Abstract
The evolution of cornified skin appendages, such as hair, feathers, and claws, is closely linked to the evolution of proteins that establish the unique mechanical stability of these epithelial structures. We hypothesized that the evolution of the limbless body anatomy of the Florida worm lizard (Rhineura floridana) and the concomitant loss of claws had led to the degeneration of genes with claw-associated functions. To test this hypothesis, we investigated the evolution of three gene families implicated in epithelial cell architecture, namely type I keratins, type II keratins, and genes of the epidermal differentiation complex in R. floridana in comparison with other squamates. We report that the orthologs of mammalian hair and nail keratins have undergone pseudogenization in R. floridana. Likewise, the epidermal differentiation complex genes tentatively named EDYM1 and EDCCs have been lost in R. floridana. The aforementioned genes are conserved in various lizards with claws, but not in snakes. Proteomic analysis of the cornified claws of the bearded dragon (Pogona vitticeps) confirmed that type I and type II hair keratin homologs, EDYM1 and EDCCs, are protein components of claws in squamates. We conclude that the convergent evolution of a limbless body was associated with the convergent loss of claw keratins and differentiation genes in squamates.
Keywords: gene family, gene loss, reptiles, worm lizard, snake
Significance.
The protein components of cornified skin appendages, such as claws, and the cornified layer of the epidermis, are encoded by three gene families, namely type I and type II keratins and epidermal differentiation complex (EDC) genes. To determine which members of these gene families are associated with functions in claws, we performed comparative genomics of reptiles with claws and limbless reptiles that lack claws. We report that both the Florida worm lizard and snakes have lost homologs of keratins and EDC genes that are expressed in the claws of limbed lizards. These results suggest that worm lizards and snakes have undergone convergent evolution at the morphological and molecular levels.
Introduction
Cornified skin appendages, such as claws, hair, and feathers, have evolved in land-dwelling vertebrates as important and partly lineage-specific adaptations (Wu et al. 2004; Alibardi et al. 2009; Fleckman et al. 2013; Chen et al. 2015; Akat et al. 2022; Eckhart et al. 2024). They are formed by terminal differentiation of epithelial cells, known as keratinocytes, which accumulate cross-linked proteins. Upon cornification, the keratinocytes undergo programmed cell death but remain interconnected by stable cell junctions (Candi et al. 2005; Harland and Plowman 2018; Matsui 2023). The main protein components of hair and nails are the so-called hair keratins, which are cysteine-rich proteins of the monophyletic subfamilies of type I and type II keratin intermediate filament proteins (Eckhart and Ehrlich 2018; Shim et al. 2022). Orthologs of hair keratins are expressed in the claws of reptiles (Eckhart et al. 2008; Alibardi et al. 2011) and amphibians (Carron et al. 2024), but do not exist in lungfish, suggesting that hair keratin subclades of type I and type II keratins have originated in basal tetrapods (Vandebergh et al. 2013; Carron et al. 2024). In the green anole lizard, some hair keratin homologs are expressed exclusively in claws, whereas others are expressed in both claws and skin (Eckhart et al. 2008), suggesting functional diversification of these keratins. Scales and feathers of sauropsids (reptiles and birds) contain additional keratins (hard acid sauropsid-specific [HAS] keratins and hard basic sauropsid-specific [HBS] keratins) that have convergently evolved a high cysteine content (Ehrlich et al. 2020). Other important protein components of cornified keratinocytes are encoded by genes of the epidermal differentiation complex (EDC) (Candi et al. 2005; Strasser et al. 2014, 2015; Alibardi et al. 2016; Holthaus and Eckhart 2024). Many of these epidermal differentiation proteins are covalently cross-linked by transglutamination under the control of transglutaminases (Candi et al. 2005; Sachslehner et al. 2023), and others have a high abundance of cysteine residues indicative of disulfide bond–dependent cross-linking (Lachner et al. 2019; Ishitsuka and Roop 2020).
Skin appendages such as claws have been lost in several lineages of tetrapods (Gans 1975; Apesteguía and Zaher 2006; Alibardi 2009; Yenmiş and Ayaz 2023). The loss of cornified skin appendages was associated with the degeneration of genes that function specifically in skin appendages (Cohn and Tickle 1999; Emerling 2017; Leal and Cohn 2018). The most prominent examples of pseudogenization and gene loss are provided by hair/claw keratin homologs in whales and dolphins (Cetacea, Mammalia) (Hecker et al. 2017; Ehrlich et al. 2019), snakes (Serpentes, Sauropsida) (Dalla Valle et al. 2011; Ehrlich et al. 2020), and caecilians (Gymnophiona, Amphibia) (Carron et al. 2024). Two types of genes of the EDC, tentatively named EDYM1 and EDCCs, were found to be conserved in diverse reptiles but not in snakes, pointing to roles of these genes in cornified claws (Holthaus et al. 2017). Recently, we have reported that the gene for transglutaminase 9 has degenerated upon the evolutionary loss of the cornified egg tooth in mammals and upon the loss of cornified claws in snakes and worm lizards (Sachslehner et al. 2024).
The Florida worm lizard (Rhineura floridana) is the first member of the clade Amphisbaenia whose genome sequence has been determined (Rhie et al. 2021). Phylogenetically, Amphisbaenia are positioned within the clade Lacertoidea (Vidal and Hedges 2005; Streicher and Wiens 2017). The fossorial lifestyle of amphisbaenians has led to several anatomical adaptations such as the degeneration of eyes, modifications of the head, the elongation of the body, and the reduction or loss of limbs and digits (Gans 1975; Kearney and Stuart 2004; Wiens et al. 2006; Foureaux et al. 2010; Allam et al. 2016). The molecular changes underlying the modifications of the skin and its appendages in relation to the fossorial adaptation of squamates are not known.
Here, we performed a comparative genomics study to determine which skin epithelium-associated genes were convergently lost during the evolutionary degeneration of limbs in the Florida worm lizard and in snakes.
Results
Homologs of Type I Hair Keratin Genes Are Disrupted by Mutations in the Worm Lizard
To test the hypothesis that evolution of a limbless body has rendered claw-specific genes dispensable and allowed for appearance of disruptive mutations or even gene loss, we investigated three clusters of genes in which candidate claw genes were identified in other squamate reptiles: type I keratin genes, type II keratin genes, and the EDC (Eckhart et al. 2008; Holthaus et al. 2017; Ehrlich et al. 2020; Alibardi 2021). Gene sequences were obtained by downloading gene predictions available in GenBank and by the identification of additional genes and pseudogenes following a published approach (Holthaus et al. 2017; Ehrlich et al. 2020) in the genome sequences of the Florida worm lizard (R. floridana) (Rhie et al. 2021) and the green anole lizard (Anolis carolinensis) (Alföldi et al. 2011; supplementary table S1, Supplementary Material online).
