Abstract
Connecting peptide (C-peptide), a byproduct of insulin biosynthesis, has diverse cellular and biological functions. Particulate matter 2.5 (PM2.5) adversely affects human skin, leading to skin thickening, wrinkle formation, skin aging, and inflammation. This study aimed to investigate the protective effects of C-peptide against PM2.5-induced damage to skin cells, focusing on oxidative stress as a key mechanism. C-peptide mitigated NADPH oxidation and intracellular reactive oxygen species (ROS) production induced by PM2.5. It also suppressed PM2.5-induced NADPH oxidase (NOX) activity and alleviated PM2.5-induced NOX1 and NOX4 expression. C-peptide protected against PM2.5-induced DNA damage, lipid peroxidation, and protein carbonylation. Additionally, C-peptide mitigated PM2.5-induced apoptosis by inhibiting intracellular ROS production. In summary, our findings suggest that C-peptide mitigates PM2.5-induced apoptosis in human HaCaT keratinocytes by inhibiting intracellular ROS production and NOX activity.
Keywords: Particulate matter 2.5, C-peptide, Reactive oxygen species, NADPH oxidase
INTRODUCTION
Connecting peptide (C-peptide) comprises 31 residue peptides that connect the A and B chains of pro-insulin. When the pancreas enzymatically breaks down pro-insulin, C-peptide is released into the bloodstream along with insulin, a multifunctional molecule (Chan et al., 2020; Dakroub et al., 2024). Proinsulin is subsequently delivered via the golgi apparatus through secretory granules, where it undergoes further processing to produce insulin and C-peptide; both are released in equimolar amounts (Washburn et al., 2021). Insulin is metabolized by the liver through its specialized internalization and degradation mechanisms, whereas C-peptide is primarily metabolized by the kidneys and cleared through renal filtration (Pina et al., 2020; Sokooti et al., 2020). The half-life of C-peptide in the plasma is longer than that of insulin (Li et al., 2020a). C-peptide replacement therapy is effective in reducing diabetes-related complications (Pinger et al., 2017) and shows promise for treating chronic complications of type 1 diabetes. Both animal and human studies have demonstrated the efficacy of C-peptide to improve kidney function, nerve conduction velocity, and blood flow in the muscles, skin, and kidneys (Novac et al., 2019). C-peptide also protects against hyperglycemia-induced endothelial cell apoptosis by inhibiting transglutaminase activation, mitochondrial dysfunction, and reactive oxygen species (ROS) production, as well as by facilitating the activation of AMP-activated protein kinase α (Lee et al., 2020). Furthermore, C-peptide increases microvascular blood circulation in the skin of patients with type 1 diabetes, likely by increasing Na+, K+-ATPase activity, and nitric oxide production (Alves et al., 2018). Particulate matter with diameters of less than 2.5 µm in size (PM2.5) has various undesirable consequences on the human skin, such as skin thickening, wrinkle formation, aging, and skin inflammation (Zhen et al., 2019a). PM2.5 is recognized as a major disruptor of normal cellular functions and can induce mitochondrial swelling, DNA damage, endoplasmic reticulum stress, and autophagy (Piao et al., 2018). In particular, PM2.5 can generate ROS, induce skin inflammation via the NFκB–IL-6 pathway, and enhance senescence through the expression of senescence-associated gene (p16INK4a) (Ryu et al., 2019a, 2019b). PM2.5-induced ROS is produced through the aryl hydrocarbon receptor-medicated NADPH oxidase (NOX) system (Kang et al., 2024).
Despite the known impacts of PM2.5 on skin health, the information on the potential of C-peptide to ameliorate these adverse effects. Therefore, in the present study, we aimed to evaluate the protective effects of C-peptide against PM2.5-mediated NADPH oxidation and related adverse effects in human HaCaT keratinocytes.
MATERIALS AND METHODS
Preparation of samples
Human C-peptide was provided by Professor Kwon-Soo Ha (Kangwon National University, Chuncheon, Korea). Standard diesel PM2.5 (SRM 1650b) was purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA) and issued by the National Institute of Standards and Technology (NIST, Gaithersburg, MD, USA). PM2.5 was dissolved in dimethyl sulfoxide (DMSO) for further assessment (Fernando et al., 2022).
Cell culture
HaCaT keratinocytes (Cell Lines Service, Eppelheim, Germany) were grown in Dulbecco’s modified Eagle medium with 10% fetal bovine serum and antibiotic-antimycotic solution and maintained at 37°C with 5% CO2 (Zhen et al., 2023).
Cell viability
Cells were grown in a 96-well cell culture plate with a cell density of 1.0×105 cells per well. After 16 h of incubation, cells were treated at various concentrations of C-peptide (10, 20, 40, 80, or 100 nM) or C-peptide (40 nM) and PM2.5 (50 µg/mL), and incubated for 24 h. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) test was performed as previously described (Shilnikova et al., 2022).
ROS detection
Cells grown at a density of 1.5×105 cells/mL were exposed to C-peptide (10, 20, 40, 80, or 100 nM) and PM2.5 (50 µg/mL) and stained with 2′,7′-dichlorodihydrofluorescin diacetate (H2DCFDA; Molecular Probes, Eugene, OR, USA) or dihydrorhodamine 123 (DHR 123; Molecular Probes) to analyze intracellular and mitochondrial ROS levels. Data were obtained using a flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA), and a confocal microscope (Olympus, Tokyo, Japan) (Zhen et al., 2023).
