testing is defined as major bleeding, renal failure, stroke, or anaphylaxis that occurred within 72 h in accordance with the PROMISE trial’s definition.18
Other pre-specified secondary outcomes were patient perception of symptoms and quality of life, repeat referrals, time to diagnosis or rule-out of CAD, lifestyle measures, initiation of medical therapy, contrast dose, radiation dose, bleedings, and adverse events related to the CAD-score measurement.
All diagnostic tests and individual components of MACE were retrieved from local administrative registries and verified using electronic healthcare records. MACE endpoints were adjudicated by a clinical event committee.
Statistical analysis
The trial design specified that 2000 patients would be needed to undergo randomization with one-year follow-up to be sufficiently powered for both the primary efficacy outcome and the composite key secondary safety outcome. From previous studies,6 a mean number of diagnostic tests of 0.94 per patient was expected in the SDE group. With 80% or greater power to detect a 15% reduction, i.e. an absolute reduction of 0.17 diagnostic tests per patient, in the primary endpoint, and a two-sided α of .05 for superiority, we would require 1042 patients. Assuming a one year rate of the key secondary outcome of 1.3%,6 we further estimated that 1914 patients would be required to provide a 90% or greater power for establishing non-inferiority between the SDE and SDE plus CAD-score groups, with a two-sided α of .05 and an upper margin set at 1.5%. These power calculations accounted for a 20% crossover from patients ruled out by the CAD-score to a diagnostic test.
The analyses followed a pre-specified statistical analysis plan. The primary endpoint was analysed using a Poisson regression with the cumulative number of diagnostic tests within one year as dependent variable, and treatment group, study site, and low PTP (yes/no) as covariates; the latter two to account for the block stratified randomization. Heterogeneity in effect sizes was assessed using interaction between allocated group and stratifying variables. Cumulative rates of diagnostic examinations were calculated and plotted. We estimated treatment effect in pre-specified subgroups of PTP, age, sex, hypertension, hyperlipidaemia, diabetes, smoking, family history of CAD, and body mass index.
The key secondary endpoint was reported as absolute rates per 100 person-years. Time-to-first event analysis was performed using the Kaplan–Meier estimator and relative treatment effect size was quantified using hazard ratios with 95% confidence intervals (CIs) estimated in a Cox proportional hazards model stratified for study site and low PTP (yes/no). Non-inferiority was established if the upper 95% CI of the absolute risk difference, using a continuity-corrected modification of the Wilson’s score method, did not exceed a pre-specified margin of 1.5%.
Seattle Angina Questionnaire summary scores and EQ-5D-5L utility score (using Danish and Swedish population weights) were reported with mean (SD) at baseline, 3 months, and 1 year. For each score, differences between treatment groups and from baseline to one-year follow-up within each treatment group were assessed using a repeated measures model accounting for study site and low PTP (yes/no).
Additional data, including baseline characteristics, were reported as mean (SD) or median (IQR) for continuous variables and count (percentage) for categorical variables. All group comparisons were conducted according to the intention-to-treat. All analyses used Statistical Software R version 4.1.0.19
Results
Study population and follow-up
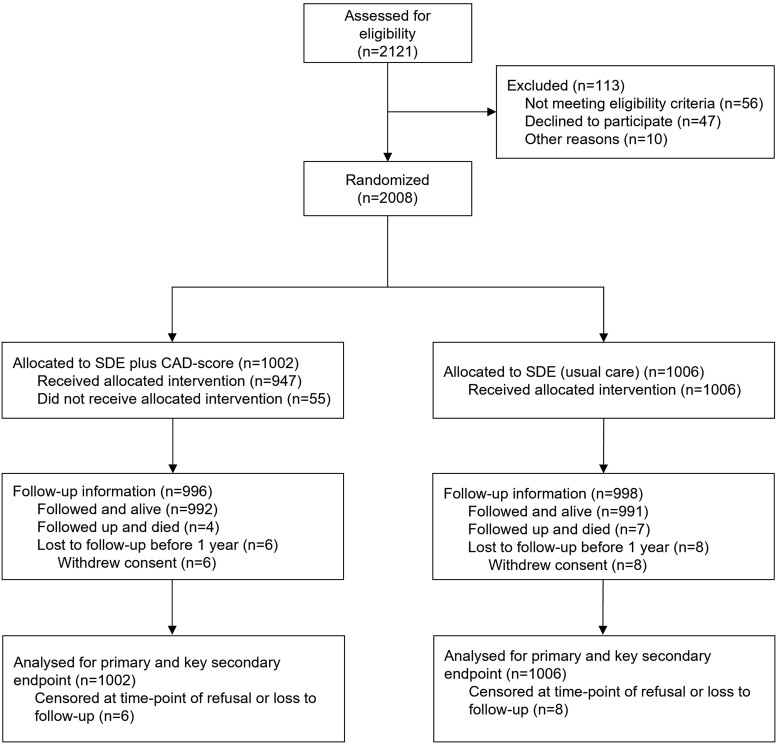
From October 2019 through September 2022, we enrolled a total of 2121 patients and 2008 underwent randomization (Figure 1). Baseline characteristics were well balanced between the two groups (Table 1). Complete follow-up of 12 months was obtained in 1994 (99%) of patients. The mean age was 62.4 ± 11.9 years, 53.9% were female, and 2.4% were ethnic minorities. The burden of CV risk factors was moderate: 43.4% of patients had hypertension, 29.6% had hyperlipidaemia, 10.1% had diabetes, 22.8% had a family history of CAD, and 61.9% were past or current tobacco users. Patients had a mean of 1.7 of these 5 risk factors, while 14.6% did not have any CV risk factors. Cerebrovascular or peripheral artery disease was present in 6.7% of patients. The median PTP of obstructive CAD according to the 2019 ESC guidelines was 14% [IQR 9–26], and overall 13.5% had a low PTP ≤ 5%. The most frequent presenting complaint was non-anginal chest pain (36.3%). Among the 1002 participants randomly assigned to the SDE plus CAD-score group, a CAD-score was successfully obtained in 94.5%. Of these, 39.5% had a CAD-score ≤ 20.
