Abstract
Current approaches to regulating osteoarthritis primarily focus on symptom management; however, these methods often have significant side effects and may not be suitable for long-term care. As an alternative to conventional treatments, injecting stem cells into knee joint cartilage is a promising option for repairing damaged cartilage. In this review, we outline the general procedure for stem cell treatment of knee joint cartilage regeneration, emphasizing the potential of intra-articular stem cell injections as a therapeutic option for osteoarthritis. We examined and summarized patient evaluation and preparation for knee joint stem cell therapy, stem cell harvesting, stem cell preparation, injection procedures for stem cell therapy, post-injection care and monitoring, potential outcomes of stem cell therapy, and considerations and risks associated with stem cell therapy. Overall, stem cell injections for knee joint cartilage damage represent a promising frontier in orthopedic care. They offer potential benefits such as pain and inflammation reduction, promotion of cartilage repair and regeneration, and the possibility of avoiding more invasive treatments such as knee surgery. Ongoing collaboration among researchers, clinicians, and regulatory organizations is crucial for advancing this field and translating scientific discoveries into effective clinical applications.
Keywords: Osteoarthritis, Cartilage, Stem cells
INTRODUCTION
Osteoarthritis (OA) is characterized by deterioration of the articular cartilage and subchondral bone. It is the most prevalent degenerative joint disease in the elderly population. Its common symptoms include joint stiffness and pain, synovial tissue inflammation, bone spur formation, joint cartilage degradation, and changes in the underlying bone structure. Etiological factors such as mechanical stress, structural injuries to the joints, inflammation, oxidative stress, and aging have been identified (Fig. 1). Despite ongoing research, a definitive cure or comprehensive management strategy for OA remains elusive, owing to the unclear molecular mechanisms underlying tissue destruction. The onset and progression of OA are influenced by inflammatory cytokines produced by chondrocytes and present in joint tissues and fluids, as well as by low-grade inflammation in intra-articular tissues. The imbalance between cartilage synthesis and degradation is the primary cause of this disease (Abramoff and Caldera, 2020; Kulkarni et al., 2021). Natural products are excellent sources for developing new drugs (Hossain et al, 2022a, 2022b; Huh et al., 2023; Hwang et al., 2023; Jang et al., 2023; Kim et al., 2023a, 2023b; Ko et al., 2023; Lee et al., 2023; Ryu et al., 2023). Therefore, we aimed to develop effective pharmacological strategies to restore balance and improve OA treatment, particularly by using novel agents derived from medicinal plants and natural products (Kang et al., 2014; Park et al., 2015; Nam et al., 2016; Park et al., 2016; Kang et al., 2017; Ra et al., 2017; Kang et al., 2018, 2019). However, neither our team nor other researchers in this field have identified or established definitive therapeutic agents. The current approaches for regulating OA focus on symptom management to alleviate pain, improve quality of life, and reduce disability. The treatment options include both pharmacological and non-pharmacological interventions (Fig. 2, Table 1). Non-pharmacological methods emphasize weight loss, exercise, use of alternative medicines, diverse anti-inflammatory medicinal herbs, nutraceuticals, and surgical interventions (Vincent et al., 2022). Pharmacological treatments include nonsteroidal anti-inflammatory drugs (NSAIDs), slow-acting drugs for OA, analgesics, potential disease-modifying drugs, bone-targeted agents, and intra-articular injections of corticosteroids and hyaluronic acid (Gregori et al., 2018). However, these therapies do not address the root cause of OA, are associated with significant side effects and may not be suitable for long-term management (Shen and Gatti, 2013). As an alternative to this conventional approach for the regulation of OA, injecting stem cells into the knee joint cartilage is a promising treatment option aimed at curing damaged cartilage and potentially delaying or avoiding the need for knee replacement surgery. Stem cells are defined as undifferentiated cells characterized by their dual capacity as follows: the ability to undergo numerous cycles of cell division while maintaining an undifferentiated state and the capacity to differentiate into specialized cell types with distinct functions and properties (Fig. 3). Stem cells have three types. First, embryonic stem cells (ESCs) are derived from the inner cell mass of blastocysts (early stage embryos). These cells can differentiate into all cell types in the three germ layers (ectoderm, mesoderm, and endoderm). ESCs can form any cell type in the body, making them highly versatile for research and therapeutic applications. Second, adult stem cells (ASCs) are present in various tissues, including the bone marrow, adipose tissue, and skin. They are limited in differentiating into cell types based on their tissues of origin. For example, hematopoietic stem cells from the bone marrow can differentiate into various blood cells, but not into neurons or muscle cells. ASCs play an important role in tissue maintenance and repair. They are less versatile than ESCs, but are less likely to cause ethical issues because they do not involve embryos. Induced pluripotent stem cells (iPSCs) are generated by reprogramming adult somatic cells (e.g., fibroblasts or keratinocytes) to a pluripotent state. They are similar to ESCs and can differentiate into almost any type of cell. iPSCs are powerful tools for research and personalized medicine. They avoid the ethical concerns associated with ESCs since they do not require the use of embryos (Tian et al., 2023). Based on the aforementioned information, this review primarily aims to explain the general procedure of stem cell treatment for knee joint cartilage regeneration, highlighting the potential of intra-articular stem cell injection for effective cartilage regeneration in the knee joint as a therapeutic option for OA (Fig. 4).
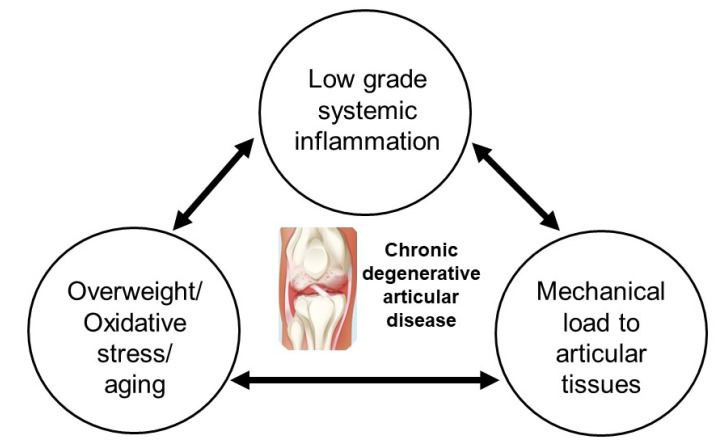
Fig. 1.
Pathophysiology of knee osteoarthritis. Osteoarthritis is the most prevalent degenerative joint disease affecting the elderly population and is characterized by the deterioration of articular cartilage and subchondral bone. Common symptoms include joint stiffness and pain, synovial tissue inflammation, bone spur formation, joint cartilage degradation, and changes in the underlying bone structure. Etiological factors such as mechanical stress, structural injuries to the joints, inflammation, oxidative stress, and aging have been identified.