NADP+/NADPH assay
The NADP+/NADPH ratio was assessed using a NADP+/NADPH assay kit (Abcam, Cambridge, MA, USA). Cells at a density of 1.5×105 cells/well were exposed to PM2.5 and C-peptide (50 µg/mL and 40 nM, respectively). NADP+/NADPH extraction was performed using 800 µL of NADP+/NADPH extraction buffer and the supernatants were analyzed following the manufacturer’s instructions (Zhen et al., 2019a).
Western blotting
Cell lysates were subjected to SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were incubated with primary antibodies against NOX1, NOX4, aryl hydrocarbon receptor (AhR), glutathione peroxidase (GPX), B-cell lymphoma-2-associated X protein (Bax), B-cell lymphoma-2 (Bcl-2), caspase-3, and caspase-9 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), nuclear factor erythroid 2-related factor 2 (NRF2) (Abcam), Cu/Zn superoxide dismutase (SOD) (Enzo Life Sciences, Farmingdale, NY, USA), or actin (Sigma-Aldrich Inc.) for 20 h. The membranes were then incubated with secondary antibodies at a 1:10000 dilution at 20°C for 1 h. Protein bands were visualized using a western blotting detection system (Amersham, Little Chalfont, Buckinghamshire, UK) (Ryu et al., 2019b).
Lipid peroxidation assay
Harvested cells were analyzed using an 8-isoprostane ELISA kit (Cayman Chemical, Ann Arbor, MI, USA) and the assessment was conducted by following the manufacturer’s instructions. Cells in the glass chamber slides were treated with C-peptide (40 nΜ) and PM2.5 for 24 h. The fluorescence of lipid peroxidation was detected by diphenyl-1-pyrenylphosphine (DPPP; Molecular Probes) and images were acquired using a confocal microscope (Olympus) (Zhen et al., 2019a).
Detection of 8-oxoguanine
The amount of 8-oxoguanine (8-OxoG) was analyzed using a Bioxytech 8-OHdG ELISA kit (Oxis International, Tampa, FL, USA) following the manufacturer’s instructions (Kim et al., 2017). Avidin-tetramethylrhodamine isothiocyanate (TRITC) conjugate (1:100) (Sigma-Aldrich Inc.) was utilized to observe 8-OxoG, and the stained cells were observed under a confocal microscope (Olympus) (Herath et al., 2024).
Protein carbonylation assay
Protein carbonylation was evaluated using an OxiSelectTM protein carbonyl ELISA kit (Cell Biolabs, San Diego, CA, USA) following the manufacturer’s instructions (Zhen et al., 2019b).
Quantification of Ca2+ level
Cells were stained using Rhod-2 acetoxymethyl ester (Rhod-2 AM; Molecular Probes) or Fluo-4 acetoxymethyl ester (Fluo-4 AM; Molecular Probes) to evaluate mitochondrial or cellular calcium levels. Images of the stained cells were captured via confocal microscopy (Olympus) (Piao et al., 2019).
Assessment of mitochondrial membrane potential (Δψm)
Cells were stained with 5,5′,6,6′-tetrachloro-1,1′,3,3′ tetraethylbenzimidazolyl-carbocyanine iodide (JC-1; Invitrogen, Carlsbad, CA, USA) for 30 min and images to assess ∆ψm were captured using a confocal microscope (Olympus) (Herath et al., 2022).
Detection of cellular apoptosis
Apoptotic bodies were observed and quantified by Hoechst 33342 staining (Sigma-Aldrich Inc.). Cells seeded in 24-well cell culture plates were treated with C-peptide for 30 min and 50 μg/mL PM2.5 for another 24 h. Cells were incubated with the Hoechst 33342 stain for 10 min and images were captured using a fluorescence microscope (Cybernetics, Silver Spring, MD, USA). Apoptotic cells were quantified by flow cytometry (Becton Dickinson), employing the Alexa Fluor 488 annexin V/dead cell apoptosis kit (Thermo Fisher Scientific Inc., Waltham, MA, USA) following the manufacturer’s instructions (Piao et al., 2019).
Statistical analysis
Groups were compared using analysis of variance and Tukey’s tests via SigmaStat version 3.5 (Systat Software Inc., San Jose, CA, USA). All data are shown as the means ± standard error. Values of p<0.05 were considered statistically significant.
RESULTS
C-peptide inhibited intracellular ROS generation, NADPH oxidation, and expressions of NOX1, NOX4, and AhR
We assessed the effect of C-peptide at various concentrations on cell viability in HaCaT keratinocytes using an MTT assay. We could not observe considerable cytotoxicity at any of the tested concentrations of C-peptide (10, 20, 40, 80, and 100 nM) (Fig. 1A). Next, we assessed the ability of C-peptide to scavenge PM2.5-induced intracellular ROS. The findings demonstrated that all tested concentrations of C-peptide resulted in a significant reduction in intracellular ROS levels induced by PM2.5 (Fig. 1B).
Fig. 1.
C-peptide inhibited intracellular ROS generation, and NADPH oxidation, NOX-related protein expression. (A) Effect of C-peptide (10, 20, 40, 80, and 100 nM, respectively) on cell viability assessed using MTT assay. (B-D) PM2.5-induced ROS scavenging ability of C-peptide assessed using H2DCFDA staining by employing (B, C) flow cytometry, and (D) confocal microscopy; NAC used as a positive control. *p<0.05 and #p<0.05, compared with the control and PM2.5-treated groups, respectively (n=3). (E) NADPH oxidation levels determined using the NADP+/NADPH ratio. *p<0.05 and #p<0.05, compared with the control and PM2.5-treated groups, respectively (n=3). (F, G) Inhibitory effects of C-peptide on (F) NOX1, NOX4, and (G) AhR expression assessed using western blotting. *p<0.05 and #p<0.05, compared with the control and PM2.5-treated groups, respectively (n=3).