Figure 1.
CONSORT diagram. Screening, randomization, and follow-up in the FILTER-SCAD trial
Table 1.
Baseline characteristics of trial participants
| Patient characteristic | SDE plus CAD-score arm (n = 1002) | SDE-only arm (n = 1006) |
|---|---|---|
| Age, mean (SD), y | 62.1 (12.1) | 62.6 (11.8) |
| Sex | ||
| Female | 558 (55.7) | 525 (52.2) |
| Male | 444 (44.3) | 481 (47.8) |
| Ethnicity | ||
| White | 975 (97.4) | 979 (97.5) |
| Other | 26 (2.5) | 25 (2.5) |
| Cardiovascular risk factors | ||
| Hypertension | 439 (43.8) | 432 (42.9) |
| Hyperlipidaemia | 299 (29.8) | 296 (29.4) |
| Family history of CAD | 231 (23.1) | 227 (22.7) |
| Current or past tobacco use | 621 (62.0) | 622 (61.8) |
| Diabetes mellitus | 94 (9.4) | 108 (10.7) |
| Body mass index, mean (SD), kg/m2 | 27.3 (5.4) | 27.3 (5.1) |
| Total burden of CV risk factorsa | ||
| No. of CV risk factors per patient, mean (SD) | 1.7 (1.1) | 1.7 (1.2) |
| Absence of any CV risk factors | 141 (14.1) | 152 (15.1) |
| Comorbidity | ||
| Peripheral artery or cerebrovascular disease | 65 (6.5) | 70 (7.0) |
| Chronic kidney disease | 17 (1.7) | 17 (1.7) |
| Risk scores | ||
| PTP, median (range)b | 13.0 (1–78) | 14.0 (1–69) |
| PTP≤5% (low risk) | 135 (13.5) | 137 (13.6) |
| PTP 5%–15% | 409 (40.8) | 397 (39.5) |
| PTP > 15% | 458 (45.7) | 472 (46.9) |
| Type of angina | ||
| Typical angina (cardiac) | 252 (25.1) | 247 (24.6) |
| Atypical angina (possible cardiac) | 301 (30.0) | 271 (26.9) |
| Non-anginal chest pain (non-cardiac) | 350 (34.9) | 379 (37.7) |
| Dyspnoea on exertion | 99 (9.9) | 109 (10.8) |
| Systolic blood pressure, mean (SD) | 135.8 (18.5) | 135.3 (18.5) |
| Diastolic blood pressure, mean (SD) | 84.5 (10.0) | 84.5 (10.6) |
| Use of relevant CV medications | ||
| Antiplatelet medication | 173 (17.3) | 175 (17.4) |
| Lipid-lowering medication | 299 (29.8) | 296 (29.4) |
| Antianginal medication | 59 (5.9) | 90 (8.9) |
| Anticoagulant medication | 47 (4.7) | 57 (5.7) |
| Seattle Angina Questionnaire, mean (SD) | ||
| Physical limitation score | 83.4 (18.8) | 82.6 (19.3) |
| Angina stability score | 50.6 (22.5) | 51.1 (22.8) |
| Angina frequency score | 74.9 (17.0) | 74.0 (17.2) |
| Treatment satisfaction score | 86.2 (16.8) | 85.7 (16.3) |
| Quality of life score | 53.5 (23.1) | 54.0 (23.4) |
| EQ-5D-5L, mean (SD) | ||
| Summary score | 0.85 (0.18) | 0.86 (0.17) |
CAD, coronary artery disease; CV, cardiovascular; PTP, pre-test probability; SD, standard deviation; SDE, standard diagnostic examination.
aRisk factors included are hypertension, hyperlipidaemia, family history of CAD, diabetes mellitus, and tobacco use.
bPTP according to 2019 ESC guidelines.
Clinical decisions and initial diagnostic testing
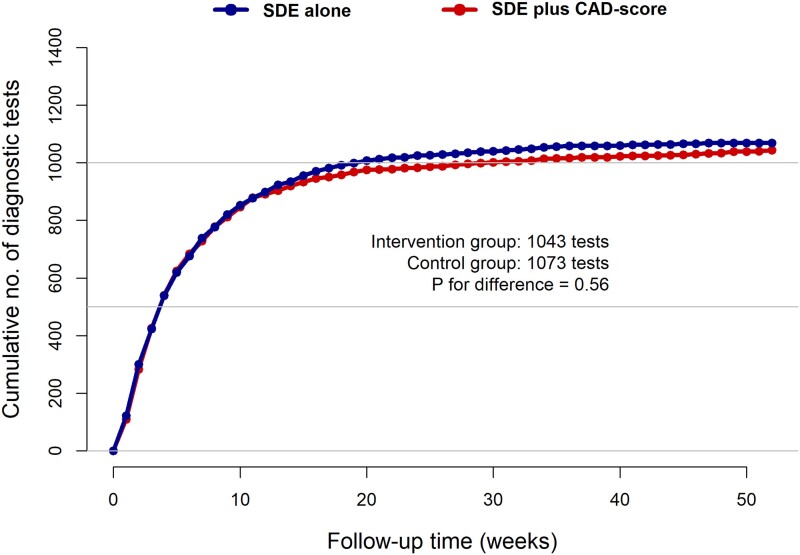
In the SDE plus CAD-score group, 234 (23.4%) had no diagnostic test performed during follow-up (Table 2). In comparison, 208 (20.7%) of the 1006 participants randomly assigned to the SDE group had no diagnostic testing performed (P = .14). The primary outcome, cumulative number of diagnostic tests within one year did not differ between groups: 1043 in the SDE plus CAD-score vs. 1073 in the SDE group (P = .56) (Figure 2). Overall, most examinations were performed shortly after randomization. Accordingly, only a few (2.0%) repeat referrals to outpatient examination were seen, with no differences between the randomization groups (see Supplementary data online, Table S2). Also, there were no differences in initiated medication after randomization between the two randomization groups.
