Abstract
We identified drugs or mechanisms targeting ABCB1 (or P-glycoprotein; P-gp)-overexpressing drug-resistant cancer populations, given that these cells play a key role in tumor recurrence. Specifically, we searched for Akt inhibitors that could increase cytotoxicity in P-gp-overexpressing drug-resistant cancer cells. We performed cytotoxicity assays using five cell lines: 1. MCF-7/ADR, 2. KBV20C cancer cells (P-gp overexpression, vincristine [VIC] resistance, and GSK690693-resistance), 3. MCF-7, 4. normal HaCaT cells (non-P-gp-overexpressing, VIC-sensitive, and GSK690693-sensitive), and 5. MDA-MB-231 cancer cells (non-P-gp overexpression, relatively VIC-resistance, and GSK690693-sensitive). Herein, we found that low-dose perifosine markedly and selectively sensitizes both MCF-7/ADR and KBV20C drug-resistant cancer cells exhibiting P-gp overexpression. Compared with other Akt inhibitors (AZD5363, BKM120, and GSK690693), low-dose perifosine specifically sensitized P-gp-overexpressing resistant MCF-7/ADR cancer cells. Conversely, Akt inhibitors (other than perifosine) could enhance sensitization effects in drug-sensitive MCF-7 and HaCaT cells. Considering that perifosine has both an alkyl-phospholipid structure and is an allosteric inhibitor for membrane-localizing Akt-targeting, we examined structurally and functionally similar Akt inhibitors (miltefosine and MK-2206). However, we found that these inhibitors were non-specific, suggesting that the specificity of perifosine in P-gp-overexpressing resistant cancer cells is unrelated to phospholipid localizing membranes or allosteric inhibition. Furthermore, we examined the molecular mechanism of low-dose perifosine in drug-resistant MCF-7/ADR cancer cells. MCF-7/ADR cells exhibited increased apoptosis via G2 arrest and autophagy induction. However, no increase in P-gp-inhibitory activity was observed in drug-resistant MCF-7/ADR cancer cells. Single low-dose perifosine treatment exerted a sensitization effect similar to co-treatment with VIC in P-gp-overexpressing drug-resistant MCF-7/ADR cancer cells, suggesting that single treatment with low-dose perifosine is a more powerful tool against P-gp-overexpressing drug-resistant cancer cells. These findings could contribute to its clinical use as a first-line treatment, explicitly targeting P-gp-overexpressing resistant cancer populations in heterogeneous tumor populations. Therefore, perifosine may be valuable in delaying or reducing cancer recurrence by targeting P-gp-overexpressing drug-resistant cancer cells.
Keywords: Pefifosine, Co-treatment, Cancer, P-gp, Drug-resistance
INTRODUCTION
Tumors are heterogeneous populations that mainly comprise antimitotic drug-sensitive cancer cells (Al-Lazikani et al., 2012; Conde et al., 2013; Brown et al., 2014). However, only a small proportion of tumors comprise drug-resistant cancer cells (Yoon and Kim, 2023). Drug-resistant cancer cells also include cancer stem cells (Cho and Kim, 2020; Robinson and Tiriveedhi, 2020; Seelig, 2020). Although initial antimitotic tumor therapeutics were found to reduce proliferation and apoptosis in drug-sensitive cancer populations, they also increased drug-resistant cancer populations (Conde et al., 2013; Yoon and Kim, 2023). In addition, an increase in drug-induced resistant cancer cell populations has been documented, resulting in tumor relapse (Gote et al., 2021; Shaikh and McClelland, 2021).
Furthermore, relapsed tumors exhibit an increased number of resistant cancer cells (Conde et al., 2013; Vucicevic et al., 2018; Yoon and Kim, 2023). Hence, antimitotic drug treatments fail to achieve tumor sensitization, affording no reduction in size or proliferation. Therefore, it is crucial to reduce drug-resistant cancer cell populations via apoptosis in heterogeneous tumor populations during the initial or first-line chemotherapeutic drug treatment.
Resistant cancer populations exhibit upregulated membrane overexpression of ABCB1 (P-glycoprotein, P-gp) (Shaikh and McClelland, 2021; Yoon et al., 2021; Yoon and Kim, 2023). One critical mechanism for evading antimitotic drug toxicity is actively pumping out antimitotic drugs as substrates within cancer cells to reduce their internal toxicity (Yang et al., 2008; Chen et al., 2016; Yoon et al., 2021). Therefore, targeting the P-gp-overexpressing-resistant cancer population could delay tumor recurrence following first-line antimitotic treatment. The selection of low-toxicity agents is important to establish a first-line treatment protocol. P-gp-inhibiting drugs are toxic to normal tissues, and treatment with these agents can simultaneously impede immune cell attacks on tumors in cancer microenvironments (Cho and Kim, 2020; Robinson and Tiriveedhi, 2020; Seelig, 2020). Therefore, the toxicity of P-gp inhibition should be minimized to target the P-gp-overexpressing resistant populations in tumors. As a potential strategy to hasten clinical trials, drug repositioning of US Food and Drug Administration (FDA)-approved drugs or drugs in clinical trials should be considered (Clark, 2013; Pantziarka and Cairns, 2014; Yoon and Kim, 2021; Yoon et al., 2021; Yoon and Kim, 2022). FDA-approved drugs identified to target P-gp-overexpressing drug-resistant cancer populations could be easily repositioned for targeting resistant cancer cells. Accordingly, we investigated repositioned drugs capable of specifically sensitizing P-gp-overexpressing resistant cancer cells compared with drug-sensitive cancer cells (Kim et al., 2019, 2020; Jiang et al., 2021; Lee et al., 2022a, 2022c; Oh et al., 2022a, 2022b; Park et al., 2023).
The PI3K/Akt/mTOR signaling pathway is highly activated in several cancers and can enhance growth-regulating signals (Keane et al., 2014; Uko et al., 2020; Toson et al., 2022). Akt-targeting inhibitors have been developed to inhibit the Akt signaling pathway, targeting ATP-binding sites as competitive inhibitors (Keane et al., 2014; Uko et al., 2020; Toson et al., 2022). Furthermore, Akt inhibitors, such as allosteric inhibitors, targeting other Akt domains (kinase catalytic domain or pleckstrin homology domain) have been developed to block their activated open structures (Keane et al., 2014; Uko et al., 2020; Toson et al., 2022). Various Akt-targeting inhibitors have also been designed to specifically localize to the cytoplasm or cellular membranes to block its activation (Keane et al., 2014; Uko et al., 2020; Toson et al., 2022).
We identified Akt inhibitors that selectively sensitized P-gp-overexpressing resistant cancer cells (Kim et al., 2013; Choi et al., 2014, 2015, 2016). In the present study, we focused on identifying low-dose Akt inhibitors with reduced toxicity for clinical application. Herein, we found that low-dose perifosine could markedly and selectively increase the cytotoxicity of P-gp-overexpressing resistant cancer cells (MCF-7/ADR and KBV20C), whereas non-P-gp-overexpressing resistant cancer cell lines (MCF-7, HaCaT, and MDA-MB-231) were considerably less sensitive to the same perifosine dose. Furthermore, we examined mechanisms through which perifosine can sensitize P-gp-overexpressing resistant cancer cells. Considering that perifosine is a phase II and III clinical drug under investigation for different cancer types (Argiris et al., 2006; Marsh Rde et al., 2007; Bendell et al., 2011; Cho et al., 2012; Fu et al., 2012), we assumed that our findings with low-dose perifosine could stimulate its application as a first-line treatment for targeting P-gp-overexpressing drug-resistant cancer populations.
MATERIALS AND METHODS
Reagents and cell culture
Perifosine, BKM120, AZD5363, GSK690693, MK-2206, miltefosine, aripiprazole, and tariquidar were purchased from Selleckchem (Houston, TX, USA). Verapamil and rhodamine 123 were purchased from Sigma-Aldrich (St. Louis, MO, USA). VIC was obtained from Enzo Life Sciences (Farmingdale, NY, USA). C-PARP and α-LC3B antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). GAPDH antibodies were acquired from Santa Cruz Biotechnology (Santa Cruz, CA, USA). KBV20C, MCF-7/ADR, P-gp-over-expressing MDR cancer cell lines, were previously described in detail and used for a long period (Lee et al., 2022a, 2022c; Oh et al., 2022a, 2022b; Park et al., 2023). HaCaT cells were previously used (Chan Kwon et al., 2020). MDA-MB-231, MCF-7 cells were also used previously (Lee et al., 2022b, 2022c; Park et al., 2023). To perform the experiments, the cells were grown in DMEM or RPMI1640 (WelGENE, Daegu, Korea) supplemented with fetal bovine serum (FBS) and antibiotics (penicillin and streptomycin).
