Abstract
Objective
For neonatal repair of coarctation of the aorta, patients may either undergo thoracotomy with extended end-to-end anastomosis or sternotomy for aortic arch reconstruction with cardiopulmonary bypass. The objective of this study was to evaluate the comparative effectiveness of the 2 approaches in patients with arch hypoplasia.
Methods
This is a single-center retrospective cohort study from July 2005 through May 2022 of patients who underwent neonatal repair for isolated coarctation of the aorta with additional arch hypoplasia. Inverse probability of treatment weighting is a statistical method for creating comparable pseudopopulations and was used to account for baseline differences in population. The primary outcome was aortic reintervention, and secondary outcomes were vocal cord dysfunction, length of stay, chylothorax, and phrenic nerve palsy.
Results
There were 130 patients who met inclusion criteria. After weighting, the interaction between distal transverse arch size and operative approach (sternotomy vs thoracotomy) was statistically significant, P < .05 for interaction. Among patients with a distal arch z-score <−3.5, patients undergoing thoracotomy with extended end-to-end anastomosis had an increased hazard for reintervention. Sternotomy was associated with an increased length of stay in the intensive care unit by 4.7 days, P < .001, and odds of vocal cord dysfunction were also greater, odds ratio 7.1 (95% confidence interval, 1.66 to 41.26; P = .01).
Conclusions
Among patients with a distal arch z-score smaller than −3.5, the hazard of reintervention was increased for patients undergoing thoracotomy with extended end-to-end anastomosis. However, length of stay and risk of vocal cord paresis was reduced in patients undergoing thoracotomy.
Key Words: neonatal arch, aortic arch reconstruction, coarctation of the aorta, aortic arch hypoplasia
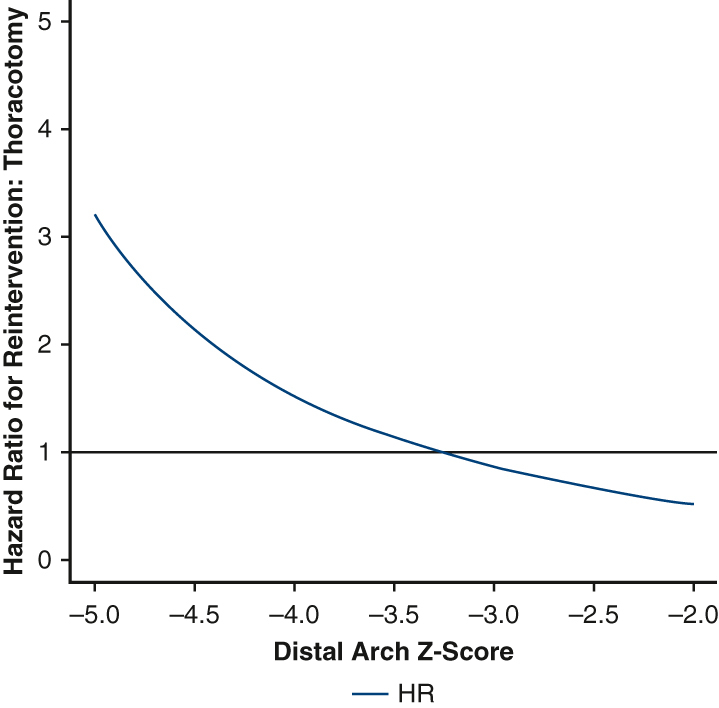
The hazard ratio for reintervention depended on distal arch z-score.
Central Message.
Patients with coarctation of the aorta and aortic arch hypoplasia with a distal arch z-score smaller than −3.5 may benefit from sternotomy over thoracotomy.
Perspective.
Repair of coarctation of the aorta with arch hypoplasia via sternotomy was associated with a reduced risk for reintervention for patients with a distal arch z-score <−3.5, but odds of recurrent laryngeal nerve injury and ICU length of stay were greater. Deciding between the sternotomy and thoracotomy should be an individualized decision after considering the relative risks of each approach.
Coarctation of the aorta accounts for 6.6% of patients with congenital heart disease.1 In many patients, the diseased segment of aorta is limited to the aortic isthmus; in others, the aortic arch may also be hypoplastic proximally. The surgical approach to repair of this lesion depends on the anticipated extent of reconstruction in order to achieve normal geometry without a residual gradient.2,3 Among patients with residual arch obstruction, potential late sequelae include the need for reintervention, hypertension, and stroke.4, 5, 6, 7, 8
An analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database has demonstrated no difference in the risk of in-hospital mortality for patients undergoing isolated repair of aortic coarctation or hypoplastic aortic arch without cardiopulmonary bypass versus patients undergoing isolated repair of aortic coarctation or hypoplastic aortic arch with cardiopulmonary bypass.9 Previous reports have suggested criteria for discriminating between patients who should undergo one approach or the other. These criteria include a distal aortic arch less than 50% the size of the ascending aorta, distal arch diameter (in millimeters) less than bodyweight in kilograms + 1, and multiple different thresholds for proximal aortic arch size.2,3,10, 11, 12, 13 However, the strength of the evidence supporting these arbitrary thresholds is limited. Moreover, the relative morbidity of the 2 approaches has not been well quantified. The recently published “The Society of Thoracic Surgeons Clinical Practice Guidelines on the Management of Neonates and Infants With Coarctation”14 offered a comprehensive review of the existing evidence. Notably, the document stressed that the existing literature was limited by significant selection bias and an opportunity existed to better elucidate variables that might favor the sternotomy approach. We undertook the current study to compare the effectiveness of thoracotomy with extended end-to-end anastomosis to sternotomy for aortic arch reconstruction in the management of neonatal patients with coarctation of the aorta and aortic arch hypoplasia.
Methods
This is a single-center retrospective cohort study of patients who underwent isolated repair of coarctation of the aorta in the neonatal period between July 2005 and May 2022 at Boston Children’s Hospital. After approval from the institutional review board at Boston Children’s Hospital (IRB-P00028876, initial approval May 2018; informed consent waived), a query of patients undergoing aortic arch repair was performed using a departmental database. Patients older than 28 days were excluded from the study in order to limit the population to newborn patients. Moreover, patients undergoing concomitant congenital heart operations other than atrial septal defect closure were excluded. Patients with genetic syndromes, heterotaxy, and right aortic arch were also excluded from the study. Data were abstracted from the medical record.
