Abstract
Objective
To evaluate the healthcare costs associated with unresolved slipping rib syndrome (SRS).
Methods
Data pertaining to patients who underwent operative repair for SRS at our academic institution were analyzed retrospectively. Duration of symptoms, previous management efforts, number of healthcare provider consultations, imaging studies, adjunctive surgical and pain management procedures performed to treat the symptoms, and prior unsuccessful SRS operations were catalogued. US Medicare billing standards were used to average costs for provider visits and overall cost of surgical and interventional pain management procedures. Analgesic medication costs were determined using generic pricing.
Results
Between February 2019 and January 2024, a total of 435 consecutive patients spent a median of 36 months searching for a diagnosis and symptom relief prior to evaluation at our institution. The median number of physicians consulted was 6 (range, 0-75). The total cost of physician visits was $2,990,434 USD. The median number of imaging studies was 5 (range, 0-55), at a total cost of $965,949. Cholecystectomy was performed in 47 patients (11%), at a cost of $716,750. Previous SRS surgery had been attempted 150 times at various institutions and accounted for $4,500,000 (estimated $30,000 per operation in billing). Intercostal nerve block, ablation, and spinal cord stimulator placement had been performed in 30%, 15%, and 5% of the patients, respectively, at a total cost of $963,821. The median number of analgesic medications used per patient was 1 (mean, 1.3; range, 0-5); the total medication cost was $1,111,860. The total preoperative healthcare cost in our series was $12,445,173, for an average of $28,610 per patient.
Conclusions
SRS remains poorly understood. Symptoms can be severe and debilitating, and patients frequently consume significant healthcare resources. With recognition and definitive surgical management, SRS may be addressed successfully. Prompt treatment has the potential for significant healthcare savings.
Key Words: slipping rib syndrome, slipped rib syndrome, healthcare cost, healthcare economy, intercostal neuralgia, costal margin reconstruction, costal cartilage excision, sutured repair
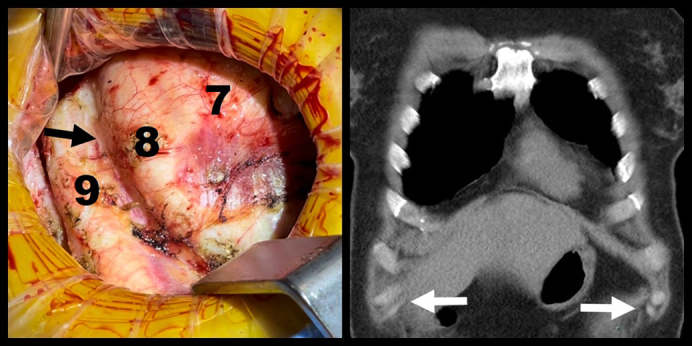
Intraoperative and coronal CT views of ninth slipped ribs. Arrows mark the sites of rib separation.
Central Message.
Slipping rib syndrome (SRS) remains poorly recognized. Symptoms may be severe and debilitating, motivating patients to utilize significant healthcare resources. With recognition and definitive surgical management, SRS can be addressed successfully. Prompt treatment has the potential for significant healthcare cost savings.
Perspective.
Slipping rib syndrome is a correctable disorder. Early recognition and proper treatment may lead to significant reduction in patient morbidity and cost savings to the patient healthcare system.
Slipping rib syndrome (SRS) is one of the most common disorders involving the false ribs. In brief, SRS can be defined as spontaneously occurring and recurrent focal pain in 1 or more false rib intercostal dermatomes that can be reproduced on physical examination and correlates with palpable subluxation of the same false rib(s).1 It is a chronic pain disorder caused by separation, or slippage, away from the costal arch of 1 or more of ribs 8 to 10.2, 3, 4 Disruption or stretching of the interchondral ligaments underlies the separation. The slipped rib becomes abnormally mobile and can repetitively traumatize and compress the intercostal nerve between it and the superior or inferior rib with only minimal movement or postural change. It occurs most commonly in rib 10 and always involves at least the rib 10.1,5,6 Concurrent rib 9 laxity or full separation is also very common.