Table 2.
Primary and key secondary endpoint according to allocated study group
| Endpoint or clinical event | SDE plus CAD-score arm (n = 1002) | SDE-only arm (n = 1006) | Hazard ratio (95% CI)a |
|---|---|---|---|
| Primary endpoint | |||
| Cumulative no. of diagnostic tests | 1043 | 1073 | 0.98 (0.90–1.06)b |
| Follow-up | |||
| Person-Years (PY) | 1002 | 1006 | |
| Months since randomization | 12 014 | 12 062 | |
| Diagnostic tests per patient, no. | |||
| 0 | 234 (23.4) | 208 (20.7) | |
| 1 | 544 (54.3) | 585 (58.2) | |
| 2 | 179 (17.9) | 157 (15.6) | |
| ≥3 | 45 (4.5) | 56 (5.6) | |
| Key secondary endpointc | |||
| Major adverse cardiac events (first event only) | 22 (2.2) | 27 (2.7) | 0.82 (0.46–1.43) |
| Death from any cause | 4 (0.4) | 7 (0.7) | 0.58 (0.17–1.98) |
| Myocardial infarction | 6 (0.6) | 6 (0.6) | 0.84 (0.26–1.16) |
| Unstable angina | 8 (0.8) | 7 (0.7) | 1.33 (0.46–3.84) |
| Heart failure hospitalization | 0 (0.0) | 2 (0.2) | NA |
| Ischaemic stroke | 1 (0.1) | 7 (0.7) | 0.14 (0.02–1.16) |
| Major procedure-related complications | 5 (0.5) | 1 (0.1) | 4.00 (0.45–35.8) |
ICA, invasive coronary angiography; NIT, non-invasive test; PY, person-years; SDE, standard diagnostic examination.
aHazard ratio for time to first event.
bIncidence rate ratio (IRR).
cNo (rates per 100 PY).
Figure 2.
Cumulative no of diagnostic tests during follow-up, by allocated treatment group. Cumulative numbers of the primary endpoint diagnostic tests over time (weeks) for the SDE group and the SDE plus CAD score group. No differences were seen between the groups (1073 vs. 1042 diagnostic tests, P = .56)
Table 3 gives an overview of the treating physicians’ justification for not pursuing the deferred testing strategy in patients with PTP ≤ 5% or CAD-score ≤ 20, respectively, and thereby deviating from the protocol-recommended diagnostic flowcharts on the clinical decision sheet (see Supplementary data online, Figure S1). The most frequent reasons given were the presence of CV risk factors and typicality of symptoms. Notably, more tests were done due to patient preference in the SDE-only arm than in patients undergoing CAD-score measurement (23% vs. 2% of patients referred).
Table 3.
Reasons for not pursuing a recommended deferred testing strategy in very low-risk patients, as determined by the treating physician
| Variable | SDE plus CAD-score arm | SDE-only arm |
|---|---|---|
| CAD score ≤ 20 | PTP ≤ 5% | |
| Very low-risk patients, n | 374 | 137 |
| Referred for a diagnostic test, n | 185 (49.5%) | 65 (47.4%) |
| PTP, median [range] | 10 [1–44] | 3 [1–5] |
| Reason for testing in very low-risk patients | ||
| CV risk factors | 137 (74%) | 42 (64.6%) |
| Typical anginal symptoms | 42 (22.7%) | 8 (12.3%) |
| Abnormal ECG, CPET, or echocardiography | 1 (0.5%) | 2 (3.1%) |
| Competing cardiac or non-cardiac condition | 7 (3.8%) | 5 (7.7%) |
| Physician preference | 4 (2.2%) | 1 (1.5%) |
| Patient preference | 4 (2.2%) | 15 (23.1%) |
| Other | 9 (4.9%) | 1 (1.5%) |
CAD, coronary artery disease; CV, cardiovascular; PTP, pre-test probability; SDE, standard diagnostic examination.
