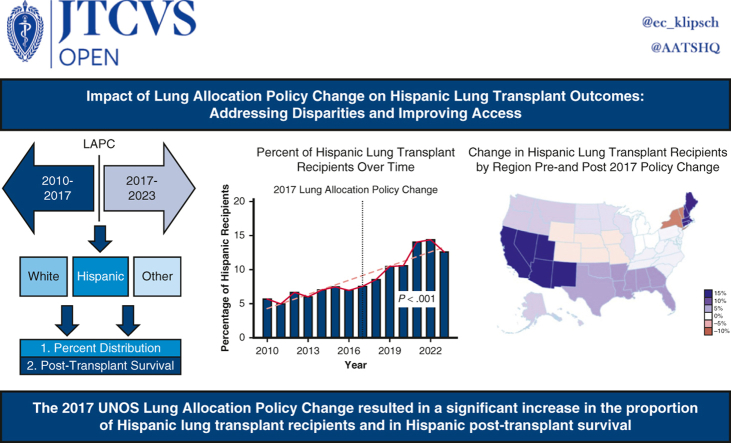
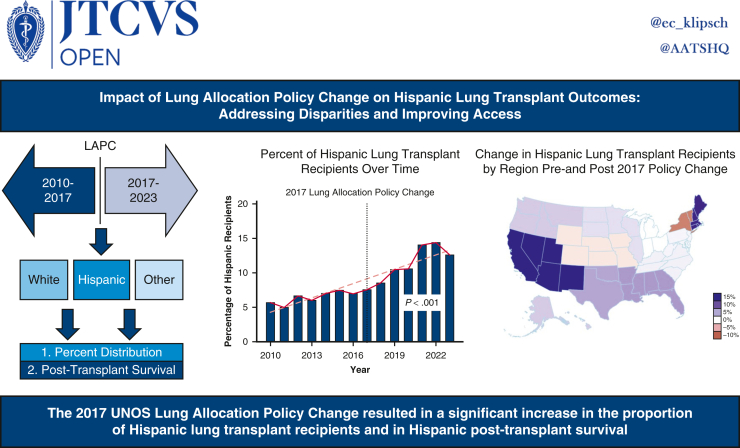
Abstract
Objective
Racial disparities in organ allocation may result in differential survival for marginalized groups. This study aims to examine the impact of the November 2017 lung allocation policy change (LAPC) on trends and outcomes of Hispanic lung transplant (LT) recipients.
Methods
The United Network for Organ Sharing database was used to identify adult (older than age 18 years) LT recipients between January 2010 and March 2023. Recipients were categorized into 3 self-identified racial groups (Hispanic, non-Hispanic White, and non-Hispanic other). The Mann-Kendall trend test was used to assess the trend in rates of Hispanic LT over 5 years pre- and 5 years post-LAPC. The primary outcome was 1-year mortality.
Results
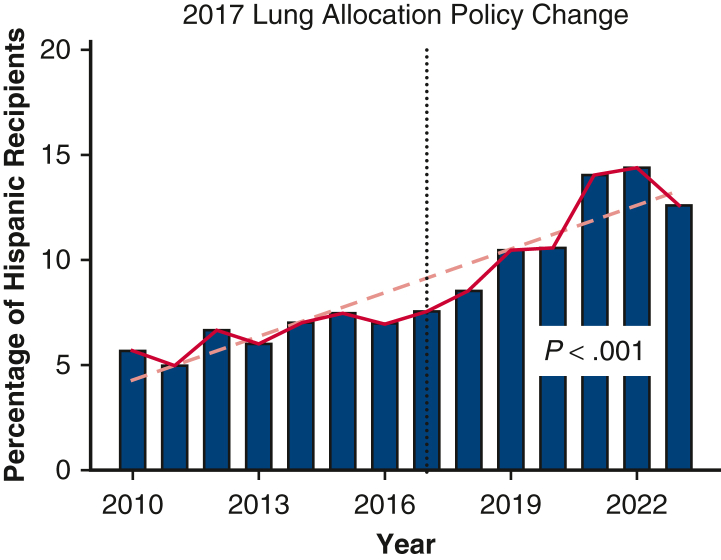
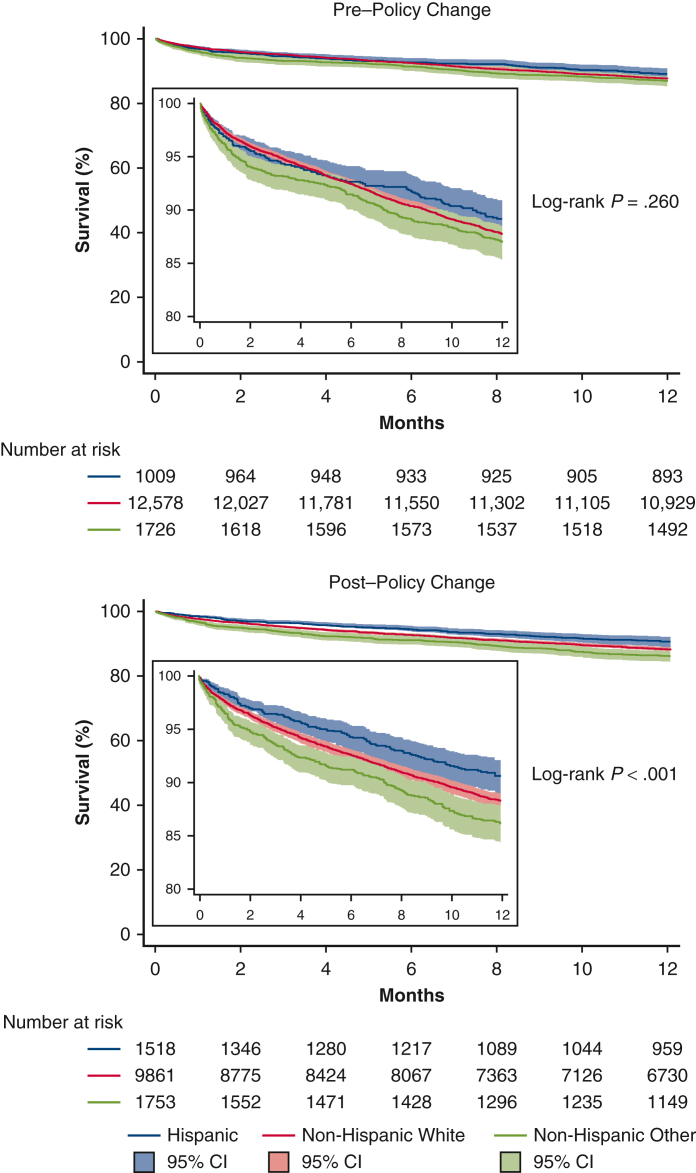
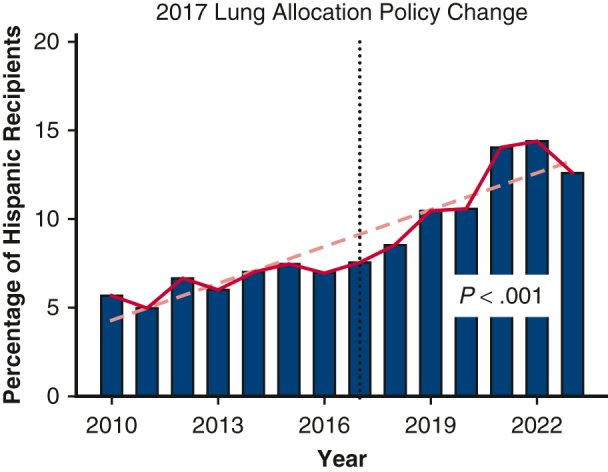
A total of 28,495 recipients from 80 centers were included, with 15,343 (53.8%) prepolicy change and 13,152 (46.2%) postpolicy change. The racial distribution of LT recipients was pre-LAPC: Hispanic: 1013 (6.6%), White: 12,601 (82.1%), Other: 1729 (11.3%) and post-LAPC: Hispanic: 1522 (11.6%), White: 9873 (75.0%), Other: 1757 (13.4%) (P < .001). Between 2013 and 2017, the proportion of Hispanic LT recipients increased from 6.0% to 7.6% (P = .221). Post-LAPC, the proportion increased from 8.5% in 2018 to 14.4% in 2022 (P < .027). Unadjusted 1-year survival rates were pre-LAPC: Hispanic: 88.8%, White: 87.6%, Other: 86.8% (log-rank P = .260) and post-LAPC: Hispanic: 90.6%, White: 88.2%, Other: 86.1% (log-rank P < .001).