Awareness of SRS has grown significantly in recent years,6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 and recognition and diagnosis are constantly improving.7,8,12,13,15,17,20, 21, 22, 23, 24, 25 Nonetheless, SRS remains a frequently overlooked diagnosis, despite often clinically apparent signs and symptoms. When patients present with complaints of rib pain, SRS often is mistaken for more commonly recognized entities. Gallbladder disease, other abdominal organ disorders, spinal radiculopathy, and shoulder injury are routinely considered first. When rib pathology is suspected, the pain of SRS is often dismissed as costochondritis and left untreated. When SRS is recognized and patients undergo surgical treatment, remotely described non-reconstructive treatments commonly are offered, frequently leading to the need for revisionary surgery.
Pain and related symptoms of instability, dyspnea, and secondary abdominal organ dysfunction can be severe and disabling. Many affected individuals are motivated to find a diagnosis and appropriate solutions, but some become apathetic and socially withdrawn, often opting for a chronic pain management strategy.1 Many patients present with a self-diagnosis of SRS after researching their own symptoms; a self-diagnosis is highly predictive and usually accurate.
A diagnosis of SRS generally is secured through a detailed history and physical examination. Imaging studies, including noncontrast computed tomography (CT) scan of the chest/abdomen and dynamic ultrasound,14,19 are useful diagnostic adjuncts. Plain films are not useful, and magnetic resonance imaging has not demonstrated utility in any published reports. Points of detachment and subluxation are often visible on CT coronal images or ultrasound images. CT scans also can identify other related intercostal impingement sites from related functional pain disorders of the lower rib cage. The only study that we require of any potential SRS patient is noncontrast CT of the chest and abdomen, and we use coronal images in the examination room and reference them, back and forth, during the physical examination to correlate physical and imaging findings.
Treatment of SRS is a subject of considerable debate. Nonsurgical modalities do not definitively address the underlying ligamentous disruption that caused the SRS but may provide at least temporary relief in some cases. Surgeons have operatively managed SRS since the early 1920s. Until recently with the advent of rib-sparing repair of SRS, costal cartilage excision (CCE) had been the option commonly used in severe cases. The theory behind excision of typically the ninth-tenth costal cartilages is that removal may lead to relief of intercostal nerve compression. Several small series have reported variable success with CCE. No definitive technique descriptions have been published, and technique varies widely among reports. Clear outcome measures are usually absent in published reports, and claims of success are difficult to interpret. Although some patients report satisfactory results after excision, many do not. Some have experienced devastating results, including severe rib cage instability, worsened or intractable pain, diminished pulmonary function, and chronic gut motility issues. Conclusions drawn in more contemporary reports1,5,13,26 suggest that costal cartilage excision without stabilization can create an iatrogenic disorder that leads to the need for often repeated revisionary surgery with additional excisions.
The typical surgical technique for CCE involves a small incision at the lower costal margin, after which the hypermobile rib tip is identified and excised to the costochondral junction. The neurovascular bundle is carefully preserved. Symptomatic relief following CCE in adult and pediatric SRS patients has been reported in several small series.7,8,12,16,17,20,22,27 However, an overarching drawback to CCE is that it does not resolve rib hypermobility. Thus, patients with SRS who undergo CCE have a reported rate of symptom recurrence as high as 26%.12,22
In 2021, we reported a novel suture repair (SR) technique for managing SRS in adult patients.6 SRS was diagnosed using clinical examination alone in 29 adult patients. The slipped ribs were sutured back to the costal margin with figure-of-eight tape sutures in 2 locations, excluding the intercostal nerve bundles. Median postoperative improvement in pain was 75% at 1 month and 80% at 6 months. Improvements in overall quality of life were similar. Among patients who received pain medication preoperatively, opioids were discontinued at 1 month in 100%, neural modulators in 86%, and nonsteroidal anti-inflammatory drugs (NSAIDs) in 92%. Pain medication use remained minimal at 6 months. The study was limited by small numbers and only a 6-month follow-up period. Similar results were achieved by Polycarpou and colleagues28 with the same SR technique.