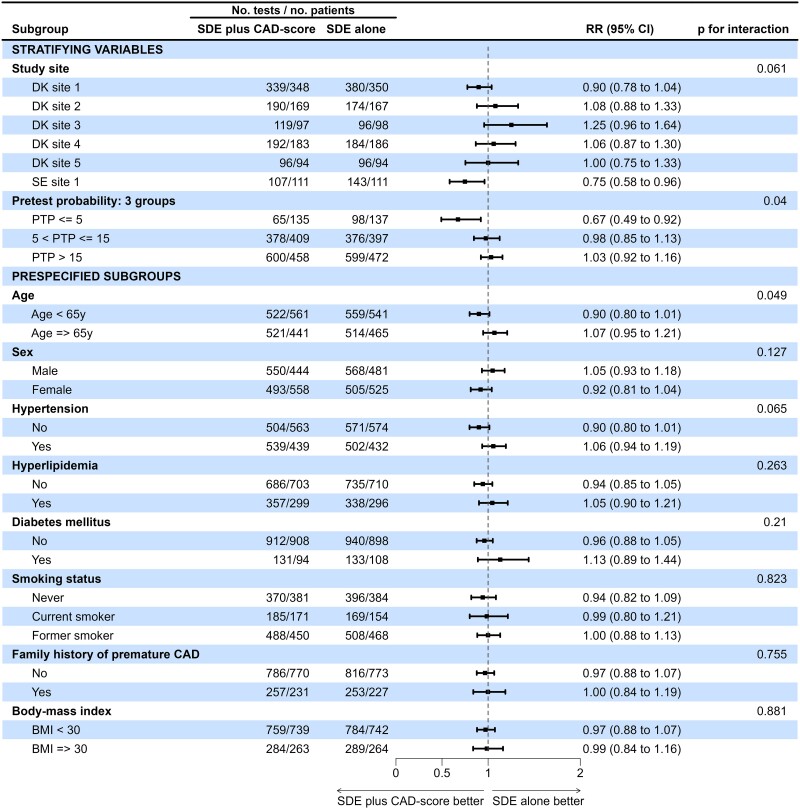
Pre-specified subgroup analyses are shown in Figure 3. No differences were found according to age, sex, hypertension, hyperlipidaemia, diabetes mellitus, smoking status, family history of premature CAD, or body mass index. In the SDE plus CAD-score group, there was a significantly lower incidence rate ratio (IRR) of diagnostic tests in patients randomized at the Swedish site and in patients with low PTP ≤ 5% compared to the SDE group (RR 0.67 [95% CI 0.49–0.92], P for interaction = .015) (Figure 3).
Figure 3.
Forest plot—subgroup analysis for the primary endpoint. Forest plot of stratifying variables (study site and PTP ≤ 5% vs. PTP > 5%) and pre-specified subgroup analysis of PTP, age, sex, hypertension, hyperlipidaemia, diabetes mellitus, smoking status, family history of premature CAD, and body mass index for the primary endpoint of cumulative numbers of diagnostic tests. P-values for interaction are unadjusted. PTP is estimated according to the 2019 European Society of Cardiology guidelines. CAD, coronary artery disease; PTP, pre-test probability
Clinical outcomes
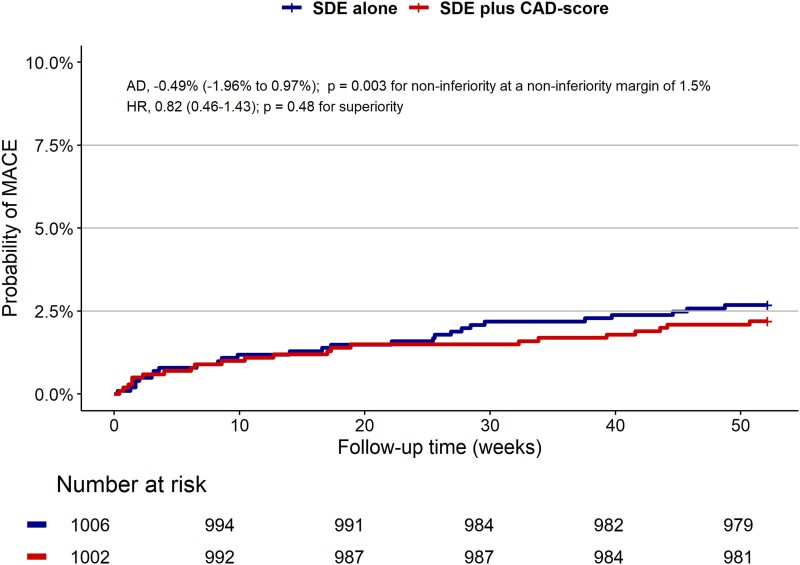
Overall, diagnostic testing was deferred in 22% of patients, and 75% of patients were referred for a NIT (see Supplementary data online, Table S2). In total, 13% of patients underwent invasive angiography and 7.5% were revascularized. Of 283 invasive angiographies performed during one-year follow-up, one-third were without significant stenosis. The total number of invasive angiography and revascularizations did not differ between groups (see Supplementary data online, Table S3). A total of 22 (2.2%) patients in the SDE plus CAD score group and 27 (2.7%) patients in the SDE group experienced a MACE one year after randomization (unadjusted HR 0.82 (95% CI 0.46–1.43), P = .48), and non-inferiority was demonstrated (absolute risk difference of −0.49% (−1.96%–0.97%), P-value for non-inferiority .003) (Table 2, Figure 4). At one year, there were four (0.4%) deaths in the SDE plus CAD-score group and seven (0.7%) deaths in the SDE group. No MACEs were seen in patients with low PTP ≤ 5%, irrespective of CAD-score (see Supplementary data online, Table S3). The prognosis in patients with CAD-score ≤ 20 was also favourable with only 0.53% experiencing MACE. No differences were seen in angina-related health status or quality of life as measured by the SAQ and the quality of life EQ-5D-5L questionnaire (Table 4).
Figure 4.
Key secondary composite endpoint of major adverse cardiac events (MACE). Probability of MACE over time (months) for the SDE group and the SDE plus CAD-score group. MACE includes all-cause death, non-fatal myocardial infarction, hospitalization for unstable angina pectoris, heart failure, or ischaemic stroke, and major complications from cardiovascular procedures adjudicated by a clinical event committee
Table 4.