Microscopic observation
Cellular morphology and density were qualitatively examined using a microscope, as previously described (Lee et al., 2022b, 2022c; Oh et al., 2022b; Park et al., 2023). MCF-7, MCF-7/ADR, HaCaT, MDA-MB-231, and KBV20C cancer cells were cultured and stimulated with 5 nM VIC, 5-10 μM perifosine, 1 μM BKM120, 10 μM AZD5363, 10 μM GSK690693, 10-20 μM miltefosine, 10 μM MK-2206, 5 μM aripiprazole, VIC+perifosine, VIC+aripiprazole, or 0.1% DMSO (negative control) for 1-2 days. The treated cells were then observed using an ECLIPSETs2 inverted routine microscope (Nikon, Tokyo, Japan) with a ×40 or ×100 objective lenses. The results were confirmed by at least two independent microscopic observations.
Cell viability assay
Cellular viability was evaluated using an EZ-CyTox cell viability assay kit (Daeil lab, Seoul, Korea). The assay has been described previously (Lee et al., 2022c; Oh et al., 2022a, 2022b; Park et al., 2022). P-gp-over-expressing KBV20C cancer cells were grown in 96-well plates and treated with 5 nM VIC, 5 μM perifosine, or 0.1% DMSO (negative control) for two days. Finally, 10 μl EZ-CyTox solution was added for 30-60 min at 37°C, and the absorbance at 450 nm was recorded using a VERSA MAX Microplate Reader (Molecular Devices Corp., Sunnyvale, CA, USA). Quantitative analysis was performed from two independent experiments in triplicate. Data are presented as mean ± standard deviation (SD).
Fluorescence-activated cell sorting (FACS) analysis
Cell cycle analysis was performed as described previously (Lee et al., 2022c; Oh et al., 2022a, 2022b; Park et al., 2022). P-gp-over-expressing MCF-7/ADR cancer cells were stimulated with 5 nM VIC, 5 μM perifosine, 10 μM perifosine, or 0.1% DMSO (negative control) for one day. Pelleted cells were then dissolved in 75% ethanol for 1 h at –20°C and stained with propidium iodide for 0.5 h. The treated cells were observed using a Novocyte Flow Cytometer (ACEA Biosciences, San Diego, CA, USA). The results were confirmed by at least two independent FACS experiments.
Annexin V analysis
Early and late apoptotic death was measured using a commercially available annexin V-fluorescein isothiocyanate (FITC) staining kit (BD Bioscience, Franklin, NJ, USA) according to previously described procedures (Lee et al., 2022c; Oh et al., 2022a, 2022b; Park et al., 2022). MCF-7, MCF-7/ADR, HaCaT, and MDA-MB-231 cancer cells were stimulated with 5 nM VIC, 5-10 μM perifosine, 1 μM BKM120, 10 μM AZD5363, 10 μM GSK690693, or 0.1% DMSO (negative control) for one day. Pelleted cells were then stained with annexin V-FITC and propidium iodide and incubated for 30 min at 25°C. The stained cells were immediately examined using a Novocyte Flow cytometer (ACEA Biosciences). The results were confirmed using at least two independent experiments.
DAPI nuclear staining
In this study, 4’,6-diamidino-2-phenylindole (DAPI) staining was performed to identify nuclear morphology and the presence of apoptotic bodies (Park et al., 2022). MCF-7/ADR cells (2×105) were seeded in confocal dishes. After 24 h of incubation, cells were treated with 0.1% DMSO as the control, 5 nM VIC, or 10 μM perifosine for 24 h. Next, the cells were fixed with acetone for 15 min, washed twice with PBS, and stained with DAPI (0.1 μg/mL in PBS) for 15 min at room temperature. After cooling, DPBS was used to thoroughly remove the dye, and morphological changes in the nuclear and apoptotic bodies were detected using a K1-fluo microscope (Nanoscope Systems, Daejeon, Korea).
Acridine orange fluorescence staining
Cells (2×105) were seeded in confocal dishes, treated with 0.1% DMSO as the control, 5 nM VIC, or 10 μM perifosine for 24 h. The cells were then washed thrice with cold PBS, fixed with 4% paraformaldehyde for 15 min, and again washed with cold PBS. After washing, the cells were stained with acridine orange (1 μg/mL) for 15 min at room temperature, washed twice with cold PBS again, and examined using a K1-fluo confocal microscope (Nanoscope Systems) equipped with a 100× objective lens.
Rhodamine uptake tests
To evaluate the P-gp-inhibitory ability of drugs, rhodamine 123 uptake tests were performed (Lee et al., 2022a, 2022c; Oh et al., 2022a; Park et al., 2023). Cells were plated in 6-well plates at a density of 3.5×105 per well for 24 h and then treated with 10 μM perifosine, 5 μM aripiprazole, 1.5 μM tariquidar (positive controls) or 0.1% DMSO (CON) for 1 h or 21 h. Then, the cells were stained with 0.5 µM rhodamine 123 and incubated in the dark for 3 h at 37°C in a humidified atmosphere containing 5%. After staining, the attached cells were harvested and analyzed using a NovoCyte Flow Cytometer (ACEA Biosciences).
Western blotting
Protein levels were examined using western blot analysis, as previously described (Lee et al., 2022a, 2022c; Oh et al., 2022a; Park et al., 2023). P-gp-over-expressing KBV20C cancer cells were grown in 100 mm dishes and treated with 5 nM VIC, 5 μM perifosine, or 0.1% DMSO (negative control) for 1 day. Total cellular protein was isolated as described below. Pelleted cells were dissolved using the PRO-PREP™ kit (iNtRON, Seongnam, Korea) and incubated on ice for at least 20 min. Protein extracts from the supernatants were collected, and the protein concentrations were measured. Equal amounts of the protein extracts were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis. The results were confirmed by at least two independent western blot experiments.
Statistical analysis
Statistical tests were performed using one-way analysis of variance (ANOVA). Statistical significance was calculated using Graph Pad Prism Software Version 5.0 (GraphPad Software, San Diego, CA, USA). Statistical significance is indicated as follows: ****p<0.0001.
RESULTS
Low-dose perifosine, a phase II Akt inhibitor with alkyl phospholipid, specifically sensitizes ABCB1-overexpressed MCF-7/ADR cancer cells
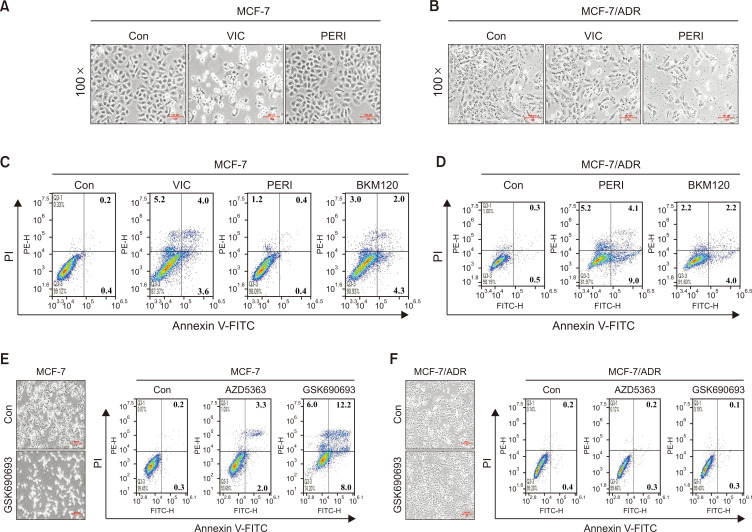
We explored the potential of enhancing the cytotoxicity in ABCB1 (or P-gp)-overexpressing drug-resistant cancer cells. The Akt signaling pathway is positively correlated with drug resistance in cancer cells (Fu et al., 2012; Tomiyasu et al., 2014; Toson et al., 2022). Therefore, we examined various Akt pathway-related inhibitors that specifically sensitized P-gp-overexpressing drug-resistant cancer cells. The IC50 value of perifosine is known to be >10 μM in various solid cancer cell lines (Patel et al., 2002; Kondapaka et al., 2003; Chiarini et al., 2008). Our preliminary studies revealed no cytotoxicity in most cancer cell lines treated with 10 µM perifosine for 24 h. As shown in Fig. 1A, drug-sensitive MCF-7 cancer cells were markedly sensitized to 5 nM VIC, although 5 μM perifosine did not impact cell proliferation. MCF-7/ADR cells are well-known P-gp-overexpressing, drug-resistant cancer cells (Tang et al., 2014; Lee et al., 2022c; Park et al., 2023). MCF-7/ADR cells exhibited a VIC-resistant phenotype (Fig. 1B). Surprisingly, as seen in microscopic observations, 5 μM perifosine reduced MCF-7/ADR cell proliferation (Fig. 1B). Comparing VIC-sensitive MCF-7 and VIC-resistant MCF-7/ADR cells, we concluded that low-dose perifosine afforded highly selective sensitization effects only in drug-resistant MCF-7/ADR cancer cells.
Fig. 1.
