Abstract
Objective
The optimal method for cerebral protection during aortic arch reconstruction in neonates and infants is unknown. We compare the outcomes of deep hypothermic circulatory arrest and selective antegrade cerebral perfusion strategies in neonatal and infant cardiac surgery.
Methods
We retrospectively identified all patients aged less than 1 year who underwent aortic arch reconstruction from 2012 to 2023. Patients were categorized on the cerebral perfusion strategy used during their procedure. Comparative analyses of perioperative and outcome variables were conducted to assess differences between cerebral protection strategies. A secondary analysis further stratifying by complexity of repair was performed. Examples of “complex” repair included the Norwood procedure, and “simple” repairs included isolated arch reconstructions. Adjusted regression models were used to identify specific outcomes associated with cerebral perfusion strategy used.
Results
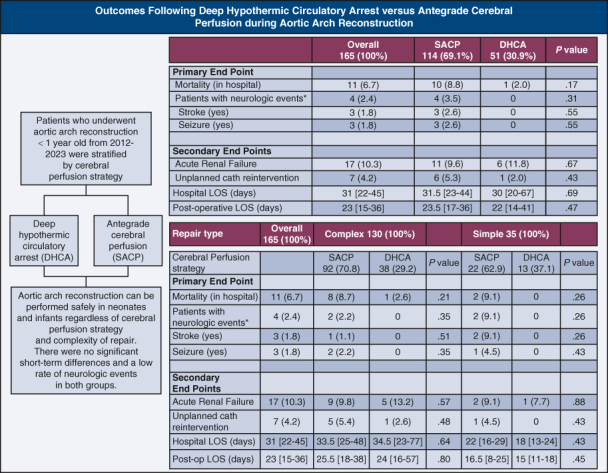
There were 165 cases included in our cohort (114 [69%] selective antegrade cerebral perfusions and 51 [31%] deep hypothermic circulatory arrests). Overall, hospital mortality was 7% (selective antegrade cerebral perfusion 9% vs deep hypothermic circulatory arrest 2%, P= .17). There were 6 total neurologic events in 4 patients after surgery in the selective antegrade cerebral perfusion group and none in the deep hypothermic circulatory arrest group. Irrespective of the cerebral perfusion strategy, there were no differences in mortality, stroke, seizures, renal failure, and catheterization reinterventions observed after surgery. This finding held true even when stratifying cerebral perfusion methods by complexity of repair. Regression analysis showed no associations for cerebral perfusion strategy with any outcome even after adjusting for age and complexity of repair.
Conclusions
There were no significant short-term differences and a low rate of neurologic events in both groups during aortic arch reconstruction among neonates and infants. Longer follow-up is necessary to evaluate the impact of cerebral perfusion strategy on neurocognitive development later in life.
Key Words: aortic arch, cerebral perfusion
Outcomes after aortic arch reconstruction by cerebral perfusion strategy.
Central Message.
There are no differences in outcomes regardless of cerebral perfusion strategy after aortic arch reconstruction in infants.
Perspective.
The optimal way to protect the brain during neonatal arch reconstruction continues to be a matter of debate. Our study shows there were no significant short-term differences and a low rate of neurologic events regardless of cerebral perfusion strategy after aortic arch reconstruction in infants.