Conclusions
The LAPC has led to increased access to LT and improved 1-year survival rates among Hispanic patients. However, efforts should continue to address disparities among other racial groups and ensure equitable outcomes for all recipients of LT.
Key Words: lung transplant, racial and ethnic disparities
Graphical Abstract
Percentage of Hispanic lung transplant recipients.
Central Message.
Following the 2017 lung allocation policy change, the number of LTs in Hispanic patients increased significantly, and there was a survival benefit in Hispanic recipients over other ethnic groups.
Perspective.
Hispanic patients have higher lung allocation scores at the time of transplant, yet waitlist outcome disparities still exist with worse outcomes in Hispanic patients compared with non-Hispanic Whites. The 2017 lung allocation score sought to make lung allograft distribution more equitable. No current studies have assessed the effect of the 2017 policy change on ethnic disparities.
See Discussion on page 519.
In 2005, a lung allocation score (LAS) was introduced to prioritize disease severity over time on the waitlist to improve lung allograft distribution and decrease racial and ethnic disparities. However, analysis of lung transplant (LT) waitlist outcomes from 2005 to 2021 found that African American and Hispanic candidates were less likely to undergo transplant compared with non-Hispanic Whites (NHW).1 Some of this disparity may be due to a combination of geographic and socioeconomic factors.2 Additionally, Hispanics are more likely to be uninsured, representing a disconnect from the medical community and making initial access to transplant centers more difficult.3 They also present to the medical system with more advanced disease and undergo transplant with higher LAS.4
The United Network for Organ Sharing (UNOS) and Organ Procurement and Transplant Network (OPTN) eliminated the use of donor service areas (DSA) in 2017. The historic use of DSAs resulted in inherent discrepancies in LT availability, with candidates in the DSAs with the lowest availability rates having significantly increased mortality while on the waitlist compared with those with the highest availability.5 The lung allocation policy change (LAPC) connected donors to recipients within a 250 nautical mile radius in an attempt to increase equitable allograft allocation.6,7
When comparing patients on the waitlist pre-LAPC and post-LAPC era, there is a significantly increased likelihood of transplantation and a decreased waitlist mortality following the change.7,8 There has also been a decrease in local transplants in favor of those farther away.9 In regard to recipient outcomes, there has been no significant difference in postoperative mortality for LT recipients following the LAPC.8 At this time, no study has been done to determine how the 2017 LAPC has affected disparity in lung allograft allocation. Considering previously mentioned ethnic and racial disparities with the use of DSAs, this present study looks to determine how the 2017 LAPC influenced distribution and outcomes of LT among Hispanics in the United States.
Methods
Study Design
This retrospective cohort study used data from the UNOS database to evaluate LT outcomes among adult recipients aged 18 years and older from January 2010 to March 2023. Institutional review board approval and individual consent was not required. Participants were stratified into three self-identified racial groups as defined in the UNOS database: Hispanic, NHW, and non-Hispanic Other, to assess disparities in transplant outcomes with a focus on the Hispanic population. The period of study was chosen to encompass data pre- and post-LAPC to allow for a comparative analysis of its influence.
Primary and secondary outcomes were identified to measure the effect of LAPC on LT recipients' survival and posttransplant complications. The primary outcome focused on 1-year mortality rates among the racial groups. Secondary outcomes included the incidence of extracorporeal membrane oxygenation within 72 hours posttransplant, occurrences of stroke, acute rejection episodes, cases requiring dialysis due to acute renal failure, duration of postoperative mechanical ventilation, and overall hospital length of stay. The UNOS database variable definitions were used to define the variables in this present study.
Statistical Analysis
Categorical variables were compared using the χ2 test or Fisher exact test when appropriate, particularly when sample sizes were small. For continuous variables, the analysis employed a 2-sided t test for variables with a normal distribution. In cases where variables deviated from a normal distribution, the Mann-Whitney U test was applied.
To assess the trend in the rates of Hispanic LT recipients over the study period, the Mann-Kendall trend test was used to compare both 5 years before and after LAPC implementation. Kaplan-Meier survival analysis was employed to estimate the 1-year survival probability across racial groups, both pre- and post-LAPC, providing a visual and statistical method to compare survival rates over time.
To assess the independent effect of race on LT mortality, multivariable Cox regression models were built. Models were stratified into pre- and post-LAPC periods to determine if the policy change had a differential influence on mortality hazard ratios (HRs) across racial groups. Variables included in the Cox regression models were chosen based on their clinical relevance and a P value < .2 on the univariable regression. Variables included in the multivariable analysis of the pre-LAPC and post-LAPC survival curves are demonstrated in Tables E1 and E2.
The study was reviewed by the institutional review board at the Medical University of South Carolina and was deemed exempt, given its retrospective design and the use of de-identified data. All statistical analyses were conducted using R software, version 4.3.1 (R Foundation for Statistical Computing).
Results
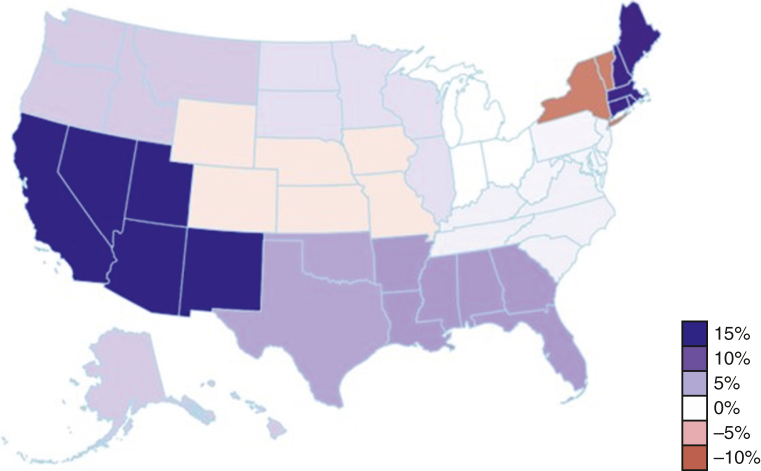
Of the 28,495 LT performed January 2010 through March 2023, 53.8% (n = 15,343) were performed pre-LAPC, and 46.2% (n = 13,152) were performed post-LAPC. Figure 1 demonstrates the change in percent of Hispanic LT recipients pre- and post-LAPC. Figure 2 shows the change by OPTN region, comparing pre- and post-LAPC eras. During the study period, we found a significant increase in Hispanic candidates listed for transplant. In 2010, only 6.37% of candidates listed were Hispanic, whereas 14.66% were Hispanic in 2023 (Pearson R = .96; P < .001). There was no significant change in the relative percent of Hispanic donors during the study period, and these data are included in Table E3.
Figure 1.
Change in percent lung transplant recipients who were Hispanic over time.
Figure 2.