Co-treatment with low-dose perifosine specifically sensitizes ABCB1-overexpressing drug-resistant MCF-7/ADR but not drug-sensitive MCF-7 cancer cells. (A, B) MCF-7 or MCF-7/ADR cells were treated with 5 nM VIC, 5 μM perifosine (PERI), or 0.1% DMSO (CON). After 1 day, all cells were observed using an inverted microscope at ×100 magnification. (C, D) MCF-7 or MCF-7/ADR cells were treated with 5 nM VIC, 5 μM perifosine (PERI), 1 μM BKM120, or 0.1% DMSO (CON). After 24 h, annexin V analyses were performed as described in Materials and Methods. (E, F) MCF-7 or MCF-7/ADR cells were treated with 10 μM AZD5363, 10 μM GSK690693, or 0.1% DMSO (CON). After 1 day, annexin V analyses were performed as described in Materials and Methods. GSK690693- or DMSO (CON)-treated cells were also observed using an inverted microscope at ×40 magnification.
Quantitative analysis of the Annexin V assay was performed to compare apoptosis between drug-sensitive MCF-7 cells and drug-resistant MCF-7/ADR cancer cells treated with low-dose perifosine. As shown in Fig. 1C and 1D, 5, 5 μM perifosine significantly increased the number of apoptotic and necrotic regions in drug-resistant MCF-7/ADR cells. Conversely, the same perifosine dose resulted in limited apoptotic regions in drug-sensitive MCF-7 cells. As expected and observed by microscopy (Fig. 1A), VIC significantly increased the cytotoxicity of drug-sensitive MCF-7 cells (Fig. 1C). This indicates that low-dose perifosine selectively increases apoptosis in drug-resistant MCF-7/ADR cells but not in sensitive MCF-7 cells.
We also tested BKM120, another inhibitor of the Akt signaling pathway, to determine its ability to sensitize drug-resistant MCF-7/ADR cancer cells (Uko et al., 2020; Toson et al., 2022). As shown in the apoptotic measurements in Fig. 1C and 1D, the same dose of BKM120 showed similar degrees of cytotoxicity in MCF-7 and MCF-7/ADR cancer cells, thereby suggesting that low-dose perifosine has selective sensitization effects on drug-resistant MCF-7/ADR cancer cells. Based on these findings, we speculated that low-dose perifosine specifically targets P-gp-overexpressing drug-resistant cancer cells.
Drug-resistant MCF-7/ADR cells are highly resistant to Akt inhibitors (AZD5363 and GSK690693)
We next examined other inhibitors of the Akt signaling pathway in drug-resistant MCF-7/ADR cancer cells. A clinical trial assessing the Akt inhibitor, AZD5363 (Choi et al., 2015), was conducted to determine whether it could increase cytotoxicity in drug-resistant MCF-7/ADR cancer cells. As shown in Fig. 1E and 1F, AZD5363 markedly increased apoptosis only in drug-sensitive MCF-7 cells, whereas the same dose of AZD5363 afforded similar levels of apoptosis in resistant MCF-7/ADR and DMSO-treated controls. Previously, GSK690693, another Akt inhibitor, was found to be a P-gp substrate and was pumped out by P-gp-overexpressing resistant cancer cells (Lee et al., 2019). As seen in both microscopic observations and Annexin V analysis, GSK690693 markedly increased cytotoxicity in drug-sensitive MCF-7 cancer cells, although it failed to increase cytotoxicity in drug-resistant MCF-7/ADR cells (Fig. 1E, 1F). Accordingly, it can be suggested that low-dose Akt-targeting inhibitors (BKM120, AZD5363, and GSK690693) did not specifically sensitize P-gp-overexpressing drug-resistant cancer cells, unlike low-dose perifosine. Collectively, low-dose perifosine could specifically sensitize P-gp-overexpressing drug-resistant cancer cells, independent of its Akt-targeting ability.
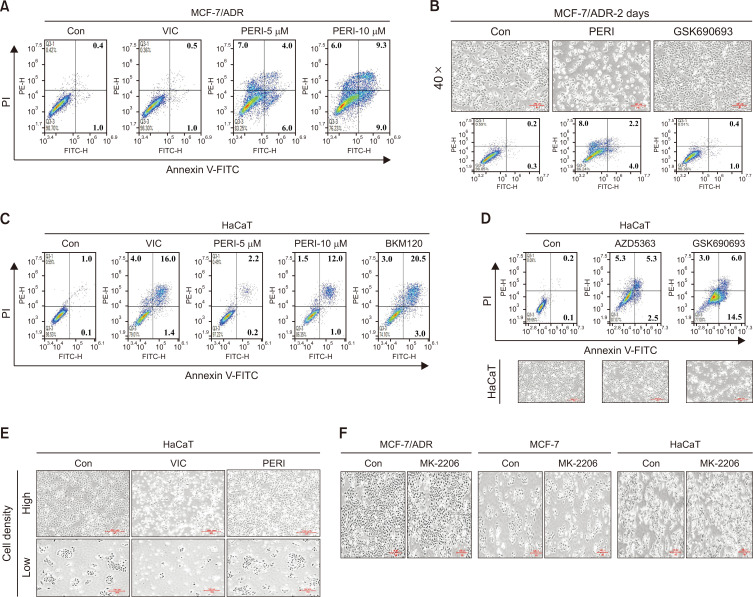
Perifosine increases apoptosis of drug-resistant MCF-7/ADR cells in a dose- and time-dependent manner
In addition, we examined whether an increased dose of perifosine could proportionally increase the cytotoxicity in drug-resistant MCF-7/ADR cancer cells. As seen in Fig. 2A, treatment with 10 μM perifosine increased both early and late apoptosis when compared with 5 μM perifosine. However, treatment with VIC failed to increase the cytotoxicity in MCF-7/ADR cells when compared to that in sensitive MCF-7 cells (Fig. 1C, 2A), suggesting that MCF-7/ADR cells are highly resistant to antimitotic drugs. We also examined whether the toxicity of perifosine could be reduced by enhancing the treatment duration. As shown in Fig. 2B, a two-day-treatment with low-dose perifosine increased the cytotoxicity in resistant MCF-7/ADR cancer cells, whereas a two-day-GSK690693 treatment did not increase the cytotoxicity. These results suggest that low-dose perifosine efficiently increased the apoptosis of resistant MCF-7/ADR cells without delaying recovery from toxicity. Therefore, low-dose perifosine may increase cytotoxicity with increased treatment duration.
Fig. 2.
Low-dose perifosine exerts minimally sensitizes normal human HaCaT cells. (A) MCF-7/ADR cells were treated with 5 nM VIC, 5 μM perifosine (PERI-5μM), 10 μM perifosine (PERI-10μM), or 0.1% DMSO (CON). After 24 h, annexin V analyses were performed as described in Materials and Methods. (B) MCF-7/ADR cells were treated with 5 μM perifosine (PERI), 10 μM GSK690693, or 0.1% DMSO (CON). After 2 day, all cells were also observed using an inverted microscope at ×40 magnification, and annexin V analyses were performed as described in Materials and Methods. (C, D) HaCaT cells were treated with 5 nM VIC, 5 μM perifosine (PERI-5μM), 10 μM perifosine (PERI-10μM), 1 μM BKM120, 10 μM AZD5363, 10 μM GSK690693, or 0.1% DMSO (CON). After 24 h, annexin V analyses were performed as described in Materials and Methods. AZD5363-, GSK690693- or DMSO (CON)-treated cells were also observed using an inverted microscope at ×40 magnification. (E) HaCaT cells were grown on 60 mm-diameter dishes with high density (upper) or low density (lower). They were then treated with 5 nM VIC, 5 μM perifosine (PERI), or 0.1% DMSO (CON). After 1 day, all cells were observed using an inverted microscope at ×40 magnification. (F) MCF-7/ADR, MCF-7, or HaCaT cells were treated with 10 μM MK-2206, or 0.1% DMSO (CON). After 1 day, all cells were observed using an inverted microscope at ×40 magnification.
Low-dose perifosine minimally sensitizes normal human keratinocytes (HaCaT cells)
It is crucial to ensure that low-dose perifosine exerts minimal toxicity toward normal cells. Therefore, we examined whether low-dose perifosine increased cytotoxicity in HaCaT cells. HaCaT is a keratinocyte-derived normal cell line routinely used to determine the effects of protective drugs on normal skin damaged by toxic agents or ultraviolet irradiation (Jeremic et al., 2016; You et al., 2019; Chan Kwon et al., 2020; Finiuk et al., 2022). Drug toxicity can be compared between normal HaCaT and cancer cells (Jeremic et al., 2016; You et al., 2019; Chan Kwon et al., 2020; Finiuk et al., 2022). Specifically, we tested whether low-dose perifosine could induce relatively higher toxicity in HaCaT cells than in drug-resistant MCF-7/ADR cancer cells. As observed in drug-sensitive MCF-7 cancer cells (Fig. 1C), treatment with 5 nM VIC primarily increased HaCaT cell apoptosis (Fig. 2C), indicating that HaCaT cells are highly antimitotic, drug-sensitive normal cells. However, treatment with 5 µM perifosine minimally sensitized HaCaT cells, suggesting that low-dose perifosine has little toxicity in drug-sensitive normal cells. However, treatment with 10 μM perifosine increased apoptosis of HaCaT cells (Fig. 2C). Considering that 10 μM perifosine can more substantially sensitize drug-resistant MCF-7/ADR cancer cells than HaCaT cells (Fig. 2A, 2C), we concluded that low-dose perifosine was less cytotoxic against normal HaCaT cells and could selectively sensitize P-gp-overexpressing drug-resistant MCF-7/ADR cancer cells.