Changes from baseline in Seattle Angina Questionnaire and EQ-5D-5L in the total population
| SDE plus CAD score | SDE only | ||
|---|---|---|---|
| Seattle Angina Questionnaire, mean (SD) | |||
| Physical limitation score | 84.0 (20.3) | 85.1 (18.8) | |
| One-year change between groupsa | −2.3 (−4.0 to −0.6) | ||
| Angina stability score | 58.3 (19.8) | 57.6 (19.5) | |
| One-year change between groupsa | 1.1 (−1.7–4.0) | ||
| Angina frequency score | 91.5 (14.0) | 90.7 (14.3) | |
| One-year change between groupsa | −0.2 (−2.0–1.5) | ||
| Treatment satisfaction score | 84.8 (19.0) | 84.2 (19.1) | |
| One-year change between groupsa | −0.03 (−2.0–1.9) | ||
| Quality of life score | 74.3 (21.0) | 72.9 (20.9) | |
| One-year change between groupsa | 1.0 (−1.2–3.3) | ||
| EQ-5D-5L, mean (SD) | |||
| Summary score | 0.88 (0.16) | 0.89 (0.16) | |
| One-year change between groupsa | −0.002 (−0.02–0.01) |
SD, standard deviation; SDE, standard diagnostic examination.
aEstimate (95% CI) for change in mean.
Discussion
Key findings
The FILTER-SCAD trial is the first to test whether integrating a simple novel acoustic analysis of heart sounds alongside a default decision algorithm could affect the inertia present in the current diagnostic approach and reduce further diagnostic testing without compromising safety or quality of life in patients with symptoms suggestive of CCS. Adding a CAD-score to SDE was safe but did not significantly reduce the overall use of diagnostic tests at one year when using our implementation strategy. Similarly, symptoms, quality of life, and lifestyle measures did not differ between the two study groups. The prognosis was very favourable, in particular among patients with PTP ≤ 5% or CAD score ≤ 20 (Structured Graphical Abstract). A CAD-score could be achieved in most patients.
Outcomes
Follow-up data on all-comer patients with stable symptoms of CAD, who are ruled out prior to diagnostic testing, are scarce in the literature. The FILTER-SCAD trial provides important long-term follow-up data on this subset of patients. Overall, only 7.6% were revascularized and the prognosis was surprisingly favourable with 2.4% of the population experiencing a MACE over 1 year follow-up. Especially patients with a low probability of CAD (PTP ≤ 5%) had a favourable prognosis with no MACE supporting the study protocol rule-out recommendation following current international guidelines. Yet, 163 diagnostic tests were performed in this subgroup. The FILTER-SCAD trial is the first to assess a true all-comer population of patients with stable symptoms of CAD referred for ambulatory cardiac assessment. The three most recent trials—PROMISE, SCOT-HEART, and PRECISE—all included younger patients with a higher burden of CV risk factors selected for CCTA or functional testing.5,7,20 Rates of all-cause death and non-fatal CV events were similarly low, supporting prior findings that these patients with stable symptoms have an overall favourable prognosis.
Implementation of rule-out strategy
The FILTER-SCAD trial was designed to address the issue of overtesting in patients with symptoms suggestive of stable CAD; a patient population shown to have an excellent prognosis and poor diagnostic yield of non-invasive testing in recent trials.5,20,21 We hoped that providing a default, diagnostic algorithm and adding the non-invasive, acoustic-based CAD-score could provide the clinician with the confidence to defer testing in patients with low likelihood of obstructive CAD as well as the necessary assurance for the patient. However, despite comprehensive training and education of site clinicians, 47.4% of patients with a PTP ≤ 5% (and no CAD score) in the SDE group and 49.5% of the patients with a CAD score ≤ 20 were referred to diagnostic testing.
However, several findings are worth highlighting. Similar to previous observational studies, we observed clear signs of overtesting.22 The 2019 ESC guidelines recommend that diagnostic testing in patients with PTP ≤ 5% should only be performed for compelling reasons, yet in our trial, 52% underwent further diagnostic testing. Importantly, among the patients with PTP ≤ 5%, only one underwent revascularization and there were no MACE. Hence, our data strongly support the 2019 ESC guidelines recommendation of deferred testing in patients with low PTP as a safe rule-out strategy.
In the subgroup with PTP ≤ 5%, the proportion undergoing further diagnostic testing was reduced from 98 to 65 diagnostic tests (72%–48%) when adding a CAD-score to SDE. In a recent substudy of the PRECISE trial, in which patients with low likelihood of obstructive CAD according to the PROMISE Minimal Risk Score (PMRS) score were randomized to deferred testing vs. usual care,23 the proportion tested was reduced from 87.5% to 35.5%. With a higher median PTP and a lower proportion of the population with very low PTP, the PRECISE populations are not directly comparable with our cohort. Furthermore, the 2103 patients enrolled in the PRECISE trial were from 65 sites and thus likely be selected whereas in the present study, all-comers were included. Nonetheless, both studies show that among patients with low likelihood of obstructive CAD, diagnostic testing can be reduced but not to the level recommended in guidelines.
An alternative strategy to increase the deferral of patients based on PTP could be the use of upfront CCTA for all patients. While this approach may require greater use of resources compared to deferring testing in low-risk individuals, it offers potential advantages such as simplicity, enhanced patient reassurance, and more effective guidance for preventive measures. The FORECAST trial, which randomized 1400 patients referred to chest pain units in the UK to either CCTA with selective fractional flow reserve (FFR) or usual care, found no significant difference in costs or clinical outcomes between the two approaches.24 Notably, testing was deferred in < 5% of patients. In contrast, the FILTER-SCAD trial encouraged deferral of testing in both intervention groups, leading to a 22% deferral rate of further testing. In both FORECAST and FILTER-SCAD, prognosis was excellent.
