Abstract
Background:
Meningioma is the most common primary intracranial tumor. This single-center study aimed to analyze the clinicopathological, radiological profile, and outcomes of patients with intracranial meningiomas in terms of functional status, morbidity, mortality, and recurrence-free survival (RFS).
Methods:
Patients of intracranial meningioma treated between January 01, 2010, and December 31, 2019, at the Department of Neurosurgery, King George’s Medical University, India, were included in this study. Retrospective data analysis of 172 patients with intracranial meningioma was done.
Results:
The majority of the patients, that is, 94 (54.65%), presented in the 4th and 5th decade. The mean size of the meningioma was 36.4 ± 4 mm (range: 26–68 mm). Of the 172 patients, 128 (74.41%) were diagnosed as non-skull base meningiomas, and in 44 patients (25.59%), meningioma originated from the skull base. Recurrence was observed on follow-up imaging in 11 patients after a mean postoperative interval of 55.2 ± 5.8 months. Radiological meningioma recurrence paralleled with clinical deterioration in seven patients. Three of these patients were subjected to the second surgery, followed by radiotherapy, and in the remaining four patients, Gamma knife or fractionated radiotherapy was given.
Conclusion:
The majority of patients had good functional outcomes (KPS >70) at discharge. Morbidity and mortality was 18.60% and 3.49%, respectively. Meningioma size ≥4 cm, age >45 years, World Health Organization Grade (II, III), non-skull base location, and Simpson grade III, IV of resection showed significantly shorter RFS.
Keywords: Intracranial meningioma, Karnofsky performance scale score, Radiotherapy, Recurrence, Simpson grade

INTRODUCTION
Meningiomas arise from the arachnoid cap cells of the arachnoid villi in the meninges and typically attach to the dural broad base. They make up 13–26% of all primary intracranial tumors, and 90% of them are benign tumors.[17,31] It is observed that females are affected more commonly than males.[33] Various studies show that meningiomas happen most often in the fourth decade of life.[6,13] Monitoring at regular intervals, surgical excision, external beam radiotherapy, and stereotactic radiosurgery are the management options.[21] Gamma knife radiosurgery (GKRS) is a good way to treat small meningioma with a tumor control rate of 97.9% at 5 years post-GKRS.[11] Complete surgical removal (Simpson Grade I–III) is still the best way to treat a meningioma.[27] Various clinicopathological and radiological factors associated with recurrence may aid in the management of patients, particularly in the use of adjuvant radiotherapy. In recurrent tumors, these factors may support in selecting the treatments including resection with or without radiotherapy, radiotherapy alone, or pharmacological intervention.
The majority of the studies and literature available pertaining to this entity are from developed countries, while this study is an effort to analyze the outcome of meningioma in relation to the Indian population and has done a comparative analysis with other relevant studies. This single-center retrospective study aimed to analyze the clinicopathological, radiological profile, and outcomes of patients with intracranial meningiomas in terms of functional status, morbidity, mortality, and recurrence-free survival (RFS).
MATERIALS AND METHODS
The study was done at the Department of Neurosurgery, King George’s Medical University (KGMU), Lucknow. This was a single-center retrospective observational study. Inclusion criteria of the study – Histologically proven patients of intracranial meningioma who were treated at the Department of Neurosurgery between January 01, 2010, and December 31, 2019. Exclusion criteria of the study – Patients who had been operated for intracranial meningioma before January 2010 presenting with recurrence in the duration mentioned above and patients with multiple meningioma. The data relevant to the study was extracted from digital records of patients from the record section of the Department of Neurosurgery, Department of Pathology, and Department of Radiotherapy. Epidemiological, clinical, and radiological data and details of surgery/radiation treatment were noted, and complications, histopathological examination, and follow-up records were analyzed with descriptive biostatistics analysis. In house software (Neuro-Patient Management System) was used for data retrieval and follow-up details.
Gross total resection (GTR) and subtotal resection (STR) of meningioma comprise Simpson grade I, II, III resection and Simpson grade IV resection, respectively, as per Response Assessment in Neuro-Oncology working group.[24]
P-value was calculated for each comparison using two-tailed analysis with significance assumed at P < 0.05. Further, the use of software such as Excel and Statistical package for the Social Sciences Version 16 was used. Univariate analysis was done with the fisher exact test. Kaplan evaluated survival functions–Meier survival analysis.
The study was approved by the Institutional Ethical Committee (Registration no. - ECR/262/Inst/UP/2013/RR-19) vide letter no 772/Ethics/2020 dated August 13, 2020.
RESULTS
A total of 172 patients treated over 10 years with proven histopathological examination report meningioma were included in this study. Patients ranged in age from 18 to 72 years. The mean age was 40.6 ± 4.1 years, with a median of 42 years. The majority of the patients that is, 94 (54.65%), presented in the 4th and 5th decade. Sixty-five were male (37.79%), and 107 were female (62.21%). The female-to-male ratio was 1.64:1. The most common symptoms were headache (77.90%) and motor deficit (59.30%) followed by seizure (55.81%).
Pre-operative magnetic resonance imaging (MRI) imaging was available in 154 patients, while 18 patients were operated on the basis of computed tomography imaging in emergency. The largest diameter in either the anterior-posterior, transverse, or craniocaudal dimension was used as an overall surrogate for meningioma size. The mean size of the meningioma was 36.4 ± 4 mm (range: 26–68 mm).