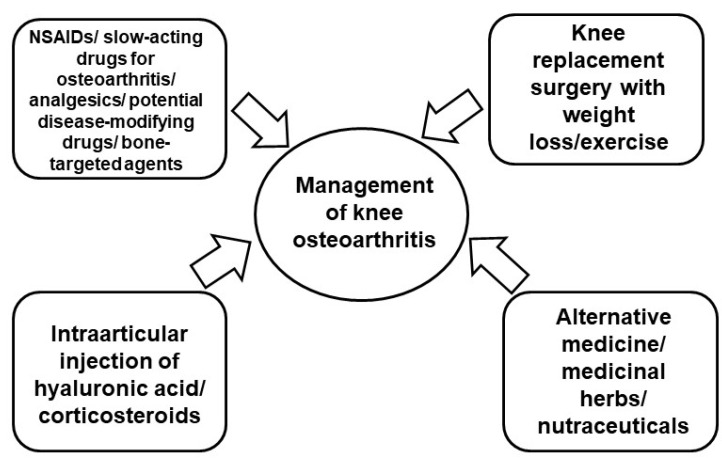
Fig. 2.
Current strategy for management of knee osteoarthritis. The current approaches for regulating osteoarthritis focus on symptom management to alleviate pain, improve quality of life, and reduce disability. Treatment options include both pharmacological and nonpharmacological interventions.
Table 1.
The conventional management of knee osteoarthritis
| Pharmacological management | Analgesics |
| Nonsteroidal anti-inflammatory drugs (NSAIDs) | |
| Symptomatic slow-acting drugs in osteoarthritis | |
| Agents for intraarticular injection including corticosteroids and hyaluronic acid | |
| Putative disease-modifying agents | |
| Bone-acting agents | |
| Non-pharmacological management | Articular surgery |
| Taking exercises | |
| Body weight loss | |
| Use of medicinal herbs and neutraceuticals |
Fig. 3.
Stem cells for knee osteoarthritis. As an alternative to the conventional approach for the regulation of osteoarthritis, injecting stem cells into knee joint cartilage is a promising treatment option aimed at curing damaged cartilage and potentially delaying or avoiding the need for knee replacement surgery. Stem cells are defined as undifferentiated cells characterized by the ability to undergo numerous cycles of cell division while maintaining an undifferentiated state and the capacity to differentiate into specialized cell types with distinct functions and properties, including chondrocytes.
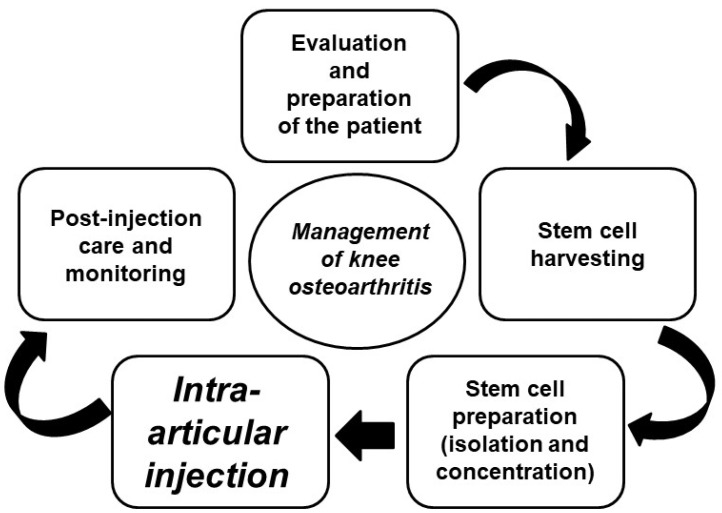
Fig. 4.
Process of stem cell therapy for knee osteoarthritis. The general procedure of stem cell treatment for knee joint cartilage regeneration is briefly described, highlighting the potential of intra-articular injection of stem cells for the effective regeneration of cartilage in the knee joint as a therapeutic option for osteoarthritis.
EVALUATION AND PREPARATION OF PATIENTS FOR KNEE JOINT STEM CELL THERAPY
Patient evaluation and preparation for stem cell therapy for knee joint cartilage damage involve a comprehensive assessment of the knee joint condition through advanced imaging studies, such as magnetic resonance imaging (MRI), coupled with careful consideration of patient-specific criteria, including age, overall health, severity of knee OA, and response to previous treatments. This systematic approach ensures that stem cell therapy is tailored to individual patient needs, maximizing potential benefits, and minimizing risks (Wei and Bao, 2022; Kyriakidis et al., 2023). A crucial aspect of evaluating the knee joint condition before stem cell therapy is the use of advanced imaging techniques. MRI is particularly favored for its ability to provide detailed images of soft tissues such as cartilage. MRI helps assess the extent and location of cartilage damage, which is essential for determining the feasibility and potential benefits of stem cell therapy. Moreover, MRI allows clinicians to visualize cartilage defects, assess the integrity of the ligaments and tendons, and evaluate the overall joint structure and alignment. This imaging modality is pivotal for identifying suitable candidates for stem cell therapy by providing comprehensive insights into the extent and severity of knee OA (Tsou et al., 2006; Trattnig et al., 2011; Menashe et al., 2012). Age is a significant factor in the selection of patients for stem cell therapy. Although no strict upper age limit is imposed, younger patients generally exhibit better outcomes because of their potentially higher regenerative capacity and better overall health. Nevertheless, older patients can still benefit from stem cell therapy depending on their general health status and knee OA severity. Ensuring that patients are in good overall health to tolerate the procedure and potential rehabilitation process is crucial (Vega et al., 2015; Spasovski et al., 2024). The severity of knee OA and extent of cartilage damage are critical factors in determining candidates for stem cell therapy. Patients with mild-to-moderate knee OA and localized cartilage defects are typically better candidates. Stem cell therapy is more effective in the early to moderate stages of OA, when some cartilage remains, allowing regeneration and repair. For patients with severe knee OA and extensive cartilage loss, the benefits of stem cell therapy may be limited, and alternative treatments, such as joint replacement surgery, may be more appropriate. Another important consideration is the patient’s response to previous treatments such as physical therapy, NSAIDs, corticosteroid injections, or hyaluronic acid injections. As an alternative strategy, stem cell therapy may be considered for patients who do not respond well to conservative treatment (Centeno et al., 2010; Diekman and Guilak, 2013).