Outcomes for aortic arch reconstruction in neonates and infants have continued to improve over the last few decades due to advances in surgical techniques and perioperative care. In patients undergoing aortic arch reconstruction, the focus has shifted away from operative and 30-day mortality to short- and long-term neurodevelopmental outcomes. Approximately 33% of adolescents who underwent cardiac surgery as a neonate have some evidence of neurodevelopmental impairment.1 It also has become apparent that 25% to 50% of neonates undergoing complex cardiac surgery have evidence of cerebral injury on preoperative imaging, and of these, approximately 75% have findings suggestive of new cerebral injury on postoperative imaging.2, 3, 4, 5
Neonates and infants with aortic arch obstruction are at increased risk for perioperative neurologic injury given that surgical reconstruction must take place under conditions that allow a bloodless field (for optimal visualization) but still provide protection for the brain and visceral organs from ischemic insult.2, 3, 4, 5 The 2 most common perfusion methods used during aortic arch reconstruction in neonates and infants are deep hypothermic circulatory arrest (DHCA) and selective antegrade cerebral perfusion (SACP).6 Over the last 2 decades, there have been multiple attempts to try and demonstrate which method (DHCA vs SACP) would provide superior cerebral and visceral protection during aortic arch reconstruction. These studies have been small retrospective, prospective, or randomized control trials that have been unable to identify an optimal perfusion method to protect the brain or visceral organs, with some arguing DHCA or SACP being superior and others demonstrating no difference.2,5,7, 8, 9, 10, 11 Additionally, a study by Meyer and colleagues,6 which used the US Society of Thoracic Surgeons (STS) Congenital Heart Surgery Database (2010-2013), had a large sample; however, this study only noted trends of the use of such strategies but did not perform comparisons to provide guidance on the use of such strategies for this population.
On this premise, we sought to evaluate our institutional data over the last decade and compare which strategy (DHCA vs SACP) is most optimal among neonates and infants undergoing aortic arch reconstruction.
Materials and Methods
This is a single-center study from 2012 to November 1, 2023. We conducted a query using our internal Society of Thoracic Surgeon database at our institution by surgery type. The list of patients was then screened to identify patients who underwent elective, urgent, or emergency aortic arch reconstruction using DHCA or SACP as perfusion strategies and were aged less than 1 year. Perfusion groups were defined as those who received SACP with less than 8 minutes of DHCA (SACP group) and those who received only DHCA (DHCA group). All patients who had truly mixed perfusion methods (>8 minutes of DHCA and SACP) were excluded. There was no difference in anesthetic management between cerebral perfusion strategies. Patients who underwent repeat or revision of previous aortic arch reconstruction were excluded. The primary outcomes of this study included in-hospital mortality and having a neurological event (stroke or seizure). Strokes were confirmed through imaging reports. Acute renal failure and having an unplanned catheter reintervention were considered secondary outcomes. The follow-up period to assess outcomes was from the time of the aortic arch repair to hospital discharge or death. Data related to outcome events were verified and manually extracted from patient electronic medical records. This protocol was approved and consent for this study was waived by our Institutional Review Board (IRB-300012193, 12/1/2023).
Cerebral Perfusion Strategy
Deep hypothermic circulatory arrest
At our institution, DHCA consists of cannulating the ductus arteriosus and cooling to a core temperature of 18 °C using a pH stat blood gas management strategy once below 32 °C. Cooling typically lasts for 30 to 35 minutes, and an 8- to 10-degree gradient is maintained between temperature extremes. The circulation is shut off once deep hypothermia is reached. Circulation is restored once the arch reconstruction and de-airing maneuvers are complete. The reconstructed aorta is recannulated, and rewarming is begun using a pH stat strategy until 32 °C and then switched to alpha stat blood gas management strategy to reach 36 °C.
Antegrade cerebral perfusion
At our institution, SACP consists of sewing a 3.5-mm Gore-Tex graft to the innominate artery and cooling to a target temperature of 20 °C to 22 °C using a pH stat blood gas management strategy once below 32 °C. Cooling typically lasts for 30 to 35 minutes, and an 8- to 10-degree gradient is maintained between temperature extremes. Flow rates range from 25 to 50 mL/kg/min with our institutional average being closer to 40 mL/kg/min. We monitor pressure via a right radial arterial line during SACP, and our goal is to maintain a mean arterial pressure between 25 and 40 mm Hg, on average being closer to 35 mm Hg. For patients undergoing an isolated arch reconstruction with a reasonable size ascending aorta, we will occasionally cannulate the side of the ascending aorta and slide the cannula up the innominate artery and snare the head vessels during SACP, thus avoiding having to sew a graft on the innominate artery. Cerebral and renal near-infrared spectroscopy are used in all patients. Rewarming is begun using a pH stat strategy until 32 °C and then switched to alpha stat blood gas management strategy to reach 36 °C.