The meningiomas exhibited radiological heterogeneity and appeared hypointense in 104 (67.53%), isointense in 47 (30.52%), and hyperintense in 3 (1.95%) on T1-weighted MRI. On T2-weighted MRI, the lesion was hypointense in 2 (1.3%), isointense in 99(64.29%), and hyperintense in 53 (34.42%). Homogeneous contrast enhancement was seen in 136 patients (88.31%), whereas heterogeneous contrast enhancement was identified in 18 patients (11.69%). Peritumoral edema was categorized into mild (presence of small halo around the tumor), moderate (edema extending to surrounding white matter tracts), and severe (edema extending to hemisphere or causing midline shift ≥5 mm. Severe peritumoral edema was observed in 53 patients (34.42%) [Figure 1]. Magnetic resonance angiography demonstrated meningioma blush and encasement of the internal carotid artery in 5 patients (3.25%) of clinoidal and medial sphenoid wing meningioma. A single lesion was present in 149 (96.75%) patients. Five patients (3.25%) had multiple meningiomas, three of whom had a diagnosis of neurofibromatosis.
Figure 1:
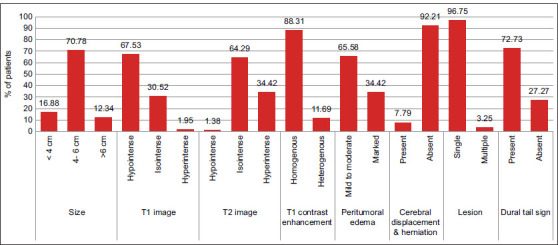
Imaging characteristics of meningioma on magnetic resonance imaging scan.
Of the 172 patients, 128 (74.41%) were diagnosed as non-skull base meningioma, including cerebral convexity (n = 79; 45.93%), falx (n = 22; 12.79%), parasagittal (n = 23; 13.37%), and cerebellar convexity (n = 4; 2.32%) meningioma. In the remaining 44 patients (25.58%), meningioma originated from the skull base [Figure 2].
Figure 2:
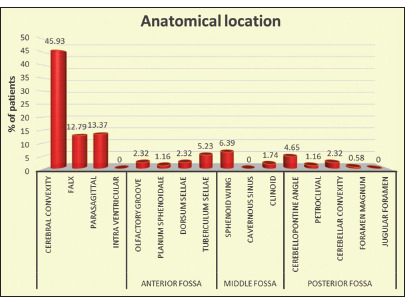
Representation of location-wise distribution of meningioma.
According to the World Health Organization (WHO), Grade I meningioma was present in 150 patients (87.21%), Grade II in 17 patients (9.88%), and Grade III meningioma in 5 patients (2.91%). Of the WHO Grade I patients, the most common was the meningothelial subtype (n = 74; 43.02%) [Figure 3].
Figure 3:
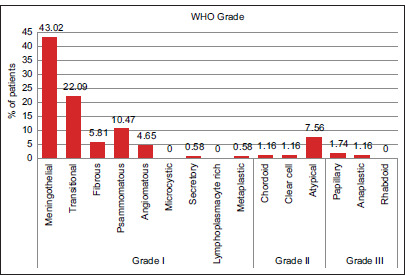
Histological types of meningioma.
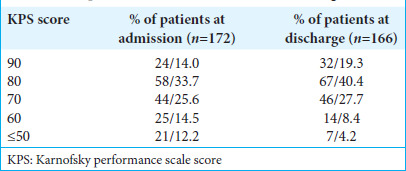
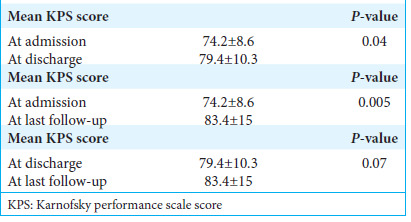
We rated the quality of life as good when the Karnofsky Performance Scale(KPS) score was >70. In contrast, the quality of life was rated poor if the KPS score was ≤70. After surgical treatment, the number of patients with a KPS score >70 at discharge was 99 (59.7%) [Table 1]. The mean KPS score at admission was 74.2 ± 8.6, and at discharge was 79.4 ± 10.3. There was a significant difference between the mean preoperative and discharge KPS scores (P = 0.04) that indicate increased survival and functional status following meningioma resection.
Table 1:
Comparison of KPS at admission and discharge.
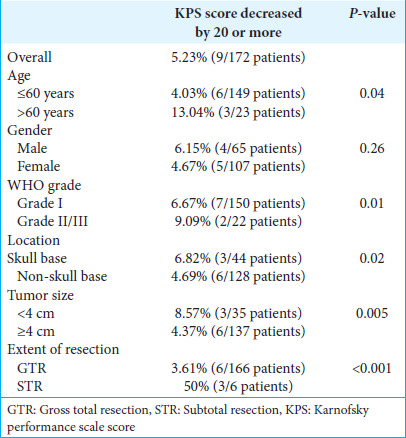
We explored the predictive factors for postoperative decrement of KPS score by 20 points or more. Among the 172 patients, 9 (5.23%) of the patients had KPS scores that were lowered by 20 points or more at the time of discharge after surgery. Lowering of KPS score was more frequently observed in patients aged more than 60 years (P = 0.04), those with tumors of WHO grade II/III (P = 0.01), those with tumors located at the skull base (P = 0.02), those with a tumor size ≥4 cm. (P = 0.005), and those for whom extent of resection was STR (P < 0.001). There was no significant correlation between the lowering of KPS score and gender (P = 0.26) [Table 2].