We also examined the cytotoxicity of other Akt inhibitors (BKM120, AZD5363, and GSK690693) in HaCaT cells. As previously described, these low-dose inhibitors exerted minimal sensitizing effects on drug-resistant MCF-7/ADR cells (Fig. 1D, 1F, 2B). However, HaCaT cells showed high levels of cytotoxicity when treated with the same doses of BKM120, AZD5363, and GSK690693 (Fig. 2C, 2D), similar to the drug-sensitive MCF-7 cells (Fig. 1C, 1E). Considering that among tested Akt signaling inhibitors, only low-dose perifosine caused minimal apoptosis in drug-sensitive HaCaT cells, we demonstrated that low-dose perifosine could specifically exert high cytotoxicity in P-gp-overexpressing drug-resistant MCF-7/ADR cancer cells. Following treatment with the same dose of anticancer drug, high-density cultured cells demonstrated fewer sensitization effects than low-density cultures (Lee et al., 2022b; Park et al., 2022). We examined whether low-dose perifosine exerts density-dependent sensitization effects on HaCaT cells. As seen in Fig. 2E, HaCaT cells exhibited similar sensitization effects at both high and low densities when treated with low-dose perifosine, indicating that low-dose perifosine exerts minimal cytotoxicity even in low-density HaCaT normal cells.
Perifosine is an allosteric inhibitor that targets the PH domain and blocks AKT activation. Therefore, we determined whether MK-2206, another allosteric inhibitor in clinical trials (Choi et al., 2014; Uko et al., 2020; Toson et al., 2022), could increase cytotoxicity in drug-resistant MCF-7/ADR cancer cells. However, treatment with 10 µM MK-2206 induced similar degrees of sensitization in MCF-7/ADR, MCF-7, and HaCaT cells (Fig. 2F), suggesting that sensitization mediated by low-dose perifosine does not involve targeting the PH domain for Akt activation. Collectively, among the tested Akt pathway inhibitors in both drug-sensitive MCF-7 and HaCaT cells and drug-resistant MCF-7/ADR cancer cells, we found that low-dose perifosine selectively and specifically increased cytotoxicity in P-gp-overexpressing drug-resistant MCF7/ADR cancer cells.
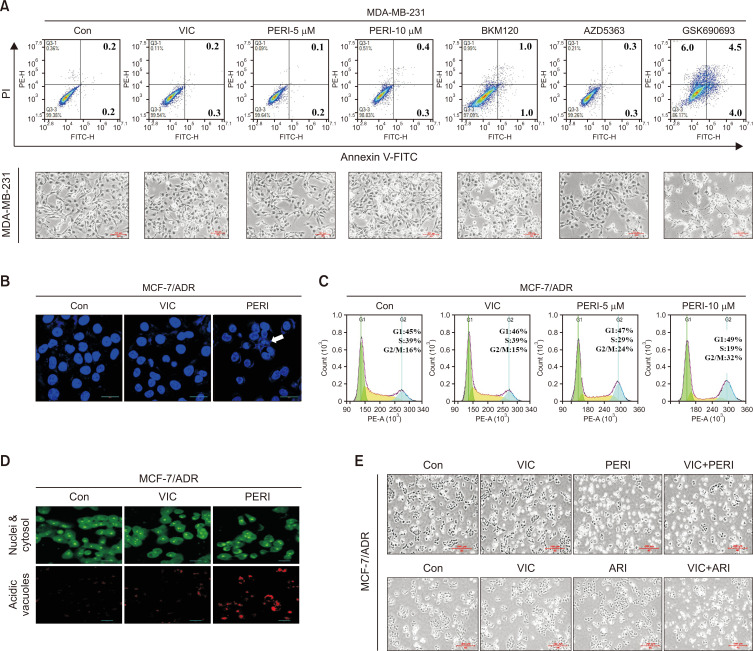
Low-dose perifosine induces minimal sensitization of MDA-MB-231 cells with non-P-gp overexpression
Next, we investigated whether low-dose perifosine sensitizes VIC-resistant cancer cells. MDA-MB-231 cancer cells, a triple-negative breast cancer (TNBC)-related cell line, show resistance to antimitotic drugs (Lee et al., 2022b). Microscopic observations and Annexin V analysis revealed that MDA-MB-231 cells were highly resistant to VIC when compared with MCF-7 and HaCaT cells (Fig. 1C, 2C, 3A). Low-dose perifosine did not increase cytotoxicity in MDA-MB-231 cells, whereas BKM120 and GSK690693 increased cytotoxicity (Fig. 3A). Given that MDA-MB-231 cells are non-P-gp overexpressing (Lee et al., 2022b), similar to MCF-7 and HaCaT cells, MDA-MB-231 cells showed high sensitization following treatment with GSK690693, a known substrate for P-gp efflux (Fig. 1E, 2D, 3A). However, MDA-MB-231 cells were resistant to AZD5363 (Fig. 3A), and the same dose of AZD5363 increased cytotoxicity in both MCF-7 and HaCaT cells (Fig. 1E, 2D). Based on the results from relatively drug-resistant and non-P-gp-overexpressing MDA-MB231 cancer cells, low-dose perifosine could specifically target cancer cells with both P-gp overexpression and drug resistance.
Fig. 3.
Perifosine increases DNA condensation, G2 arrest, and autophagy induction in P-gp overexpressing drug-resistant MCF-7/ADR cells. (A) MDA-MB-231 cells were treated with 5 nM VIC, 5 μM perifosine (PERI-5μM), 10 μM perifosine (PERI-10μM), 1 μM BKM120, 10 μM AZD5363, 10 μM GSK690693, or 0.1% DMSO (CON). After 1 day, all cells were also observed using an inverted microscope at ×40 magnification, and annexin V analyses were performed as described in Materials and Methods. (B) MCF-7/ADR cells were treated with 5 nM VIC, 10 μM perifosine (PERI), or 0.1% DMSO (CON). After 24 h, staining with DAPI was performed as described in Materials and Methods. (C) MCF-7/ADR cells were treated with 5 nM VIC, 5 μM perifosine (PERI-5μM), 10 μM perifosine (PERI-10μM), or 0.1% DMSO (CON). After 1 day, FACS analyses were performed as described in Materials and Methods. (D) MCF-7/ADR cells were treated with 5 nM VIC, 10 μM perifosine (PERI), or 0.1% DMSO (CON). After 24 h, staining with acridine orange was performed as described in Materials and Methods. (E) MCF-7/ADR cells were treated with 5 nM VIC, 5 μM perifosine (PERI), 5 μM aripiprazole (ARI), 5 nM VIC+5 μM perifosine (VIC+PERI), 5 nM VIC+5 μM aripiprazole (VIC+ARI), or 0.1% DMSO (CON). After 1 day, all cells were observed using an inverted microscope at ×40 magnification.
Perifosine increases DNA condensations, G2 arrest, and autophagy induction in drug-resistant MCF-7/ADR cells
Next, we examined the sensitization mechanism of low-dose perifosine in drug-resistant MCF-7/ADR cells. Late-stage apoptosis can be evaluated by assessing chromosomal condensation (Park et al., 2022). As seen in the DAPI staining results in Fig. 3B, 10 μM perifosine increased DAPI-stained chromosomal condensation in MCF-7/ADR cells, whereas VIC treatment resulted in minimal chromosomal condensation. This finding suggests that low-dose perifosine rapidly induces late apoptosis, resulting in the death of MCF-7 /ADR-resistant cancer cells. We also determined whether low-dose perifosine could enhance cell cycle arrest in MCF-7/ADR-resistant cancer cells. As shown in Fig. 3C, 10 μM perifosine increased G2 arrest in MCF-7/ADR cells. Treatment with 5 and 10 μM perifosine demonstrated that G2 arrest was concentration-dependent (Fig. 3C). These results indicate that low-dose perifosine increased apoptotic death via G2 arrest in MCF-7/ADR cells.
Next, we explored whether low-dose perifosine could increase autophagic death in MCF-7/ADR drug-resistant cancer cells. As shown in Fig. 3D, 10 μM perifosine induced and accumulated acridine orange staining in the acidic vacuoles of MCF-7/ADR cells, demonstrating that perifosine increased autophagy; however, VIC-treated MCF-7/ADR cells had scant acridine orange staining. Collectively, these results indicate that low-dose perifosine could induce late apoptotic death by inducing autophagic death and G2 arrest in P-gp-overexpressing drug-resistant MCF-7/ADR cancer cells.