In 2024, we reported a series of 449 patients in a study that compared a larger subset of the earlier SR patients with a second group in which a different novel technique of costal margin reconstruction (CMR) was performed.1 241 patients underwent SR. The preoperative mean pain score of 7.5 out of 10 dropped postoperatively to 4.0 at 1 month and to 2.5 at 6 months. The mean quality of life score of 38% preoperatively improved to 73% at 1 month and 83% at 6 months postoperatively. The rate of opioid use dropped postoperatively to 11% and 4% at the same intervals in the SR group. Revision was required in 66 patients, with a median time to revision of 14 months. In patients failing SR, specific failure points were identified and the new CMR technique was devised to overcome them. CMR was then performed in 247 patients, and the follow-up period was extended to 2 years to surpass the time point at which earlier SR patients had demonstrated late failure. In CMR patients, the preoperative mean pain score of 7.5 out of 10 dropped postoperatively to 4.0 at 1 month, 2.5 at 6 months, 1.9 at 12 months, 1.3 at 18 months, and 0.9 at 24 months. The mean quality of life score of 38% preoperatively improved to 73%, 83%, 88%, 93%, and 95% at the same intervals. Preoperatively, 29% of patients chronically used opioid medications. Opioid use dropped postoperatively to 11%, 4%, 4%, 0%, and 0% at the same intervals. Use of nonopioid medications followed a similar pattern. Only 2 CMR patients required full revision.
Early outcomes for all measures reported were nearly identical in the 2 groups. However, a much lower failure rate was seen in CMR patients compared to SR patients (1% vs 23%). We concluded that CMR was appropriate for all SRS patients and was significantly more durable than SR, which has a failure rate comparable to the CCE technique.
Costal margin reconstruction technique (technique video available5) uses a limited length of costal cartilage excision at the tip of each slipped rib. The excised cartilage is then fashioned into donor autograft cartilage spacers that are interposed between the slipped rib(s) and the lowest stable nonslipped rib superior to the slipped ribs(s). The ribs and intervening spacers are then sutured together snugly from top to bottom and back with a permanent tape suture into a configuration resembling a rope ladder. The construct is then semirigidly splinted with a vertically oriented overlay bioabsorbable rib plate that is sutured tightly to each slipped rib and to 1 or 2 stable ribs superior to them.
SRS patients are often heavy utilizers of medical resources. They also spend considerable effort and nonmedical financial resources traveling in search of specialists and definitive treatment. Many become disabled or limited in their capacity to work. We postulated that expeditious diagnosis and effective treatment of SRS may lead to a decreased economic burden on patients, the healthcare system, and society in general, in addition to earlier symptom resolution. To highlight this, we sought to quantify the financial burden related to treatment and management of unresolved SRS imposed on the overall healthcare system.
Patients and Methods
We performed a retrospective review of our 5-year case series of reconstruction of SRS conducted between February, 2019 to January, 2024 at a single academic institution. All patients requiring surgical reconstruction of their slipping ribs were interviewed preoperatively to elicit the duration of their SRS symptoms and details of prior clinical workup and treatment efforts. We assessed the use of medications prescribed specifically to manage SRS symptoms, including neuromodulating drugs, narcotic medications, and NSAIDs. The number of medical providers consulted and number radiologic imaging studies also were quantified. Patients were asked whether they had undergone any prior pain management procedures, including intercostal nerve block and ablation, prolotherapy injection, platelet-rich plasma injection, and spinal stimulator implantation. Previous surgical procedures performed to treat pain and other symptoms, including both nonrib operations and rib operations, were detailed for each patient.
US Medicare billing standards were used to assign a cost to each provider visit, radiologic study, interventional pain management procedure, and surgical operation. Analgesic medication costs were determined with generic pricing and extrapolated over the duration of each patient's symptoms. All results were calculated in US dollars. Obtaining exact details of types of medical providers consulted, radiologic studies used, extent and number of interventional pain management procedures undergone, and full operative details of prior surgical procedures was infeasible, and thus certain assumptions and estimations were required in our calculations. The service fees associated with the array of medical provider visits previously utilized varied. We observed that certain specialists and primary care providers were consulted by patients more frequently than others. The most commonly consulted specialty physicians included spine surgeons, gastroenterologists, orthopedic surgeons, and pain management specialists. We multiplied the service fee for each provider type by the estimated percentage of total provider visits each represented. Costs of interventional pain management procedures were calculated in the same manner.
The costs of the typical radiologic imaging studies were averaged to obtain a plausible standard imaging study cost, as our assessment indicated that each was equally likely to have been performed in the typical workup for the symptoms of SRS.