STEM CELL HARVESTING
Stem cells used for knee joint cartilage repair can originate from several locations in the body. The bone marrow contains a population of mesenchymal stem cells (MSCs), adult stem cells that can differentiate into various cell types, such as bone, cartilage, and fat cells. The procedure used to obtain bone marrow-derived stem cells involved bone marrow aspiration. Bone marrow aspiration is typically performed under local anesthesia. A needle is inserted into the bone marrow cavity, generally the iliac crest of the pelvic bone, and a small amount of bone marrow is withdrawn. The aspirate contains a mixture of cells, including MSCs, which are then processed in a laboratory to isolate and concentrate stem cells for therapeutic use. Bone marrow-derived MSCs have been extensively studied and used in clinical trials owing to their regenerative potential in the treatment of OA and cartilage defects. They are effective in promoting cartilage repair and reducing inflammation in joints affected by OA (Lee and Wang, 2017; Doyle et al., 2020; Mello et al., 2024; Pharoun et al., 2024). The adipose tissue also contains numerous MSCs, making it a viable source of stem cells. stem cells were obtained using a minimally invasive procedure known as liposuction. It involves extracting fat tissue from areas such as the abdomen or thighs using a small cannula under local anesthesia. The harvested fat is processed to isolate MSCs, which are then prepared for therapeutic use. Adipose tissue is a relatively abundant source of MSCs, and the harvesting procedure is less invasive than bone marrow aspiration. In preclinical and clinical studies, adipose-derived MSCs have shown regenerative capabilities similar to those of bone marrow-derived MSCs. Clinical trials have demonstrated the safety and efficacy of adipose-derived MSCs in improving joint function and reducing pain in patients with knee OA (Pers et al., 2016; Damia et al., 2018; Malige et al., 2024). Bone marrow aspiration and adipose tissue extraction are both minimally invasive procedures. Harvesting stem cells from bone marrow or adipose tissue offers promising therapeutic potential for knee joint cartilage repair. These minimally invasive procedures provide a source of MSCs capable of promoting cartilage regeneration and reducing the inflammation associated with OA. The choice of harvesting method may depend on factors such as patient preference, quantity of stem cells required, and specific clinical considerations. Typically performed in an outpatient setting, bone marrow aspiration involves the insertion of a needle into the bone marrow cavity. The procedure is guided by imaging (such as fluoroscopy or ultrasound) to ensure accurate placement. It is generally well tolerated with minimal discomfort (Imam et al., 2017). Liposuction for stem cell harvesting is performed under local anesthesia. A small incision is made to insert a cannula to suction the fat tissue. The procedure is relatively quick and associated with minimal recovery time (Wilson et al., 2019).
STEM CELL PREPARATION
Stem cell preparation involves the meticulous isolation and concentration of MSCs from the harvested bone marrow or adipose tissue. These processes are essential to obtain potent stem cell products that can effectively promote cartilage repair and regeneration in patients with knee OA. Quality control measures, including viability assessment, purity checks, and characterization, ensure that the stem cell preparation meets the safety and efficacy standards for clinical use. After harvesting stem cells from either the bone marrow or adipose tissue, the next critical step is processing in a laboratory to isolate and concentrate the stem cells (Zuk et al., 2002; Kemp et al., 2005; Dominici et al., 2006). Harvested tissue containing stem cells is processed to separate stem cells from other cellular components, such as red blood cells, fat cells (in the case of adipose tissue), and debris. Bone marrow aspirates are typically processed using centrifugation and density gradient separation techniques to isolate MSCs. stem cells are isolated by enzymatic digestion of the extracted fat tissue, followed by centrifugation or filtration to concentrate the MSCs. Once isolated, stem cells are concentrated to increase their potency and ensure an adequate number of cells for therapeutic use. This step involved the removal of excess fluid and non-stem cell components to obtain a concentrated suspension of MSCs. Ensuring stem cell quality and viability is crucial for their therapeutic efficacy and safety. The stem cells were rigorously tested to assess their viability and functionality after isolation. Viability tests evaluate the percentage of live versus dead cells to ensure that the majority of the isolated cells are viable and capable of performing their intended regenerative functions. Quality control measures include screening for contaminants such as bacteria, fungi, endotoxins, and other pathogens, which could compromise the safety of stem cell products. Sterility testing is essential to confirm that stem cell preparations are free of microbial contamination. Stem cells are characterized by a specific set of positive surface markers that are identified by the absence of certain negative markers. Negative markers were used to distinguish MSCs from other cell types and confirm their identity. Surface marker analysis using flow cytometry ensured that the isolated cells expressed characteristic MSC markers (e.g., CD73, CD90, and CD105) and lacked hematopoietic or endothelial cell markers. Negative markers for MSCs such as CD34, CD45, CD14, CD19, CD11b, human leukocyte antigen-DR, and CD69, are used in conjunction with positive markers to accurately identify and characterize MSCs (Pittenger et al., 1999; Barry and Murphy, 2004; Dominichi et al., 2006; Wankhade et al., 2016; Zakrzewski et al., 2019).
INJECTION PROCEDURE FOR STEM CELL THERAPY
The injection procedure for stem cell therapy involves precise guidance using ultrasound or fluoroscopic imaging techniques to ensure accurate localization of stem cells to the affected areas of the knee joint. These methods enhance the effectiveness of stem cell therapy by targeting specific regions involved in cartilage damage or OA. Additionally, adjunctive therapies, such as platelet-rich plasma (PRP), are often employed to complement stem cell treatment, harnessing their synergistic regenerative effects to promote tissue repair and improve clinical outcomes in patients with knee OA (Lungu and Moser, 2015; Kyriakidis et al., 2023). Ultrasound imaging is commonly used during stem cell injections into the knee joint. It provides real-time visualization of the joint structures and allows the clinician to accurately guide the needle to a targeted area within the knee. Ultrasound guidance ensures the precise placement of stem cells into specific regions of cartilage damage or osteoarthritic changes. It enhances the accuracy of the injection, reduces the risk of misplaced injections, and improves the overall effectiveness of the treatment (Berkoff et al., 2012; Lungu and Moser, 2015). Fluoroscopy involves real-time X-ray imaging to guide stem cell injection into the knee joint. It provides detailed visualization of bone structures and allows for precise needle placement under fluoroscopic guidance. Fluoroscopic guidance is particularly beneficial in cases where the anatomy of the knee joint is complex or when targeting specific areas within the joint affected by OA. This ensures the accurate delivery of stem cells to optimize therapeutic outcomes (Lorbach et al., 2010; Lungu and Moser, 2015; Graf et al., 2022). In addition to precise injection techniques, stem cell therapy for knee joint cartilage repair may be combined with adjunctive therapies to enhance healing (Yun et al., 2016; Ruane et al., 2021; Chen et al., 2023; Householder et al., 2023). PRP is derived from the patient’s blood and contains a concentrated source of growth factors and cytokines. When injected into the knee joint alongside stem cells, PRP enhances regenerative potential by promoting tissue repair, reducing inflammation, and improving joint function. Clinical studies have shown that combining PRP with stem cell therapy can improve outcomes in patients with knee OA, leading to reduced pain and improved joint function compared with either therapy alone (Sampson et al., 2008; Pintat et al., 2017; Gato-Calvo et al., 2019; Zhao et al., 2022). Other than PRP, growth factors such as insulin-like growth factor-1 or transforming growth factor-beta may be used to further enhance the regenerative effects of stem cell therapy. Research continues to explore the potential synergistic effects of combining stem cells with various growth factors to optimize cartilage repair and mitigate osteoarthritic symptoms.