Perifosine does not increase P-gp inhibitory activity at 4 and 24 h in drug-resistant MCF-7/ADR cells
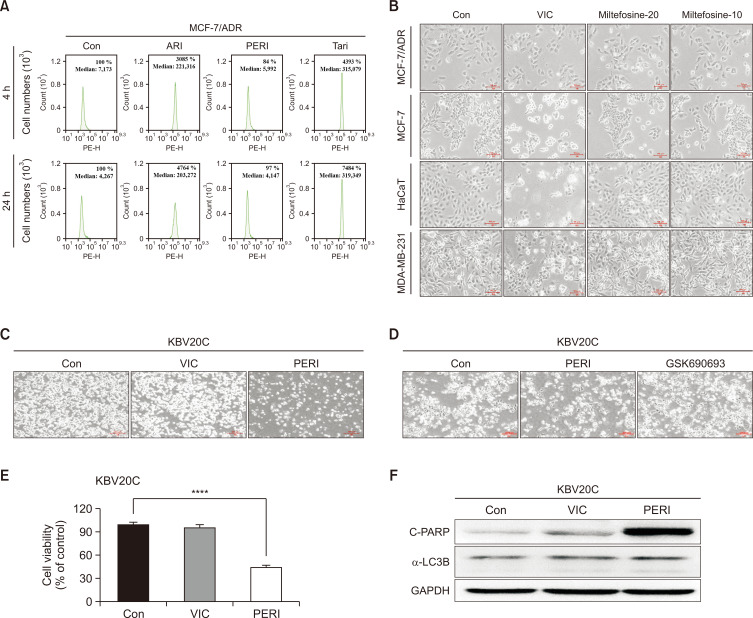
Furthermore, we evaluated whether combination treatment with perifosine could enhance the cytotoxicity in VIC-treated MCF-7/ADR cells. As seen in the microscopic observations in Fig. 3E, VIC+perifosine failed to increase the cytotoxicity in MCF-7/ADR cells when compared with perifosine treatment alone. Co-treatment with VIC+aripiprazole significantly increased the number of MCF-7/ADR cells, similar to the positive control. This suggests that a single perifosine treatment could be effective in P-gp-overexpressing, drug-resistant MCF-7/ADR cancer cells. Given that inhibition of P-gp activity contributes to the sensitization of P-gp-overexpressing drug-resistant MCF-7/ADR cells (Chiarini et al., 2008; Fu et al., 2012; Lin et al., 2012), we investigated whether low-dose perifosine could be involved in P-gp inhibitory activity. Previous studies have shown that perifosine reduces P-gp expression via transcriptional regulation (Chiarini et al., 2008; Fu et al., 2012; Lin et al., 2012). We examined the efflux ability of rhodamine 123, a well-known P-glycoprotein substrate. Considering that perifosine can impact the transcriptional regulation of P-gp (Chiarini et al., 2008; Fu et al., 2012; Lin et al., 2012), we evaluated the inhibitory activity at both 4 h (short time) and 24 h (long time). As shown in Fig. 4A, 10 μM perifosine did not increase P-gp-inhibitory activity at either 4 or 24 h, whereas low-dose positive controls (aripiprazole and tariquidar) highly increased P-gp-inhibitory activity at both time points. These results indicated that the sensitization mechanism of low-dose perifosine does not involve P-gp-inhibitory activity in drug-resistant MCF-7/ADR cancer cells.
Fig. 4.
Low-dose perifosine enhances the cytotoxicity in ABCB1-overexpressing drug-resistant KBV20C cancer cells. (A) MCF-7/ADR cells were treated with 10 μM perifosine (PERI), 5 μM aripiprazole (ARI), 1.5 μM tariquidar (Tari), or 0.1% DMSO (CON). After 1 h (left panel) or 21 h (right panel), all cells were stained with rhodamine 123 for 3 h and examined by using FACS analysis as described in Materials and Methods. (B) MCF-7/ADR, MCF-7, HaCaT, and MDA-MB-231 cells were treated with 5 nM VIC, 20 μM miltefosine, 10 μM miltefosine, or 0.1% DMSO (CON). After 1 day, all cells were also observed using an inverted microscope at ×100 magnification. (C, D) P-gp overexpressing drug-resistant KBV20C cells were treated with 5 nM VIC, 5 μM perifosine (PERI), 10 μM GSK690693, or 0.1% DMSO (CON). After 1 day, all cells were also observed using an inverted microscope at ×40 magnification. (E) KBV20C cells were treated with 5 nM VIC, 5 μM perifosine (PERI), or 0.1% DMSO (CON). Cell viability assay was performed as described in “Materials and Methods”. The data are presented as the mean ± S.D. of at least two experiments repeated in triplicate experiments. Data are presented as mean ± SD. ****p<0.0001 was considered be statistically significant. (F) KBV20C cells were treated with 5 nM VIC, 5 μM perifosine (PERI), or 0.1% DMSO (CON). After 24 h, western blot analysis was performed using antibodies against C-PARP, α-LC3B, and GAPDH.
Miltefosine has similar sensitization effects on MCF-7/ADR, MCF-7, HaCaT, and MDA-MB-231 cells
Perifosine is composed of an alkyl phospholipid with a structure similar to the Akt inhibitor miltefosine (Patel et al., 2002; Kondapaka et al., 2003; Uko et al., 2020). Considering that perifosine functions as a membrane-targeting Akt inhibitor, we examined whether miltefosine, a structurally similar inhibitor, could increase sensitization in P-gp-overexpressing drug-resistant MCF-7/ADR cancer cells. As shown by microscopic observations in Fig. 4B, VIC highly sensitized drug-sensitive MCF-7 and HaCaT cells, whereas MCF-7/ADR and MDA-MB-231 cells were resistant to VIC. However, 20 and 10 μM miltefosine exerted similar sensitization effects on MCF-7/ADR, MCF-7, HaCaT, and MDA-MB-231 cells. Low-dose perifosine specifically and highly increased cytotoxicity only in MCF-7/ADR cells, suggesting that the structural similarity between perifosine and miltefosine fails to explain the high sensitization effects in P-gp-overexpressing drug-resistant MCF-7/ADR cancer cells. Accordingly, the alkyl-phospholipid structure of perifosine may not play a substantial role in the specific sensitization of P-gp-overexpressing drug-resistant cancer cells.
Low-dose perifosine increases the cytotoxicity of other P-gp overexpressing drug-resistant KBV20C cancer cells
Finally, we determined whether low-dose perifosine could increase the cytotoxicity in other P-gp-overexpressing resistant cancer cells. KBV20C cells are an oral squamous cancer cell line highly resistant to antimitotic drugs such as VIC, vinorelbine, or eribulin (Lee et al., 2022c; Oh et al., 2022b; Park et al., 2023). MCF-7/ADR cells have been shown to demonstrate considerably higher P-gp expression on cell membranes than KBV20C cells (Tang et al., 2014; Park et al., 2023). We identified various FDA-approved repositioned drugs that could sensitize KBV20C cells at low doses (Kim et al., 2020; Lee et al., 2022a, 2022c; Oh et al., 2022b; Park et al., 2023). Based on microscopic observations and viability assays, we found that KBV20 cells were highly resistant to VIC and GSK690693 (Fig. 4C-4E). In contrast, low-dose perifosine increased cytotoxicity in P-gp-overexpressing drug-resistant KBV20C cancer cells. Considering that MCF-7/ADR and KBV20C are P-gp-overexpressing drug-resistant cancer cell lines (Tang et al., 2014; Lee et al., 2022c; Park et al., 2023), low-dose perifosine could specifically target P-gp-overexpressing drug-resistant cancer cells. We confirmed that low-dose perifosine increased apoptosis, as determined by C-PARP production (Fig. 4F). However, autophagy induction was not observed because α-LC3B levels were similar in both the control and perifosine groups (Fig. 4F). This finding suggests that autophagy induction does not significantly contribute to the cytotoxicity of low-dose perifosine in P-gp-overexpressing KBV20C cells. We assumed that autophagy can also occur without a-LC3B degradation in KBV20C cells.
In conclusion, low-dose perifosine specifically sensitizes MCF-7/ADR and KBV20C cancer cells to P-gp-overexpressing drug resistance. These results may contribute to further applications that target P-gp-overexpressing drug-resistant cancer cell populations.
DISCUSSION
Tumors comprise a heterogeneous cell population, and a small proportion of tumors include drug-resistant cancer (stem) cells (Al-Lazikani et al., 2012; Brown et al., 2014). First-line chemotherapy reduces tumor volume via apoptosis in drug-sensitive cancer populations (Al-Lazikani et al., 2012; Conde et al., 2013; Brown et al., 2014). However, it can stimulate drug-resistant cancer populations, resulting in tumor recurrence. Relapsed tumors do not respond to chemotherapeutic drugs and are primarily resistant cancers. Therefore, it is crucial to enhance the apoptosis of drug-resistant cancer cells in heterogeneous populations during first-line chemotherapy. Considering that the characteristics of these drug-resistant cancer cells include overexpression of ABCB1 (or P-gp) on their membranes (Genovese et al., 2017; Cho and Kim, 2020; Robinson and Tiriveedhi, 2020; Seelig, 2020), it is assumed that first-line combination treatments with P-gp inhibitors could enhance the possibility of delaying tumor recurrence. Herein, we explored novel drugs and their underlying mechanisms in targeting P-gp-overexpressing drug-resistant cancer cells to facilitate first-line combination chemotherapeutic treatments.