At our institution, we rarely perform dual arterial cannulation, unless there is truly an interrupted aortic arch, in that case we would add a second arterial cannula in the duct for cooling, use the Gore-Tex graft for SACP during arch reconstruction, and use the Gore-Tex graft for full-body perfusion once the arch is reconstructed while rewarming. For isolated aortic arch reconstruction, if the ascending aorta is big enough, we will cannulate the side and slide the cannula up the innominate artery for SACP. If it is too small, we will sew a graft on the innominate artery as previously described.
Statistical Analysis
Patients were categorized on the cerebral perfusion strategy used during their aortic arch repair. Comparative analyses of perioperative and outcome variables were conducted using chi-square, Fisher exact test, and Wilcoxon rank sum where appropriate to summarize and assess differences in demographic and clinical outcomes data between the 2 cerebral perfusion strategies. Adjusted regression models by age and complexity of repair were used to identify specific outcomes associated with cerebral perfusion strategy used. A secondary analysis was performed, comparing cerebral perfusion strategies and type of repair—complex (Norwood procedure) or simple (isolated arch reconstruction). Those who underwent complex repairs included patients with hypoplastic left heart syndrome, single ventricle, interrupted aortic arch with added septal defect(s), dextro-transposition of the great arteries, and atrioventricular canal. Patients who underwent simple repairs in our study included those with hypoplastic aortic arch including coarctation of the aorta with an added septal defect or other anatomic abnormalities such as mitral valve pathologies and Shone's syndrome. Statistical Analytical Software 9.4 (SAS Institute, Inc) was used for the analysis.
Results
There were 165 cases included in our cohort with neonates representing 84% (n = 139) of this group. Sixty-nine percent (n = 114) underwent SACP, and 31% (n = 51) underwent DHCA. Baseline characteristics for this cohort are shown in Table 1. The median age at the time of repair was 8 (interquartile range [IQR], 6-14) days of life (SACP 7 vs DHCA 10 days, P = .001), and median weight was 3.2 (IQR, 2.8-3.5) kg (SACP 3.1 vs DHCA 3.3 kg, P = .07). Prematurity occurred in 15% of the cohort (SACP 13% vs DHCA 19%, P = .28). Chromosomal abnormalities were identified in 25% of the cohort (SACP 24% vs DHCA 27%, P = .69), and 19% had 1 or more syndromes (SACP 19% vs DHCA 19%, P = .96). Preoperatively, 20% required mechanical ventilation (SACP 14% vs DHCA 33%, P = .004), and there were 5 neurological events (all occurring in 5 separate patients) before surgery (SACP n = 3 vs DHCA n = 2). There were 130 (79%) complex operations (SACP 81% vs DHCA 75%) and 35 simple operations (21%) (SACP 19% vs DHCA 25%). The overall median cardiopulmonary bypass time was 130 (IQR, 102-157) minutes (SACP 136 vs DHCA 102 minutes, P = .0001), and aortic crossclamp time was 59 (IQR, 48-69) minutes (SACP 60 vs DHCA 56, P = .04). The median DHCA time was 33 minutes, and SACP time was 49 minutes. Low-moderate hypothermia (20 °C-24 °C) was used in 83% of the SACP group, and all patients undergoing DHCA occurred at deep-profound (<20 °C) temperatures.
Table 1.