Table 2:
Univariate analysis of factors linked to a drop in KPS by 20 or more.
Out of 172 patients, 166 (96.51%) were discharged from the hospital, while six patients died during hospital stay after surgery. Out of the 166 patients, only 134 followed up later. During a mean follow-up period of 58.3 months (range: 3.2– 122 months), 127 patients remained alive, and seven patients succumbed after discharge. In the 127 living patients, the last follow-up mean KPS score was 83.4 ± 15.0 (range: 30–90), which was significantly different from the mean preoperative KPS score (P = 0.005). We also compared the mean discharge and the last follow-up KPS scores, and there was no significant difference between the two groups (P = 0.07) [Table 3].
Table 3:
Mean KPS scores of the patients at admission, discharge, and the last follow-up and their statistical significance.
Recurrence was observed on follow-up imaging in 11 patients after a mean post-operative interval of 55.2 ± 5.8 months. Radiological meningioma recurrence paralleled with clinical deterioration in seven patients. Three patients were subjected to a second surgery followed by radiotherapy, and the rest four were directly given GKRS or fractionated radiotherapy. Of these 11 patients, four had stable meningioma size at a mean follow-up of 32 months without any clinical deterioration [Figure 4].
Figure 4:
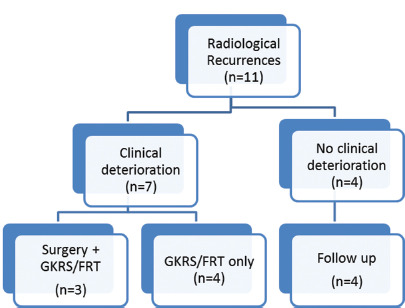
Recurrence and management flow chart.
Radiological illustration of few follow up cases have been depicted [Figures 5-7]. The histopathological examination of these 11 patients with recurrence was WHO Grade I in five patients, Grade II in five patients, and Grade III in one patient. The recurrence rate for histological WHO Grade I was 4.27%, WHO Grade II was 35.71%, and WHO Grade III was 33.33%, and the recurrence rate statistically correlates with higher grades, that is, WHO Grade II and III of meningioma (P = 0.04). The recurrence rate for Simpson grade I excision was 4.34%; for Simpson grade II was 8.47%; for Simpson grade III was 15%; and for Simpson grade IV excision was 50%. The correlation between Simpson grade excision and recurrence was statistically significant (P = 0.012).
Figure 5:
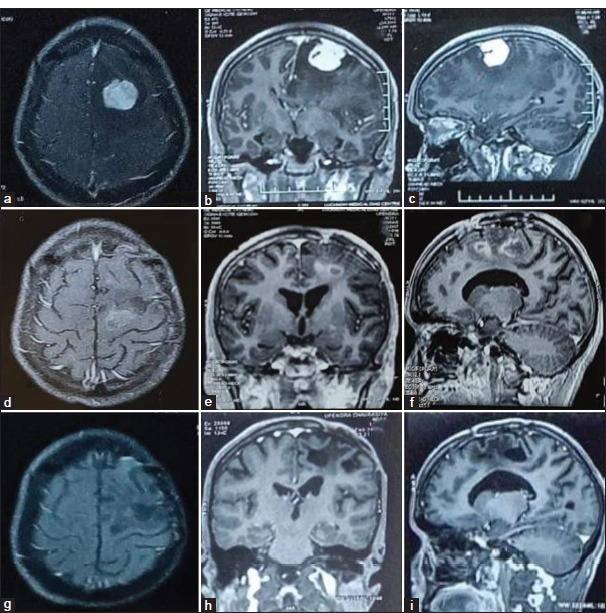
Illustrative case (a-c): Pre-operative contrast-enhanced magnetic resonance imaging (MRI) axial, sagittal, and coronal images of left frontal meningioma in a 20 year old male with seizure, headache, and right hemiparesis. The patient was operated on, and a near-total excision of the tumor was done. The histology report was suggestive of atypical meningioma. The patient received linear accelerator-based radiotherapy (60 Gy in 30 cycles) 3 months after surgery. (d-f): Follow Follow-up contrast MRI axial, sagittal, and coronal images obtained 8 months after surgery, showing few areas of ring enhancement noted in the right high frontal region – post-radiotherapy changes. (g-i): Follow-up contrast MRI axial, sagittal, and coronal images obtained 5 years after surgery showed a cystic lesion in the left frontal region with perilesional sclerosis associated with thinning of the overlying cortex suggestive of encephalomalacia and no evidence of residual or recurrent lesion noted.