Our previous studies demonstrated that 25, 50, 75, and 100 µg/mL of PM2.5 caused intracellular ROS generation in HaCaT cells in a dose-dependent manner and 50 µg/mL of PM2.5 showed significant cellular apoptosis (Piao et al., 2018). PM2.5 at 50 µg/mL dose reduced the HaCaT cell proliferation and arrested the cell cycle progression in the G0/G1 phase (Herath et al., 2022). PM2.5 at 50 µg/mL dose caused damage to cellular organelles, including endoplasmic reticulum and mitochondria (Piao et al., 2019). Therefore in this study, PM2.5 at 50 µg/mL was used to evaluate the protective ability of C-peptide against PM2.5-induced skin cell damage. Considering the effect of C-peptide on cell viability and its ROS-scavenging ability, we selected 40 nM of C-peptide for further evaluation.
Subsequently, we compared the intracellular ROS scavenging ability of 40 nM of C-peptide and NAC (a well-known antioxidant). NAC and C-peptide both showed significant reductions in PM2.5-induced intracellular ROS levels (Fig. 1C) and C-peptide showed significantly lower green fluorescence levels than PM2.5 (Fig. 1D), indicating that C-peptide mitigated intracellular ROS generation. Additionally, evaluation of NOX activity based on the NADP+/NADPH ratio revealed that PM2.5 enhanced NADPH oxidation, while C-peptide significantly attenuated this PM2.5-increased NADPH oxidation (Fig. 1E). Western blotting analysis revealed that PM2.5 increased the protein levels of NOX1, NOX4, and AhR; however, these protein levels were lower in C-peptide-treated cells than those in PM2.5-treated cells (Fig. 1F, 1G).
C-peptide attenuated PM2.5-induced lipid peroxidation, DNA damage, and protein carbonylation
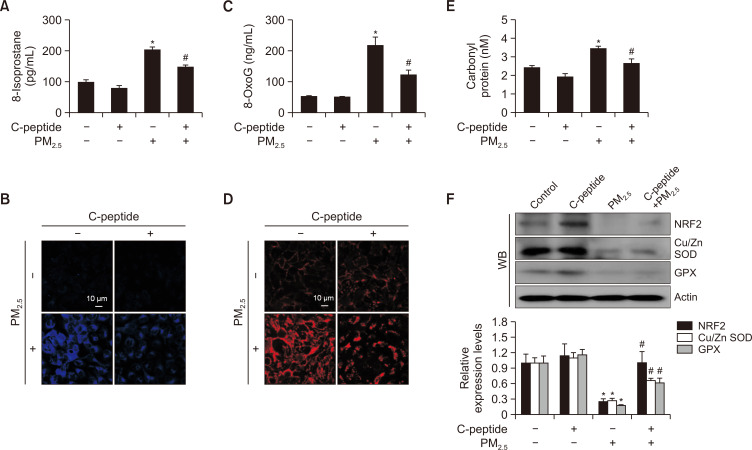
Lipid peroxidation was quantified by measuring the level of 8-isoprostane secreted into the culture medium. PM2.5-induced increase in 8-isoprostane level was attenuated by C-peptide (Fig. 2A). The levels of oxidized DPPP, an indicator of cellular lipid peroxidation, was reduced by C-peptide but increased by PM2.5 (Fig. 2B). Assessment of the cellular DNA damage using an 8-OxoG kit or avidin-TRITC staining demonstrated that C-peptide reduced PM2.5-induced 8-OxoG formation (Fig. 2C, 2D). Additionally, the increased protein carbonylation in PM2.5-treated cells was alleviated by C-peptide (Fig. 2E). NRF2 is the primary controller of the cellular defense system against oxidative stress, while Cu/Zn SOD, a cytosolic SOD, catalyzes the conversion of superoxide anion into H2O2, and GPX is essential for the breakdown of H2O2 (Shaw et al., 2020; Gusti et al., 2021; Djordjević et al., 2022). In this study, we showed that C-peptide restored the PM2.5-induced reductions in NRF2, Cu/Zn SOD, and GPX expression levels (Fig. 2F).
Fig. 2.
C-peptide suppressed the PM2.5-mediated cellular macromolecule damage. (A) Lipid peroxidation quantified by measuring the 8-isoprostane level. *p<0.05 and #p<0.05, compared to the control and PM2.5-treated groups, respectively (n=3). (B) Lipid peroxidation evaluated using confocal microscopy after DPPP staining. (C) DNA damage evaluated by measuring the 8-OxoG level. *p<0.05 and #p<0.05, compared to the control and PM2.5-treated groups, respectively (n=3). (D) Formation of 8-OxoG examined through avidin-TRITC conjugate staining; images were collected using confocal microscopy. (E) Protein oxidation investigated by assessing carbonyl formation level. *p<0.05 and #p<0.05, compared to the control and PM2.5-treated groups, respectively (n=3). (F) Expression of NRF2, Cu/Zn SOD, and GPX determined using western blotting. *p<0.05 and #p<0.05, compared with the control and PM2.5-treated groups, respectively (n=3).