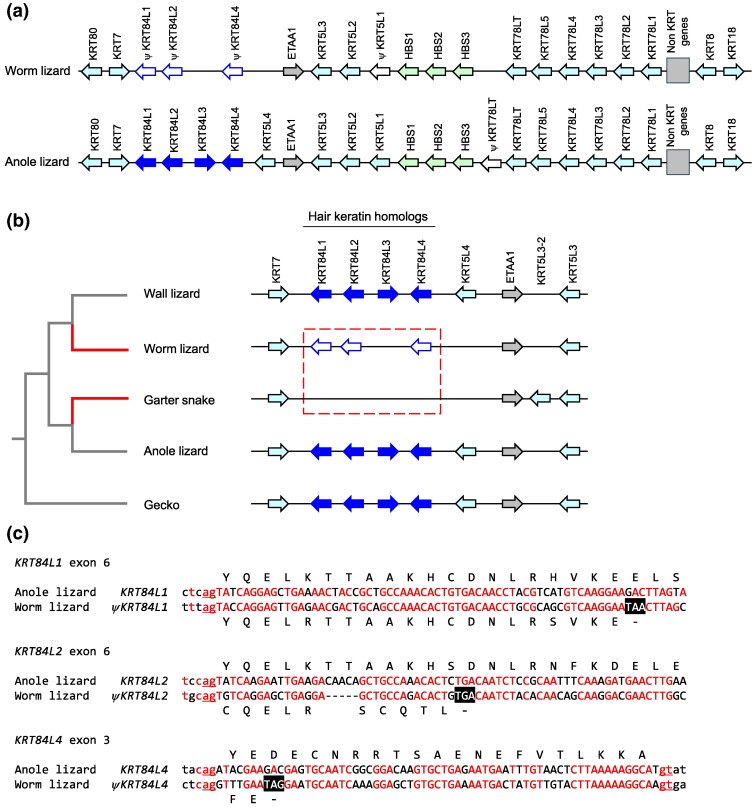
The arrangement of genes in the type I keratin cluster of the worm lizard is similar to that of the green anole lizard (Fig. 1a; supplementary table S1, Supplementary Material online). However, there is no protein-coding homolog of the anole lizard KRT36-like (KRT36L) genes, previously referred to as hard acidic (HA) 1 and HA2 keratins (Eckhart et al. 2008), between KRT23 and KRT15 in the worm lizard (Fig. 1a). Next, we compared the specific locus of KRT36L genes and their flanking genes in a phylogenetically broader group of lepidosaurs (Fig. 1b). Homologs of hair keratins (KRT36-like genes) are absent in the worm lizard and in the garter snake (supplementary table S2, Supplementary Material online), which is in agreement with the proposed loss of type I hair keratin genes at the base of Serpentes (Dalla Valle et al. 2011; Emerling 2017; Ehrlich et al. 2020). By contrast, two KRT36-like genes are present in the wall lizard, the anole lizard, and the gecko (Fig. 1b). Since the gecko is a phylogenetically basal squamate, it can be deduced that the ancestral condition for squamates was the presence of two KRT36-like genes. A gene remnant (pseudogene, ψKRT36L1) was found at the expected locus of KRT36L1 in the worm lizard (Fig. 1b). Alignment of KRT36-like nucleotide sequence stretches with the sequences of KRT36L1 genes of the wall lizard, and the anole lizard revealed mutations leading to the loss of a consensus splicing signal, a premature stop codon (Fig. 1c), and other defects in ψKRT36L1 of the worm lizard.
Fig. 1.
Mutations disrupt hair keratin homologs in the type I keratin gene cluster of the worm lizard. a) The type I keratin gene clusters of the worm lizard and the anole lizard are depicted schematically. Genes are represented by arrows pointing in the direction of transcription. Hair keratin homologs (KRT36-likes, KRT36L) are shaded dark blue, green indicates sauropsid-specific HAS keratin genes, and other keratin genes are shaded in light blue. Pseudogenes are marked with the symbol ψ and displayed as white arrows. b) Comparison of the KRT36L loci between different lepidosaur species reveals convergent loss of KRT36L genes in the worm lizard and snakes. In both sister groups and the outgroups, two KRT36L genes are present. Phylogenetic relationships of species are shown on the left. c) Nucleotide sequence alignment of a fragment of the highly degenerated KRT36L1 pseudogene of the worm lizard and homologous segments of intact KRT36L1 genes of other species. Above and below the nucleotide sequences, the amino acid sequence of the corresponding protein is shown. A premature stop codon is indicated by white fonts on black background. Red and blue letters indicate conservation in at least three and two species, respectively. Red letters indicate sequence identity. Lower case letters belong to introns. The consensus motifs of splice acceptor sites are underlined. Note the loss of the splice site in the worm lizard. The sequence of worm lizard ψKRT36L1 corresponds to GenBank accession number NC_084490.1, nucleotides 49197581 to 49197865. Keratin names were assigned according to the study by Ehrlich et al. (2020). Species: anole lizard (A. carolinensis), garter snake (T. elegans), gecko (G. japonicus), wall lizard (P. muralis), and worm lizard (R. floridana).
Homologs of Type II Hair Keratin Genes Are Disrupted by Mutations in the Worm Lizard
Like the keratin type I keratin gene cluster, the type II keratin gene cluster of the worm lizard resembles the homologous gene cluster of the anole lizard (Fig. 2a; supplementary tables S3 and S4, Supplementary Material online). Different from previously investigated species of vertebrates, both the worm lizard and the wall lizard contain nonkeratin genes that interrupt the type II keratin cluster (Fig. 2a). The worm lizard lacks orthologs of KRT5L1 and KRT5L4 of the anole lizard, and, most importantly, the genes encoding type II hair keratins are nonfunctional in the worm lizard. In contrast to the presence of four protein-coding KRT84-like genes in squamates with limbs (wall lizard, anole lizard, and gecko) (Fig. 2b), no KRT84-like genes exist in the garter snake and other snakes (Ehrlich et al. 2020), and three KRT84-like pseudogenes (ψKRT84L1, ψKRT84L2, and ψKRT84L4) are present in the worm lizard. Notably, these pseudogenes are incomplete remnants of ancestral genes with sequence segments being similar to only few of the original exons. Alignment of the nucleotide sequences showed that premature stop codons in these exon remnants and a frameshift in a degenerated exon of ψKRT84L2 disrupt the coding sequences (Fig. 2c).
Fig. 2.
Mutations disrupt hair keratin homologs in the type II keratin gene cluster of the worm lizard. a) The type II keratin gene clusters of the worm lizard and the anole lizard are depicted schematically. Genes are represented by arrows pointing in the direction of transcription. Hair keratin homologs (KRT84-likes, KRT84L) are shaded in dark blue, green indicates sauropsid-specific HBS keratin genes, and other keratin genes are shaded in light blue. Pseudogenes are marked with the symbol ψ and displayed as white arrows. b) Comparison of the KRT84L loci between different lepidosaur species reveals convergent loss of KRT84L genes in the worm lizard and snakes. In both the snake and the worm lizard sister groups, four KRT84L genes are present as well as in the outgroup (gecko). Phylogenetic relationships of species are shown on the left. c) Alignments of nucleotide sequences of partial KRT84L pseudogenes of the worm lizard and homologous segments of intact KRT84L genes of the anole lizard. Premature stop codons are highlighted by white fonts on black background. Above and below the nucleotide sequences, the amino acid sequences of the corresponding proteins are shown. Red letters indicate sequence identity. Lower case letters belong to introns. The consensus motifs of splice acceptor and donor sites are underlined. The sequences of worm lizard pseudogenes correspond to GenBank accession number NC_084482.1, nucleotides 21376268 to 21376341 (ψKRT84L1), 21360260 to 21360328 (ψKRT84L2), 21357117 to 21357190 (ψKRT84L4). Keratin names were assigned according to the study by Ehrlich et al. (2020). Species: anole lizard (A. carolinensis), garter snake (T. elegans), gecko (G. japonicus), wall lizard (P. muralis), and worm lizard (R. floridana).