Outcomes
The primary outcome of interest was aortic reintervention, either surgical or catheter-based. Reintervention was assessed as time-to-event data using Cox proportional hazards regression, and the first reintervention (either open or catheter-based) was used as the end point. Competing risks methods were not used because of the limited observed mortality. Secondary outcomes of interest included survival (both perioperative and in follow-up), postoperative intensive care unit (ICU) length of stay, total postoperative hospital length of stay, vocal cord dysfunction (defined as vocal cord immobility on postoperative direct laryngoscopy), chyle leak confirmed by pleural triglyceride evaluation, and diaphragm paresis requiring plication. Follow-up was determined by review of the medical record.
Variable of Interest
In an effort to account for differences in body size, z-scores were used. The z-score is the number of standard deviations above or below the mean size of the structure for a given infant’s body surface area, ie, a patient with a distal arch z-score of −2 is 2 standard deviations below the mean with respect to distal arch size. Normative values and z-score calculation were established with the methodology described by Sluysman and Colan15 using an online calculator (zscore.chboston.org).
Distal arch (which was defined as the aortic arch between the left common carotid and left subclavian artery) z-score was the primary variable of interest on the basis of literature review. This was confirmed using a machine-learning technique, described to follow, to identify anatomic and demographic variables that predicted whether a patient would undergo sternotomy or thoracotomy. In this machine-learning technique, risk factor variables were selected by creating 1000 bootstrap replicates, and forward selection using logistic regression was performed to predict whether a patient underwent sternotomy in each replicate. Variables that were selected in >80% of the replicates, that had consistent coefficient sign in >80% of the models, and that were statistically significant in >80% of the models were selected for the final model.16 In the complete cohort of patients (before trimming), the only observed variable that was a consistent predictor of sternotomy was distal arch z-score. Male sex, weight, bovine arch, aberrant subclavian artery, prematurity, aortic annular diameter, ascending aortic diameter, mitral annular dimension, and body surface area did not meet criteria for inclusion. In the subset of patients who were eligible for both operations (after trimming), distal arch z-score remained the only predictor.
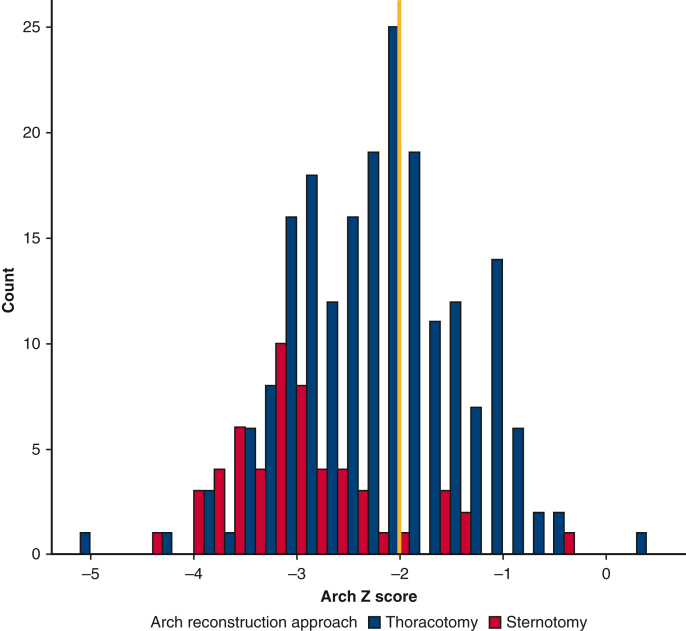
At our facility, no institutional policy or consensus has existed regarding the surgical approach. In addition, there were 13 different surgeons who performed neonatal repair of coarctation of the aorta during the time period under study. Given substantial practice variation, this created a broad region of overlap between the 2 approaches at smaller distal arch z-scores, Figure E1.
Figure E1.
Overall cohort, before trimming. In blue, patients undergoing thoracotomy with extended end-to-end anastomosis; in red, patients undergoing sternotomy with aortic arch reconstruction. The yellow line marks a distal arch z-score of −2. Above a distal arch z-score of −2, patients do not appear to routinely undergo sternotomy for aortic arch reconstruction. Below a distal arch z-score of −2, there appears to be common support.
Statistical Methods
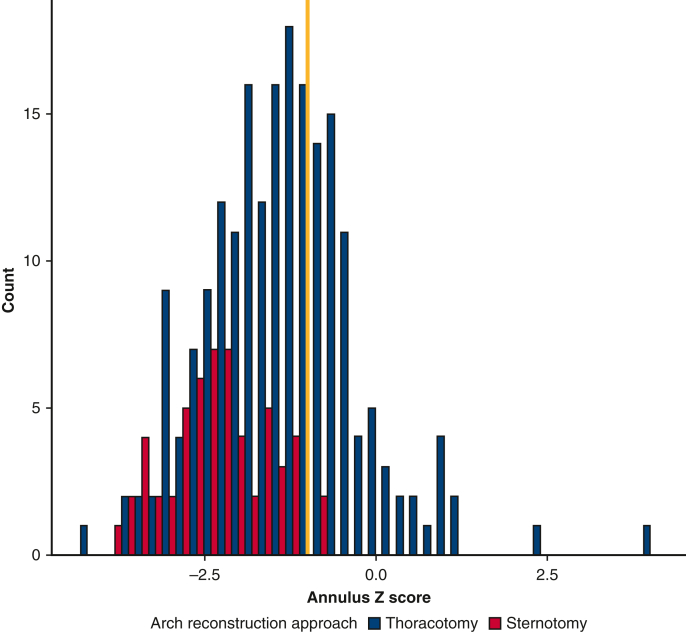
Because of a lack of exchangeability, patients with a distal arch z-score larger than −2 and those with an aortic annular z-score larger than −1 were excluded from the study in a process known as trimming (Figures E1 and E2).17 This process is critical for causal inference in that comparisons should be made between patients who are eligible for both operations.18,19 Among patients with a distal arch z-score larger than −2, only 7.6% of patients underwent sternotomy as opposed to 29.4% of patients with a distal arch z-score smaller than −2.
Figure E2.
Overall cohort, before trimming. In blue, patients undergoing thoracotomy with extended end-to-end anastomosis; in red, patients undergoing sternotomy with aortic arch reconstruction. The yellow line marks an aortic annular z-score of −1. Above an aortic annulus of z-score of −1, patients do not appear to routinely undergo sternotomy for aortic arch reconstruction. Below an aortic annular z-score of −1, there appears to be common support.