C-peptide attenuated PM2.5-induced mitochondrial stress and apoptosis
Mitochondrial ROS levels elevated by PM2.5 were attenuated by C-peptide (Fig. 3A). Confocal microscopy revealed increased green fluorescence of Fluo-4 AM in the PM2.5-treated group than that in the control group, indicating that PM2.5 elevated intracellular Ca2+ level, which was reduced in the C-peptide-treated group (Fig. 3B). Flow cytometric assessment revealed that both C-peptide and NAC reduced PM2.5-induced mitochondrial Ca2+ level (Fig. 3C). Confocal microscopy also revealed increased mitochondrial Ca2+ level in PM2.5-treated group, which was reduced in C-peptide-treated group (Fig. 3D). Additionally, PM2.5 increased mitochondria depolarization, which was notably reduced by C-peptide and NAC (Fig. 3E, 3F). PM2.5 increased the expression levels of Bax, cleaved caspase-9, and cleaved caspase-3, and decreased that of Bcl-2; however, C-peptide reversed these alterations in the expression of the pro- and anti-apoptotic markers (Fig. 3G). PM2.5 also increased nuclear condensation compared with the control cells (apoptotic index 4.4 vs. 1.0), while both C-peptide and NAC reduced nuclear condensation (apoptotic index 2.3, and 1.9, respectively; Fig. 3H). Furthermore, annexin V/PI staining indicated that C-peptide and NAC (14 and 12%, respectively) alleviated PM2.5-induced cellular apoptosis (30%) (Fig. 3I). MTT assay showed that PM2.5 reduced the cell viability to 57%, whereas C-peptide increased it to 70% (Fig. 3J).
Fig. 3.
C-peptide suppressed the PM2.5-induced mitochondrial stress and cell apoptosis. (A) The mitochondrial ROS levels assessed using confocal microscopy following DHR123 staining. (B) Total cellular Ca2+ level evaluated using Fluo-4 AM staining. (C, D) Mitochondrial Ca2+ level stained with Rhod-2 AM evaluated using (C) flow cytometer and (D) confocal microscopy. *p<0.05 and #p<0.05, compared to the control and PM2.5-treated groups, respectively (n=3). (E, F) Mitochondrial membrane potential (Δψm) assessed using JC-1 staining and evaluated by (E) confocal microscopy and (F) flow cytometry. *p<0.05 and #p<0.05, compared to the control and PM2.5-treated groups, respectively (n=3). (G) Expression of Bax, Bcl-2, cleaved caspase-9, and cleaved caspase-3 detected using western blotting. *p<0.05 and #p<0.05, compared to the control and PM2.5-treated groups, respectively (n=3). (H) Count of apoptotic bodies after Hoechst 33342 staining. *p<0.05 and #p<0.05, compared to the control and PM2.5-treated groups, respectively (n=3). (I) Cellular apoptosis detected using annexin V/PI staining. *p<0.05 and #p<0.05 compared to control and PM2.5-treated groups, respectively (n=3). (J) Cell viability evaluated using MTT assay. *p<0.05 and #p<0.05 compared to control and PM2.5-treated groups, respectively (n=3).
C-peptide reduced apoptosis by inhibiting NOX activity
Next, we evaluated the inhibitory effects of C-peptide and diphenyleneiodonium (DPI), a flavoprotein inhibitor that inhibits all NOXs (Kouki et al., 2023), on apoptosis using Hoechst 33342 staining. PM2.5 increased the apoptotic index (4.5) compared to C-peptide and DPI (2.6 and 1.8, respectively; Fig. 4A), with no significant difference between C-peptide and DPI. The findings also showed that the PM2.5-induced cellular apoptosis (17%) was alleviated by the C-peptide, DPI, and the combination of C-peptide and DPI (7, 6, and 5%, respectively; Fig. 4B). Additionally, both C-peptide and DPI reversed the PM2.5-induced increase in intracellular ROS (Fig. 4C).
Fig. 4.
C-peptide attenuated cell apoptosis by inhibiting NOX activity. (A) Count of apoptotic bodies after Hoechst 33342 staining. *p<0.05 and #p<0.05 compared to control and PM2.5-treated groups, respectively (n=3). (B) Cellular apoptosis evaluated using annexin V/PI staining. *p<0.05 and #p<0.05 compared to control and PM2.5-treated groups, respectively (n=3). (C) Intracellular ROS levels detected using H2DCFDA staining. *p<0.05 and #p<0.05 compared to control and PM2.5-treated groups, respectively (n=3).
DISCUSSION
C-peptide has demonstrated benefits in enhancing kidney function, nerve conduction velocity, and blood flow in muscles, skin, and kidneys, therefore, being considered as a potential therapy for chronic problems associated with type 1 diabetes (Novac et al., 2019). Previous studies have suggested that C-peptide augments skin conditions through its angiogenic potential and inhibits vascular permeability in the peripheral skin vessels as well as improves the wound healing ability of diabetic mice (Lim et al., 2015; Novac et al., 2019; Souto et al., 2020).
NADPH, the reduced form of NADP+, serves as a redox cofactor that plays key roles in various cellular activities such as metabolism, proliferation, and antioxidant defense (Bui et al., 2023; White and Someya, 2023). NOXs are enzymes that produce superoxide anion or hydrogen peroxide by using molecular oxygen and NADPH as an electron donor. Our study demonstrated that PM2.5 elevated the NADP+/NADPH ratio compared with the control, which was significantly attenuated by C-peptide. NOXs are a class of electron-transporting membrane enzymes that predominantly function to generate ROS (Cipriano et al., 2023). NOX1 is important in the leakage of electrons in the mitochondria and leads to O2− generation and NOX4 facilitates H2O2 production (Helfinger et al., 2019; Muñoz et al., 2020; Ganguly et al., 2023). Our findings showed that PM2.5-induced unfavorable increases in NOX1 and NOX4 activities were attenuated by C-peptide.