POST-INJECTION CARE AND MONITORING
Post-injection care and monitoring are integral components of knee joint cartilage stem cell therapy, aimed at enhancing therapeutic outcomes and ensuring patient safety and satisfaction. Rehabilitation protocols are tailored for each patient to promote joint healing and functional improvements. Regular follow-up visits allow clinicians to monitor patient progress, assess treatment effectiveness through clinical evaluation and imaging studies, and adjust management strategies as needed. Effective symptom management strategies contribute to the overall success of stem cell therapy in managing knee OA and improving patients’ quality of life (Aly, 2020). After stem cell injection into the knee joint, rehabilitation plays a crucial role in optimizing the healing process and maximizing joint function. Rehabilitation typically includes a combination of exercises, physical therapy modalities (such as ultrasound or electrical stimulation), and functional training tailored to the specific needs of the patient and extent of cartilage damage. Rehabilitation is intended to restore range of motion, strengthen the surrounding muscles to support the joint, improve stability, and enhance overall functional capacity (Centeno et al., 2008; McKay et al., 2019). Monitoring the progress through regular follow-up visits allows healthcare providers to assess the effectiveness of stem cell therapy and adjust treatment plans as necessary. During follow-up visits, the clinician evaluates changes in symptoms, joint function, and overall improvement in the patient’s quality of life. Periodic imaging studies, such as MRI or radiography, may be performed to visualize changes in the cartilage structure and assess the extent of tissue regeneration (Vangsness et al., 2014; Wiggers et al., 2021). Patients may experience temporary discomfort or mild swelling at the injection site that typically resolves within a few days. NSAIDs or analgesics may be prescribed to manage pain and inflammation as needed. Strategies for long-term symptom management include lifestyle modifications (such as weight control and physical activity), joint protection techniques, and continuation of prescribed medications (Freitag et al., 2016; Wang et al., 2017).
POTENTIAL OUTCOMES OF STEM CELL THERAPY
Stem cell therapy offers promising outcomes for patients with OA, including significant pain relief, stimulation of cartilage regeneration, and potential long-term benefits such as delaying or avoiding knee replacement surgery. Clinical evidence supports the efficacy of stem cell therapy in improving joint function and quality of life, highlighting its role as a valuable treatment option for managing knee OA (Arshi et al., 2020; Huang et al., 2020). One of the primary goals of stem cell therapy for knee joint cartilage repair is to alleviate pain associated with OA. Stem cells have anti-inflammatory properties and release growth factors that promote tissue repair and reduce joint inflammation. Studies have reported significant improvements in pain scores and reduced reliance on pain medications following stem cell therapy (Arshi et al., 2020; Thoene et al., 2023; Xie et al., 2024). Stem cells can differentiate into chondrocytes (cartilage cells) and stimulate the regeneration of damaged cartilage. MSCs secrete bioactive molecules that promote the proliferation and differentiation of nearby cells including chondrocytes, leading to cartilage repair. Research has demonstrated improvements in cartilage volume and structure on MRI following stem cell therapy, indicating the potential for cartilage regeneration (Koelling and Miosge, 2009; Borakati et al., 2018; Iturriaga et al., 2018). By reducing pain and promoting cartilage repair, stem cell therapy may delay or prevent knee replacement surgery in some patients. Longitudinal studies have suggested that patients treated with stem cell therapy experience sustained improvements in knee function and may avoid or delay invasive surgical interventions (Koh et al., 2013; Davatchi et al., 2016; Doyle et al., 2020).
CONSIDERATIONS AND RISKS OF STEM CELL THERAPY
Stem cell therapy offers potential benefits for managing OA-related symptoms, including pain relief and cartilage repair. However, factors such as the variability in treatment effectiveness among individuals, the overall safety profile of autologous stem cell therapies, and the cost and availability challenges associated with accessing these treatments should be considered. The effectiveness of stem cell therapy in knee joint cartilage repair may vary among individuals. Factors such as age, OA severity, overall health, and specific characteristics of stem cell preparations may influence treatment outcomes. Studies have shown variable responses to stem cell therapy, with some patients experiencing significant improvements in pain relief and joint function, whereas others may have more modest or transient benefits (Biazzo et al., 2020; Thoene et al., 2023). Stem cells used in knee joint cartilage therapy are typically derived from the patient’s own body (autologous), which reduces the risk of immune rejection or adverse reactions. Harvesting and injection procedures are generally considered safe, with a minimal risk of complications such as infection or tissue damage (McIntyre et al., 2018; Biazzo et al., 2020). The cost of knee joint cartilage stem cell therapy may vary widely depending on the geographic location, healthcare facility, specific protocols used, and extent of treatment required. The availability and insurance coverage of stem cell therapy may also vary, potentially limiting its accessibility for some patients (Vilquin and Rosset, 2006; Desando et al., 2013; Chen et al., 2023).
FUTURE DIRECTIONS IN STEM CELL INJECTION FOR KNEE JOINT CARTILAGE DAMAGE
Ongoing research aims to refine stem cell isolation, processing, and delivery techniques to enhance the efficacy and consistency of outcomes in the treatment of knee joint cartilage damage. Researchers are currently investigating novel methods for optimizing stem cell viability, differentiation capacity, and retention within the joint environment to promote long-term cartilage regeneration. Moreover, stem cell therapy faces evolving regulatory challenges regarding safety, efficacy standards, and ethical considerations. Regulatory agencies worldwide are developing and revising guidelines to govern the clinical application of stem cell therapies and ensure patient safety and treatment efficacy. Stem cell injection for knee joint cartilage damage represents a promising frontier in orthopedic care, offering potential benefits such as reduction in pain and inflammation, promotion of cartilage repair and regeneration, and avoidance of more invasive treatments such as knee surgery (Fig. 5, Table 2).