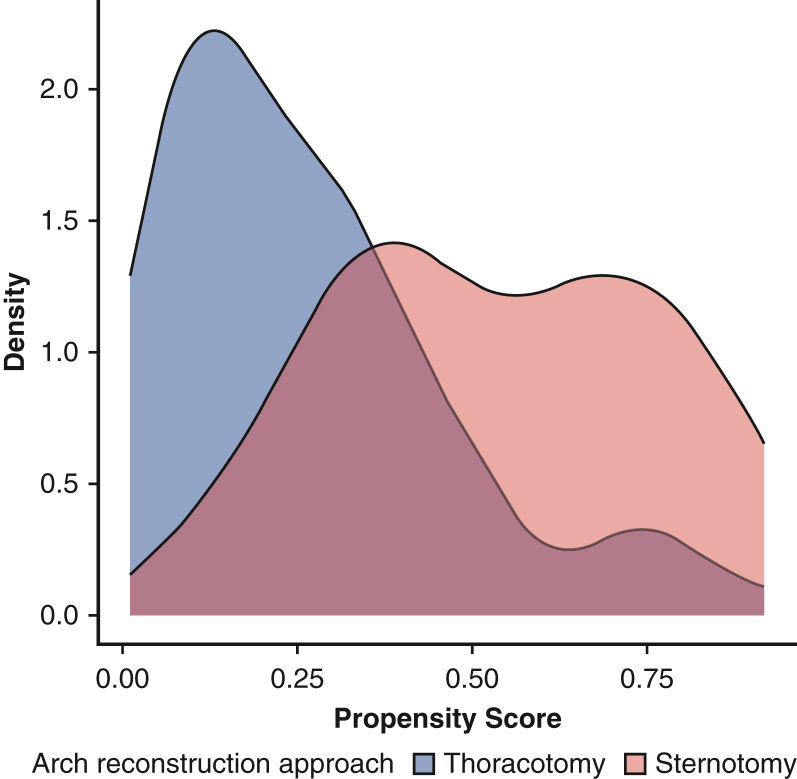
Logistic regression was used to estimate the probability of treatment assignment to either sternotomy or thoracotomy. A density plot of the propensity scores demonstrated adequate common support (Figure E3). Stabilized weights were estimated, and inverse probability of treatment weighting was performed in order to create comparable pseudopopulations that were balanced among both demographic and anatomic covariates, similar to the effect of propensity score matching.20 A standardized mean difference of >0.200 was considered to be statistically significant. Either linear or logistic regression was then used on the weighted pseudopopulations to assess the outcome of interest. Freedom from reintervention was estimated using the Kaplan-Meier method with weights. The interaction between distal arch z-score and surgical approach was evaluated using weighted Cox regression, and a plot of the distal arch size-dependent hazard was created. As a sensitivity analysis, weights were truncated at the 1st and 99th percentile in order to assess the effect of extreme weights (Figure E4).19
Figure E3.
A density plot of the 2 approaches demonstrating adequate common support.
Figure E4.
As a sensitivity analysis, extreme weights (1st and 99th percentile) were eliminated from the dataset. In doing so, stabilized weights smaller than 0.4 and greater than 3.69 were eliminated. The interaction between distal arch size and operative approach was once again tested. The interaction between surgical approach and distal arch size was once again statistically significant, P = .02. HR, Hazard ratio.
To assess the strength of existing criteria for distinguishing operative approach, we examined the overall cohort of patients who had undergone thoracotomy for extended end-to-end anastomosis, ie, including patients with a distal arch z-score larger than −2. Cox regression was used to evaluate whether the cutoff of body weight in millimeters +1 and distal arch diameter <50% of the ascending aorta diameter were risk factors for late reintervention.2,3,10,21,22 In order to assess risk factors for reintervention, 1000 bootstrap replicates were created, and risk factors were selected using forward selection on a Cox regression model. As stated previously, only variables that were selected in >80% of replicates, had consistent coefficient signs in >80% of models, and were statistically significant >80% of the time were selected for the final model.
Continuous variables are presented as mean with standard deviation or median with interquartile range (IQR). Categorical variables are presented as absolute counts with percentages. Hazard ratios (HR) and odds ratios (OR) are presented with 95% confidence intervals (CI)s. Missing data were coded as missing in order to avoid listwise deletion. Patients missing distal arch measurement (n = 8) were excluded. The analysis was conducted with R-4.2.1 (R Foundation for Statistical Computing).
Results
There were 263 patients who underwent isolated repair of coarctation of the aorta. Of these, there were 130 patients who appeared to be eligible for either sternotomy or thoracotomy on basis of the anatomic criteria used for trimming the dataset to a region of common support. There were distinct differences in age, weight, aortic annular size, and distal arch size at baseline. After inverse probability of treatment weighting with stabilized weights, the 2 weighted pseudopopulations were comparable with the exception of mitral annular diameter, which had a difference of less than 1 mm. This was considered to be statistically significant but clinically irrelevant (Table 1).
Table 1.
Before inverse probability of treatment weighting, there were distinct differences in the 2 populations
| Characteristics | Unweighted |
Weighted |
||||
|---|---|---|---|---|---|---|
| Thoracotomy |
Sternotomy |
SMD | Thoracotomy |
Sternotomy |
SMD | |
| 83 | 47 | 87.77 | 42.64 | |||
| Age, d | 7.75 (6.16) | 5.36 (3.55) | 0.474 | 6.92 (5.67) | 6.02 (3.83) | 0.188 |
| Male sex, n (%) | 56 (67.5) | 33 (70.2) | 0.059 | 63.6 (72.5) | 31.4 (73.7) | 0.027 |
| Weight, kg | 3.43 (0.53) | 3.24 (0.60) | 0.343 | 3.32 (0.55) | 3.39 (0.67) | 0.104 |
| Preoperative BSA, m2 | 0.23 (0.02) | 0.22 (0.03) | 0.346 | 0.22 (0.02) | 0.22 (0.03) | 0.054 |
| Bicuspid aortic valve, n (%) | 60 (72.3) | 29 (61.7) | 0.227 | 64.5 (73.5) | 30.1 (70.6) | 0.065 |
| Bovine arch, n (%) | 1 (1.2) | 2 (4.3) | 0.188 | 1.2 (1.4) | 1.9 (4.5) | 0.181 |
| Aberrant subclavian, n (%) | 3 (3.6) | 2 (4.3) | 0.033 | 5.0 (5.7) | 2.9 (6.8) | 0.047 |
| Aortic annulus, mm | 5.7 (0.7) | 5.3 (0.6) | 0.588 | 5.5 (0.7) | 5.4 (0.6) | 0.121 |
| Aortic annulus z-score | −2.08 (0.69) | −2.36 (0.66) | 0.413 | −2.23 (0.70) | −2.32 (0.61) | 0.125 |
| Mitral diameter (lateral), mm | 8.8 (1.3) | 8.0 (1.4) | 0.602 | 8.7 (1.2) | 8.1 (1.4) | 0.401 |
| Distal arch z-score | −2.71 (0.51) | −3.18 (0.47) | 0.968 | −2.97 (0.70) | −2.97 (0.45) | 0.006 |
After weighting, the populations were similar across all covariates except for mitral diameter, although the difference was noted to be clinically insignificant. Bold value indicates a significant difference.