PM2.5 activates AhR, leading to increased ROS generation (Peng et al., 2019), which affects the mitochondrial membrane potential and contributes to inflammation and apoptosis in the skin, specifically in epidermal keratinocytes (Aghaei-Zarch et al., 2023). Our findings indicated that PM2.5 activated AhR and increased ROS generation, while C-peptide notably reduced these effects. We also assessed the ability of C-peptide to counteract the PM2.5-mediated damage to vital cellular components. PM2.5-induced ROS results in cellular lipid peroxidation, protein carbonylation, as well as DNA damage (Herath et al., 2024). In this study, PM2.5 led to increased levels of 8-isoprostane and lipid peroxidation, both of which were reversed by C-peptide treatment. PM2.5-mediated redox imbalance can facilitate damage to the DNA and the formation of 8-OxoG, which is a biomarker of oxidative DNA damage (Shi et al., 2022). Concordantly, PM2.5 increased the 8-OxoG level in the present study. Nevertheless, C-peptide ameliorated the PM2.5-induced oxidative DNA damage, as indicated by decreased level of 8-OxoG. Additionally, the PM2.5-induced protein carbonyl formation was also significantly attenuated by C-peptide.
Oxidative stress contributes to mitochondrial oxidative damage and cell apoptosis (Zhuang et al., 2020). Our findings showed that PM2.5 increased mitochondrial ROS levels, which were significantly attenuated by C-peptide. Increased production of mitochondrial ROS may result in excessive accumulation of Ca2+ in the mitochondria, which is dependent on the mitochondrial Ca2+ uniporter, and eventually lead to cell death (Antonucci et al., 2021). Nevertheless, C-peptide also reduced both PM2.5-induced excessive mitochondrial Ca2+ accumulation and mitochondrial membrane depolarization. Excessive mitochondrial Ca2+ facilitates the opening of the mitochondrial permeability transition pore and the release of pro-apoptotic factors like cytochrome c, as well as activates the caspases (Li et al., 2020b). In the present study, PM2.5 upregulated the pro-apoptotic markers; caspase-3, caspase-9, and Bax, and downregulated the anti-apoptotic marker Bcl-2. On the contrary, C-peptide reversed these effects, suggesting that C-peptide can effectively ameliorate PM2.5-induced cellular apoptosis. This phenomenon was further confirmed by Hoechst 33342 staining; PM2.5-treated cells displayed a significantly higher apoptotic index than C-peptide-pretreated cells. Together, these findings demonstrate that C-peptide can inhibit PM2.5-induced apoptosis and restore cell viability.
We also explored the role of NOXs in the inhibitory action of C-peptide on PM2.5-induced apoptosis using DPI, a NOX inhibitor. Both C-peptide and DPI significantly ameliorated PM2.5-induced apoptosis, suggesting that the protective effects of C-peptide are primarily due to its inhibition of NOX activity. Furthermore, both C-peptide and DPI inhibited PM2.5-induced intracellular ROS generation. Therefore, the ability of C-peptide to inhibit apoptosis is largely mediated by its suppression of NOX activity and ROS generation. Previous studies have demonstrated that C-peptide inhibits protein kinase C (PKC) dependent NOX2 activity, thereby reducing the generation of ROS in both the cytosol and mitochondria (Bhatt et al., 2014), which is critical for preventing oxidative stress and cellular damage. Additionally, C-peptide has been shown to act as an endogenous antioxidant molecule in glucose-exposed endothelial cells by inhibiting the translocation of small GTP binding protein Rac1 (RAC-1), a rho family protein, to the membrane, a process necessary for the activation of NOX (Cifarelli et al., 2011). Therefore, we speculate, that C-peptide will interact with PKC and RAC-1, for the regulation of NOX activity.
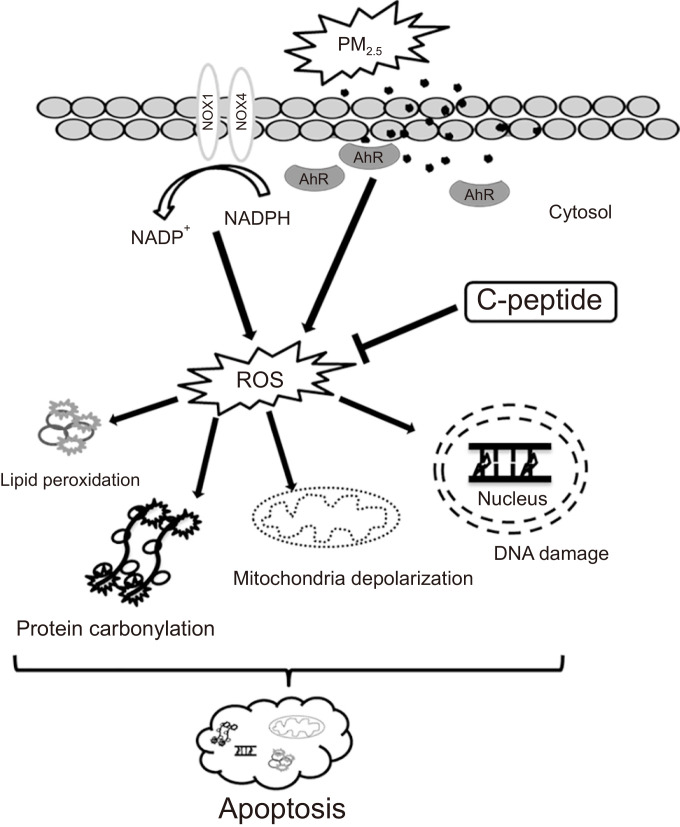
In summary, the findings of the present study suggest the potency of C-peptide to mitigate PM2.5-induced oxidative stress-mediated cellular damage in human HaCaT keratinocytes (Fig. 5). We demonstrated that C-peptide attenuated PM2.5-induced NADPH oxidation and cellular ROS generation by inhibiting PM2.5-induced NOX1 and NOX4 expressions, resulting in the inhibition of PM2.5-induced skin cell damage.