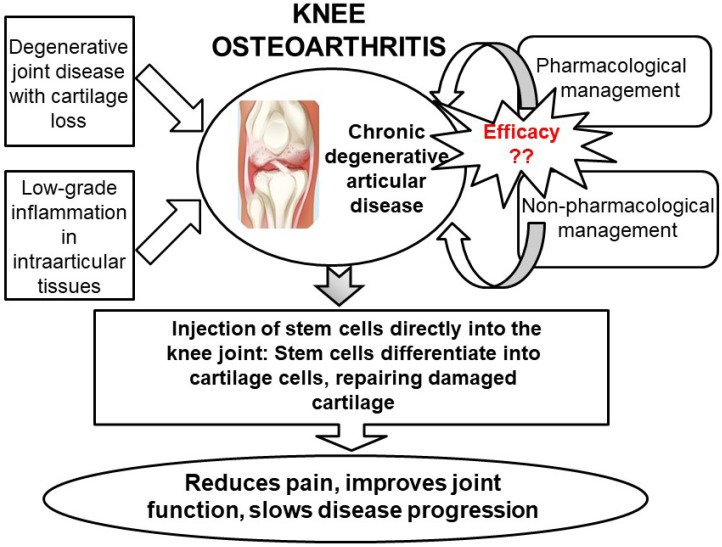
Fig. 5.
Summary of intra-articular injection of stem cells for the regeneration of knee joint cartilage as a therapeutic option for knee osteoarthritis. Treatment options for knee osteoarthritis include both pharmacological and nonpharmacological methods. However, these treatments often have significant side effects and may not be effective for long-term management. An alternative to traditional methods is the direct injection of stem cells into the knee joint cartilage. This promising approach aims to repair the damaged cartilage and potentially delay or eliminate the need for knee replacement surgery. This figure highlights the potential of intra-articular stem cell injection as a therapeutic option for regenerating knee joint cartilage in osteoarthritis.
Table 2.
Summary of intraarticular injection of stem cells for the regeneration of knee joint cartilage as a therapeutic option for knee osteoarthritis
| Objective | To evaluate the potential of stem cell injections for regenerating knee cartilage and alleviating symptoms of osteoarthritis |
| Stem Cell Types Used | - Mesenchymal Stem Cells (MSCs) - Adipose-derived Stem Cells (ADSCs) - Bone Marrow-derived Stem Cells (BMSCs) |
| Injection Site | Intraarticular (directly into the knee joint) |
| Mechanism of Action | - Promotion of cartilage repair - Modulation of inflammatory responses - Stimulation of endogenous cartilage regeneration |
| Benefits | - Pain reduction - Improved joint function - Enhanced cartilage repair |
| Challenges | - Variability in treatment outcomes - Risk of adverse effects (e.g., infection, inflammation) - High cost and limited availability |
| Current Evidence | - Mixed results in clinical trials - Promising outcomes in some studies - Need for further research to establish long-term efficacy and safety |
| Potential Advantages Over Traditional Treatments | - Less invasive compared to surgical options - Possibility of disease-modifying effects |
| Future Directions | - Standardization of stem cell preparation and administration - Long-term follow-up studies - Exploration of combination therapies and optimized protocols |
ACKNOWLEDGMENTS
This work was supported by the BK21 FOUR Program of Chungnam National University Research Grant 2024.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Abramoff B., Caldera F. E. Osteoarthritis: pathology, diagnosis, and treatment options. Med. Clin. North Am. 2020;104:293–311. doi: 10.1016/j.mcna.2019.10.007. [DOI] [PubMed] [Google Scholar]
- Aly R. M. Current state of stem cell-based therapies: an overview. Stem Cell Investig. 2020;15:7–8. doi: 10.21037/sci-2020-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshi A., Petrigliano F. A., Williams R. J., Jones K. J. stem cell treatment for knee articular cartilage defects and osteoarthritis. Curr. Rev. Musculoskelet. Med. 2020;13:20–27. doi: 10.1007/s12178-020-09598-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry F. P., Murphy J. M. Mesenchymal stem cells: clinical applications and biological characterization. Int. J. Biochem. Cell. Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Berkoff D. J., Miller L. E., Block J. E. Clinical utility of ultrasound guidance for intra-articular knee injections: a review. Clin. Interv. Aging. 2012;7:89–95. doi: 10.2147/CIA.S29265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biazzo A., D'Ambrosi R., Masia F., Izzo V., Verde F. Autologous adipose stem cell therapy for knee osteoarthritis: where are we now? Phys. Sportsmed. 2020;48:392–399. doi: 10.1080/00913847.2020.1758001. [DOI] [PubMed] [Google Scholar]
- Borakati A., Mafi R., Mafi P., Khan W. S. A systematic review and meta-analysis of clinical trials of mesenchymal stem cell therapy for cartilage repair. Curr. Stem Cell Res. Ther. 2018;13:215–225. doi: 10.2174/1574888X12666170915120620. [DOI] [PubMed] [Google Scholar]
- Centeno C. J., Busse D., Kisiday J., Keohan C., Freeman M., Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11:343–353. doi: 10.36076/ppj.2008/11/343. [DOI] [PubMed] [Google Scholar]
- Centeno C. J., Schultz J. R., Cheever M., Robinson B., Freeman M., Marasco W. Safety and complications reporting on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr. Stem Cell Res. Ther. 2010;5:81–93. doi: 10.2174/157488810790442796. [DOI] [PubMed] [Google Scholar]
- Chen Y., Cheng R. J., Wu Y., Huang D., Li Y., Liu Y. Advances in stem cell-based therapies in the treatment of osteoarthritis. Int. J. Mol. Sci. 2023;25:394. doi: 10.3390/ijms25010394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damia E., Chicharro D., Lopez S., Cuerv o B., Rubio M., Sopena J. J., Vilar J. M., Carrillo J. M. Adipose-derived mesenchymal stem cells: are they a good therapeutic strategy for osteoarthritis? Int. J. Mol. Sci. 2018;19:1926. doi: 10.3390/ijms19071926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davatchi F., Sadeghi Abdollahi B., Mohyeddin M., Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int. J. Rheum. Dis. 2016;19:219–225. doi: 10.1111/1756-185X.12670. [DOI] [PubMed] [Google Scholar]
- Desando G., Cavallo C., Sartoni F., Martini L., Parrilli A., Veronesi F., Fini M., Giardino R., Facchini A., Grigolo B. Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model. Arthritis Res. Ther. 2013;15:R22. doi: 10.1186/ar4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekman B. O., Guilak F. Stem cell-based therapies for osteoarthritis: challenges and opportunities. Curr. Opin. Rheumatol. 2013;25:119–126. doi: 10.1097/BOR.0b013e32835aa28d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D. J., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Doyle E. C., Wragg N. M., Wilson S. L. Intraarticular injection of bone marrow-derived mesenchymal stem cells enhances regeneration in knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2020;28:3827–3842. doi: 10.1007/s00167-020-05859-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag J., Bates D., Boyd R., Shah K., Barnard A., Huguenin L., Tenen A. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy - a review. BMC Musculoskelet. Disord. 2016;26:230. doi: 10.1186/s12891-016-1085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gato-Calvo L., Magalhaes J., Ruiz-Romero C., Blanco F. J., Burguera E. F. Platelet-rich plasma in osteoarthritis treatment: review of current evidence. Ther. Adv. Chronic Dis. 2019;10:2040622319825567. doi: 10.1177/2040622319825567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf D. N., Thallinger A., Zubler V., Sutter R. intraarticular steroid injection in hip and knee with fluoroscopic guidance: reassessing safety. Radiology. 2022;304:363–369. doi: 10.1148/radiol.210668. [DOI] [PubMed] [Google Scholar]