During a median follow-up time of 4.4 years (IQR, 1.5-9.4 years), there was one in-hospital death and no deaths during follow-up. After inverse probability of treatment weighting, median postoperative ICU length of stay was 2.1 days (IQR, 1.6-3.9 days) for patients who underwent thoracotomy and 5.0 days (IQR, 3.2-9.2 days) for patients who underwent sternotomy. Using weighted linear regression, sternotomy approach for aortic arch reconstruction was associated with increased ICU length of stay by 4.7 days, P < .001, and there was an inverse relationship between body weight and ICU length of stay, ie, each kilogram increase in weight reduced ICU length of stay by 1.8 days, P = .007. Total postoperative hospital length of stay for the cohort was a median of 8.1 days (IQR, 5.2-14.4). The total postoperative hospital length of stay was 6.3 days longer among patients undergoing sternotomy using weighted linear regression, P = .03, with no statistically significant effect of weight. The odds of vocal cord dysfunction were substantially greater with sternotomy, OR, 7.14 (95% CI, 1.66-41.26, P = .01) and decreased with increasing body weight, OR per kilogram increase in body weight 0.18 (95% CI, 0.04-0.62, P = .01). There was no difference in the odds of diaphragm paresis or chyle leak on the basis of surgical approach. In our sensitivity analysis, eliminating extreme weights, ie, truncating at the 1st and 99th percentile, did not affect the significance of the results.
Risk of Reintervention During Follow-up
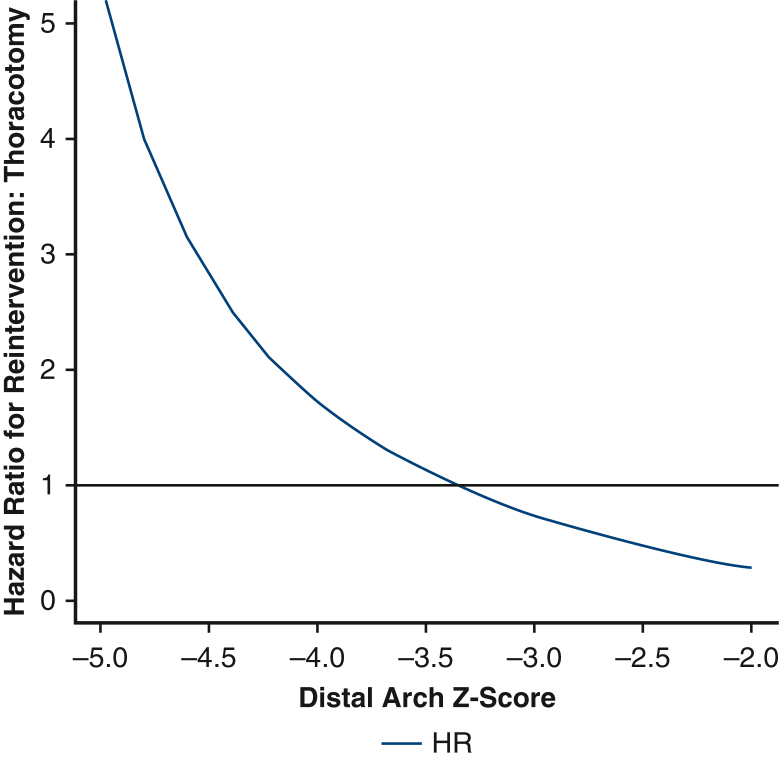