Fig. 5.
Schematic diagram of the protective ability of C-peptide against PM2.5-mediated keratinocyte cell damage. C-peptide inhibited PM2.5-induced NADPH oxidation and intracellular ROS generation. Then, C-peptide alleviated PM2.5-induced DNA damage, lipid peroxidation, protein carbonylation, mitochondrial damage, and cell apoptosis.
ACKNOWLEDGMENTS
This study was supported by the Basic Research Laboratory Program (NRF-2017R1A4A1014512) from the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning. This research was also supported by the Basic Science Research Program through NRF, funded by the Ministry of Education (RS-2023-00270936).
Footnotes
CONFLICT OF INTEREST
The authors declare no competing interests.
REFERENCES
- Aghaei-Zarch S. M., Nia A. H. S., Nouri M., Mousavinasab F., Najafi S., Bagheri-Mohammadi S., Aghaei-Zarch F., Toolabi A., Rasoulzadeh H., Ghanavi J., Moghadam M. N., Talebi M. The impact of particulate matters on apoptosis in various organs: Mechanistic and therapeutic perspectives. Biomed. Pharmacother. 2023;165:115054. doi: 10.1016/j.biopha.2023.115054. [DOI] [PubMed] [Google Scholar]
- Alves M. T., Ortiz M. M., dos Reis G. V. O. P., Dusse L. M. S., das Graças Carvalho M., Fernandes A. P., Gomes K. B. The dual effect of C-peptide on cellular activation and atherosclerosis: protective or not? Diabetes Metab. Res. Rev. 2018;35:e3071. doi: 10.1002/dmrr.3071. [DOI] [PubMed] [Google Scholar]
- Antonucci S., Di Lisa F., Kaludercic N. Mitochondrial reactive oxygen species in physiology and disease. Cell Calcium. 2021;94:102344. doi: 10.1016/j.ceca.2020.102344. [DOI] [PubMed] [Google Scholar]
- Bhatt M. P., Lim Y. C., Ha K. S. C-peptide replacement therapy as an emerging strategy for preventing diabetic vasculopathy. Cardiovasc. Res. 2014;104:234–244. doi: 10.1093/cvr/cvu211. [DOI] [PubMed] [Google Scholar]
- Bui C. V., Boswell C. W., Ciruna B., Rocheleau J. V. Apollo-NADP+ reveals in vivo adaptation of NADPH/NADP+ metabolism in electrically activated pancreatic β cells. Sci. Adv. 2023;9:eadi8317. doi: 10.1126/sciadv.adi8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K. H., Lim J., Jee J. E., Aw J. H., Lee S. S. Peptide-peptide co-assembly: A design strategy for functional detection of C-peptide, abiomarker of diabetic neuropathy. Int. J. Mol. Sci. 2020;21:9671. doi: 10.3390/ijms21249671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifarelli V., Geng X., Styche A., Lakomy R., Trucco M., Luppi P. C-peptide reduces high-glucose-induced apoptosis of endothelial cells and decreases NAD(P)H-oxidase reactive oxygen species generation in human aortic endothelial cells. Diabetologia. 2011;54:2702–2712. doi: 10.1007/s00125-011-2251-0. [DOI] [PubMed] [Google Scholar]
- Cipriano A., Viviano M., Feoli A., Milite C., Sarno G., Castellano S., Sbardella G. NADPH oxidases: from molecular mechanisms to current inhibitors. J. Med. Chem. 2023;66:11632–11655. doi: 10.1021/acs.jmedchem.3c00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakroub A., Dbouk A., Asfour A., Nasser S. A., El-Yazbi A. F., Sahebkar A., Eid A. A., Iratni R., Eid A. H. C-peptide in diabetes: a player in a dual hormone disorder? J. Cell Physiol. 2024;239:e31212. doi: 10.1002/jcp.31212. [DOI] [PubMed] [Google Scholar]
- Djordjević V. V., Kostić J., Krivokapić Ž., Krtinić D., Ranković M., Petković M., Ćosić V. Decreased activity of erythrocyte catalase and glutathione peroxidase in patients with schizophrenia. Medicina. 2022;58:1491. doi: 10.3390/medicina58101491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando P. D. S. M., Piao M. J., Kang K. A., Zhen A. X., Herath H. M. U. L., Kang H. K., Choi Y. H., Hyun J. W. Hesperidin protects human HaCaT keratinocytes from particulate matter 2.5-induced apoptosis via the inhibition of oxidative stress and autophagy. Antioxidants. 2022;11:1363. doi: 10.3390/antiox11071363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly R., Ngoruh A., Ingty P., Yadav S. K., Bhattacharjee A. Identification of an inhibitor for atherosclerotic enzyme NOX-1 to inhibit ROS production. Futur. J. Pharm. Sci. 2023;9:24. doi: 10.1186/s43094-023-00474-4. [DOI] [Google Scholar]