Figure 7:
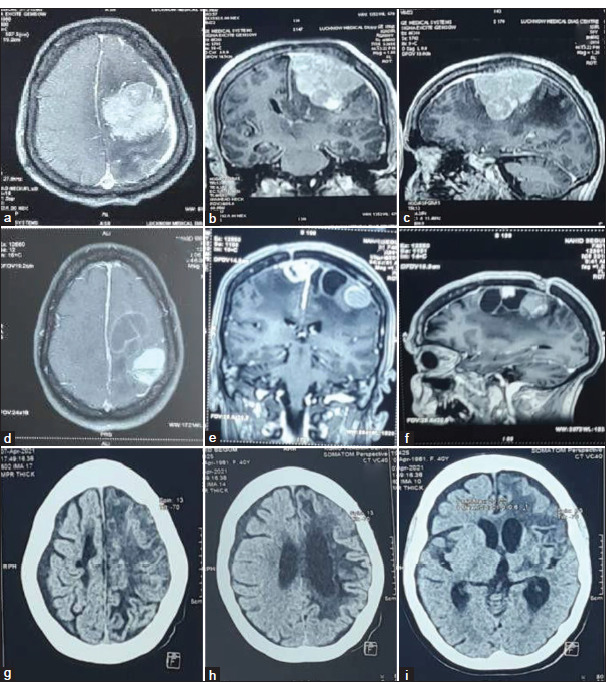
Illustrative case (a-c): Pre-operative contrast enhanced magnetic resonance imaging (MRI) axial, sagittal, and coronal images of the left middle 1/3rd parasagittal meningioma in 35 years female with complex partial seizure, headache, right hemiparesis and slurring of speech. The patient underwent Simpson grade II excision of the tumor. The histology report was suggestive of secretory meningioma (WHO grades I). Seven years after surgery, the Patient came with complaints of right hemiparesis, seizure, and aphasia. (d-f): MRI axial, sagittal, and coronal images showed well defined extra-axial solid cystic lesion with septal enhancement in cystic lesion and homogeneous contrast enhancement in solid component along the left parasagittal region with surrounding mild intraparenchymal edema suggestive of recurrent meningioma. As the patient had poor pre-treatment factors for surgery, external beam radiotherapy was given (54 Gy in 30 cycles). (g-i): Post-radiotherapy non-contrast computed tomography head was suggestive of hypodensities in the left frontal and parietal regions with ex-vacuo dilatation of ipsilateral lateral ventricle and adjoining sulcal spaces suggestive of gliotic changes.
Figure 6:
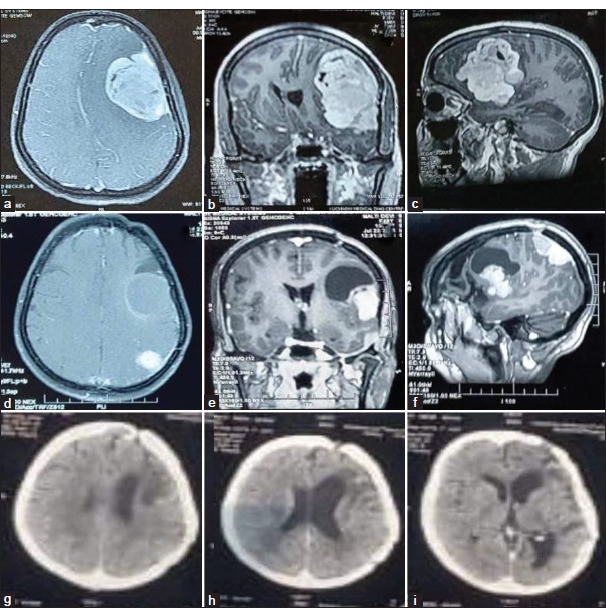
Illustrative case (a-c): Pre-operative contrast enhanced magnetic resonance imaging (MRI) axial, sagittal, and coronal images showed left fronto-temporal meningioma in a 45-year female with symptoms of headache, vomiting, and altered sensorium. The patient had undergone Simpson grade II excision of the tumor. The histology report was suggestive of transitional meningioma (WHO grades I). The patient improved symptomatically. After 4 years of follow-up, the patient came back with complaints of right hemiparesis, generalized seizure, and dysarthria. (d-f): Follow-up MRI axial, sagittal, and coronal images showed a well-defined extra-axial solid cystic lesion with a solid component showing homogeneous contrast enhancement. Another well-defined extra-axial solid lesion with homogenous contrast enhancement is noted along the left parietal lobe without perilesional edema, suggestive of – recurrent meningioma. The patient was operated on the second time, and Simpson grade II excision of the tumor was done. After surgery, external beam radiotherapy was given (54 Gy in 30 cycles). (g-i): Post-radiotherapy non-contrast computed tomography head was done, suggestive of small to moderate sized hypodensities in the left frontal, temporal, and parietal regions with ex-vacuo dilatation of ipsilateral ventricle and adjoining sulcal spaces suggestive of gliotic changes.
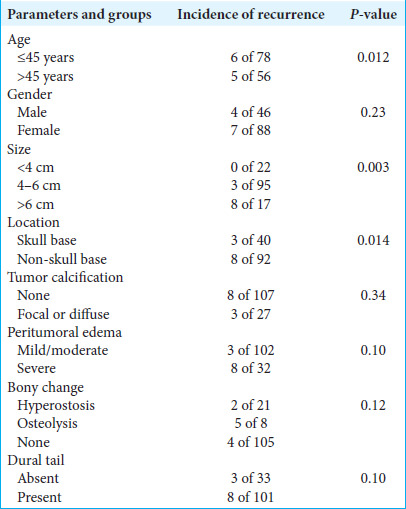
It was observed in our study that the correlation of recurrence with higher age group patients (age >45 years), large tumor size group (>4 cm), and skull base meningioma group was statistically significant, while the correlation of recurrence with gender, tumor calcification, cerebral edema, bony changes, and dural tail was not statistically significant [Table 4].