The West Virginia University Institutional Review Board approved this study with a waiver of consent (protocol 1908679868; approved August 29, 2019).
Results
The study cohort comprised 435 consecutive patients with SRS who underwent surgical treatment. Their mean age was 42 years (range, 14-84 years). Female patients accounted for 71% of the cohort. The mean body mass index was 27 (range, 17-45). Approximately 60% of the patients seen in the study period had self-diagnosed and self-referred for SRS. The remainder were referred by medical providers for a suspected SRS diagnosis or simply for rib pain. We did not track patients who did not undergo surgical treatment. There were additional patients referred or self-referred for SRS who did not have SRS, but this was represented <10% of cases. Approximately 30% of the patients diagnosed with SRS during the study period either chose not to undergo surgical repair or were not offered surgery because their symptoms were not severe enough to warrant surgery.
Prior to surgical treatment, most patients had been prescribed at least 1 analgesic medication to manage their symptoms at the time of initial consultation. Almost one-half routinely used NSAIDs, muscle relaxants, benzodiazepines, or nerve stabilizing drugs; 29% used narcotic medication; 32% regularly used 1 analgesic medication; 27% used 2 analgesic medications, 11% used 3, and 2% used 4. Only 27% took no analgesic medication preoperatively. Weighted costs by provider are presented in Table 1, and weighted costs by interventional pain procedure are presented in Table 2. Imaging costs are listed in Table 3. Details of monthly medical treatment cost of analgesic medications and extrapolated cost over the span of treatment before definitive surgical therapy are shown in Table 4. The total cost of analgesic medications over the span of treatment in our series was $1,111,860.
Table 1.
Cost of physician encounters
| Type of visit | Medicare average cost | Proportion of encounters, % | Cost factor by provider type |
|---|---|---|---|
| Primary care level 3 visit | $104 | 10 | $10 |
| Primary care level 5 visit | $234 | 10 | $23 |
| Average medical specialist consultation | $335 | 50 | $168 |
| Average physician encounter cost | $861 | ||
| Total extrapolated cost of 3472 physician encounters | $2,990,434 |
ER, Emergency room.
Table 2.
Cost of interventional pain management procedures
| Interventional pain procedure | Medicare average cost | Proportion of patients who underwent procedure, % | No. of patients | Extrapolated cost |
|---|---|---|---|---|
| Nerve block | $876 | 30 | 131 | $114,318 |
| Prolotherapy | $600 | 5 | 22 | $13,050 |
| Platelet-rich plasma injection | $630 | 5 | 22 | $13,703 |
| Nerve ablation | $1030 | 15 | 65 | $67,208 |
| Spinal stimulator | $57,896 | 3 | 13 | $755,543 |
| Total extrapolated procedure cost | $963,821 |
Table 3.
Costs of radiologic imaging
| Imaging study | Medicare average |
|---|---|
| Chest X-ray, 2 views | $96 |
| CT chest with IV contrast | $267 |
| CT abdomen/pelvis w/wo IV contrast | $463 |
| CT thoracic spine w/wo IV contrast | $240 |
| CT lumbar spine w/wo IV contrast | $240 |
| Ultrasound abdomen, limited | $134 |
| HIDA scan | $546 |
| MRI thoracic spine | $453 |
| Electrocardiography | $588 |
| Average study cost | $336 |
| Total extrapolated cost of 2872 imaging studies | $965,949 |
CT, Computed tomography; IV, intravenous; HIDA, hepatobiliary iminodiacetic acid; MRI, magnetic resonance imaging.
Table 4.
Cost of analgesic medications
| Parameter | Medications per patient | Monthly cost per patient | Cost over span of treatment |
|---|---|---|---|
| Mean | 1.3 | $36 | $2556 |
| Median | 1 | $20 | $684 |
| Range low | 0 | — | — |
| Range high | 5 | $967 | $69,120 |
| Total extrapolated analgesic medication cost | $1,111,860 |
The patients had seen a median of 6 physicians (mean, 8; range, 1-75) in prior consultation, without definitive diagnosis or treatment in most cases. An estimated 50% of the physician visits were initial specialist consultations, 30% were emergency department visits, and the remainder were with primary care providers. The total extrapolated cost of the 3472 medical provider visits in the cohort was $2,990,434 (Table 1). The extrapolated cost of 2872 radiologic imaging studies and electrocardiograms was $965,949 (Table 3).