- Gregori D., Giacovelli G., Minto C., Barbetta B., Gualtieri F., Azzolina D., Vaghi P., Rovati L. C. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: a systematic review and meta-analysis. JAMA. 2018;320:2564–2579. doi: 10.1001/jama.2018.19319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain R., Kim K. I., Jin F., Lee H. J., Lee C. J. Betulin, an anti-inflammatory triterpenoid compound, regulates MUC5AC mucin gene expression through NF-kB signaling in human airway epithelial cells. Biomol. Ther. (Seoul) 2022a;30:540–545. doi: 10.4062/biomolther.2022.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain R., Kim K. I., Li X., Lee H. J., Lee C. J. Involvement of IKK/IkBα/NF-kB p65 signaling into the regulative effect of engeletin on MUC5AC mucin gene expression in human airway epithelial cells. Biomol. Ther. (Seoul) 2022b;30:473–478. doi: 10.4062/biomolther.2022.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Householder N. A., Raghuram A., Agyare K., Thipaphay S., Zumwalt M. A review of recent innovations in cartilage regeneration strategies for the treatment of primary osteoarthritis of the knee: intra-articular injections. Orthop. J. Sports Med. 2023;11:23259671231155950. doi: 10.1177/23259671231155950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R., Li W., Zhao Y., Yang F., Xu M. Clinical efficacy and safety of stem cell therapy for knee osteoarthritis: a meta-analysis. Medicine (Baltimore) 2020;99:e19434. doi: 10.1097/MD.0000000000019434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh E., Choi J. G., Choi Y., Ju I. G., Noh D., Shin D. Y., Kim D. H., Park H. J., Oh M. S. 6-Shogaol, an active ingredient of ginger, improves intestinal and brain abnormalities in proteus mirabilis-induced Parkinson's disease mouse model. Biomol. Ther. (Seoul) 2023;31:417–424. doi: 10.4062/biomolther.2023.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. Y., Wi K., Yoon G., Lee C. J., Lee S. I., Jung J. G., Jeong H. W., Kim J. S., Choi C. H., Na C. S., Shim J. H., Lee M. H. Licochalcone D inhibits skin epidermal cells transformation through the regulation of AKT signaling pathways. Biomol. Ther. (Seoul) 2023;31:682–691. doi: 10.4062/biomolther.2023.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam M. A., Mahmoud S. S. S., Holton J., Abouelmaati D., Elsherbini Y., Snow M. A. Systematic review of the concept and clinical applications of bone marrow aspirate concentrate in orthopaedics. SICOT J. 2017;3:17. doi: 10.1051/sicotj/2017007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturriaga L., Hernáez-Moya R., Erezuma I., Dolatshahi-Pirouz A., Orive G. Advances in stem cell therapy for cartilage regeneration in osteoarthritis. Expert Opin. Biol. Ther. 2018;18:883–896. doi: 10.1080/14712598.2018.1502266. [DOI] [PubMed] [Google Scholar]
- Jang J. H., Yang G., Seok J. K., Kang H. C., Cho Y. Y., Lee H. S., Lee J. Y. Loganin prevents hepatic steatosis by blocking NLRP3 inflammasome activation. Biomol. Ther. (Seoul) 2023;31:40–47. doi: 10.4062/biomolther.2022.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y. H., Lee H. J., Lee C. J., Park J. S. Natural products as sources of novel drug candidates for the pharmacological management of osteoarthritis: a narrative review. Biomol. Ther. (Seoul) 2019;27:503–513. doi: 10.4062/biomolther.2019.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B. J., Ryu J., Lee C. J., Hwang S. C. Luteolin inhibits the activity, secretion and gene expression of MMP-3 in cultured articular chondrocytes and production of MMP-3 in the rat knee. Biomol. Ther. (Seoul) 2014;22:239–245. doi: 10.4062/biomolther.2014.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D. G., Lee H. J., Kim K. T., Hwang S. C., Lee C. J., Park J. S. Effect of oleanolic acid on the activity, secretion and gene expression of matrix metalloproteinase-3 in articular chondrocytes in vitro and the production of matrix metalloproteinase-3 in vivo. Korean J. Physiol. Pharmacol. 2017;21:197–204. doi: 10.4196/kjpp.2017.21.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D. G., Lee H. J., Lee C. J., Park J. S. Inhibition of the expression of matrix metalloproteinases in articular chondrocytes by resveratrol through affecting nuclear factor-kappa B signaling pathway. Biomol. Ther. (Seoul) 2018;26:560–567. doi: 10.4062/biomolther.2018.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp K. C., Hows J., Donaldson C. Bone marrow-derived mesenchymal stem cells. Leuk. Lymphoma. 2005;46:1531–1544. doi: 10.1080/10428190500215076. [DOI] [PubMed] [Google Scholar]
- Kim K. I., Hossain R., Ryu J., Lee H. J., Lee C. J. Regulation of the gene expression of airway MUC5AC mucin through NF-κB signaling pathway by artesunate, an antimalarial agent. Biomol. Ther. (Seoul) 2023a;31:544–549. doi: 10.4062/biomolther.2023.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N., Kwon J., Shin U. S., Jung J. Stimulatory anticancer effect of resveratrol mediated by G protein-coupled estrogen receptor in colorectal cancer. Biomol. Ther. (Seoul) 2023b;31:655–660. doi: 10.4062/biomolther.2023.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H., An S., Jang H., Ahn S., Park I. G., Hwang S. Y., Gong J., Oh S., Kwak S. Y., Choi W. J., Kim H., Noh M. Macakurzin C derivatives as a novel pharmacophore for pan-peroxisome proliferator-activated receptor modulator. Biomol. Ther. (Seoul) 2023;31:312–318. doi: 10.4062/biomolther.2022.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelling S., Miosge N. Stem cell therapy for cartilage regeneration in osteoarthritis. Expert Opin. Biol. Ther. 2009;9:1399–1405. doi: 10.1517/14712590903246370. [DOI] [PubMed] [Google Scholar]