Table 4:
Correlation of clinico-radiological parameters with recurrence of meningiomas.
Various clinical and histologic factors were analyzed for their influence on RFS. Univariate analysis was performed to identify predictors of RFS for patients with intracranial meningiomas. The following variants were included in the models: sex (male, female), age (>45, ≤45 years), location (skull base, non-skull base), Simpson Grades (I, II, III, and IV), WHO Grades (I, II, III), and meningioma size (<4, 4–6, >6 cm). The mean RFS period among 134 patients was 55.2 ± 5.8 months.
The more aggressive pathology group (WHO Grade II/III) manifested a shorter RFS than the benign group (WHO Grade I) (P = 0.04). Meningioma location was an influential factor for RFS, skull base meningioma showed longer RFS than non-skull base meningioma (P = 0.014). The size of the meningioma and the patient’s age were also important variables in predicting RFS time. No recurrence was noted in meningioma size <4 cm (P = 0.003), and RFS was shorter in patients of age >45 years (P = 0.012) [Figure 8].
Figure 8:
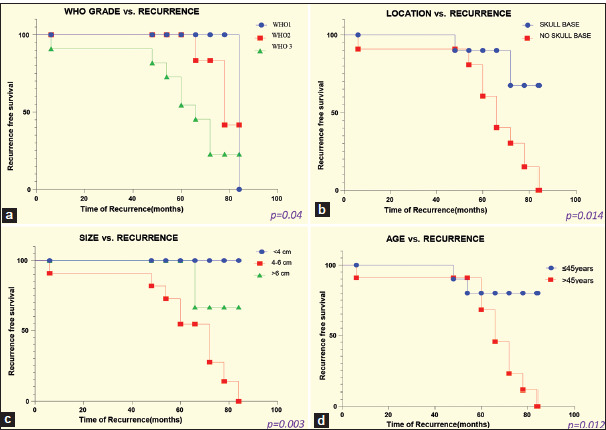
Kaplan–Meier analysis for recurrence free survival as stratified by (a) WHO classification of meningioma, (b) location, (c) size, and (d) patient’s age.
DISCUSSION
Surgical resection of accessible symptomatic lesions has been the mainstay of primary treatment. This study was an honest attempt to review the clinicopathological and radiological profile of patients at a tertiary care center in an Indian scenario, functional status in terms of KPS score, outcome based on RFS, and their management with an aim to shed light on the difficulties faced.
In our study, the age of the patients ranged from 18 years to 72 years, with the majority of patients, that is, 54.65%, presented in the 4th and 5th decade. The mean age of the patients was 40.6 ± 4.1 years, and this was in accordance with a study done by Goyal, Gupta, and Lakshmi, where meningiomas occurred commonly in the fourth decade of life.[6,13] The overall female-to-male (F: M) ratio in our study was 1.6:1, which was compliant with findings in various studies with ratios ranging from 1.5:1 to 3:1.[6,9,28] Rohringer et al. reported that the F: M ratio was 2:1, while in the study by Howng and Kwan et al., the ratio was 1.94:1.[2,10,25]
Our study concluded that the mean size of the meningioma was 36.4 ± 4 mm. Kong et al. reported the mean size of the meningioma as 47.6 ± 11.7 mm, while Magill et al. reported the mean size of the meningioma as 38 ± 18 mm.[12,14] Tumor size is an important prognostic factor as larger tumor size may associated with a greater likelihood of a higher grade of meningioma.[14,22] In the present study, homogeneous and heterogeneous contrast enhancement was identified in 88.31% and 11.69%, respectively, whereas Gasparetto et al. reported homogenous contrast enhancement in 83% of cases.[2,4]
We rated the quality of life as good when the last follow-up KPS score was >70, while it was rated poor if the last follow-up KPS score was ≤70. We found that 81.10% of patients had good outcomes, whereas 18.90% of patients had poor outcomes based on the last follow-up KPS score. Chen et al. rated 82.3% of patients as good (i.e., KPS score >70), whereas 17.7% of patients were rated as poor (i.e., KPS ≤70) in their study.[1]
Clinicopathological factors affecting recurrence
Our study found that 8.21% of patients had radiological evidence of recurrence (median follow-up period 55.2 ± 5.8 months; range 48–84 months). Most of the patients presented in the 5th and 6th decade. Out of 11 patients, 7 (63.64%) were female and 4 (36.36%) were male. Mubeen et al. observed that the elderly population (>60 years) constituted the majority of recurrent meningiomas in their study; however, they did not notice any role of gender in the recurrence pattern.[2,19] Gupta et al. reported higher recurrence rates for males than for females, while Mahmood et al. and Nakasu et al. found no association between meningioma in young patients (<40 years) and a high likelihood of recurrence.[7,15,20] In our study, the majority of recurrent meningiomas were seen in the 5th and 6th decade of life and there was no statistically significant difference noted between gender and meningioma recurrence.