Baseline characteristics for neonates and infants undergoing aortic arch reconstruction by cerebral perfusion strategy (2012 to November 2023)
| Variables | Overall 165 (100%) |
SACP 114 (69.1%) |
DHCA 51 (30.9%) |
P value |
|---|---|---|---|---|
| Male sex | 106 (64.2) | 76 (66.7) | 30 (58.8) | .33 |
| Age (d) | 8 [6-14] | 7 [5-12] | 10 [7-38] | .001 |
| Weight (kg) | 3.2 [2.8-3.5] | 3.1 [2.7-3.5] | 3.3 [2.9-4.0] | .07 |
| Premature birth (yes) | 25 (15.1) | 15 (13.2) | 10 (19.6) | .28 |
| Gestational age at birth (wk) | 38 [37-39] | 38 [37-39] | 38 [36-39] | .23 |
| Chromosomal abnormality (yes) | 42 (25.4) | 28 (24.6) | 14 (27.5) | .69 |
| Syndrome (yes) | 32 (19.4) | 22 (19.3) | 10 (19.6) | .96 |
| Preoperative seizures | 3 (1.8) | 1 (0.9) | 2 (3.9) | .22 |
| Preoperative CVA or IVH grade >2 | 2 (1.2) | 2 (1.8) | 0 | 1.0 |
| Mechanical ventilation preoperatively | 33 (20.0) | 16 (14.0) | 17 (33.3) | .004 |
| Complex repair | 130 (78.8) | 92 (80.7) | 38 (74.5) | |
| Underlying diagnosis for complex repairs | ||||
| HLHS/SV | 100 (76.9) | 77 (83.7) | 23 (60.5) | |
| IAA/VSD/ASD | 16 (12.3) | 8 (8.7) | 8 (21.1) | |
| d-TGA/VSD | 13 (10.0) | 6 (6.5) | 7 (18.4) | |
| AV Canal | 1 (0.8) | 1 (1.1) | 0 | |
| Simple repair | 35 (21.2) | 22 (19.3) | 13 (25.5) | |
| Underlying diagnosis of simple repairs | ||||
| Hypoplastic arch/VSD | 16 (45.7) | 11 (50.0) | 5 (38.5) | |
| Hypoplastic arch/ASD | 9 (25.7) | 7 (31.8) | 2 (15.4) | |
| Hypoplastic arch/other | 10 (28.6) | 4 (18.2) | 6 (46.1) | |
| Perioperative variables | ||||
| Ventilator use (d) | 4 [3-7] | 4 [3-7] | 4 [2-7] | .56 |
| Delayed sternal closure (yes) | 113 (68.5) | 88 (77.2) | 25 (49) | .0003 |
| G-tube placement at discharge (yes) | 55 (33.3) | 32 (28.1) | 23 (45.1) | .03 |
| Intraoperative variables | ||||
| CPB time (min) | 130 [102-157] | 136 [117-161] | 102 [76-132] | <.0001 |
| Crossclamp time (min) | 59 [48-69] | 60 [51-69] | 56 [33-69] | .04 |
| DHCA time (min) | 2.5 [0-18.5] | 0 [0-3] | 33.5 [22-45] | <.0001 |
| SACP time (min) | - | 49.5 [37-58] | - | - |
| Hypothermia | - | |||
| Deep hypothermia (≤20 °C) | - | 18 (17.1) | 51 (100) | |
| Low-moderate hypothermia (20.1 °C-24 °C) | - | 87 (82.9) | 0 |
N (%); Median [Q1-Q3]. SACP, Selective antegrade cerebral perfusion; DHCA, deep hypothermic circulatory arrest; CVA, cerebral vascular accident; IVH, intraventricular hemorrhage; HLHS, hypoplastic left heart syndrome; SV, single ventricle; IAA, interrupted aortic arch; VSD, ventricular septal defect; ASD, atrial septal defect; d-TGA, dextro-transposition of the great arteries; AV, atrioventricular; CPB, cardiopulmonary bypass.
Outcomes after repair and during hospitalization are shown in Table 2. The overall hospital mortality was 7% (SACP 9% vs DHCA 2%, P = .17). There were 6 neurologic events occurring in 4 patients after surgery in the SACP group and none among the DHCA group (P = .31). None of the patients who had a neurologic event in the postoperative period had evidence of a preoperative neurologic event. Two of the 4 patients with neurological events died of hemorrhagic strokes during hospitalization. The other 2 patients with neurologic strokes seemed to be embolic in nature and survived their hospitalization. Both surviving patients with stroke were discharged on antiepileptics due to subclinical seizure activity noted on their electroencephalogram during hospitalization, and only 1 of these patients had neurologic symptoms of tongue thrusting and right lower-extremity twitching at discharge. However, irrespective of the cerebral perfusion strategy performed, there were no significant differences in mortality, stroke, seizures, renal failure, and catheterization reinterventions observed after surgery. This finding held true even when stratifying the data based on cerebral perfusion method and the type of repair (complex vs simple, Table 3).