The EDC of the Worm Lizard Is Largely Syntenic With the EDC of the Green Anole Lizard
In contrast to the availability of reliable predictions for most keratin genes of the worm lizard, only few genes of the EDC were correctly predicted prior to our study. To enable the comparative analysis of the EDC, we applied a combination of tBLASTn searches using EDC proteins of other squamates as queries and de novo gene predictions from translated nucleotides sequences (Strasser et al. 2014) in the EDC regions of the Florida worm lizard (R. floridana). In particular, we used sequences of EDC genes of the common wall lizard (Podarcis muralis) (Holthaus et al. 2024). Preliminary names were assigned to the numerous newly identified genes of the EDC. Full gene names with brief explanation are listed in supplementary table S5, Supplementary Material online, whereas in the text and figure, abbreviations are used starting with ED (epidermal differentiation) followed by a term indicating either a specific amino acid composition or motif of the protein encoded. The genomic locations of EDC genes identified in the worm lizard are listed in supplementary table S6, Supplementary Material online. Amino acid sequences of EDC proteins are listed in supplementary fig. S1, Supplementary Material online.
The general organization of the EDC of the worm lizard is similar to that of other lepidosaurs (Strasser et al. 2014; Holthaus et al. 2017, 2020) with the exception of the wall lizard in which EDC genes are rearranged (Fig. 3). S100A genes at the extremes of the EDC, PGLYRP3, S100 fused-type protein (SFTP), and single-coding exon EDC (SEDC) genes were identified in the worm lizard like in the EDC of other amniotes. Like other lepidosaurs, the worm lizard has a cluster of corneous beta protein (CBP) or beta-keratin genes in the central region of the EDC with SEDC genes encoding proline-rich proteins on one side and different types of SEDC genes on the other side (Fig. 3). Most of the EDC genes of the wall lizard are conserved in the worm lizard, but there are notable exceptions, namely EDCATM, EDCC, EDCP, EDHEM, EDPAML, EDPCS, EDPQ2, EDQM, EDQSG, EDWM2, EDWM3, and EDYM1. The EDYM1 and EDCC genes are also absent in the python and cobra (Holthaus et al. 2017). As the latter genes represent candidates for an evolutionary association with limbs, we investigated them further.
Fig. 3.
The EDC of the worm lizard in comparison with the EDC of other lepidosaurs. The EDC of the worm lizard (R. floridana) is schematically depicted, whereby the genes are aligned to those in the EDC of the wall lizard (P. muralis), a snake (Ophiophagus hannah), and the anole lizard (A. carolinensis). Phylogenetic relationships of species are shown on the left. Single-copy genes are represented by arrows pointing in the direction of transcription, whereas arrays of three or more paralogous genes are shown as boxes, with the number of genes being indicated after the symbol #. Colors highlight the following groups of EDC genes: red, genes missing in both worm lizard and snakes; yellow, corneous beta-proteins; green, genes located between CBPs and SFTPs; blue, SFTPs; and violet, genes located between PGLYRP3 and CBPs. Note that the EDC of the wall lizard has undergone a rearrangement with three segments, here connected by broken lines, being syntenic with segments of the EDC in other lepidosaurs (Holthaus et al. 2024). Non-EDC genes are shown in black. Members of gene families are numbered in each species without inferring 1:1 orthology to genes of the same number in other species. The symbol ψ indicates pseudogenes. The symbol // is used to indicate gaps in the sequence assembly. The symbol § indicates separation of genes on different scaffolds. The schematic depiction is not drawn to scale.
EDC Genes Associated With Claws in Lepidosaurs Have Been Lost in the Worm Lizard
EDYM1 and EDCC were previously found in the anole lizard but not in snakes, leading to the hypothesis that they might have a specific function in claws (Holthaus et al. 2017). Here, we show that EDYM1 and EDCC are also absent from the EDC of the worm lizard (Fig. 4). By contrast, EDYM1 is conserved as a single-copy gene, and three to nine EDCC paralogs are present in other lepidosaurs except snakes (Fig. 4).
Fig. 4.
EDC genes associated with claws in lepidosaurs have been lost in the worm lizard. Comparison of the EDCC and EDYM1 gene locus between the worm lizard and other lepidosaurs. In both the limbless worm lizard and snakes, EDCC and EDYM1 genes have been lost while they are present in the wall and anole lizard, gecko, and tuatara. Phylogenetic relationships of species are shown on the left. Genes are represented by arrows pointing in the direction of transcription. The symbol // is used to indicate gaps in the sequence assembly. The symbol § indicates separation of genes on different scaffolds.
Having identified EDYM1 and EDCCs as potentially claw-associated genes, we investigated their expression by reverse transcription polymerase chain reaction (RT-PCR) and mass spectrometry–based proteomics of claws of the green anole lizard. RT-PCR showed that EDYM1, EDCC1, EDCC2, and EDCC3 are expressed in clawed toes (supplementary fig. S2, Supplementary Material online). The proteomic analysis confirmed the expression of EDCC1 and EDCC2 in clawed toes but not in the skin of the back (supplementary tables S7 and S8, Supplementary Material online).
Finally, we performed a proteomic analysis of claws and skin of the bearded dragon (Pogona vitticeps), in which claws could be separated from adjacent tissue more efficiently than from the small toes of A. carolinensis. This analysis showed that EDYM1- and EDCC-like proteins were present in claws, but not in the skin (Table 1). Likewise, homologs of hair keratins were detected in the claws and not in the skin (Table 1). Among all proteins detected, a type I hair keratin homolog (GenBank gene LOC110074843) and a type II hair keratin homolog (GenBank gene LOC110070014) had the highest Sequest score, which is a measure of confidence of detection (supplementary tables S9 and S10, Supplementary Material online). These data show that orthologs of genes that have been lost or pseudogenized in the worm lizard are expressed in the claws of limbed lizards.
Table 1.