Given that FDA-approved drugs have long been used in clinics and their toxicities are well reported (Yoon et al., 2021; Yoon and Kim, 2022, 2023), these drugs can be easily applied as first-line combination treatments for targeting resistant cancer populations. Identifying novel mechanisms of FDA-approved agents could facilitate drug repositioning to target P-gp-overexpressing drug-resistant cancer cells. We previously reported various FDA-approved (or clinical trial) repositioned drugs for sensitizing P-gp-overexpressing drug-resistant cancer cells with either single treatment or co-treatment (Kim et al., 2020; Lee et al., 2022a, 2022c; Oh et al., 2022a; Park et al., 2023). We focused on identifying whether the repositioned drugs could sensitize drug-resistant cancer cells at low doses, given that toxicity in normal tissues could impact the efficacy of low-dose treatment.
Herein, we investigated various Akt inhibitors that could increase the cytotoxicity in P-glycoprotein (P-gp)-overexpressing drug-resistant cancer cells. We aimed to identify drugs that could be repositioned at low doses from among FDA-approved drugs or those in clinical trials. Based on our comparison of drug-sensitive cancer (or normal) cells and other Akt inhibitors, we found that low-dose perifosine specifically targeted the sensitization of P-gp-overexpressing drug-resistant cancer cells. We next provide a detailed discussion and speculate on our findings.
Importantly, we found that P-gp-overexpressing drug-resistant cancer cells had a substantially lower IC50 value for perifosine than drug-sensitive cancer or normal cells. Relatively low doses of perifosine could increase the cytotoxicity in P-gp-overexpressing drug-resistant MCF-7/ADR cancer cells, whereas drug-sensitive MCF-7 cancer cells showed minimal sensitization effect at these concentrations. This finding suggests that low-dose perifosine specifically targets P-gp-overexpressing, drug-resistant cancer cells. Furthermore, low-dose perifosine had a limited sensitization effect on normal HaCaT cells, suggesting that low-dose perifosine could reduce drug toxicity in normal cells when used in patients with cancer. Moreover, we demonstrated that low-dose perifosine could sensitize KBV20C cells (another P-gp-overexpressing drug-resistant cancer cell line), ensuring that low-dose perifosine can specifically target P-gp-overexpressing drug-resistant cancer cells. However, low-dose perifosine had a minimal sensitizing effect on MDA-MB-231 breast cancer cells (relatively VIC-resistant but non-P-gp-overexpressing).
Accordingly, the results in MDA-MB-231 breast cancer cells strongly support the idea that low-dose perifosine can specifically sensitize P-gp-overexpressing drug-resistant cancer cells. Previous studies have also reported that perifosine increases cytotoxicity in resistant cancer cells (Chiarini et al., 2008; Fu et al., 2012; Lin et al., 2012). However, these studies largely focused on leukemia cell types and explored prolonged time durations (48 h) for sensitization. One study used perifosine at a dose exceeding 10 μM to sensitize drug-resistant leukemia cells (Chiarini et al., 2008). Herein, we used breast cancer cell lines or normal cells (HaCaT) as solid tumor models and examined relatively low-dose perifosine (5 μM) for a short time duration (24 h) to sensitize P-gp-overexpressing resistant cancer cells. Our findings could reveal novel mechanisms for the repositioning of low-dose perifosine.
Several inhibitors targeting the Akt signaling pathway have been developed owing to their close association with cancer cell proliferation (Kondapaka et al., 2003; Keane et al., 2014; Uko et al., 2020; Toson et al., 2022). Therefore, it is crucial to determine whether other Akt-targeting drugs can also sensitize P-gp-overexpressing drug-resistant cancer cells with a specificity similar to that of low-dose perifosine. In drug-sensitive MCF-7 cancer cells and normal HaCaT cells, two Akt inhibitors, 1 μM BKM120 and 10 μM AZD5363, induced higher levels of apoptosis than 5 μM perifosine. However, the same doses of BKM120 and AZD5363 induced far less cytotoxicity than low-dose perifosine in P-gp-overexpressing drug-resistant MCF-7/ADR cancer cells. These findings indicate that the selective specificity of low-dose perifosine in MCF-7/ADR cells could be independent of Akt inhibition mechanisms in P-gp-overexpressing drug-resistant cancer cells. GSK690693, another Akt inhibitor, is a P-gp substrate and can be pumped out by P-gp-overexpressing drug-resistant cancer cells (Lee et al., 2019). We confirmed that P-gp overexpressing drug-resistant MCF-7/ADR and KBV20C cancer cells were highly resistant to 20 μM GSK690693. In contrast, the same dose of GSK690693 could highly sensitize non-P-gp-overexpressing cells (MCF-7, HaCaT, and MDA-MB231 cells). These results also support the speculations that low-dose perifosine can selectively increase cytotoxicity only in P-gp-overexpressing-resistant cancer cells.
The Akt-targeting inhibitors were divided as follows: (1) competitive inhibitors as blocking agents for ATP binding, and (2) allosteric inhibitors that target the PH domain to prevent Akt activation and downstream signaling pathways (Kondapaka et al., 2003; Keane et al., 2014; Uko et al., 2020; Toson et al., 2022). Given that perifosine is an allosteric inhibitor of Akt activation, we examined its potential in combination with MK-2206, an Akt allosteric inhibitor, in clinical trials. Our results revealed that sensitization with low-dose perifosine was not mediated by targeting the PH domain for Akt activation, given that 10 μM MK-2206 treatment showed similar degrees of sensitization in MCF-7/ADR, MCF-7, and HaCaT cells.
As perifosine is an alkyl phospholipid, we also examined miltefosine, a structurally similar Akt inhibitor (Kondapaka et al., 2003; Chiarini et al., 2008; Uko et al., 2020; Toson et al., 2022). However, we found that miltefosine had similar cytotoxicity in MCF-7/ADR, MCF-7, HaCaT, and MDA-MB231 cells. On comparing Akt inhibitors (BKM120, AZD5363, GSK690693, MK-206, and miltefosine) with perifosine, we concluded that low-dose perifosine selectively sensitizes P-gp-overexpressing drug-resistant cancer cells, independent of Akt inhibition. Further investigations are needed to elucidate the sensitization mechanisms that underlie the selective specificity of P-gp-overexpressing drug-resistant cancer cells.
Considering a more detailed analysis of sensitization mechanisms, low-dose perifosine increased G2 arrest in P-gp-overexpressing, drug-resistant MCF-7/ADR cells. Perifosine increased G2 arrest in a dose-dependent manner, suggesting that G2 arrest plays a major role in apoptotic cell death. Low-dose perifosine primarily facilitated the final steps of apoptotic death, as demonstrated by chromosomal condensation detected by DAPI staining. Considering that low-dose perifosine could increase cytotoxic death in MCF-7/ADR cells, we assumed that most G2-arrested MCF-7/ADR cells could be in states of cellular death, not in recovery from toxicity. We compared one- and two-day apoptotic effects induced by low-dose perifosine and found that apoptotic death increased with the duration of perifosine treatment. This supports the notion that drug-resistant MCF-7/ADR cells could not circumvent the toxicity of low-dose perifosine over time. Autophagy induction involves an increase in apoptosis (Park et al., 2022). We found that perifosine increased the number of acidic vacuoles in drug-resistant MCF-7/ADR cells, indicating that perifosine could induce autophagy. Therefore, we concluded that low-dose perifosine specifically increased final apoptotic death in P-gp-overexpressing drug-resistant MCF-7/ADR cells through G2 arrest and autophagy induction.
Previous reports have shown that co-treatment with perifosine can synergistically increase cytotoxicity in cancer cells (Fu et al., 2012; Guidetti et al., 2014). Perifosine was shown to reduce P-gp expression via an mRNA-regulating mechanism (Chiarini et al., 2008; Lin et al., 2012). P-gp inhibition mediated by low-dose perifosine may increase cellular VIC accumulation, enhancing toxicity in P-gp-overexpressing resistant cancer cells. Therefore, we examined whether co-treatment with low-dose perifosine could increase the number of VIC-treated MCF-7/ADR cells. However, we noted minimal differences between low-dose perifosine treatment and VIC+perifosine combination treatment.