Patients with SRS had commonly undergone 1 or more interventional pain management procedures. Using our estimate of the percentage of patients that had undergone each type of procedure (Table 2), we calculated a total cost of $963,821.
Nontherapeutic cholecystectomy was performed in 11% of patients to treat symptoms, amounting to an extrapolated cost of $716,750. Some patients had undergone cholecystectomy and had improved symptomatology thereafter and corresponding imaging or pathology reports evidencing actual gallbladder disease. Only those with fully negative findings on imaging and final gallbladder pathology and who did not improve after cholecystectomy were included in our determination of nontherapeutic cholecystectomy. Two percent had undergone other nonrib operations, such as hysterectomy or bowel resection in an attempt to treat the same symptoms, but without resolution thereafter. However, given the variability in the types of operations performed, we did not quantify a total cost.
Some form of surgical treatment for SRS was performed in 23%, including costal cartilage excision (CCE) alone, CCE with vertical bioabsorbable plating, sutured rib repair (including 66 of our own patients with failed sutured repair), and other various repair techniques. Using an estimate of $30,000 for each of the 150 prior rib operations reported by patients in the series, extrapolated surgical costs totaled $4,500,000.
Table 5 summarizes the total costs of each type of medical expenditure in the series. The total cost of all healthcare efforts to diagnose, manage, and treat the symptoms of SRS prior to definitive reconstruction in our 435 patients amounted to $12,445,173, for an average cost per patient of $28,610.
Table 5.
Preoperative medical costs
| Category | Total cost |
|---|---|
| Doctor visits | $3,683,098 |
| Pain procedures | $1,467,516 |
| Imaging studies | $965,949 |
| Cholecystectomies | $716,750 |
| Previous rib operations | $4,500,000 |
| Pain medication | $1,111,860 |
| Total costs in series of 435 patients | $12,445,173 |
| Total costs per patient | $28,610 |
Discussion
Our series represents the largest cohort of patients with SRS reported to date and thus provides insight into the economic burden on the healthcare system that undiagnosed or unresolved cases imposes. SRS is considered a rare disorder, but we contend that it is much more common than previously acknowledged. Given the broad region of the torso affected, we suspect that many patients with chronic pain may suffer from SRS. Our series includes patients referred or self-referred from all over the United States and internationally. Interestingly, the series also includes a significant number of local patients seeking thoracic surgical treatment of different diagnoses that were indeed SRS masquerading as other disorders. A high index of suspicion and awareness of the associated symptoms of SRS led to identification and accurate diagnosis of these patients. We assessed the medical expenditures in a group of 435 patients. If the same calculations were applied to the actual population of patients suffering with SRS, the financial ramifications might be quite significant.
Our baseline premise is that SRS is correctable, and that the costs of ongoing treatment diminish or end once it is surgically corrected and the patient recovers fully. The cost of CMR, a surgical technique that we have used with a high success rate, and appropriate postoperative care is approximately $30,000 per patient. This, combined with the cost of a CT chest and abdomen and surgical consultation, would total $30,602 as the total cost of “effective treatment” in a straightforward patient case of SRS. A short course of pain medications would add nominal cost as well. The cost of expedient and effective care is still significant but similar to that of other surgical procedures that patients had undergone that did not fully correct their symptoms.
This study has several inherent limitations. First, data were collected retrospectively. Prior medical records were reviewed when available, but the historical data gathered directly from patients is subject to recall bias. Second, many of the cost figures that we calculated were based on assumptions and estimates. Further detailed assessment of actual costs of prior medical care would not be feasible in the clinical setting from which we gathered the data. Third, the calculated cost of interventional pain management procedures likely underestimates the actual cost, given that our binary assessment of whether each patient had undergone a procedure did not factor in the extent or number of each type of procedure performed. We were unable to assess the financial burden to patients in terms of costs of travel, out-of-pocket medical expenses, lost work productivity, or other intangible costs. We suspect that these costs would be significant if calculable. Nevertheless, this is the first attempt at quantifying the financial burden of this poorly understood pathological entity and the first step toward mitigating this burden through awareness and recognition.