Change in percent of Hispanic lung transplant recipients by Organ Procurement and Transplant Network region before and after the 2017 lung allocation policy change (n = 15,343 [53.8%] from January 2010 to November 2017 vs n = 13,152 [46.2%] from November 2017 to March 2023).
During the pre-LAPC era, out of 15,343 recipients, 82.1% (12,601) were NHW, 6.6% (1013) were Hispanic, and 11.3% (1729) were non-Hispanic Other. Recipient and donor demographics as well as secondary outcomes for the pre-LAPC era are included in Table 1. The analysis showed significant demographic differences among the groups. Hispanic patients were younger compared with NHW and non-Hispanic Other (mean age, 53.10 vs 56.54 vs 54.40 years; P < .001) and had a higher average body mass index (25.91 vs 25.14 vs 25.65; P < .001). Hispanic patients had a higher prevalence of diabetes (27.3% vs 19.4% vs 22.8%; P < .001), and a higher incidence of significant disability with Karnofsky Index ≤40% compared with NHW but not non-Hispanic Other (59% vs 55.2% vs 62.1%; P < .001). Hispanic patients had higher mean LAS (53.55 vs 47.73 vs 50.87; P < .001). They were more likely to require intensive care unit admission before surgery (15.3% vs 11.4% vs 12.2%; P < .001). Additionally, Hispanic patients were more likely to have a tracheostomy placed before transplant (5.7% vs 4.1% vs 2.8%; P = .004).
Table 1.
Donor and recipient demographics and secondary outcomes before the 2017 lung allocation policy change
| Variables | Hispanic (n = 1013) | Non-Hispanic White (n = 12,601) | Non-Hispanic Other (n = 1729) | P value |
|---|---|---|---|---|
| Recipient | ||||
| Age (y) | 53.10 ± 13.77 | 56.54 ± 13.33 | 54.40 ± 11.22 | <.001 |
| Male sex | 589 (58.1) | 7711 (61.2) | 874 (50.5) | <.001 |
| BMI | 25.91 ± 4.44 | 25.14 ± 4.60 | 25.65 ± 4.49 | <.001 |
| Dialysis before transplant | 11 (1.9) | 57 (0.8) | 6 (0.7) | .018 |
| Diabetes | 277 (27.3) | 2440 (19.4) | 395 (22.8) | <.001 |
| Cause of lung failure | <.001 | |||
| Obstructive | 81 (8.0) | 3835 (30.4) | 362 (20.9) | |
| Pulmonary vascular | 68 (6.7) | 416 (3.3) | 130 (7.5) | |
| Cystic fibrosis/infectious | 122 (12.1) | 2056 (16.3) | 145 (8.4) | |
| Restrictive/interstitial | 661 (65.3) | 5696 (45.2) | 1038 (60.0) | |
| Lung retransplant | 42 (4.2) | 372 (3.0) | 35 (2.0) | |
| Other | 38 (3.8) | 225 (1.8) | 19 (1.1) | |
| LAS score | 53.55 ± 18.64 | 47.73 ± 18.04 | 50.87 ± 18.77 | <.001 |
| ICU at time of transplant | 155 (15.3) | 1451 (11.5) | 211 (12.2) | .001 |
| On mechanical ventilation | 86 (8.5) | 881 (7.0) | 113 (6.5) | .137 |
| Pretransplant tracheostomy | 58 (5.7) | 511 (4.1) | 49 (2.8) | .004 |
| Karnofsky Index | <.001 | |||
| ≥80% | 35 (4.4) | 532 (5.6) | 69 (5.2) | |
| 50%-70% | 288 (36.6) | 3700 (39.2) | 437 (32.8) | |
| ≤40% | 464 (59.0) | 5215 (55.2) | 828 (62.1) | |
| Days on waitlist | 151.30 ± 301.58 | 171.53 ± 331.21 | 165.07 ± 300.48 | .138 |
| Ischemic time (h) | 4.98 ± 1.61 | 5.22 ± 1.80 | 5.20 ± 1.76 | <.001 |
| Donors | ||||
| Age (y) | 35.77 ± 14.11 | 34.78 ± 14.05 | 35.16 ± 13.76 | .064 |
| Male sex | 473 (46.7) | 7861 (62.4) | 909 (52.6) | <.001 |
| Race | <.001 | |||
| White | 331 (32.7) | 1839 (14.6) | 293 (16.9) | |
| Black | 464 (45.8) | 7954 (63.1) | 940 (54.4) | |
| Other | 218 (21.5) | 2808 (22.3) | 496 (28.7) | |
| BMI | 26.24 ± 5.61 | 26.21 ± 5.40 | 26.42 ± 5.74 | .330 |
| Diabetes | 75 (7.5) | 930 (7.4) | 120 (7.0) | .799 |
| Secondary outcomes | ||||
| Mechanical ventilation | <.001 | |||
| None | 33 (3.3) | 500 (4.0) | 53 (3.1) | |
| ≤48 h | 564 (55.7) | 7624 (60.5) | 827 (47.8) | |
| Between 48 h and 5 d | 171 (16.9) | 1972 (15.6) | 337 (19.5) | |
| >5 d | 223 (22.0) | 2240 (17.8) | 468 (27.1) | |
| Acute renal failure dialysis | 74 (7.3) | 744 (5.9) | 182 (10.5) | <.001 |
| Acute rejection | 76 (7.5) | 1008 (8.0) | 153 (8.8) | .379 |
| Length of stay (d) | 23.29 ± 27.26 | 24.90 ± 29.42 | 30.22 ± 36.32 | <.001 |
| ECMO at 72 h | 36 (7.0) | 298 (5.5) | 89 (10.4) | <.001 |
Values are presented as n (%) or mean ± SD. BMI, Body mass index; LAS, lung allocation score; ICU, intensive care unit; ECMO, extracorporeal membrane oxygenation.
During the post-LAPC era, out of 13,152 recipients, 75.1% (9873) were NHW, 11.6% (1522) were Hispanic, and 13.4% (1757) were non-Hispanic Other. Recipient and donor demographics as well as secondary outcomes for the post-LAPC era are included in Table 2. Similar to pre-LAPC, the study found that Hispanic patients tended to be younger (mean age, 55.10 vs 60.08 vs 55.43 years; P < .001) and had a higher prevalence of diabetes (28.3% vs 17.6% vs 23.7%; P < .001). Hispanic patients' LAS remained higher than their NHW counterparts (57.43 vs 48.11 vs 53.95; P < .001), as did intensive care unit admission before transplant (25.3% vs 13.2% vs 19.4%; P < .001) and tracheostomy placement (9.3% vs 3.6% vs 5.9%; P < .001). Additionally, a greater percentage of post-LAPC Hispanic patients required mechanical ventilation before transplant (10.1% vs 4.9% vs 6.9%; P < .001).
Table 2.