- Koh Y. G., Jo S. B., Kwon O. R., Suh D. S., Lee S. W., Park S. H., Choi Y. J. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29:748–755. doi: 10.1016/j.arthro.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Kulkarni P., Martson A., Vidya R., Chitnavis S., Harsulkar A. Pathophysiological landscape of osteoarthritis. Adv. Clin. Chem. 2021;100:37–90. doi: 10.1016/bs.acc.2020.04.002. [DOI] [PubMed] [Google Scholar]
- Kyriakidis T., Pitsilos C., Iosifidou M., Tzaveas A., Gigis I., Ditsios K., Iosifidis M. Stem cells for the treatment of early to moderate osteoarthritis of the knee: a systematic review. J. Exp. Orthop. 2023;10:102. doi: 10.1186/s40634-023-00665-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. O., Lee M. H., Kwak A. W., Lee J. Y., Yoon G., Joo S. H., Choi Y. H., Park J. W., Shim J. H. Licochalcone H targets EGFR and AKT to suppress the growth of oxaliplatin -sensitive and -resistant colorectal cancer cells. Biomol. Ther. (Seoul) 2023;31:661–673. doi: 10.4062/biomolther.2023.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. Y., Wang B. Cartilage repair by mesenchymal stem cells: clinical trial update and perspectives. J. Orthop. Translat. 2017;9:76–88. doi: 10.1016/j.jot.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorbach O., Kieb M., Scherf C., Seil R., Kohn D., Pape D. Good results after fluoroscopic-guided intra-articular injections in the treatment of adhesive capsulitis of the shoulder. Knee Surg. Sports Traumatol. Arthrosc. 2010;18:1435–1441. doi: 10.1007/s00167-009-1030-7. [DOI] [PubMed] [Google Scholar]
- Lungu E., Moser T. P. A practical guide for performing arthrography under fluoroscopic or ultrasound guidance. Insights Imaging. 2015;6:601–610. doi: 10.1007/s13244-015-0442-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malige A., Gates C., Cook J. L. Mesenchymal stem cells in orthopaedics: a systematic review of applications to practice. J. Orthop. 2024;58:1–9. doi: 10.1016/j.jor.2024.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre J. A., Jones I. A., Han B., Vangsness C. T., Jr Intra-articular mesenchymal stem cell therapy for the human joint: a systematic review. Am. J. Sports Med. 2018;46:3550–3563. doi: 10.1177/0363546517735844. [DOI] [PubMed] [Google Scholar]
- McKay J., Frantzen K., Vercruyssen N., Hafsi K., Opitz T., Davis A., Murrell W. Rehabilitation following regenerative medicine treatment for knee osteoarthritis-current concept review. J. Clin. Orthop. Trauma. 2019;10:59–66. doi: 10.1016/j.jcot.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello D. B., Mesquita F. C. P., Silva Dos Santos D., Asensi K. D., Dias M. L., Campos, de Carvalho A. C., Goldenberg R. C. D. S., Kasai-Brunswick T. H. Mesenchymal stromal cell-based products: challenges and clinical therapeutic options. Int. J. Mol. Sci. 2024;25:6063. doi: 10.3390/ijms25116063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menashe L., Hirko K., Losina E., Kloppenburg M., Zhang W., Li L., Hunter D. J. The diagnostic performance of MRI in osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2012;20:13–21. doi: 10.1016/j.joca.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam D. C., Kim B. K., Lee H. J., Shin H. D., Lee C. J., Hwang S. C. Effects of prunetin on the proteolytic activity, secretion and gene expression of MMP-3 in vitro and production of MMP-3 in vivo. Korean J. Physiol. Pharmacol. 2016;20:221–228. doi: 10.4196/kjpp.2016.20.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. S., Lee H. J., Lee D. Y., Jo H. S., Jeong J. H., Kim D. H., Nam D. C., Lee C. J., Hwang S. C. Chondroprotective effects of wogonin in experimental models of osteoarthritis in vitro and in vivo. Biomol. Ther. (Seoul) 2015;23:442–448. doi: 10.4062/biomolther.2015.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. S., Kim D. K., Shin H. D., Lee H. J., Jo H. S., Jeong J. H., Choi Y. L., Lee C. J., Hwang S. C. Apigenin regulates interleukin-1β-induced production of matrix metalloproteinase both in the knee joint of rat and in primary cultured articular chondrocytes. Biomol. Ther. (Seoul) 2016;24:163–170. doi: 10.4062/biomolther.2015.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pers Y. M., Rackwitz L., Ferreira R., Pullig O., Delfour C., Barry F., Sensebe L., Casteilla L., Fleury S., Bourin P., Noel D., Canovas F., Cyteval C., Lisignoli G., Schrauth J., Haddad D., Domergue S., Noeth U., Jorgensen C. ADIPOA Consortium, author. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial. Stem Cells Transl. Med. 2016;5:847–856. doi: 10.5966/sctm.2015-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharoun J., Berro J., Sobh J., Abou-Younes M. M., Nasr L., Majed A., Khalil A., Joseph, Stephan, Faour W. H. Mesenchymal stem cells biological and biotechnological advances: Implications for clinical applications. Eur. J. Pharmacol. 2024;977:176719. doi: 10.1016/j.ejphar.2024.176719. [DOI] [PubMed] [Google Scholar]
- Pintat J., Silvestre A., Magalon G., Gadeau A. P., Pesquer L., Perozziello A., Peuchant A., Mounayer C., Dallaudière B. Intra-articular injection of mesenchymal stem cells and platelet-rich plasma to treat patellofemoral osteoarthritis: preliminary results of a long-term pilot study. J. Vasc. Interv. Radiol. 2017;28:1708–1713. doi: 10.1016/j.jvir.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Pittenger M. F., Mackay A. M., Beck S. C., Jaiswal R. K., Douglas R., Mosca J. D., Moorman M. A., Simonetti D. W., Craig S., Marshak D. R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Ra H. J., Lee H. J., Jo H. S., Nam D. C., Lee Y. B., Kang B. H., Moon D. K., Kim D. H., Lee C. J., Hwang S. C. Betulin suppressed interleukin-1β-induced gene expression, secretion and proteolytic activity of matrix metalloproteinase in cultured articular chondrocytes and production of matrix metalloproteinase in the knee joint of rat. Korean J. Physiol. Pharmacol. 2017;21:19–26. doi: 10.4196/kjpp.2017.21.