Proteomic analysis of claws and skin of the bearded dragon (P. vitticeps)
| Protein type | Protein | Accession (UniProt) | Gene symbol (GenBank) | Description (GenBank) | Expression sites (detection by MS) | Evolutionary fate of ortholog in worm lizard | |
|---|---|---|---|---|---|---|---|
| Claws | Skin | ||||||
| Keratins, type I | KRT36L1 | A0A6J0T4Z5 | LOC110074843 | Keratin, type I cuticular Ha6-like | + | − | Loss |
| KRT36L2 | A0A6J0T2L7 | LOC110074851 | Keratin, type I cuticular Ha6-like | + | − | Loss | |
| KRT14L2 | A0A6J0T2V3 | LOC110074892 | Keratin, type I cytoskeletal 14-like | + | − | ||
| KRT14L1 | A0A6J0T2W2 | LOC110074898 | Keratin, type I cytoskeletal 14-like | + | + | ||
| KRT15 | A0A6J0SXS2 | LOC110074850 | Keratin, type I cytoskeletal 15-like isoform X1 | + | + | ||
| KRT24L | A0A6J0T561 | LOC110074871 | Keratin, type I cytoskeletal 15-like isoform X2 | − | + | ||
| KRT24 | A0A6J0SY29 | LOC110074904 | Keratin, type I cytoskeletal 24-like isoform X2 | − | + | ||
| KRT9L1a | A0A6J0SXS9 | LOC110074853 | Keratin, type I cytoskeletal 10-like | − | + | ||
| HAS4/KRT9LC4 | A0A6J0SXW5 | LOC110074873 | Keratin, type I cytoskeletal 10-like | − | + | ||
| Keratins, type II | KRT84L1 | A0A6J0SDW8 | LOC110070014 | Keratin, type II cuticular Hb5-like | + | − | Loss |
| KRT84L2 | A0A6J0SEM6 | LOC110070016 | Keratin, type II cytoskeletal 8-like | + | − | Loss | |
| KRT84L3 | predicted | LOC110070017 | Keratin, type II cuticular Hb2-like | + | − | Loss | |
| KRT84L4 | A0A6J0SA49 | LOC110070015 | Keratin, type II cytoskeletal cochleal-like isoform X1 | + | − | ||
| KRT5L4 | A0A6J0SA54 | LOC110070018 | Keratin, type II cytoskeletal cochleal-like | + | + | ||
| KRT5L2 | A0A6J0VBD4 | LOC110090296 | Keratin, type II cytoskeletal 5-like isoform X2 | + | + | ||
| KRT78L2 | A0A6J0VFV2 | LOC110090297 | Keratin, type II cytoskeletal cochleal-like | + | + | ||
| KRT78L5 | A0A6J0VFU1 | LOC110090289 | Keratin, type II cytoskeletal 5 | − | + | ||
| HBS2/KRT78LC2 | A0A6J0VBD1 | LOC110090293 | Keratin, type II cytoskeletal 7-like | − | + | ||
| KRT78L4 | A0A6J0VFV7 | LOC110090299 | Keratin, type II cytoskeletal 5 | − | + | ||
| EDC proteins | EDYM1 | n.a. | n.a. | NW_018151906.1:92820-93404 (coding sequence) | + | − | Loss |
| EDCCL1 | n.a. | n.a. | NW_018151906.1:c70382-70750 (coding sequence) | + | − | Loss | |
| EDCCL2 | n.a. | n.a. | NW_018151906.1:c80185-80565 (coding sequence) | + | − | Loss | |
| EDCCL4 | n.a. | n.a. | NW_018151906.1:84888-85103 (coding sequence) | + | − | Loss | |
| CBP1 | n.a. | n.a. | NW_018151906.1:54057-55130 (coding sequence) | + | − | ||
| EDGY1 | n.a. | n.a. | NW_018151895.1:c48244-48633 (coding sequence) | + | − | ||
| EDCP | n.a. | n.a. | NW_018151765.1:13812-14501 (coding sequence) | + | − | ||
| Desmosomal proteins | DSP | A0A6J0TYQ4 | DSP | Desmoplakin | + | + | |
| PKP1 | A0A6J0SNS3 | PKP1 | Plakophilin-1 | + | + | ||
| DSG1 | A0A6J0TDZ5 | LOC110077646 | Desmoglein-1-beta-like | + | + | ||
| DSC1 | A0A6J0UWW8 | LOC110087251 | Desmocollin-1-like | + | + | ||
| JUP | A0A6J0T2Q4 | JUP | Junction plakoglobin | + | + | ||
| PKP3 | A0A6J0STP6 | PKP3 | Plakophilin-3 | + | + | ||
Proteins were named according to the nomenclature used previously for other sauropsids (Ehrlich et al. 2020). Orthology is uncertain for some proteins (a). Amino acid sequences of EDC proteins were obtained by translation of predicted genes. GenBank accession numbers of coding sequences are provided under “Description.” Detailed information on results of proteomics is provided in supplementary tables S7 and S8, Supplementary Material online. In the column “Evolutionary fate of ortholog in worm lizard,” the loss of proteins is highlighted. Other evolutionary changes are not indicated.
MS, mass spectrometry; n.a., not applicable.
Taken together, the comparative analysis of keratin and EDC genes in the selected panel of squamate species leads to a model for the evolutionary degeneration of cornified claws at the molecular level (Fig. 5). The Florida worm lizard and snakes lack homologs of type I hair/claw keratins (KRT36-likes), type II hair/claw keratins (KRT84-likes), and specific EDC genes (EDYM1 and EDCCs), which are conserved in other squamates investigated and which are expressed in the claws of at least one lizard, the bearded dragon. This indicates that all the aforementioned genes were present in the last common ancestor of squamates and that they underwent convergent loss or pseudogenization in two lineages that lost limbs and also claws (Fig. 5).
Fig. 5.
Model of convergent evolution in limbless reptiles: snakes and the worm lizard. The phylogenetic tree shows relationships of lepidosaurs investigated in this study and divergence times (Ma) (Kumar et al. 2022) of the corresponding lineages. In both the worm lizard and snakes, limbs and claws have been lost. Xs on the branches indicate the loss of at least two genes of the following groups: type I claw keratins (orthologs of mammalian hair keratin KRT36), type II claw keratins (orthologs of mammalian hair keratin KRT84), and EDC genes (EDYM1 and EDCC). Species: worm lizard (R. floridana), wall lizard (P. muralis), snake (T. elegans), anole lizard (A. carolinensis), gecko (G. japonicus), and tuatara (Sphenodon punctatus).
Discussion
The loss of a phenotypic trait is an intriguing evolutionary process that allows to identify trait-specific genes by comparative genomics and gene expression analyses. The loss of pigments in cave-dwelling animals of diverse taxa (Aardema et al. 2020); the loss of teeth as adaptation to specializations in food uptake in frogs, birds, turtles, baleen whales, anteaters, and others (Meredith et al. 2013, 2014; Paluh et al. 2021); and the loss of limbs in cetaceans, snakes, and caecilians (Cohn and Tickle 1999; Thewissen et al. 2006; Kvon et al. 2016; Leal and Cohn 2016, 2018; Roscito et al. 2018, 2022; Sun et al. 2022; Ovchinnikov et al. 2023) have been studied extensively, leading to important insights into the molecular regulation of traits and dynamics of evolution. Importantly, the independent loss of a particular trait in different lineages is predicted to lead to the parallel loss of those genes that are required for the development and function of this trait, but not for other traits under selective constraints. The present study contributes another example to this concept by showing that parallel loss of claws manifests in loss-of-function mutations in claw-specific genes.
Our results show that specific intermediate filament proteins of the type I and type II keratin families and a subgroup of EDC proteins have been lost in the Florida worm lizard. The keratins affected by pseudogenization are homologs of the so-called hair keratins, which actually evolved as claw keratins (Eckhart et al. 2008; Langbein et al. 2010; Carron et al. 2024) and were co-opted to hair only in mammals. Type I and type II hair/claw keratins originated in amphibians and were demonstrated to be components of cornified claws of clawed frogs (Carron et al. 2024), anole lizards (Eckhart et al. 2008), mice (Jaeger et al. 2019), and humans (Perrin et al. 2004). In the first report on hair keratin homologs in the lizard A. carolinensis, two genes (then termed HA1 and HB1) were detected by RT-PCR exclusively in claws, whereas the other homologs of hair keratins (HA2, HB2, and HB3) were expressed in the claws, abdominal skin, and the tongue (Eckhart et al. 2008). In line with these mRNA data, we detected the protein Krt36L2, which is equivalent to HA2 (Eckhart et al. 2008), not only in claws (supplementary table S7, Supplementary Material online) but also in back skin (supplementary table S8, Supplementary Material online). Given the apparently pleiotropic roles of some hair keratin homologs, it cannot be predicted with certainty that the evolutionary loss of claws (and hair in mammals) will make the corresponding genes dispensable and therefore prone to pseudogenization. A disruptive point mutation was detected in a type I hair keratin gene of the limbless slowworm (Anguis fragilis) (Dalla Valle et al. 2011). The results of the present study reveal degeneration of all hair keratin orthologs in the Florida worm lizard, indicating that the main ancestral function of these proteins was the molecular architecture of claws, which are absent in this limbless lizard. The identification in the Florida worm lizard of pseudogenes, representing gene fossils, at loci syntenic with orthologous genes of other species, confirms that genes were not missed by our search, but indeed inactivated by mutations. Notably, the genes flanking hair keratin homologs were conserved—with variable distances between them—in the species investigated (supplementary table S11, Supplementary Material online).