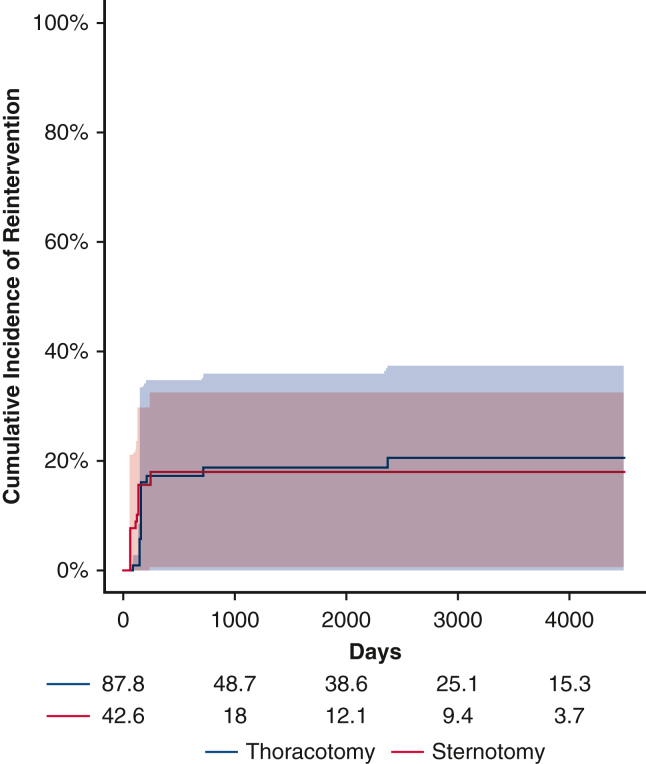
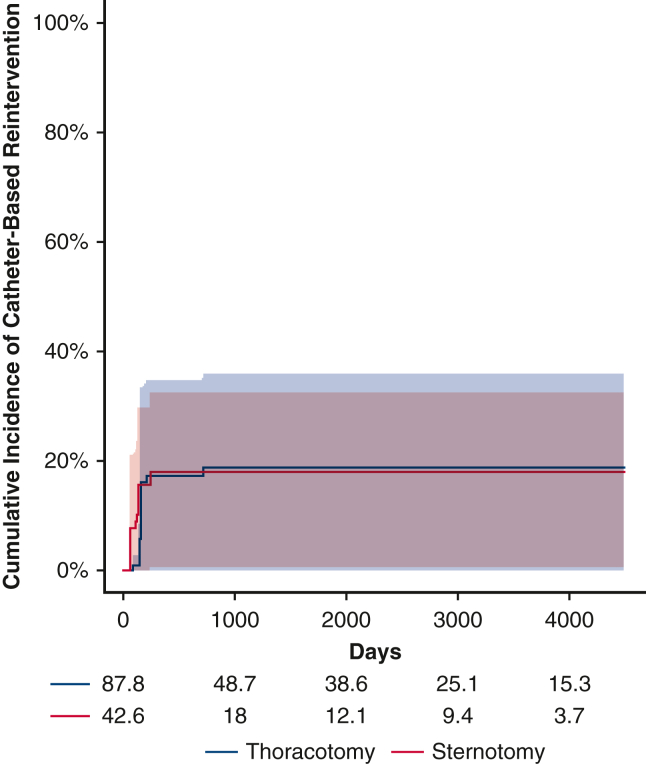
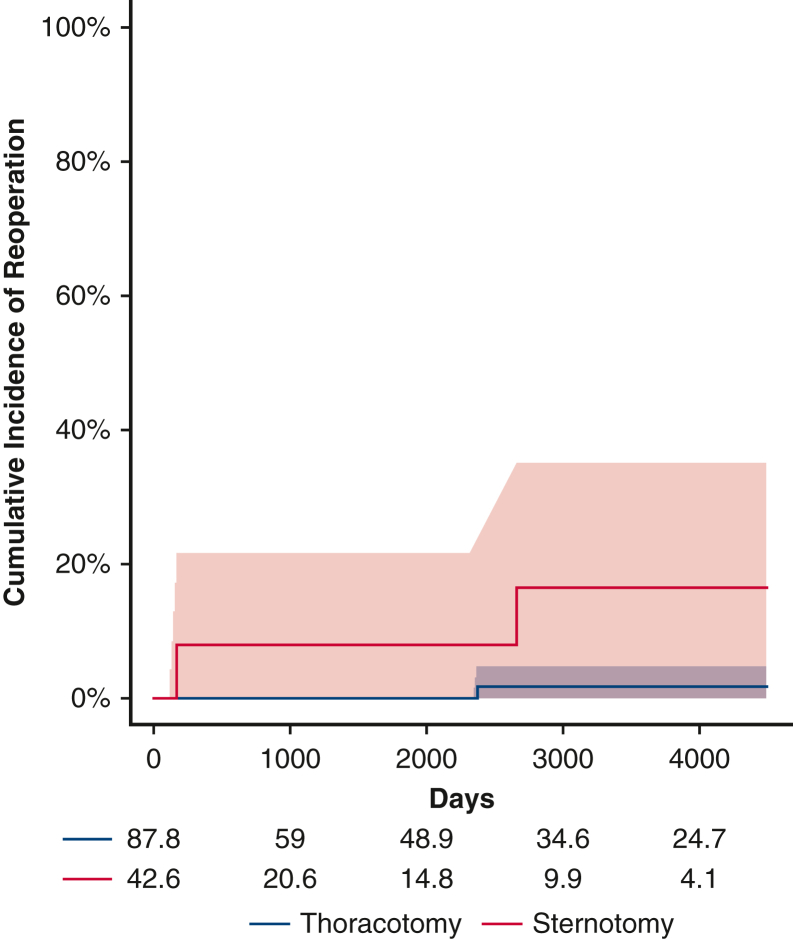
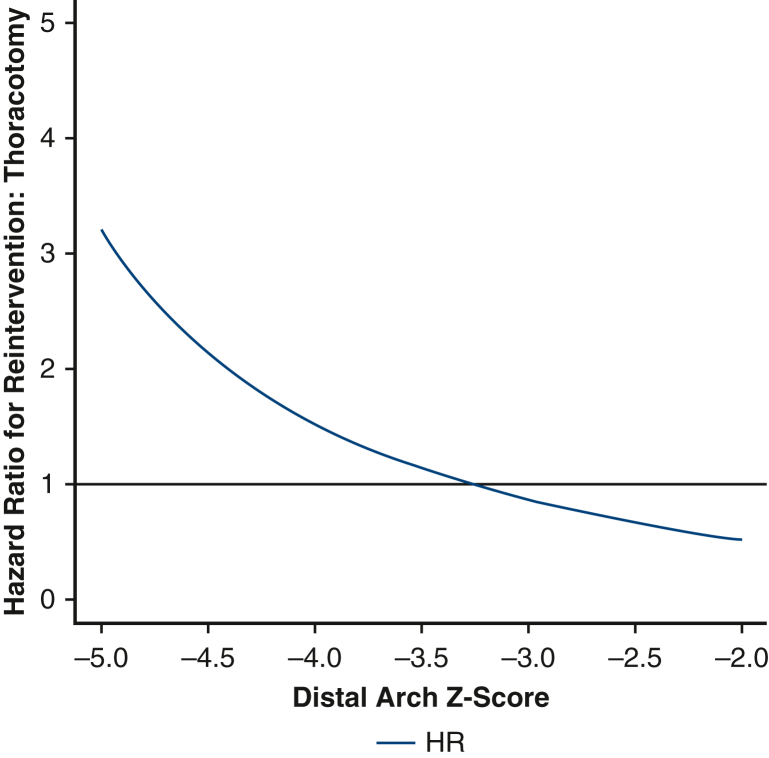
Before weighting, there were a total of 12 first-time reinterventions. Of these, 11 were catheter-based and 1 was open. Two patients subsequently underwent an open operation after an initial catheter-based procedure. After inverse probability weighting, there were 14.5 first-time reinterventions in the thoracotomy group and 7.2 first-time reinterventions in the sternotomy group. The 5-year cumulative incidence of reintervention in the weighted pseudopopulations, irrespective of distal arch z-score, was 18.7% (95% CI, 0%-35.7%) for patients undergoing thoracotomy with extended end-to-end anastomosis and 18.0% (95% CI, 0.5%-32.4%) for patients undergoing sternotomy with aortic arch reconstruction, Figure 1, the cumulative incidence of catheter-based and open reoperation are separately reported (Figures E5 and E6). With respect to hazard for reintervention, the interaction between distal arch z-score and operative approach was statistically significant, P = .03 (Figure 2). The HR was >1 with a distal arch z-score <−3.5 suggesting an increased risk of reintervention associated with thoracotomy approach. Conversely, when the distal arch z-score was >−3.0, the HR was <1, suggesting a reduced risk associated with thoracotomy approach compared with sternotomy. In the sensitivity analysis, eliminating extreme weights, ie, truncating at the 1st and 99th percentile, once again did not affect the significance of the results.