In the present study, eight non-skull base meningiomas recurred, out of which three were parasagittal meningiomas (27.27%), three were convexity meningiomas (18.19%), and two were falx meningioma (18.19%). The other three recurrences were seen at the skull base location, one each at the sphenoid ridge (9.09%) and tuberculum sellae and dorsum sellae (9.09%). The factor that may explain higher recurrence in skull base meningiomas is that aggressive resection is not possible in many cases due to the surrounding vital neurovascular structures. Sun et al. observed that recurrent meningiomas were more frequently found at the occipital convexity, tentorium, sellar regions, parasagittal sinus, and left sphenoid wing, whereas Yang et al. observed in their study that location was not found to influence meningioma recurrence.[29,32]
In our study, the recurrence rate for Simpson grade I excision was 4.34%; for Simpson grade II, it was 8.47%; for Simpson grade III was 15%; and for Simpson grade IV excision was 50%. The correlation between Simpson grade excision and recurrence was statistically significant (P = 0.012). Nanda et al. reported overall meningioma recurrence rates for Simpson resection Grades I, II, III, and IV were 5%, 22%, 31%, and 35%, respectively, while in a study done by Ehresman et al.[3] observed that even after GTR is achieved, the risk of recurrence in their study is highest in WHO Grade I & II meningiomas and those with preoperative neurological deficits.[2,4,21] Vob et al. observed that the risk of recurrence was similar after Simpson grade I and II resections, tended to increase after grade III, and was higher after grade IV resections.[30]
Our study reported that the recurrence rate for histological WHO Grade I was 4.27%, for WHO Grade II was 35.71%, and for WHO Grade III was 33.33%, and the recurrence rate was statistically correlated with higher grades, that is, WHO grade II and III of meningioma (P = 0.04). Moon et al. reported that atypical and malignant meningiomas have been reported to exhibit a higher recurrence rate compared with that of benign meningioma (WHO Grade I).[18] Perry et al. reported benign meningiomas have recurrence rates of about 7–25%, atypical meningiomas recur in 29–52% of cases, and anaplastic meningiomas at rates of 50–94%, whereas Ehresman et al. reported that WHO Grade 2 and 3 meningiomas had 3.6 times more recurrences than their WHO Grade I counterparts [Table 5].[3,23]
Table 5:
Various studies on clinicopathological factors affecting meningioma recurrence.
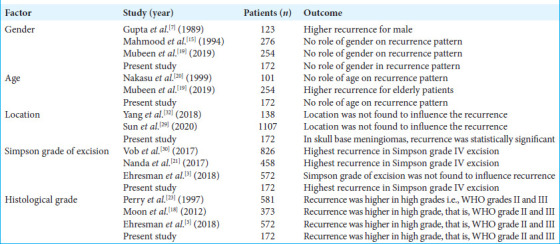
Radiological factors affecting recurrence
Mantle et al. observed that the presence of brain edema was a predictive factor of recurrence after complete resection; 36% of meningiomas with edema recurred, and 1% of meningiomas without edema recurred. The peritumoral edema grade correlated linearly with the chance of brain invasion and recurrence.[16] In another study by Souto et al.[28] and Hirashima et al.[8] suggest that peritumoral edema is one of the factors for recurrence. In the present study, there was no significant correlation between recurrence and peritumoral edema (P = 0.10). We believe that the cause of peritumoral edema was a long-term compression of the tumor in the veins, which obstructed normal venous drainage or due to brain parenchymal invasion.
Nakasu et al. observed that there was no recurrence in meningioma with either focal or diffuse calcification.[20] In the present study, the correlation between recurrence and tumor calcification was statistically non-significant (P = 0.34). Nakasu et al. observed more recurrence in osteolytic meningioma as compared to meningiomas associated with hyperostosis, as the hyperostotic bone was removed completely in a higher percentage of cases[20], whereas Goyal et al. reported a significant number of patients with radiological hyperostosis have meningioma invasion of the bone.[5] In our study, there was no significant correlation between recurrence and bony changes (P = 0.12). A study by Magill et al. reported larger meningioma (size >6 cm) is associated with a greater likelihood of a recurrence,[14] whereas Yang et al. reported that meningioma size was found not to influence meningioma recurrence.[32] It was observed in our study that the correlation of recurrence with a large tumor size group (>4 cm) was statistically significant (P = 0.003). Nakasu et al. could not find a significant relationship between meningioma recurrence and the existence of dural tails on MR images, as there was a policy to remove the area of dural enhancement[20], whereas Rokni-Yazdi et al. observed that MRI findings failed to predict tumoral invasion of the dural tail in histologic samples.[26] In our study, we found that there was no significant correlation between recurrence and dural tail on radiology (P = 0.1) [Table 6].
Table 6:
Various studies on radiological factors affecting meningioma recurrence.
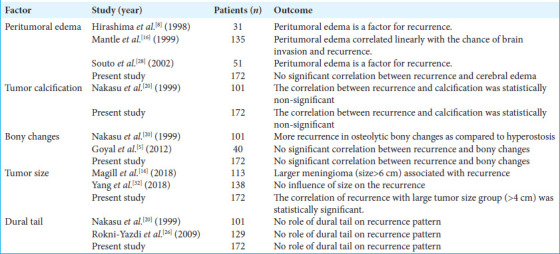
Limitations of the study
First, it is a retrospective study which has its inherent disadvantages. Second, the 2016 WHO classification of tumors of the central nervous system had changes in the grading of meningioma, whereas the present study patient’s histopathological examination is based on pre 2016 classification, which might cause an impact on recurrence. Finally, this is a monocentric study.
CONCLUSION
This current study is one of the largest series containing datasets for factors related to recurrence. The large meningioma (size ≥4 cm), old age (age >45 years), high histological grade (WHO Grade II, III), non-skull base location, and Simpson grade III, IV of resection showed significantly shorter RFS while gender, tumor calcification, peritumoral edema, and dural tail sign have no association with recurrence. The study suggests that surgical resection is safe for meningioma and has a low rate of surgical complications and mortality.