Low-dose perifosine treatment for either 4 h (short) or 24 h (long) did not enhance rhodamine 123 accumulation. In contrast, treatment with positive controls (aripiprazole and tariquidar) markedly increased rhodamine 123 accumulation, indicating that low-dose perifosine did not correlate with P-gp activity in terms of sensitizing P-gp-overexpressing drug-resistant MCF-7/ADR cancer cells. Considering that low-dose perifosine exerts minimal P-gp inhibitory activity and no synergistic effects when combined with anticancer drugs in drug-resistant MCF-7/ADR cancer cells, we conclude that low-dose perifosine could be practical as a monotherapy, despite the lack of synergistic effects when combined with anticancer drugs.
Collectively, our findings revealed that low-dose perifosine could specifically sensitize P-gp-overexpressing drug-resistant cancer cells, which facilitates the subsequent targeting by anticancer therapeutics. Our results could contribute to targeting P-gp-overexpressing drug-resistant (stem) cancer cell populations among heterogeneous cancer populations in tumors.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2022R1A4A1018930).
Footnotes
CONFLICT OF INTEREST
The Authors declare no conflicts of interest regarding this study.
REFERENCES
- Al-Lazikani B., Banerji U., Workman P. Combinatorial drug therapy for cancer in the post-genomic era. Nat. Biotechnol. 2012;30:679–692. doi: 10.1038/nbt.2284. [DOI] [PubMed] [Google Scholar]
- Argiris A., Cohen E., Karrison T., Esparaz B., Mauer A., Ansari R., Wong S., Lu Y., Pins M., Dancey J., Vokes E. A phase II trial of perifosine, an oral alkylphospholipid, in recurrent or metastatic head and neck cancer. Cancer Biol. Ther. 2006;5:766–770. doi: 10.4161/cbt.5.7.2874. [DOI] [PubMed] [Google Scholar]
- Bendell J. C., Nemunaitis J., Vukelja S. J., Hagenstad C., Campos L. T., Hermann R. C., Sportelli P., Gardner L., Richards D. A. Randomized placebo-controlled phase II trial of perifosine plus capecitabine as second- or third-line therapy in patients with metastatic colorectal cancer. J. Clin. Oncol. 2011;29:4394–4400. doi: 10.1200/JCO.2011.36.1980. [DOI] [PubMed] [Google Scholar]
- Brown R., Curry E., Magnani L., Wilhelm-Benartzi C. S., Borley J. Poised epigenetic states and acquired drug resistance in cancer. Nat. Rev. Cancer. 2014;14:747–753. doi: 10.1038/nrc3819. [DOI] [PubMed] [Google Scholar]
- Chan Kwon Y., Sik Kim H., Lee B. M. Detoxifying effects of optimal hyperoxia (40% oxygenation) exposure on benzo[a]pyrene-induced toxicity in human keratinocytes. J. Toxicol. Environ. Health A. 2020;83:82–94. doi: 10.1080/15287394.2020.1730083. [DOI] [PubMed] [Google Scholar]
- Chen Z., Shi T., Zhang L., Zhu P., Deng M., Huang C., Hu T., Jiang L., Li J. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: a review of the past decade. Cancer Lett. 2016;370:153–164. doi: 10.1016/j.canlet.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Chiarini F., Del Sole M., Mongiorgi S., Gaboardi G. C., Cappellini A., Mantovani I., Follo M. Y., McCubrey J. A., Martelli A. M. The novel Akt inhibitor, perifosine, induces caspase-dependent apoptosis and downregulates P-glycoprotein expression in multidrug-resistant human T-acute leukemia cells by a JNK-dependent mechanism. Leukemia. 2008;22:1106–1116. doi: 10.1038/leu.2008.79. [DOI] [PubMed] [Google Scholar]
- Cho D. C., Hutson T. E., Samlowski W., Sportelli P., Somer B., Richards P., Sosman J. A., Puzanov I., Michaelson M. D., Flaherty K. T., Figlin R. A., Vogelzang N. J. Two phase 2 trials of the novel Akt inhibitor perifosine in patients with advanced renal cell carcinoma after progression on vascular endothelial growth factor-targeted therapy. Cancer. 2012;118:6055–6062. doi: 10.1002/cncr.27668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y., Kim Y. K. Cancer stem cells as a potential target to overcome multidrug resistance. Front. Oncol. 2020;10:764. doi: 10.3389/fonc.2020.00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi A. R., Jung M. J., Kim J. H., Yoon S. Co-treatment of salinomycin sensitizes AZD5363-treated cancer cells through increased apoptosis. Anticancer Res. 2015;35:4741–4747. [PubMed] [Google Scholar]
- Choi A. R., Kim J. H., Woo Y. H., Cheon J. H., Kim H. S., Yoon S. Co-treatment of LY294002 or MK-2206 with AZD5363 attenuates AZD5363-induced increase in the level of phosphorylated AKT. Anticancer Res. 2016;36:5849–5858. doi: 10.21873/anticanres.11170. [DOI] [PubMed] [Google Scholar]
- Choi A. R., Kim J. H., Yoon S. Sensitization of cancer cells through reduction of total Akt and downregulation of salinomycin-induced pAkt, pGSk3beta, pTSC2, and p4EBP1 by cotreatment with MK-2206. Biomed Res. Int. 2014;2014:295760. doi: 10.1155/2014/295760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K. B. New therapeutic bearings for repositioned drugs. Curr. Top. Med. Chem. 2013;13:2281–2282. doi: 10.2174/15680266113136660159. [DOI] [PubMed] [Google Scholar]
- Conde J., de la Fuente J. M., Baptista P. V. Nanomaterials for reversion of multidrug resistance in cancer: a new hope for an old idea? Front. Pharmacol. 2013;4:134. doi: 10.3389/fphar.2013.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finiuk N., Zelisko N., Klyuchivska O., Yushyn I., Lozynskyi A., Cherniienko A., Manko N., Senkiv J., Stoika R., Lesyk R. Thiopyrano[2,3-d]thiazole structures as promising scaffold with anticancer potential. Chem. Biol. Interact. 2022;368:110246. doi: 10.1016/j.cbi.2022.110246. [DOI] [PubMed] [Google Scholar]
- Fu S., Hennessy B. T., Ng C. S., Ju Z., Coombes K. R., Wolf J. K., Sood A. K., Levenback C. F., Coleman R. L., Kavanagh J. J., Gershenson D. M., Markman M., Dice K., Howard A., Li J., Li Y., Stemke-Hale K., Dyer M., Atkinson E., Jackson E., Kundra V., Kurzrock R., Bast R. C., Jr, Mills G. B. Perifosine plus docetaxel in patients with platinum and taxane resistant or refractory high-grade epithelial ovarian cancer. Gynecol. Oncol. 2012;126:47–53. doi: 10.1016/j.ygyno.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese I., Ilari A., Assaraf Y. G., Fazi F., Colotti G. Not only P-glycoprotein: amplification of the ABCB1-containing chromosome region 7q21 confers multidrug resistance upon cancer cells by coordinated overexpression of an assortment of resistance-related proteins. Drug Resist. Updat. 2017;32:23–46. doi: 10.1016/j.drup.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Gote V., Nookala A. R., Bolla P. K., Pal D. Drug resistance in metastatic breast cancer: tumor targeted nanomedicine to the rescue. Int. J. Mol. Sci. 2021;22:4673. doi: 10.3390/ijms22094673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidetti A., Carlo-Stella C., Locatelli S. L., Malorni W., Mortarini R., Viviani S., Russo D., Marchiano A., Sorasio R., Dodero A., Farina L., Giordano L., Di Nicola M., Anichini A., Corradini P., Gianni A. M. Phase II study of perifosine and sorafenib dual-targeted therapy in patients with relapsed or refractory lymphoproliferative diseases. Clin. Cancer Res. 2014;20:5641–5651. doi: 10.1158/1078-0432.CCR-14-0770. [DOI] [PubMed] [Google Scholar]
- Jeremic M., Pesic M., Dinic J., Bankovic J., Novakovic I., Segan D., Sladic D. Simple avarone mimetics as selective agents against multidrug resistant cancer cells. Eur. J. Med. Chem. 2016;118:107–120. doi: 10.1016/j.ejmech.2016.04.011. [DOI] [PubMed] [Google Scholar]
- Jiang C., Lee S. H., Park J. H., Lee J. S., Park J. W., Kim J. R., Lee S. H., Kim H. S., Yoon S. A low dose of aripiprazole has the strongest sensitization effect among 19 repositioned bipolar drugs in P-gp-overexpressing drug-resistant cancer cells. Anticancer Res. 2021;41:687–697. doi: 10.21873/anticanres.14820. [DOI] [PubMed] [Google Scholar]
- Keane N. A., Glavey S. V., Krawczyk J., O'Dwyer M. AKT as a therapeutic target in multiple myeloma. Expert Opin. Ther. Targets. 2014;18:897–915. doi: 10.1517/14728222.2014.924507. [DOI] [PubMed] [Google Scholar]