- Gusti A. M. T., Qusti S. Y., Alshammari E. M., Toraih E. A., Fawzy M. S. Antioxidants-related superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), glutathione-s-transferase (GST), and nitric oxide synthase (NOS) gene variants analysis in an obese population: a preliminary case-control study. Antioxidants. 2021;10:595. doi: 10.3390/antiox10040595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfinger V., Palfi K., Weigert A., Schröder K. The NADPH oxidase Nox4 controls macrophage polarization in an NFκB-dependent manner. Oxid. Med. Cell. Longev. 2019;2019:3264858. doi: 10.1155/2019/3264858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herath H. M. U. L., Piao M. J., Kang K. A., Fernando P. D. S. M., Hyun J. W. Rosmarinic acid protects skin keratinocytes from particulate matter 2.5-induced apoptosis. Int. J. Med. Sci. 2024;21:681–689. doi: 10.7150/ijms.90814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herath H. M. U. L., Piao M. J., Kang K. A., Zhen A. X., Fernando P. D. S. M., Kang H. K., Yi J. M., Hyun J. W. Hesperidin exhibits protective effects against PM2.5-mediated mitochondrial damage, cell cycle arrest, and cellular senescence in human HaCaT keratinocytes. Molecules. 2022;27:4800. doi: 10.3390/molecules27154800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K. A., Piao M. J., Fernando P. D. S. M., Herath H. M. U. L., Yi J. M., Choi Y. H., Hyun Y. M., Zhang K., Park C. O., Hyun J. W. Particulate matter stimulates the NADPH oxidase system via AhR-mediated epigenetic modifications. Environ. Poll. 2024;347:123675. doi: 10.1016/j.envpol.2024.123675. [DOI] [PubMed] [Google Scholar]
- Kim K. C., Ruwan Kumara M. H. S., Kang K. A., Piao M. J., Oh M. C., Ryu Y. S., Jo J. O., Mok Y. S., Shin J. H., Park Y., Kim S. B., Yoo S. J., Hyun J. W. Exposure of keratinocytes to non-thermal dielectric barrier discharge plasma increases the level of 8-oxoguanine via inhibition of its repair enzyme. Mol. Med. Rep. 2017;16:6870–6875. doi: 10.3892/mmr.2017.7454. [DOI] [PubMed] [Google Scholar]
- Kouki A., Ferjani W., Ghanem-Boughanmi N., Ben-Attia M., Dang P. M., Souli A., El-Benna J. The NADPH oxidase inhibitors apocynin and diphenyleneiodonium protect rats from LPS-induced pulmonary inflammation. Antioxidants. 2023;12:770. doi: 10.3390/antiox12030770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. J., Lee Y. J., Jeon H. Y., Kim M., Han E. T., Park W. S., Hong S. H., Kim Y. M., Ha K. S. Application of elastin-like biopolymer-conjugated C-peptide hydrogel for systemic long-term delivery against diabetic aortic dysfunction. Acta Biomater. 2020;118:32–43. doi: 10.1016/j.actbio.2020.09.055. [DOI] [PubMed] [Google Scholar]
- Li M., Song L., Yuan J., Zhang D., Zhang C., Liu Y., Lin Q., Wang H., Su K., Li Y., Ma Z., Liu D., Dong J. Association between serum insulin and C-peptide levels and breast cancer: An updated systematic review and meta-analysis. Front. Oncol. 2020a;10:553332. doi: 10.3389/fonc.2020.553332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wang C., Lian Y., Zhang H., Meng X., Yu M., Li Y., Xie N. Role of the mitochondrial calcium uniporter in Mg2+-free-induced epileptic hippocampal neuronal apoptosis. Int. J. Neurosci. 2020b;130:1024–1032. doi: 10.1080/00207454.2020.1715978. [DOI] [PubMed] [Google Scholar]
- Lim Y. C., Bhatt M. P., Kwon M. H., Park D., Na S., Kim Y. M., Ha K. S. Proinsulin C-peptide prevents impaired wound healing by activating angiogenesis in diabetes. J. Invest. Dermatol. 2015;135:269–278. doi: 10.1038/jid.2014.285. [DOI] [PubMed] [Google Scholar]
- Muñoz M., López-Oliva M. E., Rodríguez C., Martínez M. P., Sáenz-Medina J., Sánchez A., Climent B., Benedito S., García-Sacristán A., Rivera L., Hernández M., Prieto D. Differential contribution of Nox1, Nox2 and Nox4 to kidney vascular oxidative stress and endothelial dysfunction in obesity. Redox Biol. 2020;28:101330. doi: 10.1016/j.redox.2019.101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novac C., Radulian G., Orzan A., Balgradean M. Short update on C-peptide and its clinical value. Maedica. 2019;14:53–58. doi: 10.26574/maedica.2019.14.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng F., Tsuji G., Zhang J., Chen Z., Furue M. Potential role of PM2.5 in melanogenesis. Environ. Int. 2019;132:105063. doi: 10.1016/j.envint.2019.105063. [DOI] [PubMed] [Google Scholar]
- Piao M. J., Ahn M. J., Kang K. A., Ryu Y. S., Hyun Y. J., Shilinikova K., Zhen A. O., Jeong J. W., Choi Y. H., Kang H. K., Koh Y. S., Hyun J. W. Particulate matter 2.5 damages skin cells by inducing oxidative stress, subcellular organelle dysfunction, and apoptosis. Arch. Toxicol. 2018;92:2077–2091. doi: 10.1007/s00204-018-2197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao M. J., Kang K. A., Zhen A. X., Fernando P. D. S. M., Ahn M. J., Koh Y. S., Kang H. K., Yi J. M., Choi Y. H., Hyun J. W. Particulate matter 2.5 mediates cutaneous cellular injury by inducing mitochondria-associated endoplasmic reticulum stress: protective effects of ginsenoside Rb1. Antioxidants. 2019;8:383–393. doi: 10.3390/antiox8090383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pina A. F., Borges D. O., Meneses M. J., Branco P., Birne R., Vilasi A., Macedo M. P. Insulin: trigger and target of renal functions. Front. Cell Dev. Biol. 2020;8:519. doi: 10.3389/fcell.2020.