Donor and recipient demographics and secondary outcomes after 2017 lung allocation policy change
| Variables | Hispanic (n = 1522) | Non-Hispanic White (n = 9873) | Non-Hispanic Other (n = 1757) | P value |
|---|---|---|---|---|
| Recipient | ||||
| Age (y) | 55.10 ± 12.67 | 60.08 ± 11.48 | 55.43 ± 11.89 | <.001 |
| Male sex | 877 (57.6) | 6249 (63.3) | 890 (50.7) | <.001 |
| BMI | 26.16 ± 4.28 | 25.99 ± 4.38 | 26.02 ± 4.68 | .346 |
| Dialysis before transplant | 11 (0.72) | 41 (0.42) | 14 (0.80) | .466 |
| Diabetes | 431 (28.3) | 1736 (17.6) | 417 (23.7) | <.001 |
| Cause of lung failure | <.001 | |||
| Obstructive | 88 (5.8) | 2586 (26.2) | 272 (15.5) | |
| Pulmonary vascular | 100 (6.6) | 442 (4.5) | 154 (8.8) | |
| Cystic fibrosis/infectious | 221 (14.5) | 1080 (10.9) | 194 (11.0) | |
| Restrictive/interstitial | 971 (63.8) | 5218 (52.9) | 1038 (59.1) | |
| Lung retransplant | 43 (2.8) | 247 (2.5) | 33 (1.9) | |
| Other | 99 (6.5) | 300 (3.0) | 66 (3.8) | |
| LAS score | 57.43 ± 20.70 | 48.11 ± 18.14 | 53.95 ± 20.27 | <.001 |
| ICU at time of transplant | 385 (25.3) | 1300 (13.2) | 341 (19.4) | <.001 |
| On mechanical ventilation | 154 (10.1) | 488 (4.9) | 122 (6.9) | <.001 |
| Pretransplant tracheostomy | 141 (9.3) | 355 (3.6) | 104 (5.9) | <.001 |
| Karnofsky Index | <.001 | |||
| ≥80% | 48 (4.1) | 364 (5.0) | 37 (2.8) | |
| 50%-70% | 336 (28.8) | 2648 (36.3) | 375 (28.2) | |
| ≤40% | 783 (67.1) | 4292 (58.8) | 918 (69.0) | |
| Days on waitlist | 127.42 ± 253.72 | 112.06 ± 235.73 | 127.04 ± 218.46 | .006 |
| Ischemic time (h) | 5.68 ± 2.20 | 6.04 ± 2.53 | 6.11 ± 2.56 | <.001 |
| Donors | ||||
| Age (y) | 36.30 ± 14.07 | 35.81 ± 13.50 | 35.98 ± 13.49 | .408 |
| Male sex | 757 (49.7) | 6287 (63.7) | 931 (53.0) | <.001 |
| Race | <.001 | |||
| White | 455 (29.9) | 1562 (15.8) | 333 (19.0) | |
| Black | 755 (49.6) | 6158 (62.4) | 991 (56.4) | |
| Other | 312 (20.5) | 2153 (21.8) | 433 (24.6) | |
| Diabetes | 161 (10.8) | 848 (8.7) | 146 (8.4) | .022 |
| Secondary outcome | ||||
| Mechanical ventilation | <.001 | |||
| None | 37 (2.5) | 133 (1.3) | 25 (1.4) | |
| ≤48 h | 743 (49.2) | 5868 (59.5) | 788 (45.1) | |
| Between 48 h and 5 d | 288 (19.1) | 1613 (16.4) | 369 (21.1) | |
| >5 d | 424 (28.1) | 2189 (22.2) | 522 (31.6) | |
| Acute renal failure dialysis | 121 (8.0) | 838 (8.5) | 237 (13.6) | <.001 |
| Acute rejection | 95 (6.3) | 695 (7.0) | 130 (7.4) | .416 |
| Length of stay (d) | 29.97 ± 34.76 | 29.13 ± 34.59 | 34.33 ± 39.70 | <.001 |
| ECMO at 72 h | 176 (11.6) | 794 (8.1) | 264 (15.1) | <.001 |
BMI, Body mass index; LAS, lung allocation score; ICU, intensive care unit; ECMO, extracorporeal membrane oxygenation.
Figure 3 shows the 1-year Kaplan-Meier survival curve by ethnicity pre-LAPC (log-rank P = .260) and post-LAPC (log-rank P < .001). In multivariable Cox regression models, there was no association between recipient height or LAS and 1-year posttransplant mortality both pre- and post-LAPC, detailed in Tables E4 and E5. Additionally, there was no association between height or LAS and waitlist time in either era. Waitlist times decreased for all groups in the post-LAPC era. Adjusted HRs of 1-year mortality compared with Hispanic recipients were pre-LAPC: NHW HR, 1.18 (95% CI, 0.96-1.44; P = .107), Non-Hispanic Other HR, 1.25 (95% CI, 0.98-1.59; P = .071) and post-LAPC: NHW HR, 1.49 (95% CI, 1.21-1.83; P < .001), Non-Hispanic Other HR, 1.47 (95% CI, 1.16-1.85; P = .001). Secondary outcomes are seen in Tables 1 and 2.
Figure 3.
Posttransplant survival: Before and after the 2017 lung allocation policy change (95% CI).
Discussion
According to previous studies of the Scientific Registry of Transplant Recipients, self-identified Hispanic patients with end-stage lung disease have worse waitlist outcomes compared with other ethnic groups awaiting LT.1 They also undergo transplant with higher LAS on average and are more commonly in critical condition.4 In this nationwide retrospective cohort analysis, we found that the number of Hispanic LT recipients increased significantly during the 5-year period following the 2017 LAPC. Additionally, despite still having higher LAS compared with other ethnic groups, Hispanics had higher 1-year survival rates by univariable and multivariable analysis.
The many barriers preventing Hispanics from timely access to the health care system have been well documented.10 Recent US census data show that 17.7% of Hispanics do not have health insurance compared with overall average of 8.6%.11 Hispanic patients have been previously shown to have lower odds to be allocated lung allografts for transplant both in the pre- and post-LAS eras.12, 13, 14 A recent study has shown “adjusted wait list access to transplant was lower in non-white candidates” with Hispanic patients having a HR of 0.87 compared with NHW patients.15 This present study showed that the relative percent of Hispanic candidates listed for LT increased over the study period, demonstrating how access to LT has improved for Hispanics over the past decade. Additionally, this change in the wait list demographics could have contributed to the increase in the number of Hispanic transplant recipients in the years following the 2017 LAPC. Days on the waitlist decreased across all ethnic groups following the 2017 policy change. During the pre-LAPC era, there was no significant difference between days on the waitlist when comparing ethnic groups. However, there was a difference seen post-LAPC favoring NHW, showing that persistent disparities exist. There are multiple factors influencing waitlist time, and patients with higher LAS have higher risk for waitlist mortality.16 Additionally, although NHW patients had a greater decrease in waitlist mortality compared with Hispanic patients, it does not account for regional factors. Hispanic patients saw the most percentage increase in number of transplants in areas with high Hispanic populations, and they may encounter a bottleneck effect in these areas, meaning there may have been more competition for the regionally restricted limited resource of donor lungs. NHW remain the majority in most all regions of the country and would experience similar benefits of wider access to donors and thus improved waitlist times, especially in areas with low Hispanic populations. Overall, the results seen in this present analysis show that the 2017 LAPC and elimination of DSA aided in increasing the number Hispanics undergoing transplant, as seen in Figure 1. The OPTN Thoracic Transplantation Committee released a 6-month report following the removal of DSAs. The committee showed the mean match LAS at transplant increased following the 2017 policy change.17 It is possible that the overall increase in Hispanic LT seen postpolicy change is due to their higher average LAS.