Finally, 15% of the patients in our series suffered from other significant concurrent painful functional disorders of the lower ribcage, including 12th rib syndrome, L1 syndrome, and costoiliac impingement. These patients required simultaneous or staged surgical procedures, which added additional cost to their overall care. CMR for SRS is not always the sole intervention that patients need for satisfactory symptom relief, as many have a complex set of related rib disorders.
Undiagnosed or unresolved SRS imposes a heavy burden, not only an economic burden on the healthcare system, but also a burden on patients, both financially and personally. We conclude that expeditious diagnosis and effective surgical management has a strong potential for reducing this burden.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
Supported by National Heart, Blood, and Lung Institute Grant 2UM1 HL088925 12 (to V.B. and J.W.A.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The West Virginia University Institutional Review Board approved this study with a waiver of consent (protocol 1908679868; approved August 29, 2019).
References
- 1.Hansen A.J., Hayanga J., Toker A., Badhwar V. Costal margin reconstruction for slipping rib syndrome: outcomes of more than 500 cases and advancements beyond earlier sutured repair technique. JTCS Open. 2024;19:347–354. doi: 10.1016/j.xjon.2024.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinz G.J., Zavala D.C. Slipping rib syndrome. JAMA. 1977;237(8):794–795. [PubMed] [Google Scholar]
- 3.Telford K.M. The slipping rib syndrome. Can Med Assoc J. 1950;62(5):463–465. [PMC free article] [PubMed] [Google Scholar]
- 4.Wright J.T. Slipping-rib syndrome. Lancet. 1980;2(8195 Pt 1):632–634. doi: 10.1016/s0140-6736(80)90294-9. [DOI] [PubMed] [Google Scholar]
- 5.Hansen A., Hayanga J., Toker A. Costal margin reconstruction for complex slipping rib syndrome [Video] 2023. www.CTSNet.org [DOI] [PMC free article] [PubMed]
- 6.Hansen A.J., Toker A., Hayanga J., Buenaventura P., Spear C., Abbas G. Minimally invasive repair of adult slipped rib syndrome without costal cartilage excision. Ann Thorac Surg. 2020;110(3):1030–1035. doi: 10.1016/j.athoracsur.2020.02.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudolph H.C., Nam B.T. Combined excision of costal cartilage and rib plating for slipped rib syndrome. Ann Thorac Surg. 2022;113(3):e207–e209. doi: 10.1016/j.athoracsur.2021.05.019. [DOI] [PubMed] [Google Scholar]
- 8.MacGregor R.M., Schulte L.J., Merritt T.C., Keller M.S., Aubuchon J.D., Abarbanell A.M. Slipping rib syndrome in children: natural history and outcomes following costal cartilage excision. J Surg Res. 2022;280:204–208. doi: 10.1016/j.jss.2022.06.061. [DOI] [PubMed] [Google Scholar]
- 9.Girbau A., Álvarez-Rey G., Cano-Herrera C.L., Balius R. Slipping rib syndrome: a clinical and dynamic-sonographic entity. A serial cases report. J Back Musculoskelet Rehabil. 2022;35(2):253–259. doi: 10.3233/BMR-200273. [DOI] [PubMed] [Google Scholar]
- 10.Bong J., Healey D. Slipping rib syndrome. J Med Imaging Radiat Oncol. 2022;66(3):409–410. doi: 10.1111/1754-9485.13247. [DOI] [PubMed] [Google Scholar]
- 11.Foley Davelaar C.M. A clinical review of slipping rib syndrome. Curr Sports Med Rep. 2021;20(3):164–168. doi: 10.1249/JSR.0000000000000821. [DOI] [PubMed] [Google Scholar]
- 12.Fraser J.A., Briggs K.B., Svetanoff W.J., St Peter S.D. Long-term outcomes and satisfaction rates after costal cartilage resection for slipping rib syndrome. J Pediatr Surg. 2021;56(12):2258–2262. doi: 10.1016/j.jpedsurg.2021.01.034. [DOI] [PubMed] [Google Scholar]