The role of EDC proteins in claws has been much less investigated than the role of keratins (Alibardi 2021). Several papers refer to a subgroup of EDC proteins as claw beta-keratins or “claw keratins” (Whitbread et al. 1991). These proteins are CBPs, the corresponding genes are located in the EDC, and their expression was detected in cornified claws of birds (Holthaus et al. 2018). However, “claw keratins” of birds are not specifically expressed in claws (Wu et al. 2015). In squamates, some CBPs are expressed predominantly in claws (Dalla Valle et al. 2010). Moreover, a relatively high number of presumably redundant CBP paralogs is present in all sauropsid species investigated (Holthaus et al. 2018), including snakes (Holthaus et al. 2017), and CBP pseudogenes have been identified in many sauropsids with and without claws (Holthaus et al. 2016, 2017, 2020). Therefore, we screened other EDC genes of the worm lizard for inactivating mutations. We did not find loss-of-function mutations in SFTP genes, which are expressed in epithelial compartments, such as the subunguis of the claw (Mlitz et al. 2014; Alibardi et al. 2015). Possibly, the conservation of SFTPs in worm lizards and snakes is due to additional functions of SFTPs in the periderm during embryonic development (Eckhart et al. 2024). However, we did detect the loss of particular EDC genes in worm lizards, and strikingly, two of these genes, EDYM1 and EDCC, have also been lost in snakes (Holthaus et al. 2017). The expression of EDYM1 and EDCC in the clawed toes of the green anole lizard supports the hypothesis that they play a role in claws; however, the toe samples comprised the claw-forming epithelium and adjacent tissue. Strong evidence for functions of these proteins in claws is provided by the detection of EDYM1 and EDCC homologs in the isolated claws of the bearded dragon. Notably, the avian ortholog of EDYM1 was detected by proteomics in the claws, but also in the beak and scales of the chicken (Strasser et al. 2014). EDYM1 and EDCC are not conserved in the python (Holthaus et al. 2017), although this snake develops claw-like structures on vestigial hind limbs, known as pelvic spurs (Gillingham and Chambers 1982). The molecular composition of the epithelium on pelvic spurs and its evolutionary relationship to the cornified epithelium of claws should be investigated in future studies.
The EDC proteins convergently lost in the worm lizard and in snakes do not feature particular amino acid sequences that would suggest specific functions. More cysteine-rich proteins (tentatively named EDCRPs and EDPCCCs), which are assumed to be preferred substrates of disulfide bond–mediated cross-linking, are conserved in both worm lizard and snakes. This suggests that cysteine-dependent protein cross-linking of EDC proteins is not limited to claws. Although the precise contribution of EDYM1 and EDCCs to the cornification of claws remains to be determined, the apparent association of these EDC-encoded proteins with the evolution of claws establishes a predominant role of these EDC proteins in claw formation of squamates. Previously, EDC-encoded CBPs were identified as components of lizard claws (Dalla Valle et al. 2010), and mRNAs of other EDC genes were detected in the toes of A. carolinensis (Strasser et al. 2014).
In summary, the results of this study suggest convergent evolution toward loss of claw proteins in limbless squamates. The convergent loss of specific keratins and EDC proteins upon loss of claws supports the concept that the ancestral and predominant function of these proteins was to build cornified claws.
Materials and Methods
Comparative Analysis of Keratin Type I and Type II Gene Loci
The complete keratin gene clusters were analyzed in the genomes of the Florida worm lizard (R. floridana) and the green anole lizard. For the analysis of keratin type I and type II gene clusters, gene predictions of the Florida worm lizard in GenBank (chromosome accession numbers NC_084490.1 and NC_084482.1, respectively) (Rhie et al. 2021) were used and compared with keratin sequences of the green anole lizard (A. carolinensis) (Alföldi et al. 2011; Ehrlich et al. 2020). The sequences of type I and type II keratins (Ehrlich et al. 2020) were corrected, where necessary, based on the current genome sequence version (GenBank accession numbers NC_085846.1 and NC_085842.1, respectively) (supplementary fig. S3, Supplementary Material online). Basic Local Alignment Search Tool (Altschul et al. 1990) searches using keratin sequences of other species were performed to identify the gene segments not predicted in GenBank and pseudogenes. GenBank accession numbers of type I and type II keratins are shown in supplementary tables S1 and S3, Supplementary Material online, respectively.
For comparison of homologous loci, in addition to the above-mentioned species, we investigated the common wall lizard (P. muralis) (Andrade et al. 2019), the western terrestrial garter snake (Thamnophis elegans) (Vertebrate Genomes Project 2019), and the Japanese gecko (Gekko japonicus) (Liu et al. 2015). Accession numbers of genes at the KRT36L and KRT84L loci are listed in supplementary tables S2 and S4, Supplementary Material online. Sequence alignments were performed with MUSCLE and MultAlin (Corpet 1988).
EDC Gene Identification in Genome Sequences
A multiple-step approach was used to identify the sequences of EDC genes in the Florida worm lizard (R. floridana) (Rhie et al. 2021). EDC genes predicted in the GenBank were compared with EDC genes of other lepidosaurs (Strasser et al. 2014; Holthaus et al. 2017, 2020, 2024) with regard to exon–intron structure and sequence conservation. Genes conforming to the consensus structures of SEDC and SFTPs were used for further studies. Additional EDC genes were identified by tBLASTn searches and de novo prediction of coding sequences according to a published approach (Strasser et al. 2014). The filter for low-complexity regions was deactivated to avoid erroneous exclusion of typical EDC genes containing segments of low sequence complexity. De novo-predicted EDC genes were used as queries to identify similar proteins in the same and in other species. The EDC gene predictions were validated by RNA-seq evidence, if available in the NCBI GenBank browser for “genomic regions, transcripts, and products” (last accessed on 2024 June 5). The loci of EDYM1, EDCCs, and flanking genes were compared using previously reported EDC gene data (Strasser et al. 2014; Holthaus et al. 2017, 2020, 2024) and new gene predictions in the worm lizard.
RT-PCR Analysis of Putative Claw-Associated EDC mRNAs of A. carolinensis
RNA was prepared from tissues of A. carolinensis and reverse-transcribed into cDNA as described previously (Strasser et al. 2014). The following cDNAs of A. carolinensis were amplified with intron-spanning primers as indicated: EDYM1, 5′-ATCCGCTCGAGAAGGCTTC-3′ and 5′-TGAGGGCTTCATCATACCATACT-3′, EDCC1, 5′-GTCTCCAAACGTTGCATTCCACG-3′ and 5′-CAGGGGTGACGTGGATATCT-3′, EDCC2, 5′-ACTGTTTCTTCTCCAGACGTCT-3′ and 5′-GTGCAACCATGATCTCGGAC-3′, EDCC3, 5′-CCGTTGATGTCCATCTCCATCC-3′ and 5′-ATTCTGAAGGGCTGGTTGGT-3′, EDQM, 5′-ACCTTGCTTCAATTTCCAGAGA-3′ and 5′-CTGATCCTGTGACGGCTTTG-3′, EDCM, 5′-CGAACTTCCTACACTTGAGCA-3′ and 5′-TGGCAAGATGAGCACAAAAG-3′, and EEF1A1, 5′-TTGCCACACTGCCCATATTG-3′ and 5′-CGCTTTCTTGTCAACTGCCT-3′. The PCR products were analyzed by agarose gel electrophoresis in comparison with DNA length marker VI (Sigma Aldrich). The identity of the bands was confirmed by Sanger sequencing.