The distribution of change seen in Figure 2 is similar to the overall percent distribution of Hispanics in the United States. Areas of the United States with larger Hispanic populations saw the largest percent increases in LT. However, the changes in transplant seen in each state do not completely align with the Hispanic population of that given state. For example, the state of New York saw a decrease in the percent of Hispanic LT despite a large Hispanic population. One possible explanation for this phenomenon is the COVID-19 pandemic, which took place in the post-LAPC era. Descriptive analysis of the UNOS registry from March 2020 through May 2020 showed a 10% decrease in LT volume nationally and a 50% decrease in median transplants in areas with high numbers of COVID-19 cases, such as large cities with high-population densities.18
Previous studies have found that NHW had improved functional capacity before LT compared with Hispanic patients, suggesting that Hispanic patients present with more advanced lung disease.15 Tables 1 and 2 further strengthen this finding by demonstrating that both pre- and post-LAPC, Hispanic patients had higher LAS compared with NHW. Additionally, Hispanic patients were more likely to have restrictive or interstitial lung disease, which has a lower median survival compared with other pathologies.19 Despite multiple poor prognostic factors seen in the Hispanic population both pre- and post-policy change, this analysis found that after the 2017 LAPC Hispanic patients had higher 1-year survival rates compared with other ethnic groups, shown in Figure 3. The finding of similar 1-year survival pre-LAPC despite similar patient demographics and illness severity could be explained by the low percentage of Hispanic recipients compared with NHW recipients in the pre-LAPC era. As the number of Hispanic transplant recipients increased, the survival difference becomes evident. These findings are consistent with another study that has analyzed LT outcomes between 2005 and 2019 and found that Hispanic recipients have higher 1- and 3-year survival.4 These results are also consistent with the well-documented Hispanic paradox seen in multiple areas of health outcomes.20, 21, 22, 23, 24, 25, 26
The Hispanic paradox is an epidemiological finding where Hispanics have improved health outcomes compared with other ethnic groups despite the presence of worse prognostic factors. For example, a 2010 study of the National Center for Health Statistics found Hispanics had a life expectancy 2 years longer than NHW, and another systematic review published in 2013 found a 17.5% lower risk of mortality in Hispanics compared with NHW.21,22 This finding has been replicated in an analysis of the National Surgical Quality Improvement Program database that found that compared with other ethnicities Hispanics had lower odds of 30-day postoperative mortality and major morbidity.23 A study of the National Cancer Database determined that Hispanics with non–small cell lung cancer have overall better survival across all stages of disease.24 Additionally, there are studies in both kidney and liver transplantation that have found Hispanics have improved survival at up to 5 years posttransplant.25,26 Given that Hispanic recipients had worse prognostic indicators but still had similar to improved survival compared with other ethnic groups, this analysis could represent another instance of the Hispanic paradox.
There have been multiple previous explanations of the Hispanic paradox. One such explanation, the salmon bias, states that Hispanic immigrants were not included in United States mortality numbers because immigrants often return to their country of origin near the end of their lives, and although a prior study has confirmed its existence, analysis of data from the Social Security Administration show that it is a small factor.27 The healthy immigrant bias proposes that immigrants who come to the United States are unusually fit seeing as they were able to traverse the many obstacles making immigration difficult, and similar to the salmon bias, it has been shown to be true but fails to fully explain the paradox.28 The Barrio effect provides further explanation, postulating that Hispanics in high-density Hispanic neighborhoods have better health outcomes despite worse socioeconomic status due to the support they receive from the individuals in their immediate surroundings.29 The cultural collectivism commonly seen in the Hispanic population could also prove to be an important aspect of their survival advantage.30 The latter 2 mechanisms mentioned here, the Barrio effect and cultural collectivism, may play a particularly important role in the differences seen in Hispanic LT recipients when compared with other ethnicities because low social support has been well documented as a potential risk factor for poor posttransplant outcomes.31,32
There were multiple limitations of this study. First, it is a retrospective analysis of a large-scale database that does not account for all factors that contribute to survival outcomes. The analysis is also unable to account for missing variables. One notable missing variable is the recipients’ panel reactive antibody. Unfortunately, a large portion of patients was missing this data point in the database used in this study. Another key outcome not included in this study is waitlist mortality, but the complexity of the analysis to address how waitlist mortality changed pre- and post-LAPC unfortunately relegates this analysis beyond the scope of this present study. Ideally, this analysis would be conducted by comparing the percent of Hispanic and NHW recipients with comparable LAS who are offered transplant but given the variables in the database this analysis was not possible. Although there may be a contribution of the overall demographics of the United States to our study findings, unfortunately we are unable to adjust for these changes due to the intervals of available census data. Nevertheless, this is the first study to our knowledge to examine the potential relationship between the 2017 LAPC and LT outcomes for Hispanic patients in the United States. Additionally, it should be noted as a limitation that the groups presented in this study do not address the specific background of each patient, and the term Hispanic as it is defined in the UNOS database is self-identified and encompasses a wide breadth of people with varying biological and genetic backgrounds. The authors of this present study seek to specifically assess the self-identified Hispanic population of the United States to better understand disparities affecting this minority group as a social determinant of health. These results should not be extrapolated outside of this group of self-identified Hispanics in the United States, and further they should be viewed with the knowledge that there are a myriad of socioeconomic factors influencing health outcomes in this group, the full analysis of which are beyond the scope of this present study. Of note, this study does not account for the more recent 2023 policy change and the implementation of the lung composite allocation score. However, the findings presented here remain relevant as they demonstrate the effects of the 2017 LAPC and show how elimination of DSA influenced LT recipient outcomes. This information is important as it can further inform future policy through understanding the incremental effects of changes made previously.
Conclusions
This study found that there was a significant increase in the number of Hispanic LT following the 2017 LAPC. Hispanic LT recipients also had improved survival compared with other ethnic groups following the policy change (Figure 4). Given these findings, it appears that the 2017 LAPC had an overall positive effect on Hispanic LT recipients, suggesting that the 2017 LAPC succeeded in its mission of increasing access to LT.
Figure 4.
Graphical Abstract.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://www.aats.org/resources/impact-of-lung-allocation-poli-6987.

Conflict of Interest Statement
Dr Arman Kilic is a speaker/consultant for Abbott, Abiomed, 3ive, and LivaNova. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
Institutional Review Board approval and Informed consent statement: This study was exempt from review and individual consent by the Institutional Review Board of the Medical University of South Carolina.
Appendix E1
Table E1.