Figure 1.
Cumulative incidence with 95% confidence intervals of reintervention for patients undergoing repair via thoracotomy (blue) versus sternotomy (red). There was no difference between the populations before accounting for the interaction between operative approach and distal arch size.
Figure E5.
Catheter-based reintervention. There was no significant difference in the cumulative incidence of catheter-based reintervention when comparing thoracotomy (blue) with sternotomy (red); shaded areas represent 95% confidence intervals, before accounting for the interaction between operative approach and distal arch size.
Figure E6.
Open reoperation. Before weighting, there were 3 open surgical reoperations with 2 occurring in the sternotomy group (red) and 1 in the thoracotomy group (blue); shaded areas represent 95% confidence intervals. Both patients who eventually underwent reoperation from the sternotomy group had previously undergone catheter based intervention. This makes inference somewhat challenging, but it may be the case that when an intervention is required after the sternotomy approach, it is more likely that a catheter-based reintervention will be inadequate.
Figure 2.
Plot demonstrating the relative hazard of thoracotomy compared with sternotomy as a function of distal transverse arch size. Patients with a distal arch z-score <−3.5 appear to have lower risk of reintervention via sternotomy; patients with a distal arch z-score larger than −3 appeared to have reduced risk of reintervention via thoracotomy. HR, Hazard ratio.
Additional Analyses
In our analysis examining the entire cohort of patients who had undergone thoracotomy and extended end-to-end anastomosis during the entire time period under investigation (n = 197), our machine-learning technique for variable selection did not identify distal arch z-score as a risk factor for reintervention. The only variable that was a consistent predictor of reintervention was presence of a bovine arch (HR, 9.4; 95% CI, 3.8-23.1; P < .001), which was present in 12 patients (6.1%). There was no difference in the distal arch z-score when comparing patients with and without bovine arch in this cohort, P = .3.
Applied to the entire cohort of patients who had undergone thoracotomy for extended end-to-end anastomosis (n = 197), there were 83 patients who would have undergone sternotomy with aortic reconstruction on the basis of body weight + 1 criterion. This criterion was not an independent predictor of reintervention on Cox proportional hazards regression, P = .4. Using the <50% ascending aorta criterion, there were 64 patients who would have been considered for sternotomy with aortic arch reconstruction, and this criterion also was not an independent predictor of reintervention in Cox proportional hazards regression, P = .5. Using these alternative criteria would have increased the total ICU length of stay for the cohort by approximately 390.1 and 300.8 bed-days, respectively.
Discussion
Coarctation of the aorta may be addressed via thoracotomy with extended end-to-end anastomosis or sternotomy for cardiopulmonary bypass and aortic arch reconstruction. Short-term outcomes for both of these operations have acceptable rates of aortic reintervention ranging from 4% to 14% at a median follow-up ranging from 1 year to 6 years.21,23, 24, 25, 26, 27 However, limited guidance has been provided regarding optimal patient selection. We found that among patients with a distal arch z-score smaller than −3.5, left thoracotomy with extended end-to-end anastomosis was associated with an increased hazard for reintervention compared with sternotomy with aortic arch reconstruction using cardiopulmonary bypass. Moreover, there was substantial morbidity associated with sternotomy and aortic arch reconstruction including prolonged postoperative ICU and total postoperative hospital length of stay in addition to an increased odds of recurrent laryngeal nerve injury.
When comparing the 2 approaches, previous studies have not made distinct efforts to create comparable groups insomuch that patients who were not truly candidates for sternotomy with aortic arch reconstruction were included in the comparator group having undergone thoracotomy,12,22,23,25,28, 29, 30, 31 ie, the 2 groups lacked exchangeability.18,19 Moreover, many of the studies were underpowered to detect differences between groups and also included patients across a broad age range. For these reasons, discrepant findings published in the literature may be attributable to differences in baseline populations with failure to account for confounding.
Several of the studies suggesting alternative distal arch criteria were restricted to the subset of patients who had undergone thoracotomy for extended end-to-end anastomosis. Tulzer and colleagues12 have suggested a lower limit of z −4.6 for the proximal arch, and Kotani and colleagues11 have gone so far as to suggest z −6 for the proximal arch. Moreover, Callahan and colleagues13 have conjectured that the lower limit of size may not be defined. Indeed, repair of interrupted aortic arch is technically possible via thoracotomy without the use of cardiopulmonary bypass.32 Despite these suggestions, these studies may suffer from selection bias, as the subset of patients who underwent sternotomy—presumably with some elevated risk for residual obstruction if performed via thoracotomy—had already been removed from the at-risk population. Mirroring this phenomenon, in our exploratory analysis including only those patients who had undergone left thoracotomy for extended end-to-end anastomosis, distal arch z-score did not prove to be an independent predictor of reintervention.
The increased risk for reintervention seen among our patients with bovine arch undergoing thoracotomy for repair of coarctation of the aorta is consistent with previous reports.33,34 Although only 6.1% of the patients in our cohort undergoing thoracotomy for repair of coarctation of the aorta had bovine arch, the reported prevalence of bovine arch in other series has ranged from 17% to 28.6%.33, 34, 35 As such, this may be an added consideration when deciding between sternotomy and thoracotomy.