Proteomic Analysis of Claws and Skin of Anole Lizard and Bearded Dragon
Claw-bearing toes and back skin of A. carolinensis (Strasser et al. 2014) and claws and back skin of P. vitticeps were dissected and incubated in lysis buffer containing 30 mM Tris, 7 M urea (VWR), 2 M thiourea (Sigma Aldrich), 4% CHAPSO (Pierce), and 0.2 M dithiothreitol (DTT, Roth) at 70 °C for 3 h (Holthaus et al. 2024). Subsequently, the samples were homogenized in Precellys tubes prefilled with CK14 ceramic beads in a Precellys (VWR) homogenizer. Specimens were centrifuged at 4 °C and 18,000 × g for 15 min to remove insoluble debris. The samples were afterwards reduced (0.5 M DTT, Roth), alkylated with iodoacetamide (1 M, Sigma), and bound to sp3 beads (Preomics) at a 10:1 ratio of beads:protein. Specimens were supplemented with 50% acetonitrile solution. After a wash with 80% ethanol, the samples were digested with trypsin/LysC and incubated overnight at 37 °C. Then, the samples were acidified with 30% trifluoroacetic acid (TFA) and washed with acetonitrile and 0.1% TFA. Solid phase extraction was done with 90% acetonitrile and 0.4% formic acid. An Orbitrap Exploris 480 MS with FAIMS (Thermo Fisher) connected to an Ultimate 3000 (Thermo Fisher) nano-high-performance liquid chromatography system was used for the proteomic analysis as described previously (Holthaus et al. 2024). The protein reference databases for A. carolinensis (taxonomy ID: 28377, reference proteome: UP000001646) and P. vitticeps (taxonomy ID: 103695, reference proteome: UP000504619) were downloaded from UniProt. Peptides were identified with Proteome Discoverer Software (Version: 2.4.1.15).
Supplementary Material
Acknowledgments
We thank the Proteomics Core Facility (Anja Wagner, Markus Unterwurzacher, and Klaus Kratochwill) of the Medical University of Vienna for excellent support.
Contributor Information
Karin Brigit Holthaus, Department of Dermatology, Medical University of Vienna, Vienna 1090, Austria.
Julia Steinbinder, Department of Dermatology, Medical University of Vienna, Vienna 1090, Austria.
Attila Placido Sachslehner, Department of Dermatology, Medical University of Vienna, Vienna 1090, Austria.
Leopold Eckhart, Department of Dermatology, Medical University of Vienna, Vienna 1090, Austria.
Supplementary Material
Supplementary material is available at Genome Biology and Evolution online.
Funding
This research was funded in part by the Austrian Science Fund (FWF): grant-DOI: 10.55776/P32777 and grant-DOI: 10.55776/P36596. For open access purposes, the authors have applied a CC BY public copyright license to any author accepted manuscript version arising from this submission.
Data Availability
Proteome data were deposited in the PRIDE database under the accession number PXD054063. Other data are incorporated into the article and its online Supplementary material.
Literature Cited
- Aardema ML, Stiassny MLJ, Alter SE. Genomic analysis of the only blind cichlid reveals extensive inactivation in eye and pigment formation genes. Genome Biol Evol. 2020:12(8):1392–1406. 10.1093/gbe/evaa144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akat E, Yenmiş M, Pombal MA, Molist P, Megías M, Arman S, Veselỳ M, Anderson R, Ayaz D. Comparison of vertebrate skin structure at class level: a review. Anat Rec (Hoboken). 2022:305(12):3543–3608. 10.1002/ar.24908. [DOI] [PubMed] [Google Scholar]
- Alföldi J, Di Palma F, Grabherr M, Williams C, Kong L, Mauceli E, Russell P, Lowe CB, Glor RE, Jaffe JD, et al. The genome of the green anole lizard and a comparative analysis with birds and mammals. Nature. 2011:477(7366):587–591. 10.1038/nature10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alibardi L. Development, comparative morphology and cornification of reptilian claws in relation to claws evolution in tetrapods. Contr Zool. 2009:78(1):25–42. 10.1163/18759866-07801003. [DOI] [Google Scholar]
- Alibardi L. Development, structure, and protein composition of reptilian claws and hypotheses of their evolution. Anat Rec (Hoboken). 2021:304(4):732–757. 10.1002/ar.24515. [DOI] [PubMed] [Google Scholar]
- Alibardi L, Dalla Valle L, Nardi A, Toni M. Evolution of hard proteins in the sauropsid integument in relation to the cornification of skin derivatives in amniotes. J Anat. 2009:214(4):560–586. 10.1111/j.1469-7580.2009.01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alibardi L, Holthaus KB, Sukseree S, Hermann M, Tschachler E, Eckhart L. Immunolocalization of a histidine-rich epidermal differentiation protein in the chicken supports the hypothesis of an evolutionary developmental link between the embryonic subperiderm and feather barbs and barbules. PLoS One. 2016:11(12):e0167789. 10.1371/journal.pone.0167789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alibardi L, Jaeger K, Dalla Valle L, Eckhart L. Ultrastructural localization of hair keratin homologs in the claw of the lizard Anolis carolinensis. J Morphol. 2011:272(3):363–370. 10.1002/jmor.10920. [DOI] [PubMed] [Google Scholar]
- Alibardi L, Mlitz V, Eckhart L. Immunolocalization of scaffoldin, a trichohyalin-like protein, in the epidermis of the chicken embryo. Anat Rec (Hoboken). 2015:298(2):479–487. 10.1002/ar.23039. [DOI] [PubMed] [Google Scholar]
- Allam AA, Daza JD, Abo-Eleneen RE. Histology of the skin of three limbless squamates dwelling in mesic and arid environments. Anat Rec (Hoboken). 2016:299(7):979–989. 10.1002/ar.23356. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990:215(3):403–410. 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Andrade P, Pinho C, Pérez I de Lanuza G, Afonso S, Brejcha J, Rubin CJ, Wallerman O, Pereira P, Sabatino SJ, Bellati A, et al. Regulatory changes in pterin and carotenoid genes underlie balanced color polymorphisms in the wall lizard. Proc Natl Acad Sci U S A. 2019:116(12):5633–5642. 10.1073/pnas.1820320116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apesteguía S, Zaher H. A cretaceous terrestrial snake with robust hindlimbs and a sacrum. Nature. 2006:440(7087):1037–1040. 10.1038/nature04413. [DOI] [PubMed] [Google Scholar]
- Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005:6(4):328–340. 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- Carron M, Sachslehner AP, Cicekdal MB, Bruggeman I, Demuynck S, Golabi B, De Baere E, Declercq W, Tschachler E, Vleminckx K, et al. Evolutionary origin of Hoxc13-dependent skin appendages in amphibians. Nat Commun. 2024:15(1):2328. 10.1038/s41467-024-46373-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CF, Foley J, Tang PC, Li A, Jiang TX, Wu P, Widelitz RB, Chuong CM. Development, regeneration, and evolution of feathers. Annu Rev Anim Biosci. 2015:3(1):169–195. 10.1146/annurev-animal-022513-114127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn MJ, Tickle C. Developmental basis of limblessness and axial patterning in snakes. Nature. 1999:399(6735):474–479. 10.1038/20944. [DOI] [PubMed] [Google Scholar]
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988:16(22):10881–10890. 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla Valle L, Benato F, Rossi C, Alibardi L, Tschachler E, Eckhart L. Deleterious mutations of a claw keratin in multiple taxa of reptiles. J Mol Evol. 2011:72(3):265–273. 10.1007/s00239-010-9427-y. [DOI] [PubMed] [Google Scholar]