Details of pre-2017 lung allocation policy multivariable analysis
| Variables | 1-y mortality (univariable) | 1-y mortality (multivariable) |
|---|---|---|
| Race/ethnicity | ||
| Hispanic | – | – |
| White | 1.11 (0.92-1.35) (P = .270) | 1.18 (0.96-1.44) (P = .107) |
| Other | 1.20 (0.96-1.51) (P = .108) | 1.25 (0.98-1.59) (P = .071) |
| Center volume | 1.00 (1.00-1.00) (P = .847) | – |
| Age (y) | 1.01 (1.01-1.02) (P < .001) | 1.02 (1.01-1.02) (P < .001) |
| Sex | ||
| Female | – | – |
| Male | 1.17 (1.06-1.28) (P = .001) | 1.11 (1.00-1.24) (P = .045) |
| BMI | 1.02 (1.01-1.03) (P < .001) | 1.01 (1.00-1.02) (P = .196) |
| Creatinine (mg/dL) | 1.13 (1.08-1.18) (P < .001) | 1.10 (1.03-1.17) (P = .004) |
| Total bilirubin (mg/dL) | 1.06 (1.04-1.08) (P < .001) | 1.05 (1.03-1.07) (P < .001) |
| Diabetes | ||
| No | – | – |
| Yes | 1.07 (0.96-1.19) (P = .225) | – |
| Lung failure etiology | ||
| Obstructive | – | – |
| Pulmonary vascular | 1.52 (1.22-1.89) (P < .001) | 1.51 (1.20-1.90) (P < .001) |
| CF/infectious | 0.86 (0.73-1.01) (P = .058) | 1.06 (0.88-1.28) (P = .563) |
| Restrictive/interstitial | 1.23 (1.10-1.37) (P < .001) | 1.02 (0.90-1.17) (P = .752) |
| Lung retransplant | 2.26 (1.82-2.79) (P < .001) | 2.13 (1.67-2.71) (P < .001) |
| Other | 1.23 (0.88-1.72) (P = .221) | 1.28 (0.91-1.81) (P = .152) |
| LAS score | ||
| Mean ± SD | 1.01 (1.01-1.01) (P < .001) | 1.00 (1.00-1.01) (P = .016) |
| ICU at time of transplant | ||
| No | – | – |
| Yes | 1.68 (1.49-1.89) (P < .001) | 1.25 (1.04-1.51) (P = .019) |
| Mechanical ventilation | ||
| No | – | – |
| Yes | 1.85 (1.61-2.13) (P < .001) | 1.40 (1.16-1.70) (P < .001) |
| Time on waitlist (d) | 1.00 (1.00-1.00) (P = .060) | 1.00 (1.00-1.00) (P = .649) |
| Lung ischemic time (h) | 1.04 (1.02-1.07) (P = .001) | 1.05 (1.02-1.07) (P < .001) |
| Donor age (y) | 1.01 (1.00-1.01) (P = .001) | 1.00 (1.00-1.01) (P = .012) |
| Donor sex | ||
| Female | - | - |
| Male | 0.92 (0.84-1.01) (P = .068) | 0.91 (0.82-1.01) (P = .083) |
| Donor race | ||
| Hispanic | – | – |
| White | 0.89 (0.78-1.00) (P = .058) | 0.89 (0.74-1.07) (P = .199) |
| Other | 1.10 (0.96-1.27) (P = .169) | 1.10 (0.95-1.27) (P = .216) |
| Donor BMI | ||
| Mean (SD) | 0.99 (0.98-1.00) (P = .071) | 0.99 (0.98-0.99) (P = .001) |
| Donor diabetes | ||
| No | – | - |
| Yes | 1.29 (1.11-1.51) (P = .001) | 1.21 (1.02-1.42) (P = .025) |
| Sex-matched | ||
| No | – | – |
| Yes | 0.93 (0.85-1.03) (P = .154) | 0.96 (0.87-1.06) (P = .428) |
| Race-matched | ||
| No | – | – |
| Yes | 0.84 (0.76-0.92) (P < .001) | 0.96 (0.80-1.15) (P = .653) |
| HLA-matched | ||
| No | – | – |
| Yes | 0.94 (0.82-1.07) (P = .325) | – |
| ABO-identical | ||
| No | – | – |
| Yes | 0.83 (0.70-0.98) (P = .026) | 0.82 (0.69-0.97) (P = .019) |
| CMV-matched | ||
| No | – | – |
| Yes | 1.05 (0.96-1.15) (P = .286) | – |
Values are presented as hazard ratio (95% Confidence Interval) unless otherwise noted. BMI, Body mass index; CF, cystic fibrosis; LAS, lung allocation score; ICU, intensive care unit; HLA, human leukocyte antigen; ABO, Blood Grouping System; CMV, cytomegalovirus.
Table E2.
Details of post-2017 lung allocation policy multivariable analysis interaction terms of height and lung allocation score
| Variables | 1-y mortality (univariable) | 1-y mortality (multivariable) |
|---|---|---|
| Race/ethnicity | ||
| Hispanic | – | – |
| White | 1.25 (1.04-1.51) (P = .017) | 1.49 (1.21-1.83) (P < .001) |
| Other | 1.51 (1.21-1.88) (P < .001) | 1.47 (1.16-1.85) (P = .001) |
| Center volume | 1.00 (1.00-1.00) (P < .001) | 1.00 (0.99-1.00) (P < .001) |
| Age (y) | 1.01 (1.00-1.01) (P < .001) | 1.02 (1.01-1.02) (P < .001) |
| Sex | ||
| Female | – | – |
| Male | 0.93 (0.84-1.04) (P = .184) | 0.82 (0.72-0.93) (P = .001) |
| BMI | 1.02 (1.01-1.03) (P < .001) | 1.02 (1.00-1.03) (P = .021) |
| Creatinine (mg/dL) | 1.33 (1.23-1.44) (P < .001) | 1.29 (1.18-1.40) (P < .001) |
| Total bilirubin (mg/dL) | 1.09 (1.07-1.11) (P < .001) | 1.08 (1.06-1.10) (P < .001) |
| Diabetes | ||
| No | – | – |
| Yes | 1.22 (1.07-1.38) (P = .002) | 1.15 (1.01-1.32) (P = .032) |
| Lung failure etiology | ||
| Obstructive | – | – |
| Pulmonary vascular | 1.47 (1.15-1.89) (P = .002) | 1.36 (1.05-1.76) (P = .022) |
| CF/infectious | 1.14 (0.93-1.40) (P = .201) | 1.13 (0.90-1.42) (P = .280) |
| Restrictive/interstitial | 1.37 (1.19-1.58) (P < .001) | 1.18 (1.01-1.39) (P = .041) |
| Lung retransplant | 2.05 (1.52-2.76) (P < .001) | 2.08 (1.51-2.87) (P < .001) |
| Other | 1.52 (1.15-2.03) (P = .004) | 1.40 (1.03-1.91) (P = .031) |
| LAS score | ||
| Mean ± SD | 1.01 (1.01-1.01) (P < .001) | 1.01 (1.00-1.01) (P = .002) |
| ICU at time of transplant | ||
| No | – | – |
| Yes | 1.54 (1.36-1.76) (P < .001) | 1.24 (1.01-1.52) (P = .039) |
| Mechanical ventilation | ||
| No | – | – |
| Yes | 1.44 (1.18-1.74) (P < .001) | 0.98 (0.77-1.24) (P = .871) |
| Time on waitlist (d) | 1.00 (1.00-1.00) (P = .669) | – |
| Lung ischemic time (h) | 1.06 (1.04-1.08) (P < .001) | 1.07 (1.05-1.09) (P < .001) |
| Donor age (y) | 1.01 (1.00-1.01) (P < .001) | 1.01 (1.00-1.01) (P < .001) |
| Donor sex | ||
| Female | – | – |
| Male | 0.87 (0.78-0.97) (P = .011) | 0.95 (0.84-1.07) (P = .384) |
| Donor race | ||
| Hispanic | – | – |
| White | 1.04 (0.90-1.21) (P = .561) | 1.13 (0.93-1.37) (P = .226) |
| Other | 1.31 (1.11-1.55) (P = .001) | 1.20 (1.01-1.42) (P = .040) |
| Donor BMI | ||
| Mean ± SD | 0.99 (0.98-1.00) (P = .022) | 0.98 (0.97-0.99) (P < .001) |
| Donor diabetes | ||
| No | – | – |
| Yes | 1.23 (1.04-1.47) (P = .016) | 1.15 (0.96-1.38) (P = .130) |
| Sex-matched | ||
| No | – | – |
| Yes | 0.98 (0.87-1.10) (P = .685) | – |
| Race-matched | ||
| No | – | – |
| Yes | 0.82 (0.74-0.91) (P < .001) | 0.81 (0.67-0.99) (P = .037) |
| HLA-matched | ||
| No | – | – |
| Yes | 0.91 (0.78-1.07) (P = .265) | – |
| ABO-identical | ||
| No | – | – |
| Yes | 1.01 (0.82-1.24) (P = .957) | – |
| CMV-matched | ||
| No | – | – |
| Yes | 0.99 (0.89-1.11) (P = .898) | – |
Values are presented as hazard ratio (95% Confidence Interval) unless otherwise noted. BMI, Body mass index; CF, cystic fibrosis; LAS, lung allocation score; ICU, intensive care unit; HLA, human leukocyte antigen; ABO, Blood Grouping System; CMV, cytomegalovirus.
Table E3.
Percentage of Hispanic-organ donors 2010 to 2023
| Year | Hispanic donors all solid organs (%) | Hispanic lung donors (%) |
|---|---|---|
| 2010 | 13.85 | 17.32 |
| 2011 | 14.42 | 16.8 |
| 2012 | 13.64 | 14.4 |
| 2013 | 14.22 | 15.82 |
| 2014 | 14.32 | 16.28 |
| 2015 | 14.47 | 16.5 |
| 2016 | 13.97 | 15.21 |
| 2017 | 14.82 | 16.48 |
| 2018 | 14.8 | 15.93 |
| 2019 | 15.29 | 16.26 |
| 2020 | 14.87 | 18.11 |
| 2021 | 15.03 | 19.46 |
| 2022 | 14.85 | 17.6 |
| 2023 | 13.82 | 18.69 |
Table E4.