The FILTER-SCAD was carried out on six sites in two countries with some site differences in study set-up. This is expressed in the heterogenicity of the site-specific findings. At the Swedish site (SE site 1, Figure 3), a significant difference was seen in the IRR between the intervention and SDE, with the intervention being better. This might be because the inclusion was more nurse-driven than at the Danish sites and only a few different doctors were involved in the study. In contrast, a large number of doctors were trained to be sub-investigators at the Danish sites.
Inertia in reducing diagnostic testing in chronic coronary syndrome
It is well known that changing clinical practice is hard, and the average time from evidence available to clinically implement spans more than 10 years.25–27 While cardiologists are aware of the low diagnostic yield associated with current testing strategies, there is significant inertia in reducing testing of patients with low likelihood of CAD. Therefore, before incorporating the acoustic CADScor®System device as a part of everyday clinical practice, we performed this implementation trial to assess its acceptability among clinicians and patients in routine real-life clinical settings. With the FILTER-SCAD trial, we tried to influence the inertia by re-enforcing the recommended 2019 ESC PTP rule-out strategy combined with the simple acoustic-driven CAD-score testing with proven abilities to rule out obstructive CAD10,11 as a default documented diagnostic algorithm. Despite thorough instructions, guidance and diagnostic work-up nudging, our implementation strategy resulted in more deferred testing in the lowest PTP (≤ 5%) group but overall no reduction in testing. Apparently, neither providing PTP nor addition of the CAD-score provided the physician with sufficient overall confidence to defer further diagnostic testing, except perhaps among patients with PTP < 5%. Noteworthily, 23% of patients in the control arm patient preference were the reason for further testing, as opposed to only 2% in the intervention arm. Despite small numbers, these findings suggest a potential reassuring effect of the CAD-score strategy meriting further study.
This inertia in reducing diagnostic testing is likely influenced by multiple factors, including both clinicians and patients being familiar with existing diagnostic testing methods and having specific expectations from the assessments, expectations from referring entities and from patients, the fear of missing obstructive CAD, the ready availability of precise diagnostic methods, and the reassurance and therapeutic guidance. Notably, financial incentives are not relevant under the Scandinavian universal healthcare systems. It should be recognized that diagnostic testing, even when yield is low, may be perceived as advantageous, including patient reassurance, potential of guided medical therapy following e.g. calcium score, and avoidance of treatment lethargy. However, excessive diagnostic testing also comes with some challenges and disadvantages including radiation, complications, incidental findings leading to more tests, economic costs, and patient anxiety. This in combination with a low diagnostic yield, a good prognosis, and the fact that angina pectoris often recedes without intervention, calls for a deferred testing strategy combined with optimal medical therapy and appropriate follow-up. However, to identify and address apparent resistance to changing the diagnostic pathway towards more deferred testing, a focus on implementation science and testing of other implementation strategies is needed.
Limitations
Several limitations of the trial need to be considered. Firstly, a CAD-score > 20 may inappropriately lead to a referral to a diagnostic test. The CAD-score displays favourable properties in ruling out but should not be used to rule in. However, this study, adding of CAD-score to SDE did not increase but reduced the numbers of diagnostic test in the PTP ≤ 5% group. Secondly, crossovers among participants with PTP > 5% who did not undergo diagnostic tests were not accounted for in our sample size calculation. These reduced the observed difference in cumulative number of diagnostic tests and thus effectively reduce the power of our trial increasing the likelihood of a type 2 error. However, the study was highly overpowered for the primary endpoint to reach power for the key secondary safety endpoint. Thirdly, we did not have data on redeemed prescriptions for guideline-directed medication. Finally, the CAD-score has not been tested in relation to microvascular angina/ischaemia with non-obstructive CAD.
Generalizability
The generalizability of our results to most patients with stable symptoms of CAD is likely good; only limited by the inclusion of almost exclusively self-identified white participants within a Scandinavian healthcare system setting. The pragmatic design resembles everyday clinical practice in Denmark and Sweden.
Conclusions
In this pragmatic randomized implementation study, we found that adding a CAD score to the 2019 ESC PTP-guided rule-out strategy implemented as part of a standardized diagnostic algorithm was safe but did not result in an overall reduction in number of diagnostic tests. Despite the favourable prognosis, confirmed by a very low MACE rate, clinician and patient resistance to modifying current pathways persisted. As a result, implementation of a PTP ± CAD-score based strategy did not achieve the expected increase in test deferral with the current approach.
The rule-out properties of the CAD-score have been well documented previously,9–13 and the score has the potential to increase test deferral with a different implementation strategy. Further implementation studies may benefit from a more selective use of the CAD-score as an add-on to PTP, potentially within a nurse-led framework. Additionally, a more rigorous implementation approach, possibly with institutional financial incentives, might improve test deferral rates. Further detailed analysis of our data could also identify specific subgroups where a selective PTP + CAD-score strategy would be particularly relevant.
Supplementary Material
Acknowledgements
The authors would like to thank all involved study personnel for their contribution to the study.
Contributor Information
Louise Hougesen Bjerking, Department of Cardiology, Copenhagen University Hospital—Bispebjerg and Frederiksberg, Bispebjerg Bakke 23, 2400 Copenhagen NV, Denmark.
Kim Wadt Skak-Hansen, Department of Cardiology, Copenhagen University Hospital—Bispebjerg and Frederiksberg, Bispebjerg Bakke 23, 2400 Copenhagen NV, Denmark.