We observed an increased odds of recurrent laryngeal nerve injury in patients undergoing sternotomy for aortic arch reconstruction. Importantly, recurrent laryngeal nerve injury may necessitate additional procedures including injection laryngoplasty, thyroplasty, tracheostomy, gastrostomy, or prolonged nasoenteric feeding.36 For this reason, recurrent laryngeal nerve monitoring may be beneficial in this population. Notably, recurrent laryngeal nerve monitoring requires that the patient not be paralyzed during the mobilization of the aortic arch in the process of identifying and preserving the recurrent laryngeal nerve.37, 38, 39 Despite these precautions, however, the recurrent laryngeal nerve may still be vulnerable to stretch injury either during the dissection or from an overly patulous arch patch.
Limitations
This was a retrospective study, which may have been subject to confounding. Our efforts to create comparable groups using inverse probability of treatment weighting appeared to achieve similar pseudopopulations. However, inverse probability of treatment weighting is only able to account for observable variables and does not completely eliminate the possibility of confounding. Because our facility serves a geographical area that contains many pediatric cardiology programs, patients most commonly follow-up at outside institutions. Consequently, another limitation of our study is the duration of follow-up. However, as patients are typically repatriated to our facility for reintervention because of the close partnership between our group and regional pediatric cardiology practices, the reintervention rate is likely to be accurate. Reintervention, however, is an imperfect means of accounting for residual lesions, as clinically significant residual lesions contributing to elevated risk for cardiovascular disease may not rise to the level of reintervention.6 Despite these limitations, this is the first study to enumerate the distinct risks and benefits with respect to recurrent laryngeal nerve dysfunction, hospital length of stay, and reintervention using causal inference techniques.
Conclusions
In this study comparing patients with hypoplastic distal arch, the risk for reintervention depended on both the surgical approach and the size of the distal arch. Although sternotomy for aortic arch reconstruction was associated with a lower risk for reintervention in patients with distal arch z-score smaller than −3.5, there was an associated increase in the odds of recurrent laryngeal nerve injury and longer hospital length of stay. These results suggest that the risk for reintervention must be weighed against the potential morbidity of the surgical approach. Furthermore, this understanding of the risk of reintervention may be a means of identifying patients who require more frequent clinical follow-up.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
This work was supported by a grant to Dr Chiu from the Congenital Heart Defect Coalition.
Drs Chiu and Gearhart contributed equally to this article.
Appendix E1
References
- 1.Mitchell S.C., Korones S.B., Berendes H.W. Congenital heart disease in 56,109 births incidence and natural history. Circulation. 1971;43(3):323–332. doi: 10.1161/01.cir.43.3.323. [DOI] [PubMed] [Google Scholar]
- 2.Kaushal S., Backer C.L., Patel J.N., et al. Coarctation of the aorta: midterm outcomes of resection with extended end-to-end anastomosis. Ann Thorac Surg. 2009;88(6):1932–1938. doi: 10.1016/j.athoracsur.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 3.Karl T.R., Sano S., Brawn W., Mee R.B.B. Repair of hypoplastic or interrupted aortic arch via sternotomy. J Thorac Cardiovasc Surg. 1992;104(3):688–695. [PubMed] [Google Scholar]
- 4.Wu M.H., Chen H.C., Kao F.Y., Huang S.K. Risk of systemic hypertension and cerebrovascular accident in patients with aortic coarctation aged <60 years (from a national database study) Am J Cardiol. 2015;116(5):779–784. doi: 10.1016/j.amjcard.2015.05.052. [DOI] [PubMed] [Google Scholar]
- 5.Oster M.E., McCracken C., Kiener A., et al. Long-term survival of patients with coarctation repaired during infancy (from the Pediatric Cardiac Care Consortium) Am J Cardiol. 2019;124(5):795–802. doi: 10.1016/j.amjcard.2019.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pickard S.S., Gauvreau K., Gurvitz M., et al. Stroke in adults with coarctation of the aorta: a national population-based Study. J Am Heart Assoc. 2018;7(11) doi: 10.1161/JAHA.118.009072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meijs T.A., Minderhoud S.C.S., Muller S.A., et al. Cardiovascular morbidity and mortality in adult patients with repaired aortic coarctation. J Am Heart Assoc. 2021;10(22) doi: 10.1161/JAHA.121.023199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ylinen M.K., Pihkala J.I., Salminen J.T., Sarkola T. Predictors of blood pressure and hypertension long-term after treatment of isolated coarctation of the aorta in children—a population-based study. Interact Cardiovasc Thorac Surg. 2022;35(3) doi: 10.1093/icvts/ivac212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ungerleider R.M., Pasquali S.K., Welke K.F., et al. Contemporary patterns of surgery and outcomes for aortic coarctation: an analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. J Thorac Cardiovasc Surg. 2013;145(1):150–158. doi: 10.1016/j.jtcvs.2012.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moulaert A.J., Bruins C.C., Oppenheimer-Dekker A. Anomalies of the aortic arch and ventricular septal defects. Circulation. 1976;53(6):1011–1015. doi: 10.1161/01.cir.53.6.1011. [DOI] [PubMed] [Google Scholar]
- 11.Kotani Y., Anggriawan S., Chetan D., et al. Fate of the hypoplastic proximal aortic arch in infants undergoing repair for coarctation of the aorta through a left thoracotomy. Ann Thorac Surg. 2014;98(4):1386–1393. doi: 10.1016/j.athoracsur.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 12.Tulzer A., Mair R., Kreuzer M., Tulzer G. Outcome of aortic arch reconstruction in infants with coarctation: importance of operative approach. J Thorac Cardiovasc Surg. 2016;152(6):1506–1513.e1. doi: 10.1016/j.jtcvs.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 13.Callahan C.P., Saudek D., Creighton S., et al. Proximal arch in left thoracotomy repair of neonatal and infant coarctation—how small is too small? World J Pediatr Congenit Heart Surg. 2019;10(4):469–474. doi: 10.1177/2150135119852329. [DOI] [PubMed] [Google Scholar]