One-year posttransplant mortality pre-2017 lung allocation policy change multivariable Cox Regression with interaction terms of height and lung allocation score
| Variables | Hazard ratio | SE | z | P>z | 95% CI |
|
|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||
| Height | 1.000219 | 0.0071329 | 0.03 | .976 | 0.9863359 | 1.014297 |
| LAS | 0.9944152 | 0.0201031 | −0.28 | .782 | 0.9557842 | 1.034608 |
| Height and LAS interaction term | 1.000056 | 0.0001179 | 0.48 | .635 | 0.999825 | 1.000287 |
| Race, Hispanic as reference | ||||||
| White | 1.195926 | 0.1271013 | 1.68 | .092 | 0.9710464 | 1.472885 |
| Other | 1.243666 | 0.1559274 | 1.74 | .082 | 0.9727083 | 1.590103 |
| Age | 1.018139 | 0.0025106 | 7.29 | .000 | 1.01323 | 1.023072 |
| Male sex | 1.067264 | 0.0745227 | 0.93 | .351 | 0.9307564 | 1.223793 |
| BMI | 1.006555 | 0.0058144 | 1.13 | .258 | 0.9952231 | 1.018016 |
| Creatinine | 1.097001 | 0.0358171 | 2.84 | .005 | 1.029 | 1.169496 |
| Bilirubin | 1.05018 | 0.0117874 | 4.36 | .000 | 1.02733 | 1.073539 |
| Lung failure etiology | ||||||
| Obstructive | 1.538459 | 0.1806525 | 3.67 | .000 | 1.222177 | 1.93659 |
| CF/infectious | 1.064179 | 0.1031539 | 0.64 | .521 | 0.8800454 | 1.286838 |
| Restrictive/interstitial | 1.03031 | 0.0699767 | 0.44 | .660 | 0.9018952 | 1.17701 |
| Lung retransplant | 2.068848 | 0.2586848 | 5.81 | .000 | 1.619183 | 2.64339 |
| Other | 1.239322 | 0.2223915 | 1.2 | .232 | 0.8718453 | 1.761688 |
| ICU at transplant | 1.250321 | 0.1203596 | 2.32 | .020 | 1.035339 | 1.509944 |
| Mechanical ventilation | 1.419904 | 0.1386134 | 3.59 | .000 | 1.172636 | 1.719312 |
| Time on waitlist | 0.9999982 | 0.0000787 | −0.02 | .982 | 0.999844 | 1.000152 |
| Lung ischemic time | 1.048523 | 0.0135443 | 3.67 | .000 | 1.02231 | 1.075408 |
| Donor age | 1.004113 | 0.0017808 | 2.31 | .021 | 1.000629 | 1.007609 |
| Donor male sex | 0.9012594 | 0.0499326 | −1.88 | .061 | 0.8085197 | 1.004637 |
| Donor race (Hispanic as reference) | ||||||
| White | 0.899057 | 0.0863185 | −1.11 | .268 | 0.7448406 | 1.085203 |
| Other | 1.087023 | 0.08051 | 1.13 | .260 | 0.9401446 | 1.256848 |
| Donor BMI | 0.985692 | 0.0044881 | −3.17 | .002 | 0.9769346 | 0.9945278 |
| Donor diabetes | 1.192757 | 0.1011569 | 2.08 | .038 | 1.010095 | 1.408451 |
| Sex-matched | 0.9555358 | 0.0487922 | −0.89 | .373 | 0.8645346 | 1.056116 |
| Race-matched | 0.9277557 | 0.08891 | −0.78 | .434 | 0.7688827 | 1.119456 |
| ABO-identical | 0.8302194 | 0.0716435 | −2.16 | .031 | 0.7010335 | 0.9832116 |
LAS, Lung allocation score; BMI, body mass index; CF, cystic fibrosis; ICU, intensive care unit; ABO, Blood Grouping System.
Table E5.
One-year posttransplant mortality after the 2017 lung allocation policy change multivariable Cox regression with interaction terms of height and lung allocation score
| Variables | Hazard ratio | SE | z | P>z | 95% CI |
|
|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||
| Height | 0.9925084 | 0.008208 | −0.91 | .363 | 0.9765506 | 1.008727 |
| LAS | 1.021652 | 0.0231537 | 0.95 | .345 | 0.9772648 | 1.068056 |
| Height and LAS interaction term | 0.9999119 | 0.0001333 | −0.66 | .509 | 0.9996505 | 1.000173 |
| Race (Hispanic as reference) | ||||||
| White | 1.590798 | 0.1706145 | 4.33 | .000 | 1.289208 | 1.962941 |
| Other | 1.548331 | 0.1851228 | 3.66 | .000 | 1.224875 | 1.957203 |
| Center volume | 0.9963468 | 0.0008082 | −4.51 | .000 | 0.9947641 | 0.997932 |
| Age | 1.017143 | 0.0029497 | 5.86 | .000 | 1.011378 | 1.022941 |
| Male sex | 0.9493163 | 0.0764396 | −0.65 | .518 | 0.8107213 | 1.111605 |
| BMI | 1.016189 | 0.006614 | 2.47 | .014 | 1.003309 | 1.029236 |
| Creatinine | 1.300347 | 0.0570466 | 5.99 | .000 | 1.19321 | 1.417104 |
| Bilirubin | 1.076732 | 0.0105225 | 7.57 | .000 | 1.056304 | 1.097554 |
| Diabetes | 1.14674 | 0.0770357 | 2.04 | .042 | 1.005271 | 1.308118 |
| Lung failure etiology | ||||||
| Obstructive | 1.371707 | 0.1828651 | 2.37 | .018 | 1.056297 | 1.781299 |
| CF/infectious | 1.132718 | 0.1303952 | 1.08 | .279 | 0.903928 | 1.419416 |
| Restrictive/interstitial | 1.190032 | 0.0981056 | 2.11 | .035 | 1.012479 | 1.398721 |
| Lung retransplant | 2.101508 | 0.3436763 | 4.54 | .000 | 1.525202 | 2.895575 |
| Other | 1.436204 | 0.2251475 | 2.31 | .021 | 1.056275 | 1.952789 |
| ICU at transplant | 1.237241 | 0.1289255 | 2.04 | .041 | 1.008685 | 1.517584 |
| Mechanical ventilation | 0.9697121 | 0.1184022 | −0.25 | .801 | 0.7633272 | 1.231898 |
| Lung ischemic time | 1.066193 | 0.0101595 | 6.73 | .000 | 1.046466 | 1.086293 |
| Donor age | 1.00838 | 0.002101 | 4.01 | .000 | 1.00427 | 1.012506 |
| Donor male sex | 0.9830393 | 0.0627241 | −0.27 | .789 | 0.8674789 | 1.113994 |
| Donor race, Hispanic as reference | ||||||
| White | 1.16496 | 0.1164707 | 1.53 | .127 | 0.9576552 | 1.417139 |
| Other | 1.232002 | 0.1085664 | 2.37 | .018 | 1.036578 | 1.464269 |
| Donor BMI | 0.9809876 | 0.0049598 | −3.8 | .000 | 0.9713147 | 0.9907569 |
| Donor diabetes | 1.160692 | 0.1074784 | 1.61 | .108 | 0.9680486 | 1.391673 |
| Race-matched | 0.8086317 | 0.080767 | −2.13 | .033 | 0.6648625 | 0.9834894 |
LAS, Lung allocation score; BMI, body mass index; CF, cystic fibrosis; ICU, intensive care unit.