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruane J. J., Ross A., Zigmont V., McClure D., Gascon G. A single-blinded randomized controlled trial of mesenchymal stem cell therapy for the treatment of osteoarthritis of the knee with active control. J. Stem Cells Regen. Med. 2021;17:3–17. doi: 10.46582/jsrm.1701002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu J., Kim K. I., Hossain R., Lee M., Hong J. T., Lee H. J., Lee C. J. Meclofenamate suppresses MUC5AC mucin gene expression by regulating the NF-kB signaling pathway in human pulmonary mucoepidermoid NCI-H292 cells. Biomol. Ther. (Seoul) 2023;31:306–311. doi: 10.4062/biomolther.2023.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson S., Gerhardt M., Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr. Rev. Musculoskelet. Med. 2008;1:165–174. doi: 10.1007/s12178-008-9032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Gatti R. The safety and efficacy of intra-articular dual molecular weighted hyaluronic acid in the treatment of knee osteoarthritis: the I.D.E.H.A. study. Orthop. Rev. (Pavia) 2013;5:e33. doi: 10.4081/or.2013.e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasovski D., Spasovski V., Bascarevic Z., Stojiljkovic M., Andjelkovic M., Pavlovic S. Seven-year longitudinal study: clinical evaluation of knee osteoarthritic patients treated with mesenchymal stem cells. J. Clin. Med. 2024;13:3861. doi: 10.3390/jcm13133861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoene M., Bejer-Olenska E., Wojtkiewicz J. The current state of osteoarthritis treatment options using stem cells for regenerative therapy: a review. Int. J. Mol. Sci. 2023;24:8925. doi: 10.3390/ijms24108925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Z., Yu T., Liu J., Wang T., Higuchi A. Introduction to stem cells. Prog. Mol. Biol. Transl. Sci. 2023;199:3–32. doi: 10.1016/bs.pmbts.2023.02.012. [DOI] [PubMed] [Google Scholar]
- Trattnig S., Winalski C. S., Marlovits S., Jurvelin J. S., Welsch G. H., Potter H. G. Magnetic resonance imaging of cartilage repair: a review. Cartilage. 2011;2:5–26. doi: 10.1177/1947603509360209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou I. Y., Yegappan M., Ong W. S., Goh P. O., Tan J. L., Chee T. S. Cartilage injury and repair: assessment with magnetic resonance imaging. Singapore Med. J. 2006;47:80–87. [PubMed] [Google Scholar]
- Vangsness C. T., Jr, Farr J., Boyd J., Dellaero D. T., Mills C. R., LeRoux-Williams M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J. Bone Joint Surg. Am. 2014;96:90–98. doi: 10.2106/JBJS.M.00058. [DOI] [PubMed] [Google Scholar]
- Vega A., Martín-Ferrero M. A., Del Canto F., Alberca M., García V., Munar A., Orozco L., Soler R., Fuertes J. J., Huguet M., Sánchez A., García-Sancho J. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99:1681–1690. doi: 10.1097/TP.0000000000000678. [DOI] [PubMed] [Google Scholar]
- Vilquin J. T., Rosset P. Mesenchymal stem cells in bone and cartilage repair: current status. Regen. Med. 2006;1:589–604. doi: 10.2217/17460751.1.4.589. [DOI] [PubMed] [Google Scholar]
- Vincent T. L., Alliston T., Kapoor M., Loeser R. F., Troeberg L., Little C. B. Osteoarthritis pathophysiology: therapeutic target discovery may require a multifaceted approach. Clin. Geriatr. Med. 2022;38:193–219. doi: 10.1016/j.cger.2021.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Shimmin A., Ghosh P., Marks P., Linklater J., Connell D., Hall S., Skerrett D., Itescu S., Cicuttini F. M. Safety, tolerability, clinical, and joint structural outcomes of a single intra-articular injection of allogeneic mesenchymal precursor cells in patients following anterior cruciate ligament reconstruction: a controlled double-blind randomised trial. Arthritis Res. Ther. 2017;19:180. doi: 10.1186/s13075-017-1391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wankhade U. D., Shen M., Kolhe R., Fulzele S. Advances in adipose-derived stem cells isolation, characterization, and application in regenerative tissue engineering. Stem Cells Int. 2016;2016:3206807. doi: 10.1155/2016/3206807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P., Bao R. Intra-articular mesenchymal stem cell injection for knee osteoarthritis: mechanisms and clinical evidence. Int. J. Mol. Sci. 2022;24:59. doi: 10.3390/ijms24010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggers T. G., Winters M., Van, den Boom N. A., Haisma H. J., Moen M. H. Autologous stem cell therapy in knee osteoarthritis: a systematic review of randomised controlled trials. Br. J. Sports Med. 2021;55:1161–1169. doi: 10.1136/bjsports-2020-103671. [DOI] [PubMed] [Google Scholar]
- Wilson A., Chee M., Butler P., Boyd A. S. Isolation and characterisation of human adipose-derived stem cells. Methods Mol. Biol. 2019;1899:3–13. doi: 10.1007/978-1-4939-8938-6_1. [DOI] [PubMed] [Google Scholar]
- Xie R. H., Gong S. G., Song J., Wu P. P., Hu W. L. Effect of mesenchymal stromal cells transplantation on the outcomes of patients with knee osteoarthritis: a systematic review and meta-analysis. J. Orthop. Res. 2024;42:753–768. doi: 10.1002/jor.25724. [DOI] [PubMed] [Google Scholar]
- Yun S., Ku S. K., Kwon Y. S. Adipose-derived mesenchymal stem cells and platelet-rich plasma synergistically ameliorate the surgical-induced osteoarthritis in Beagle dogs. J. Orthop. Surg. Res. 2016;11:9. doi: 10.1186/s13018-016-0342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewski W., Dobrzyński M., Szymonowicz M., Rybak Z. Stem cells. past, present, and future. Stem Cell Res. Ther. 2019;10:68. doi: 10.1186/s13287-019-1165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Liang G., Han Y., Yang W., Xu N., Luo M., Pan J., Liu J., Zeng L. F. Combination of mesenchymal stem cells (MSCs) and platelet-rich plasma (PRP) in the treatment of knee osteoarthritis: a meta-analysis of randomised controlled trials. BMJ Open. 2022;12:e061008. doi: 10.1136/bmjopen-2022-061008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk P. A., Zhu M., Ashjian P., De Ugarte D. A., Huang J. I., Mizuno H., Alfonso Z. C., Fraser J. K., Benhaim, Hedrick M. H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell. 2002;13:4279–4295. doi: 10.1091/mbc.e02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]