- Dalla Valle L, Nardi A, Bonazza G, Zucal C, Emera D, Alibardi L. Forty keratin-associated beta-proteins (beta-keratins) form the hard layers of scales, claws, and adhesive pads in the green anole lizard, Anolis carolinensis. J Exp Zool B Mol Dev Evol. 2010:314(1):11–32. 10.1002/jez.b.21306. [DOI] [PubMed] [Google Scholar]
- Eckhart L, Dalla Valle L, Jaeger K, Ballaun C, Szabo S, Nardi A, Buchberger M, Hermann M, Alibardi L, Tschachler E. Identification of reptilian genes encoding hair keratin-like proteins suggests a new scenario for the evolutionary origin of hair. Proc Natl Acad Sci U S A. 2008:105(47):18419–18423. 10.1073/pnas.0805154105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart L, Ehrlich F. Evolution of trichocyte keratins. Adv Exp Med Biol. 2018:1054:33–45. 10.1007/978-981-10-8195-8_4. [DOI] [PubMed] [Google Scholar]
- Eckhart L, Holthaus KB, Sachslehner AP. Cell differentiation in the embryonic periderm and in scaffolding epithelia of skin appendages. Dev Biol. 2024:515:60–66. 10.1016/j.ydbio.2024.07.002. [DOI] [PubMed] [Google Scholar]
- Ehrlich F, Fischer H, Langbein L, Praetzel-Wunder S, Ebner B, Figlak K, Weissenbacher A, Sipos W, Tschachler E, Eckhart L. Differential evolution of the epidermal keratin cytoskeleton in terrestrial and aquatic mammals. Mol Biol Evol. 2019:36(2):328–340. 10.1093/molbev/msy214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich F, Lachner J, Hermann M, Tschachler E, Eckhart L. Convergent evolution of cysteine-rich keratins in hard skin appendages of terrestrial vertebrates. Mol Biol Evol. 2020:37(4):982–993. 10.1093/molbev/msz279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerling CA. Genomic regression of claw keratin, taste receptor and light-associated genes provides insights into biology and evolutionary origins of snakes. Mol Phylogenet Evol. 2017:115:40–49. 10.1016/j.ympev.2017.07.014. [DOI] [PubMed] [Google Scholar]
- Fleckman P, Jaeger K, Silva KA, Sundberg JP. Comparative anatomy of mouse and human nail units. Anat Rec (Hoboken). 2013:296(3):521–532. 10.1002/ar.22660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foureaux G, Egami MI, Jared C, Antoniazzi MM, Gutierre RC, Smith RL. Rudimentary eyes of squamate fossorial reptiles (Amphisbaenia and Serpentes). Anat Rec (Hoboken). 2010:293(2):351–357. 10.1002/ar.21021. [DOI] [PubMed] [Google Scholar]
- Gans C. Tetrapod limblessness: evolution and functional corollaries. Am Zool. 1975:15(2):455–467. 10.1093/icb/15.2.455. [DOI] [Google Scholar]
- Gillingham JC, Chambers JA. Courtship and pelvic spur use in the Burmese python, python Molurus bivittatus. Copeia. 1982:1982(1):193. 10.2307/144429. [DOI] [Google Scholar]
- Harland DP, Plowman JE. Development of hair fibres. Adv Exp Med Biol. 2018:1054:109–154. 10.1007/978-981-10-8195-8_10. [DOI] [PubMed] [Google Scholar]
- Hecker N, Sharma V, Hiller M. Transition to an aquatic habitat permitted the repeated loss of the pleiotropic KLK8 gene in mammals. Genome Biol Evol. 2017:9(11):3179–3188. 10.1093/gbe/evx239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthaus KB, Alibardi L, Tschachler E, Eckhart L. Identification of epidermal differentiation genes of the tuatara provides insights into the early evolution of lepidosaurian skin. Sci Rep. 2020:10(1):12844. 10.1038/s41598-020-69885-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthaus KB, Eckhart L. Development-associated genes of the epidermal differentiation complex (EDC). J Dev Biol. 2024:12(1):4. 10.3390/jdb12010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthaus KB, Eckhart L, Dalla Valle L, Alibardi L. Review: evolution and diversification of corneous beta-proteins, the characteristic epidermal proteins of reptiles and birds. J Exp Zool B Mol Dev Evol. 2018:330(8):438–453. 10.1002/jez.b.22840. [DOI] [PubMed] [Google Scholar]
- Holthaus KB, Mlitz V, Strasser B, Tschachler E, Alibardi L, Eckhart L. Identification and comparative analysis of the epidermal differentiation complex in snakes. Sci Rep. 2017:7(1):45338. 10.1038/srep45338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthaus KB, Sachslehner AP, Steinbinder J, Eckhart L. Epidermal differentiation genes of the common wall lizard encode proteins with extremely biased amino acid contents. Genes (Basel). 2024:15(9):1136. 10.3390/genes15091136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthaus KB, Strasser B, Sipos W, Schmidt HA, Mlitz V, Sukseree S, Weissenbacher A, Tschachler E, Alibardi L, Eckhart L. Comparative genomics identifies epidermal proteins associated with the evolution of the turtle shell. Mol Biol Evol. 2016:33(3):726–737. 10.1093/molbev/msv265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitsuka Y, Roop DR. Loricrin: past, present, and future. Int J Mol Sci. 2020:21(7):2271. 10.3390/ijms21072271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger K, Sukseree S, Zhong S, Phinney BS, Mlitz V, Buchberger M, Narzt MS, Gruber F, Tschachler E, Rice RH, et al. Cornification of nail keratinocytes requires autophagy for bulk degradation of intracellular proteins while sparing components of the cytoskeleton. Apoptosis. 2019:24(1–2):62–73. 10.1007/s10495-018-1505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney M, Stuart BL. Repeated evolution of limblessness and digging heads in worm lizards revealed by DNA from old bones. Proc Biol Sci. 2004:271(1549):1677–1683. 10.1098/rspb.2004.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Suleski M, Craig JM, Kasprowicz AE, Sanderford M, Li M, Stecher G, Hedges SB. TimeTree 5: an expanded resource for species divergence times. Mol Biol Evol. 2022:39(8):msac174. 10.1093/molbev/msac174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvon EZ, Kamneva OK, Melo US, Barozzi I, Osterwalder M, Mannion BJ, Tissières V, Pickle CS, Plajzer-Frick I, Lee EA, et al. Progressive loss of function in a limb enhancer during snake evolution. Cell. 2016:167(3):633–642.e11. 10.1016/j.cell.2016.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachner J, Ehrlich F, Mlitz V, Hermann M, Alibardi L, Tschachler E, Eckhart L. Immunolocalization and phylogenetic profiling of the feather protein with the highest cysteine content. Protoplasma. 2019:256(5):1257–1265. 10.1007/s00709-019-01381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langbein L, Yoshida H, Praetzel-Wunder S, Parry DA, Schweizer J. The keratins of the human beard hair medulla: the riddle in the middle. J Invest Dermatol. 2010:130(1):55–73. 10.1038/jid.2009.192. [DOI] [PubMed] [Google Scholar]
- Leal F, Cohn MJ. Loss and re-emergence of legs in snakes by modular evolution of sonic hedgehog and HOXD enhancers. Curr Biol. 2016:26(21):2966–2973. 10.1016/j.cub.2016.09.020. [DOI] [PubMed] [Google Scholar]