Merete Heitmann, Department of Cardiology, Copenhagen University Hospital—Bispebjerg and Frederiksberg, Bispebjerg Bakke 23, 2400 Copenhagen NV, Denmark.
Jens Dahlgaard Hove, Department of Cardiology, Copenhagen University Hospital—Amager and Hvidovre, Denmark; Center of Functional Imaging and Research, Copenhagen University Hospital—Amager and Hvidovre, Denmark.
Sune Ammentorp Haahr-Pedersen, Department of Cardiology, Copenhagen University Hospital—Herlev and Gentofte, Denmark.
Henrik Engblom, Department of Clinical Physiology, Clinical Science, Skåne University Hospital, Lund, Sweden.
David Erlinge, Department of Cardiology, Clinical Science, Skåne University Hospital, Lund, Sweden.
Sune Bernd Emil Werner Räder, Department of Cardiology, Copenhagen University Hospital—North Zealand, Hillerød, Denmark.
Jens Brønnum-Schou, Center of Functional Imaging and Research, Copenhagen University Hospital—Amager and Hvidovre, Denmark.
Tor Biering-Sørensen, Department of Cardiology, Copenhagen University Hospital—Herlev and Gentofte, Denmark; Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark.
Camilla Lyngby Kjærgaard, Department of Cardiology, Copenhagen University Hospital—Bispebjerg and Frederiksberg, Bispebjerg Bakke 23, 2400 Copenhagen NV, Denmark.
Søren Strange, The Danish Association of Practicing Medical Specialists, Denmark.
Søren Galatius, Department of Cardiology, Copenhagen University Hospital—Bispebjerg and Frederiksberg, Bispebjerg Bakke 23, 2400 Copenhagen NV, Denmark.
Eva Irene Bossano Prescott, Department of Cardiology, Copenhagen University Hospital—Bispebjerg and Frederiksberg, Bispebjerg Bakke 23, 2400 Copenhagen NV, Denmark.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
L.H.B.: none. K.W.S.-H.: none. M.H.: speaker for Novartis, Sanofi, Amgen, and Astra-Zeneca. J.D.H.: advisory board member and speaker for Novo Nordisk and Astra-Zeneca. Co-owner of O2matic. S.A.H.-P.: none. H.E.: none. D.E.: has received honorarium for advisory board/speaker fees from Amgen, AstraZeneca, Chiesi, Sanofi, Novo Nordisk, InfraredX/Nipro, and Kaminari Medical. S.B.E.W.R.: none. J.B-S.: none. T.B-S.: has received research grants from Sanofi Pasteur, GSK, Novo Nordisk, AstraZeneca, Boston Scientific, and GE Healthcare, consulting fees from Novo Nordisk, IQVIA, Parexel, Amgen, CSL Seqirus, GSK, and Sanofi Pasteur, and lecture fees from Bayer, Novartis, Sanofi Pasteur, GE Healthcare, and GSK. C.L.K.: none. S.S.: none. S.G.: holds shares in Acarix. E.I.B.P.: none.
Data Availability
Data cannot be shared for ethical/privacy reasons.
Funding
Fonden for Faglig Udvikling i Speciallægepraksis (grant number R117-A3068-B1692, R210-A4752-B1692, R253-A5907-B1692); Acarix A/S (unrestricted grant); Helsefonden (grant number 21-B-0348); Kai Hansens Fond; and Kai Houmann Nielsens Fond.
Ethical Approval
The study protocol was approved by the Danish Medical Agency (2019024326), the Danish National Committee on Health Research Ethics (H-19012579), and the Swedish Ethical Review Authority (Dnr 2019-04252). ClinicalTrial.gov ID is NCT04121949.
Pre-registered Clinical Trial Number
The pre-registered clinical trial number is NCT04121949 (ClinicalTrial.gov).
References
- 1. Hemingway H, Langenberg C, Damant J, Frost C, Pyörälä K, Barrett-Connor E. Prevalence of angina in women versus men: a systematic review and meta-analysis of international variations across 31 countries. Circulation 2008;117:1526–36. 10.1161/CIRCULATIONAHA.107.720953 [DOI] [PubMed] [Google Scholar]
- 2. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2019;40(6):508–17. 10.1093/eurheartj/ehy848 [DOI] [PubMed] [Google Scholar]
- 3. Barnett ML, Song Z, Landon BE. Trends in physician referrals in the US, 1999–2009. Arch Intern Med 2012;172:163–70. 10.1001/archinternmed.2011.722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jordan KP, Timmis A, Croft P, van der Windt DA, Denaxas S, González-Izquierdo A, et al. Prognosis of undiagnosed chest pain: linked electronic health record cohort study. BMJ 2017;357:j1194. 10.1136/bmj.j1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015;372:1291–300. 10.1056/NEJMoa1415516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Therming C, Galatius S, Heitmann M, Højberg S, Sørum C, Bech J, et al. Low diagnostic yield of non-invasive testing in patients with suspected coronary artery disease: results from a large unselected hospital-based sample. Eur Heart J Qual Care Clin Outcomes 2018;4:301–8. 10.1093/ehjqcco/qcx048 [DOI] [PubMed] [Google Scholar]
- 7. SCOT-HEART investigators . CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet Lond Engl 2015;385:2383–91. 10.1016/S0140-6736(15)60291-4 [DOI] [PubMed] [Google Scholar]
- 8. Thomas JL, Winther S, Wilson RF, Bøttcher M. A novel approach to diagnosing coronary artery disease: acoustic detection of coronary turbulence. Int J Cardiovasc Imaging 2017;33:129–36. 10.1007/s10554-016-0970-5 [DOI] [PubMed] [Google Scholar]