Footnotes
How to cite this article: Varshney A, Jaiswal S, Bajaj A, Yadav A, Srivastava C, Chandra A, et al. Management of intracranial meningioma: Outcome analysis and clinico radiological profile of 172 patients. Surg Neurol Int. 2024;15:464. doi: 10.25259/SNI_556_2024
Contributor Information
Aditya Varshney, Email: itsadityavarshney@gmail.com.
Somil Jaiswal, Email: dr.somil26@gmail.com.
Ankur Bajaj, Email: ankurbajaj@kgmcindia.edu.
Awdhesh Yadav, Email: awkymail@gmail.com.
Chhitij Srivastava, Email: drcsrivastava@gmail.com.
Anil Chandra, Email: neurochandra@gmail.com.
Bal Krishna Ojha, Email: drbkojha@gmail.com.
Shalini Bhalla, Email: shalinibhalla@kgmcindia.edu.
Pooja Jaiswal, Email: drpj1983@gmail.com.
Brijesh Pratap Singh, Email: brijeshmbbs@gmail.com.
Manish Kumar Singh, Email: manishsingh.brdmc@gmail.com.
Ethical approval
The Institutional Review Board approved the research/study at King George’s Medical University, number 772/Ethics/2020, dated August 13, 2020.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
REFERENCES
- 1.Chen ZY, Zheng CH, Tang Li, Su XY, Lu GH, Zhang CY, et al. Intracranial meningioma surgery in the elderly (over 65 years): Prognostic factors and outcome. Acta Neurochir (Wien) 2015;157:1549–57. doi: 10.1007/s00701-015-2502-9. discussion 1557. [DOI] [PubMed] [Google Scholar]
- 2.Corell A, Thurin E, Skoglund T, Farahmand D, Henriksson R, Rydenhag B, et al. Neurosurgical treatment and outcome patterns of meningioma in Sweden: A nationwide registry-based study. Acta Neurochir (Wien) 2019;161:333–41. doi: 10.1007/s00701-019-03799-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehresman JS, Garzon-Muvdi T, Rogers D, Lim M, Gallia GL, Weingart J, et al. The relevance of simpson grade resections in modern neurosurgical treatment of World Health Organization grade I, II, and III meningiomas. World Neurosurg. 2018;109:e588–93. doi: 10.1016/j.wneu.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 4.Gasparetto EL, Leite CC, Lucato LT, de Barros CV, Marie SK, Santana P, et al. Intracranial meningiomas: Magnetic resonance imaging findings in 78 cases. Arq Neuropsiquiatr. 2007;65:610–4. doi: 10.1590/s0004-282x2007000400012. [DOI] [PubMed] [Google Scholar]
- 5.Goyal N, Kakkar A, Sarkar C, Agrawal D. Does bony hyperostosis in intracranial meningioma signify tumor invasion? A radio-pathologic study. Neurol India. 2012;60:50. doi: 10.4103/0028-3886.93589. [DOI] [PubMed] [Google Scholar]
- 6.Goyal R, Gupta P. Clinicopathological study of meningioma from rural setup of central India : A 5 year experience. Indian J Pathol Oncol. 2019;6:539–42. [Google Scholar]
- 7.Gupta PK, Sastry Kolluri VR, Das S, Chandra Mouli BA, Narayana Swamy KS, Das BS. Recurrences in meningioma after surgery. Acta Neurochir (Wien) 1989;100:104–7. doi: 10.1007/BF01403594. [DOI] [PubMed] [Google Scholar]
- 8.Hirashima Y, Hayashi N, Fukuda O, Ito H, Endo S, Takaku A. Platelet-activating factor and edema surrounding meningiomas. J Neurosurg. 1998;88:304–7. doi: 10.3171/jns.1998.88.2.0304. [DOI] [PubMed] [Google Scholar]
- 9.Holleczek B, Zampella D, Urbschat S, Sahm F, von Deimling A, Oertel J, et al. Incidence, mortality and outcome of meningiomas: A population-based study from Germany. Cancer Epidemiol. 2019;62:101562. doi: 10.1016/j.canep.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Howng SL, Kwan AL. Intracranial meningioma. Gaoxiong Yi Xue Ke Xue Za Zhi. 1992;8:312–9. [PubMed] [Google Scholar]
- 11.Kollova A, Liscak R, Novotny J, Vladyka V, Simonova G, Janouskova L. Gamma knife surgery for benign meningioma. J Neurosurg. 2007;107:325–36. doi: 10.3171/JNS-07/08/0325. [DOI] [PubMed] [Google Scholar]
- 12.Kong CC, Kandasamy R, Haspani S, Idris Z, Abdullah JM. Incidence, clinico-radiological features and outcome of skull base versus non-skull base meningiomas treated in kuala lumpur general hospital: A five-year experience. Malays J Med Sci. 2018;25:88–102. doi: 10.21315/mjms2018.25.3.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakshmi SS. Meningiomas: A clinicopathological study. Int J Med Res Health Sci. 2015;4:827. [Google Scholar]
- 14.Magill ST, Young JS, Chae R, Aghi MK, Theodosopoulos PV, McDermott MW. Relationship between tumor location, size, and WHO grade in meningioma. Neurosurg Focus. 2018;44:E4. doi: 10.3171/2018.1.FOCUS17752. [DOI] [PubMed] [Google Scholar]
- 15.Mahmood A, Qureshi NH, Malik GM. Intracranial meningiomas: Analysis of recurrence after surgical treatment. Acta Neurochir (Wien) 1994;126:53–8. doi: 10.1007/BF01476410. [DOI] [PubMed] [Google Scholar]