- Kim J. H., Choi A. R., Kim Y. K., Kim H. S., Yoon S. Low amount of salinomycin greatly increases Akt activation, but reduces activated p70S6K levels. Int. J. Mol. Sci. 2013;14:17304–17318. doi: 10.3390/ijms140917304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. Y., Park Y. J., Lee B. M., Yoon S. Co-treatment with HIV protease inhibitor nelfinavir greatly increases late-phase apoptosis of drug-resistant KBV20C cancer cells independently of P-glycoprotein inhibition. Anticancer Res. 2019;39:3757–3765. doi: 10.21873/anticanres.13524. [DOI] [PubMed] [Google Scholar]
- Kim K. S., Jiang C., Kim J. Y., Park J. H., Kim H. R., Lee S. H., Kim H. S., Yoon S. Low-dose crizotinib, a tyrosine kinase inhibitor, highly and specifically sensitizes P-glycoprotein-overexpressing chemoresistant cancer cells through induction of late apoptosis in vivo and in vitro. Front. Oncol. 2020;10:696. doi: 10.3389/fonc.2020.00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondapaka S. B., Singh S. S., Dasmahapatra G. P., Sausville E. A., Roy K. K. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol. Cancer Ther. 2003;2:1093–1103. [PubMed] [Google Scholar]
- Lee J. S., Oh Y., Kim H. S., Yoon S. Low-dose rifabutin increases cytotoxicity in antimitotic-drug-treated resistant cancer cells by exhibiting strong P-gp-inhibitory activity. Int. J. Mol. Sci. 2022a;23:7383. doi: 10.3390/ijms23137383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Oh Y., Lee J. S., Park J. H., Shin J. K., Han J. H., Kim H. S., Yoon S. Combination treatment using pyruvate kinase M2 inhibitors for the sensitization of high density triple-negative breast cancer cells. In Vivo. 2022b;36:2105–2115. doi: 10.21873/invivo.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Oh Y., Park J. H., Kyung S. Y., Kim H. S., Yoon S. Terconazole, an azole antifungal drug, increases cytotoxicity in antimitotic drug-treated resistant cancer cells with substrate-specific P-gp inhibitory activity. Int. J. Mol. Sci. 2022c;23:13809. doi: 10.3390/ijms232213809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. D., Lee O. W., Brimacombe K. R., Chen L., Guha R., Lusvarghi S., Tebase B. G., Klumpp-Thomas C., Robey R. W., Ambudkar S. V., Shen M., Gottesman M. M., Hall M. D. A high-throughput screen of a library of therapeutics identifies cytotoxic substrates of P-glycoprotein. Mol. Pharmacol. 2019;96:629–640. doi: 10.1124/mol.119.115964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Zhang X., Wang Q., Li J., Zhang P., Zhao M., Li X. Perifosine downregulates MDR1 gene expression and reverses multidrug-resistant phenotype by inhibiting PI3K/Akt/NF-kappaB signaling pathway in a human breast cancer cell line. Neoplasma. 2012;59:248–256. doi: 10.4149/neo_2012_032. [DOI] [PubMed] [Google Scholar]
- Marsh Rde W., Rocha Lima C. M., Levy D. E., Mitchell E. P., Rowland K. M., Jr, Benson A. B. A phase II trial of perifosine in locally advanced, unresectable, or metastatic pancreatic adenocarcinoma. Am. J. Clin. Oncol. 2007;30:26–31. doi: 10.1097/01.coc.0000251235.46149.43. [DOI] [PubMed] [Google Scholar]
- Oh Y., Lee J. S., Lee J. S., Park J. H., Kim H. S., Yoon S. Co-treatment of low dose pacritinib, a phase III Jak2 inhibitor, greatly increases apoptosis of P-gp over-expressing cancer cells with multidrug resistance. Anticancer Res. 2022a;42:2433–2442. doi: 10.21873/anticanres.15722. [DOI] [PubMed] [Google Scholar]
- Oh Y., Lee J. S., Lee J. S., Park J. H., Kim H. S., Yoon S. JAK2 inhibitor, fedratinib, inhibits P-gp activity and co-treatment induces cytotoxicity in antimitotic drug-treated P-gp overexpressing resistant KBV20C cancer cells. Int. J. Mol. Sci. 2022b;23:4597. doi: 10.3390/ijms23094597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantziarka P., Cairns L. Recycling existing drugs for cancer therapy: delivering low cost cancer care. Ecancermedicalscience. 2014;8:ed40. doi: 10.3332/ecancer.2014.ed40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. H., Lee J. S., Oh Y., Lee J. S., Park H. E., Lee H., Park Y. S., Kyung S. Y., Kim H. S., Yoon S. PKM2 is overexpressed in glioma tissues, and its inhibition highly increases late apoptosis in U87MG cells with low-density specificity. In Vivo. 2022;36:694–703. doi: 10.21873/invivo.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. H., Lee J. S., Shin J. K., Sharma S., Kim H. S., Yoon S. Low-dose pimecrolimus, an FDA-approved calcineurin inhibitor, sensitizes drug-resistant cancer cells via strong P-gp inhibition. Anticancer Res. 2023;43:1103–1112. doi: 10.21873/anticanres.16255. [DOI] [PubMed] [Google Scholar]
- Patel V., Lahusen T., Sy T., Sausville E. A., Gutkind J. S., Senderowicz A. M. Perifosine, a novel alkylphospholipid, induces p21(WAF1) expression in squamous carcinoma cells through a p53-independent pathway, leading to loss in cyclin-dependent kinase activity and cell cycle arrest. Cancer Res. 2002;62:1401–1409. [PubMed] [Google Scholar]
- Robinson K., Tiriveedhi V. Perplexing role of P-glycoprotein in tumor microenvironment. Front. Oncol. 2020;10:265. doi: 10.3389/fonc.2020.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig A. P-glycoprotein: one mechanism, many tasks and the consequences for pharmacotherapy of cancers. Front. Oncol. 2020;10:576559. doi: 10.3389/fonc.2020.576559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh N., McClelland S. E. Diversity in chromosome numbers promotes resistance to chemotherapeutics. Dev. Cell. 2021;56:2399–2400. doi: 10.1016/j.devcel.2021.08.017. [DOI] [PubMed] [Google Scholar]
- Tang S. J., Chen L. K., Wang F., Zhang Y. K., Huang Z. C., To K. K., Wang X. K., Talele T. T., Chen Z. S., Chen W. Q., Fu L. W. CEP-33779 antagonizes ATP-binding cassette subfamily B member 1 mediated multidrug resistance by inhibiting its transport function. Biochem. Pharmacol. 2014;91:144–156. doi: 10.1016/j.bcp.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Tomiyasu H., Goto-Koshino Y., Fujino Y., Ohno K., Tsujimoto H. Antitumour effect and modulation of expression of the ABCB1 gene by perifosine in canine lymphoid tumour cell lines. Vet. J. 2014;201:83–90. doi: 10.1016/j.tvjl.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Toson B., Fortes I. S., Roesler R., Andrade S. F. Targeting Akt/PKB in pediatric tumors: a review from preclinical to clinical trials. Pharmacol. Res. 2022;183:106403. doi: 10.1016/j.phrs.2022.106403. [DOI] [PubMed] [Google Scholar]
- Uko N. E., Guner O. F., Matesic D. F., Bowen J. P. Akt pathway inhibitors. Curr. Top. Med. Chem. 2020;20:883–900. doi: 10.2174/1568026620666200224101808. [DOI] [PubMed] [Google Scholar]
- Vucicevic L., Misirkic-Marjanovic M., Harhaji-Trajkovic L., Maric N., Trajkovic V. Mechanisms and therapeutic significance of autophagy modulation by antipsychotic drugs. Cell Stress. 2018;2:282–291. doi: 10.15698/cst2018.11.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K., Wu J., Li X. Recent advances in the research of P-glycoprotein inhibitors. Biosci. Trends. 2008;2:137–146. [PubMed] [Google Scholar]
- Yoon S., Kim H. S. Drug repositioning with an anticancer effect: contributions to reduced cancer incidence in susceptible individuals. In Vivo. 2021;35:3039–3044. doi: 10.21873/invivo.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S., Kim H. S. Contribution of cancer-targeting drugs toward faster clinical application. Int. J. Mol. Sci. 2022;23:6445. doi: 10.3390/ijms23126445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S., Kim H. S. First-line combination treatment with low-dose bipolar drugs for ABCB1-overexpressing drug-resistant cancer populations. Int. J. Mol. Sci. 2023;24:8389. doi: 10.3390/ijms24098389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S., Wang X., Vongpunsawad S., Tromp G., Kuivaniemi H. Editorial: FDA-approved drug repositioning for P-glycoprotein overexpressing resistant cancer. Front. Oncol. 2021;11:632657. doi: 10.3389/fonc.2021.632657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Y. H., Lin Y. F., Nirosha B., Chang H. T., Huang Y. F. Polydopamine-coated gold nanostar for combined antitumor and antiangiogenic therapy in multidrug-resistant breast cancer. Nanotheranostics. 2019;3:266–283. doi: 10.7150/ntno.36842. [DOI] [PMC free article] [PubMed] [Google Scholar]