- 9. Winther S, Schmidt SE, Holm NR, Toft E, Struijk JJ, Bøtker HE, et al. Diagnosing coronary artery disease by sound analysis from coronary stenosis induced turbulent blood flow: diagnostic performance in patients with stable angina pectoris. Int J Cardiovasc Imaging 2016;32:235–45. 10.1007/s10554-015-0753-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Winther S, Nissen L, Schmidt SE, Westra JS, Rasmussen LD, Knudsen LL, et al. Diagnostic performance of an acoustic-based system for coronary artery disease risk stratification. Heart Br Card Soc 2018;104:928–35. 10.1136/heartjnl-2017-311944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmidt SE, Winther S, Larsen BS, Groenhoej MH, Nissen L, Westra J, et al. Coronary artery disease risk reclassification by a new acoustic-based score. Int J Cardiovasc Imaging 2019;35:2019–28. 10.1007/s10554-019-01662-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmidt SE, Winther S, Boettcher M. Coronary artery disease risk reclassification using an acoustic-based score in view of the new European Society of Cardiology 2019 guidelines on chronic coronary syndromes. Int J Cardiovasc Imaging 2020;36:383–4. 10.1007/s10554-019-01746-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rasmussen LD, Winther S, Karim SR, Westra J, Johansen JK, Søndergaard HM, et al. Likelihood reclassification by an acoustic-based score in suspected coronary artery disease. Heart 2023;109:1223–30. 10.1136/heartjnl-2023-322357 [DOI] [PubMed] [Google Scholar]
- 14. Bjerking L, Hansen K, Biering-Soerensen T, Engblom H, Erlinge D, Haarh-Pedersen S, et al. Cost-effectiveness of adding a non-invasive acoustic rule-out test in the evaluation of patients with suspected stable angina pectoris. Design of the randomized multicenter FILTER-SCAD trial. Eur Heart J 2020;41:ehaa946.3569. 10.1093/ehjci/ehaa946.3569 [DOI] [Google Scholar]
- 15. Task Force Members, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003.. 10.1093/eurheartj/eht296 [DOI] [PubMed] [Google Scholar]
- 16. Rasmussen L, Winther S, Karim SR, Westra J, Kheyr M, Johansen JK, et al. Diagnostic accuracy and reclassification potential of the acoustic CADScor algorithm in intermediate risk patients with suspected coronary artery disease. Eur Heart J 2021;42:ehab724.1174. 10.1093/eurheartj/ehab724.1174 [DOI] [Google Scholar]
- 17. Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association task force on clinical data standards (writing committee to develop cardiovascular endpoints data standards). J Am Coll Cardiol 2015;66:403–69. 10.1016/j.jacc.2014.12.018 [DOI] [PubMed] [Google Scholar]
- 18. Douglas PS, Hoffmann U, Lee KL, Mark DB, Al-Khalidi HR, Anstrom K, et al. PROspective Multicenter Imaging Study for Evaluation of chest pain: rationale and design of the PROMISE trial. Am Heart J 2014;167:796–803.e1. 10.1016/j.ahj.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. R Core Team . R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2023. Available from: https://www.R-project.org/ [Google Scholar]
- 20. Douglas PS, Nanna MG, Kelsey MD, Yow E, Mark DB, Patel MR, et al. Comparison of an initial risk-based testing strategy vs usual testing in stable symptomatic patients with suspected coronary artery disease: the PRECISE randomized clinical trial. JAMA Cardiol 2023;8(10):904–14. 10.1001/jamacardio.2023.2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. SCOT-HEART Investigators, Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med 2018;379:924–33. 10.1056/NEJMoa1805971 [DOI] [PubMed] [Google Scholar]
- 22. Reeh J, Therming CB, Heitmann M, Højberg S, Sørum C, Bech J, et al. Prediction of obstructive coronary artery disease and prognosis in patients with suspected stable angina. Eur Heart J 2018;40:1426–35. 10.1093/eurheartj/ehy806 [DOI] [PubMed] [Google Scholar]
- 23. Udelson JE, Kelsey MD, Nanna MG, Fordyce CB, Yow E, Clare RM, et al. Deferred testing in stable outpatients with suspected coronary artery disease: a prespecified secondary analysis of the PRECISE randomized clinical trial. JAMA Cardiol 2023;8:915–24. 10.1001/jamacardio.2023.2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Curzen N, Nicholas Z, Stuart B, Wilding S, Hill K, Shambrook J, et al. Fractional flow reserve derived from computed tomography coronary angiography in the assessment and management of stable chest pain: the FORECAST randomized trial. Eur Heart J 2021;42:3844–85. 10.1093/eurheartj/ehab444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rubin R. It takes an average of 17 years for evidence to change practice—the burgeoning field of implementation science seeks to speed things up. JAMA 2023;329:1333–6. 10.1001/jama.2023.4387 [DOI] [PubMed] [Google Scholar]
- 26. Bauer MS, Kirchner J. Implementation science: what is it and why should I care? Psychiatry Res 2020;283:112376. 10.1016/j.psychres.2019.04.025 [DOI] [PubMed] [Google Scholar]
- 27. Borg J, Gustafsson C, Landerdahl Stridsberg S, Zander V. Implementation of welfare technology: a state-of-the-art review of knowledge gaps and research needs. Disabil Rehabil Assist Technol 2023;18:227–39. 10.1080/17483107.2022.2120104 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data cannot be shared for ethical/privacy reasons.