- 16.Mantle RE, Lach B, Delgado MR, Baeesa S, Belanger G. Predicting the probability of meningioma recurrence based on the quantity of peritumoral brain edema on computerized tomography scanning. J Neurosurg. 1999;91:375–83. doi: 10.3171/jns.1999.91.3.0375. [DOI] [PubMed] [Google Scholar]
- 17.Marosi C, Hassler M, Roessler K, Reni M, Sant M, Mazza E, et al. Meningioma. Crit Rev Oncol Hematol. 2008;67:153–71. doi: 10.1016/j.critrevonc.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Moon HS, Jung S, Jang WY, Jung TY, Moon KS, Kim IY. Intracranial meningiomas, WHO Grade Il : Prognostic implications of clinicopathologic features. J Korean Neurosurg Soc. 2012;52:14–20. doi: 10.3340/jkns.2012.52.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mubeen B, Makhdoomi R, Nayil K, Rafiq D, Kirmani A, Salim O, et al. Clinicopathological characteristics of meningiomas: Experience from a tertiary care hospital in the Kashmir Valley. Asian J Neurosurg. 2019;14:41–6. doi: 10.4103/ajns.AJNS_228_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakasu S, Nakasu Y, Nakajima M, Matsuda M, Handa J. Preoperative identification of meningiomas that are highly likely to recur. J Neurosurg. 1999;90:455–62. doi: 10.3171/jns.1999.90.3.0455. [DOI] [PubMed] [Google Scholar]
- 21.Nanda A, Bir SC, Maiti TK, Konar SK, Missios S, Guthikonda B. Relevance of Simpson grading system and recurrence-free survival after surgery for World Health Organization Grade I meningioma. J Neurosurg. 2017;126:201–11. doi: 10.3171/2016.1.JNS151842. [DOI] [PubMed] [Google Scholar]
- 22.Oya S, Ikawa F, Ichihara N, Wanibuchi M, Akiyama Y, Nakatomi H, et al. Nation-wide brain tumor registry-based study of intracranial meningioma in Japan: Analysis of surgery-related risks. Neurol Med Chir (Tokyo) 2021;61:98–106. doi: 10.2176/nmc.oa.2020-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perry A, Stafford SL, Scheithauer BW, Suman VJ, Lohse CM. Meningioma grading: An analysis of histologic parameters. Am J Surg Pathol. 1997;21:1455–65. doi: 10.1097/00000478-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Rogers L, Barani I, Chamberlain M, Kaley TJ, McDermott M, Raizer J, et al. Meningiomas: Knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015;122:4–23. doi: 10.3171/2014.7.JNS131644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rohringer M, Sutherland GR, Louw DF, Sima AA. Incidence and clinicopathological features of meningioma. J Neurosurg. 1989;71:665–72. doi: 10.3171/jns.1989.71.5.0665. [DOI] [PubMed] [Google Scholar]
- 26.Rokni-Yazdi H, Azmoudeh Ardalan F, Asadzandi Z, Sotoudeh H, Shakiba M, Adibi A, et al. Pathologic significance of the “dural tail sign. ” Eur J Radiol. 2009;70:10–6. doi: 10.1016/j.ejrad.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Ryu HS, Moon KS, Lee KH, Jang WY, Jung TY, Kim IY, et al. Recurred intracranial meningioma: A retrospective analysis for treatment outcome and prognostic factor. Brain Tumor Res Treat. 2017;5:54–63. doi: 10.14791/btrt.2017.5.2.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Souto AA, Chimelli L, Takya CM, Souza JM, Fonseca AL, Silva LF. Brain edema in meningiomas: Radiological and histological factors. Arq Neuropsiquiatr. 2002;60:807–17. [PubMed] [Google Scholar]
- 29.Sun C, Dou Z, Wu J, Jiang B, Iranmanesh Y, Yu X, et al. The preferred locations of meningioma according to different biological characteristics based on voxel-wise analysis. Front Oncol. 2020;10:1412. doi: 10.3389/fonc.2020.01412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vob KM, Spille DC, Sauerland C, Suero Molina E, Brokinkel C, Paulus W, et al. The Simpson grading in meningioma surgery: Does the tumor location influence the prognostic value? J Neurooncol. 2017;133:641–51. doi: 10.1007/s11060-017-2481-1. [DOI] [PubMed] [Google Scholar]
- 31.Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99:307–14. doi: 10.1007/s11060-010-0386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang CC, Tsai CC, Chen SJ, Chiang MF, Lin JF, Hu CK, et al. Factors associated with recurrence of intracranial meningiomas after surgical resection: A retrospective single-center study. Int J Gerontol. 2018;12:57–61. [Google Scholar]
- 33.Zang KD. Meningioma: A cytogenetic model of a complex benign human tumor, including data on 394 karyotyped cases. Cytogenet Cell Genet. 2001;93:207–20. doi: 10.1159/000056986. [DOI] [PubMed] [Google Scholar]