- 13.McMahon L.E., Salevitz N.A., Notrica D.M. Vertical rib plating for the treatment of slipping rib syndrome. J Pediatr Surg. 2021;56(10):1852–1856. doi: 10.1016/j.jpedsurg.2020.09.062. [DOI] [PubMed] [Google Scholar]
- 14.Patel N.G., Patel D.M., Patel M.V., Patel M.M., Patel T.R., Patel S.Y. Diagnostic value of dynamic high-frequency ultrasound for the slipping rib and twelfth rib syndrome: a case series with review. Curr Med Imaging. 2021;17(4):459–463. doi: 10.2174/1573405616666201005114406. [DOI] [PubMed] [Google Scholar]
- 15.Gress K., Charipova K., Kassem H., et al. A comprehensive review of slipping rib syndrome: treatment and management. Psychopharmacol Bull. 2020;50(4 Suppl 1):189–196. [PMC free article] [PubMed] [Google Scholar]
- 16.Squillaro A.I., Sanders K., Onwubiko C., Chang C.J., Kim S. Laparoscopic treatment of slipping rib syndrome in pediatric patients. J Laparoendosc Adv Surg Tech A. 2020;30(11):1253–1256. doi: 10.1089/lap.2020.0314. [DOI] [PubMed] [Google Scholar]
- 17.Mazzella A., Fournel L., Bobbio A., et al. Costal cartilage resection for the treatment of slipping rib syndrome (cyriax syndrome) in adults. J Thorac Dis. 2020;12(1):10–16. doi: 10.21037/jtd.2019.07.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMahon L.E. Recurrent slipping rib syndrome: initial experience with vertical rib stabilization using bioabsorbable plating. J Laparoendosc Adv Surg Tech A. 2020;30(3):334–337. doi: 10.1089/lap.2019.0519. [DOI] [PubMed] [Google Scholar]
- 19.Van Tassel D., McMahon L.E., Riemann M., Wong K., Barnes C.E. Dynamic ultrasound in the evaluation of patients with suspected slipping rib syndrome. Skeletal Radiol. 2019;48(5):741–751. doi: 10.1007/s00256-018-3133-z. [DOI] [PubMed] [Google Scholar]
- 20.Foley C.M., Sugimoto D., Mooney D.P., Meehan W.P., III, Stracciolini A. Diagnosis and treatment of slipping rib syndrome. Clin J Sport Med. 2019;29(1):18–23. doi: 10.1097/JSM.0000000000000506. [DOI] [PubMed] [Google Scholar]
- 21.McMahon L.E. Slipping rib syndrome: a review of evaluation, diagnosis and treatment. Semin Pediatr Surg. 2018;27(3):183–188. doi: 10.1053/j.sempedsurg.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Gould J.L., Rentea R.M., Poola A.S., Aguayo P., St Peter S.D. The effectiveness of costal cartilage excision in children for slipping rib syndrome. J Pediatr Surg. 2016;51(12):2030–2032. doi: 10.1016/j.jpedsurg.2016.09.032. [DOI] [PubMed] [Google Scholar]
- 23.Fares M.Y., Dimassi Z., Baydoun H., Musharrafieh U. Slipping rib syndrome: solving the mystery of the shooting pain. Am J Med Sci. 2019;357(2):168–173. doi: 10.1016/j.amjms.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Kuehn R., Muncie C., Berch B. Recurrent pain after resection for slipping rib syndrome: report of a difficult case. Am Surg. 2018;84(9):e346–e347. [PubMed] [Google Scholar]
- 25.Turcios N.L. Slipping rib syndrome in an adolescent: an elusive diagnosis. Clin Pediatr (Phila) 2013;52(9):879–881. doi: 10.1177/0009922812469290. [DOI] [PubMed] [Google Scholar]
- 26.Hansen A, Hayanga J, Toker A, Badhwar V. Healthcare economic burden of unresolved slipping rib syndrome. Poster presentation at 104th Annual Meeting of the American Association for Thoracic Surgery; April 29, 2024; Toronto, Ontario, Canada.
- 27.Fu R., Iqbal C.W., Jaroszewski D.E., St Peter S.D. Costal cartilage excision for the treatment of pediatric slipping rib syndrome. J Pediatr Surg. 2012;47(10):1825–1827. doi: 10.1016/j.jpedsurg.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Polycarpou A., Chopko T.C., Glasgow A.E., et al. One-year results of minimally invasive sutured fixation of the slipped ribs in the pediatric population. J Pediatr Surg. 2024;59(9):1703–1707. doi: 10.1016/j.jpedsurg.2024.02.027. [DOI] [PubMed] [Google Scholar]