References
- 1.Florissi I., Chidi A.P., Liu Y., et al. Racial disparities in waiting list outcomes of patients listed for lung transplantation. Ann Thorac Surg. 2024;117(3):619–626. doi: 10.1016/j.athoracsur.2023.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swaminathan A.C., Hellkamp A.S., Neely M.L., et al. Disparities in lung transplant among patients with idiopathic pulmonary fibrosis: an analysis of the IPF-PRO Registry. Ann Am Thorac Soc. 2022;19(6):981–990. doi: 10.1513/AnnalsATS.202105-589OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eguia E., Cobb A.N., Kirshenbaum E.J., Afshar M., Kuo P.C. Racial and ethnic postoperative outcomes after surgery: the Hispanic Paradox. J Surg Res. 2018;232:88–93. doi: 10.1016/j.jss.2018.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bremer M.N., Gama G.J., Rao Omkar, et al. Robust outcomes for Hispanic lung transplant recipients in the United States. OBM Transplantation. 2023;7(2):180. doi: 10.21926/obm.transplant.2302180. [DOI] [Google Scholar]
- 5.Benvenuto L.J., Anderson D.R., Kim H.P., et al. Geographic disparities in donor lung supply and lung transplant waitlist outcomes: a cohort study. Am J Transplant. 2018;18(6):1471–1480. doi: 10.1111/ajt.14630. [DOI] [PubMed] [Google Scholar]
- 6.Kosztowski M., Zhou S., Bush E., et al. Geographic disparities in lung transplant rates. Am J Transplant. 2019;19(5):1491–1497. doi: 10.1111/ajt.15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benvenuto L.J., Arcasoy S.M. The new allocation era and policy. J Thorac Dis. 2021;13(11):6504–6513. doi: 10.21037/jtd-2021-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin M., Iyengar A., Helmers M.R., et al. Modern outcomes of heart-lung transplantation: assessing the impact of the updated US allocation system. Eur J Cardiothorac Surg. 2022;63(1) doi: 10.1093/ejcts/ezac559. [DOI] [PubMed] [Google Scholar]
- 9.Puri V., Hachem R.R., Frye C.C., et al. Unintended consequences of changes to lung allocation policy. Am J Transplant. 2019;19(8):2164–2167. doi: 10.1111/ajt.15307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escarce JJ, Kapur K. Access to and quality of health care. In: National Research Council (US) Panel on Hispanics in the United States; Tienda M, Mitchell F, editors. Hispanics and the Future of America. 2006. https://www.ncbi.nlm.nih.gov/books/NBK19910/
- 11.Branch B., Conway D. Health insurance coverage by race and Hispanic origin: 2021. 2022. https://www.census.gov/library/publications/2022/acs/acsbr-012.html
- 12.Lederer D.J., Caplan-Shaw C.E., O'Shea M.K., et al. Racial and ethnic disparities in survival in lung transplant candidates with idiopathic pulmonary fibrosis. Am J Transplant. 2006;6(2):398–403. doi: 10.1111/j.1600-6143.2005.01205.x. [DOI] [PubMed] [Google Scholar]
- 13.Lederer D.J., Benn E.K., Barr R.G., et al. Racial differences in waiting list outcomes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177(4):450–454. doi: 10.1164/rccm.200708-1260OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riley L.E., Lascano J. Gender and racial disparities in lung transplantation in the United States. J Heart Lung Transplant. 2021;40(9):963–969. doi: 10.1016/j.healun.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Mooney J.J., Hedlin H., Mohabir P., et al. Racial and ethnic disparities in lung transplant listing and waitlist outcomes. J Heart Lung Transplant. 2018;37(3):394–400. doi: 10.1016/j.healun.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirji A., Zhao H., Ospina M.B., et al. Clinical judgment versus lung allocation score in predicting lung transplant waitlist mortality. Clin Transplant. 2020;34(7) doi: 10.1111/ctr.13870. [DOI] [PubMed] [Google Scholar]
- 17.Committee O.T. Monitoring of the lung allocation change, 6-month report removal of DSA as a unit of allocation. 2018. https://optn.transplant.hrsa.gov/media/2517/20180621_thoracic_committee_report_lung.pdf
- 18.Benvenuto L., Snyder M.E., Aversa M., et al. Geographic differences in lung transplant volume and donor availability during the COVID-19 pandemic. Transplantation. 2021;105(4):861–866. doi: 10.1097/TP.0000000000003600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thabut G., Mal H. Outcomes after lung transplantation. J Thorac Dis. 2017;9(8):2684–2691. doi: 10.21037/jtd.2017.07.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markides K.S., Coreil J. The health of Hispanics in the southwestern United States: an epidemiologic paradox. Public Health Rep. 1986;101(3):253–265. [PMC free article] [PubMed] [Google Scholar]
- 21.Ruiz J.M., Steffen P., Smith T.B. Hispanic mortality paradox: a systematic review and meta-analysis of the longitudinal literature. Am J Public Health. 2013;103(3):e52–e60. doi: 10.2105/AJPH.2012.301103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arias E. United States life tables by Hispanic origin. Vital Health Stat 2. 2010;2(152):1–33. [PubMed] [Google Scholar]
- 23.Betancourt-Garcia M.M., Vatcheva K., Gupta P.K., et al. The effect of Hispanic ethnicity on surgical outcomes: an analysis of the NSQIP database. Am J Surg. 2019;217(4):618–633. doi: 10.1016/j.amjsurg.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Kumar R., Castillero F., Bhandari S., et al. The Hispanic paradox in non-small cell lung cancer. Hematol Oncol Stem Cell Ther. 2022;15(2):21–29. doi: 10.1016/j.hemonc.2021.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Gordon E.J., Caicedo J.C. Ethnic advantages in kidney transplant outcomes: the Hispanic paradox at work? Nephrol Dial Transplant. 2009;24(4):1103–1109. doi: 10.1093/ndt/gfn691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Althoff A.L., Ali M.S., O'Sullivan D.M., et al. Short- and long-term outcomes for ethnic minorities in the United States after liver transplantation: parsing the Hispanic paradox. Transplant Proc. 2022;54(8):2263–2269. doi: 10.1016/j.transproceed.2022.08.038. [DOI] [PubMed] [Google Scholar]
- 27.Turra C.M., Elo I.T. The impact of salmon bias on the Hispanic mortality advantage: new evidence from social security data. Popul Res Policy Rev. 2008;27(5):515–530. doi: 10.1007/s11113-008-9087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez J., García-Pérez M., Orozco-Aleman S. Unraveling the hispanic health paradox. J Econ Perspect. 2023;37(1):145–168. doi: 10.1257/jep.37.1.145. [DOI] [Google Scholar]
- 29.Eschbach K., Ostir G.V., Patel K.V., Markides K.S., Goodwin J.S. Neighborhood context and mortality among older Mexican Americans: is there a barrio advantage? Am J Public Health. 2004;94(10):1807–1812. doi: 10.2105/ajph.94.10.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campos B., Kim H.S. Incorporating the cultural diversity of family and close relationships into the study of health. Am Psychol. 2017;72(6):543–554. doi: 10.1037/amp0000122. [DOI] [PubMed] [Google Scholar]
- 31.Maldonado J.R. Why it is important to consider social support when assessing organ transplant candidates? Am J Bioeth. 2019;19(11):1–8. doi: 10.1080/15265161.2019.1671689. [DOI] [PubMed] [Google Scholar]
- 32.Smith P.J., Snyder L.D., Palmer S.M., et al. Depression, social support, and clinical outcomes following lung transplantation: a single-center cohort study. Transpl Int. 2018;31(5):495–502. doi: 10.1111/tri.13094. [DOI] [PubMed] [Google Scholar]