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinger C. W., Entwistle K. E., Bell T. M., Liu Y., Spence D. M. C-Peptide replacement therapy in type 1 diabetes: are we in the trough of disillusionment? Mol. Biosyst. 2017;13:1432–1437. doi: 10.1039/C7MB00199A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu Y. S., Kang K. A., Piao M. J., Ahn M. J., Yi J. M., Bossis G., Hyun Y. M., Park C. O., Hyun J. W. Particulate matter-induced senescence of skin keratinocytes involves oxidative stress-dependent epigenetic modifications. Exp. Mol. Med. 2019b;51:1–14. doi: 10.1038/s12276-019-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu Y. S., Kang K. A., Piao M. J., Ahn M. J., Yi J. M., Hyun Y. M., Kim S. H., Ko M. K., Park C. O., Hyun J. W. Particulate matter induces inflammatory cytokine production via activation of NFκB by TLR5-NOX4-ROS signaling in human skin keratinocyte and mouse skin. Redox Biol. 2019a;21:101080. doi: 10.1016/j.redox.2018.101080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Chattopadhyay A. Nrf2-ARE signaling in cellular protection: mechanism of action and the regulatory mechanisms. J. Cell. Physiol. 2020;235:3119–3130. doi: 10.1002/jcp.29219. [DOI] [PubMed] [Google Scholar]
- Shi F., Zhang Z., Cui H., Wang J., Wang Y., Tang Y., Yang W., Zou P., Ling X., Han F., Liu J., Chen Q., Liu C., Cao J., Ao L. Analysis by transcriptomics and metabolomics for the proliferation inhibition and dysfunction through redox imbalance-mediated DNA damage response and ferroptosis in male reproduction of mice and TM4 Sertoli cells exposed to PM2.5. Ecotoxicol. Environ. Saf. 2022;238:113569. doi: 10.1016/j.ecoenv.2022.113569. [DOI] [PubMed] [Google Scholar]
- Shilnikova K., Piao M. J., Kang K. A., Fernando P. D. S. M., Herath H. M. U. L., Cho S. J., Hyun J. W. Natural compound shikonin induces apoptosis and attenuates epithelial to mesenchymal transition in radiation-resistant human colon cancer cells. Biomol. Ther. (Seoul) 2022;30:137–144. doi: 10.4062/biomolther.2021.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokooti S., Kieneker L. M., Borst M. H., Muller Kobold A., Kootstra-Ros J. E., Gloerich J., van Gool A. J., Heerspink H. J. L., Gansevoort R., Dullaart R. P. F., Bakker S. J. L. Plasma C-peptide and risk of developing type 2 diabetes in the general population. J. Clin. Med. 2020;9:3001. doi: 10.3390/jcm9093001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souto S. B., Campos J. R., Fangueiro J. F., Silva A. M., Cicero N., Lucarini M., Durazzo A., Santini A., Souto E. B. Multiple cell signalling pathways of human proinsulin C-peptide in vasculopathy protection. Int. J. Mol. Sci. 2020;21:645. doi: 10.3390/ijms21020645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn R. L., Mueller K., Kaur G., Moreno T., Moustaid-Moussa N., Ramalingam L., Dufour J. M. C-peptide as a therapy for type 1 diabetes mellitus. Biomedicines. 2021;9:270. doi: 10.3390/biomedicines9030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K., Someya S. The roles of NADPH and isocitrate dehydrogenase in cochlear mitochondrial antioxidant defense and aging. Hear. Res. 2023;427:108659. doi: 10.1016/j.heares.2022.108659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen A. X., Piao M. J., Hyun Y. J., Kang K. A., Ryu Y. S., Cho S. J., Kang H. K., Koh Y. S., Ahn M. J., Kim T. H., Hyun J. W. Purpurogallin protects keratinocytes from damage and apoptosis induced by ultraviolet B radiation and particulate matter 2.5. Biomol. Ther. (Seoul) 2019b;27:395–403. doi: 10.4062/biomolther.2018.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen A. X., Piao M. J., Kang K. A., Fernando P. D. S. M., Herath H. M. U. L., Cho S. J., Hyun J. W. 3-Bromo-4,5-dihydroxybenzaldehyde protects keratinocytes from particulate matter 2.5-induced damages. Antioxidants. 2023;12:1307. doi: 10.3390/antiox12061307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen A. X., Piao M. J., Kang K. A., Fernando P. D. S. M., Kang H. K., Koh Y. S., Yi J. M., Hyun J. W. Niacinamide protects skin cells from oxidative stress induced by particulate matter. Biomol. Ther. (Seoul) 2019a;27:562–569. doi: 10.4062/biomolther.2019.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang C., Ni S., Yang Z. C., Liu R. P. Oxidative stress induces chondrocyte apoptosis through caspase-dependent and caspase-independent mitochondrial pathways and the antioxidant mechanism of angelica sinensis polysaccharide. Oxid. Med. Cell. Longev. 2020;2020:3240820. doi: 10.1155/2020/3240820. [DOI] [PMC free article] [PubMed] [Google Scholar]