- Leal F, Cohn MJ. Developmental, genetic, and genomic insights into the evolutionary loss of limbs in snakes. Genesis. 2018:56(Suppl 1):e23077. 10.1002/dvg.23077. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhou Q, Wang Y, Luo L, Yang J, Yang L, Liu M, Li Y, Qian T, Zheng Y, et al. Gekko japonicus genome reveals evolution of adhesive toe pads and tail regeneration. Nat Commun. 2015:6(1):10033. 10.1038/ncomms10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T. Epidermal barrier development via corneoptosis: a unique form of cell death in stratum granulosum cells. J Dev Biol. 2023:11(4):43. 10.3390/jdb11040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith RW, Gatesy J, Springer MS. Molecular decay of enamel matrix protein genes in turtles and other edentulous amniotes. BMC Evol Biol. 2013:13(1):20. 10.1186/1471-2148-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith RW, Zhang G, Gilbert MT, Jarvis ED, Springer MS. Evidence for a single loss of mineralized teeth in the common avian ancestor. Science. 2014:346(6215):1254390. 10.1126/science.1254390. [DOI] [PubMed] [Google Scholar]
- Mlitz V, Strasser B, Jaeger K, Hermann M, Ghannadan M, Buchberger M, Alibardi L, Tschachler E, Eckhart L. Trichohyalin-like proteins have evolutionarily conserved roles in the morphogenesis of skin appendages. J Invest Dermatol. 2014:134(11):2685–2692. 10.1038/jid.2014.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov V, Uliano-Silva M, Wilkinson M, Wood J, Smith M, Oliver K, Sims Y, Torrance J, Suh A, McCarthy SA, et al. Caecilian genomes reveal the molecular basis of adaptation and convergent evolution of limblessness in snakes and caecilians. Mol Biol Evol. 2023:40(5):msad102. 10.1093/molbev/msad102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluh DJ, Riddell K, Early CM, Hantak MM, Jongsma GF, Keeffe RM, Magalhães Silva F, Nielsen SV, Vallejo-Pareja MC, Stanley EL, et al. Rampant tooth loss across 200 million years of frog evolution. Elife. 2021:10:e66926. 10.7554/eLife.66926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin C, Langbein L, Schweizer J. Expression of hair keratins in the adult nail unit: an immunohistochemical analysis of the onychogenesis in the proximal nail fold, matrix and nail bed. Br J Dermatol. 2004:151(2):362–371. 10.1111/j.1365-2133.2004.06108.x. [DOI] [PubMed] [Google Scholar]
- Rhie A, McCarthy SA, Fedrigo O, Damas J, Formenti G, Koren S, Uliano-Silva M, Chow W, Fungtammasan A, Kim J, et al. Towards complete and error-free genome assemblies of all vertebrate species. Nature. 2021:592(7856):737–746. 10.1038/s41586-021-03451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscito JG, Sameith K, Kirilenko BM, Hecker N, Winkler S, Dahl A, Rodrigues MT, Hiller M. Convergent and lineage-specific genomic differences in limb regulatory elements in limbless reptile lineages. Cell Rep. 2022:38(3):110280. 10.1016/j.celrep.2021.110280. [DOI] [PubMed] [Google Scholar]
- Roscito JG, Sameith K, Parra G, Langer BE, Petzold A, Moebius C, Bickle M, Rodrigues MT, Hiller M. Phenotype loss is associated with widespread divergence of the gene regulatory landscape in evolution. Nat Commun. 2018:9(1):4737. 10.1038/s41467-018-07122-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachslehner AP, Surbek M, Golabi B, Geiselhofer M, Jäger K, Hess C, Kuchler U, Gruber R, Eckhart L. Transglutaminase activity is conserved in stratified epithelia and skin appendages of mammals and birds. Int J Mol Sci. 2023:24(3):2193. 10.3390/ijms24032193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachslehner AP, Surbek M, Holthaus KB, Steinbinder J, Golabi B, Hess C, Eckhart L. The evolution of transglutaminases underlies the origin and loss of cornified skin appendages in vertebrates. Mol Biol Evol. 2024:41(6):msae100. 10.1093/molbev/msae100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J, Park J, Abudureyimu G, Kim MH, Shim JS, Jang KT, Kwon EJ, Jang HS, Yeo E, Lee JH, et al. Comparative spatial transcriptomic and single-cell analyses of human nail units and hair follicles show transcriptional similarities between the onychodermis and follicular dermal papilla. J Invest Dermatol. 2022:142(12):3146–3157.e12. 10.1016/j.jid.2022.06.022. [DOI] [PubMed] [Google Scholar]
- Strasser B, Mlitz V, Hermann M, Rice RH, Eigenheer RA, Alibardi L, Tschachler E, Eckhart L. Evolutionary origin and diversification of epidermal barrier proteins in amniotes. Mol Biol Evol. 2014:31(12):3194–3205. 10.1093/molbev/msu251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser B, Mlitz V, Hermann M, Tschachler E, Eckhart L. Convergent evolution of cysteine-rich proteins in feathers and hair. BMC Evol Biol. 2015:15(1):82. 10.1186/s12862-015-0360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicher JW, Wiens JJ. Phylogenomic analyses of more than 4000 nuclear loci resolve the origin of snakes among lizard families. Biol Lett. 2017:13(9):20170393. 10.1098/rsbl.2017.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Rong X, Liu X, Yu Z, Zhang Q, Ren W, Yang G, Xu S. Evolutionary genetics of flipper forelimb and hindlimb loss from limb development-related genes in cetaceans. BMC Genomics. 2022:23(1):797. 10.1186/s12864-022-09024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thewissen JG, Cohn MJ, Stevens LS, Bajpai S, Heyning J, Horton WE Jr. Developmental basis for hind-limb loss in dolphins and origin of the cetacean bodyplan. Proc Natl Acad Sci U S A. 2006:103(22):8414–8418. 10.1073/pnas.0602920103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandebergh W, Maex M, Bossuyt F, Van Bocxlaer I. Recurrent functional divergence of early tetrapod keratins in amphibian toe pads and mammalian hair. Biol Lett. 2013:9(3):20130051. 10.1098/rsbl.2013.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal N, Hedges SB. The phylogeny of squamate reptiles (lizards, snakes, and amphisbaenians) inferred from nine nuclear protein-coding genes. C R Biol. 2005:328(10-11):1000–1008. 10.1016/j.crvi.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Whitbread LA, Gregg K, Rogers GE. The structure and expression of a gene encoding chick claw keratin. Gene. 1991:101(2):223–229. 10.1016/0378-1119(91)90415-8. [DOI] [PubMed] [Google Scholar]
- Wiens JJ, Brandley MC, Reeder TW. Why does a trait evolve multiple times within a clade? Repeated evolution of snakelike body form in squamate reptiles. Evolution. 2006:60(1):123–141. 10.1111/j.0014-3820.2006.tb01088.x. [DOI] [PubMed] [Google Scholar]
- Wu P, Hou L, Plikus M, Hughes M, Scehnet J, Suksaweang S, Widelitz R, Jiang TX, Chuong CM. Evo-devo of amniote integuments and appendages. Int J Dev Biol. 2004:48(2-3):249–270. 10.1387/ijdb.041825pw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Ng CS, Yan J, Lai YC, Chen CK, Lai YT, Wu SM, Chen JJ, Luo W, Widelitz RB, et al. Topographical mapping of α- and β-keratins on developing chicken skin integuments: functional interaction and evolutionary perspectives. Proc Natl Acad Sci U S A. 2015:112(49):E6770–E6779. 10.1073/pnas.1520566112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenmiş M, Ayaz D. The story of the finest armor: developmental aspects of reptile skin. J Dev Biol. 2023:11(1):5. 10.3390/jdb11010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Proteome data were deposited in the PRIDE database under the accession number PXD054063. Other data are incorporated into the article and its online Supplementary material.