- 14.Stephens E.H., Feins E.N., Karamlou T., et al. The Society of Thoracic Surgeons clinical practice guidelines on the management of neonates and infants with coarctation. Ann Thorac Surg. 2024;118(3):527–544. doi: 10.1016/j.athoracsur.2024.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Sluysmans T., Colan S.D. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99(2):445–457. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 16.Austin P.C., Tu J.V. Bootstrap methods for developing predictive models. Am Stat. 2004;58(2):131–1317. [Google Scholar]
- 17.Crump R.K., Hotz V.J., Imbens G.W., Mitnik O.A. Dealing with limited overlap in estimation of average treatment effects. Biometrika. 2009;96(1):187–199. [Google Scholar]
- 18.Greenland S., Robins J.M. Identifiability, exchangeability, and epidemiological confounding. Int J Epidemiol. 1986;15(3):413–419. doi: 10.1093/ije/15.3.413. [DOI] [PubMed] [Google Scholar]
- 19.Cole S.R., Hernan M.A. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robins J.M., Hernán M.Á., Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Mery C.M., Guzmán-Pruneda F.A., Trost J.G., et al. Contemporary results of aortic coarctation repair through left thoracotomy. Ann Thorac Surg. 2015;100(3):1039–1046. doi: 10.1016/j.athoracsur.2015.04.129. [DOI] [PubMed] [Google Scholar]
- 22.Sakurai T., Stickley J., Stumper O., et al. Repair of isolated aortic coarctation over two decades: impact of surgical approach and associated arch hypoplasia. Interact Cardiovasc Thorac Surg. 2012;15(5):865–870. doi: 10.1093/icvts/ivs265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karamlou T., Bernasconi A., Jaeggi E., et al. Factors associated with arch reintervention and growth of the aortic arch after coarctation repair in neonates weighing less than 2.5 kg. J Thorac Cardiovasc Surg. 2009;137(5):1163–1167. doi: 10.1016/j.jtcvs.2008.07.065. [DOI] [PubMed] [Google Scholar]
- 24.IJsselhof R., Liu H., Pigula F., et al. Rates of interventions in isolated coarctation repair in neonates versus infants: does age matter? Ann Thorac Surg. 2019;107(1):180–186. doi: 10.1016/j.athoracsur.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Lehnert A., Villemain O., Gaudin R., Méot M., Raisky O., Bonnet D. Risk factors of mortality and recoarctation after coarctation repair in infancy. Interact Cardiovasc Thorac Surg. 2019;29(3):469–475. doi: 10.1093/icvts/ivz117. [DOI] [PubMed] [Google Scholar]
- 26.Ghani M.O.A., Raees M.A., Harris G.R., Shannon C.N., Nicholson G.T., Bichell D.P. Reintervention after infant aortic arch repair using a tailored autologous pericardial patch. Ann Thorac Surg. 2021;111(3):973–979. doi: 10.1016/j.athoracsur.2020.04.091. [DOI] [PubMed] [Google Scholar]
- 27.Costopoulos K., Philip J., Lopez-Colon D., Kaliki G., Chandran A., Bleiweis M. A single centre experience with an evolving approach for the repair of coarctation of the aorta. Cardiol Young. 2019;29(7):885–887. doi: 10.1017/S104795111900101X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conte S., Lacour-Gayet F., Serraf A., et al. Surgical management of neonatal coarctation. J Thorac Cardiovasc Surg. 1995;109(4):663–675. doi: 10.1016/S0022-5223(95)70347-0. [DOI] [PubMed] [Google Scholar]
- 29.Wright G.E., Nowak C.A., Goldberg C.S., Ohye R.G., Bove E.L., Rocchini A.P. Extended resection and end-to-end anastomosis for aortic coarctation in infants: results of a tailored surgical approach. Ann Thorac Surg. 2005;80(4):1453–1459. doi: 10.1016/j.athoracsur.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Parikh K.J., Fundora M.P., Sasaki N., Rossi A.F., Burke R.P., Sasaki J. Use of aortic arch measurements in evaluating significant arch hypoplasia in neonates with coarctation. Prog Pediatr Cardiol. 2021;62 [Google Scholar]
- 31.Minotti C., Scioni M., Castaldi B., et al. Effectiveness of repair of aortic coarctation in neonates: a long-term experience. Pediatr Cardiol. 2022;43:17–26. doi: 10.1007/s00246-021-02685-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCrindle B.W., Tchervenkov C.I., Konstantinov I.E., et al. Risk factors associated with mortality and interventions in 472 neonates with interrupted aortic arch: a Congenital Heart Surgeons Society study. J Thorac Cardiovasc Surg. 2005;129(2):343–350. doi: 10.1016/j.jtcvs.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Turek J.W., Conway B.D., Cavanaugh N.B., et al. Bovine arch anatomy influences recoarctation rates in the era of the extended end-to-end anastomosis. J Thorac Cardiovasc Surg. 2018;155(3):1178–1183. doi: 10.1016/j.jtcvs.2017.10.055. [DOI] [PubMed] [Google Scholar]
- 34.Miwa K., Iwai S., Tsumura S., et al. Influence of bovine arch anatomy on surgical outcomes of coarctation of the aorta. Pediatr Cardiol. 2023;44(4):933–939. doi: 10.1007/s00246-022-03072-y. [DOI] [PubMed] [Google Scholar]
- 35.Reinshagen L., Vodiskar J., Mühler E., Hövels-Gürich H.H., Vazquez-Jimenez J.F. Bicarotid trunk: how much is “not uncommon”? Ann Thorac Surg. 2014;97(3):945–949. doi: 10.1016/j.athoracsur.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 36.Jabbour J., Martin T., Beste D., Robey T. Pediatric vocal fold immobility: natural history and the need for long-term follow-up. JAMA Otolaryngol Head Neck Surg. 2014;140(5):428. doi: 10.1001/jamaoto.2014.81. [DOI] [PubMed] [Google Scholar]
- 37.Lawlor C.M., Meisner J., Jennings R.W., Zendejas B., Choi S.S. Comparative effectiveness of recurrent laryngeal nerve monitoring techniques in pediatric surgery. Laryngoscope. 2022;132(4):889–994. doi: 10.1002/lary.29837. [DOI] [PubMed] [Google Scholar]
- 38.Lawlor C.M., Zendejas B., Baird C., Munoz-San Julian C., Jennings R.W., Choi S.S. Intraoperative recurrent laryngeal nerve monitoring during pediatric cardiac and thoracic surgery: a mini review. Front Pediatr. 2020;8 doi: 10.3389/fped.2020.587177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiu P., Zendejas B., Baird C. Multidisciplinary approach to vascular rings and vascular-related aerodigestive compression: a clinical practice review. Transl Pediatr. 2023;12(6):1258–1277. doi: 10.21037/tp-23-39. [DOI] [PMC free article] [PubMed] [Google Scholar]