Table 2.
Outcomes for neonates and infants after undergoing aortic arch reconstruction by cerebral perfusion strategy
| Variables | Overall 165 (100%) |
SACP 114 (69.1%) |
DHCA 51 (30.9%) |
P value |
|---|---|---|---|---|
| Primary end point | ||||
| Mortality (in hospital) | 11 (6.7) | 10 (8.8) | 1 (2.0) | .17 |
| Patients with neurologic events∗ | 4 (2.4) | 4 (3.5) | 0 | .31 |
| Stroke (yes) | 3 (1.8) | 3 (2.6) | 0 | .55 |
| Seizure (yes) | 3 (1.8) | 3 (2.6) | 0 | .55 |
| Secondary end points | ||||
| Acute renal failure | 17 (10.3) | 11 (9.6) | 6 (11.8) | .67 |
| Unplanned catheter reintervention | 7 (4.2) | 6 (5.3) | 1 (2.0) | .43 |
| Hospital LOS (d) | 31 [22-45] | 31.5 [23-44] | 30 [20-67] | .69 |
| Postoperative LOS (d) | 23 [15-36] | 23.5 [17-36] | 22 [14-41] | .47 |
N (%); Median [Q1-Q3]. SACP, Selective antegrade cerebral perfusion; DHCA, deep hypothermic circulatory arrest; LOS, length of stay.
Six total neurologic events occurring in 4 patients after surgery.
Table 3.
Outcomes for neonates and infants after undergoing aortic arch reconstruction by type of repair and cerebral perfusion strategy
| Variables | Complex 130 (100%) |
P value | Simple 35 (100%) |
P value | ||
|---|---|---|---|---|---|---|
| SACP 92 (70.8) |
DHCA 38 (29.2) |
SACP 22 (62.9) |
DHCA 13 (37.1) |
|||
| Intraoperative variables | ||||||
| CPB time (min) | 145 [125-163] | 112 [84-142] | .001 | 113 [98-131] | 81 [65-92] | .003 |
| Crossclamp time (min) | 61 [54-69] | 60 [39-76] | .88 | 48 [39-66] | 37 [24-55] | .36 |
| DHCA time (min) | 0 [0-3] | 40 [28-46] | <.0001 | 0 [0-0] | 22 [16-31] | <.0001 |
| SACP time (min) | 51 [42-59] | - | - | 30 [23-38] | - | - |
| Primary end point | ||||||
| Mortality (in hospital) | 8 (8.7) | 1 (2.6) | .21 | 2 (9.1) | 0 | .26 |
| Patients with neurologic events∗ | 2 (2.2) | 0 | .35 | 2 (9.1) | 0 | .26 |
| Stroke (yes) | 1 (1.1) | 0 | .51 | 2 (9.1) | 0 | .26 |
| Seizure (yes) | 2 (2.2) | 0 | .35 | 1 (4.5) | 0 | .43 |
| Secondary end points | ||||||
| Acute renal failure | 9 (9.8) | 5 (13.2) | .57 | 2 (9.1) | 1 (7.7) | .88 |
| Unplanned catheter reintervention | 5 (5.4) | 1 (2.6) | .48 | 1 (4.5) | 0 | .43 |
| Hospital LOS (d) | 33.5 [25-48] | 34.5 [23-77] | .64 | 22 [16-29] | 18 [13-24] | .43 |
| Postoperative LOS (d) | 25.5 [18.5-38.5] | 24 [16-57] | .80 | 16.5 [8-25] | 15 [11-18] | .45 |
N (%); Median [Q1-Q3]. SACP, Selective antegrade cerebral perfusion; DHCA, deep hypothermic circulatory arrest; CPB, cardiopulmonary bypass; LOS, length of stay.
Six total neurologic events occurring in 4 patients after surgery.
Regression analysis showed no associations for cerebral perfusion strategy with any outcome even after adjusting for age and complexity of repair. We were unable to compute odds for neurologic events because there are no events in the DHCA group (Table 4).
Table 4.
Regression analysis for associations between cerebral perfusion strategy and outcomes
| Variables | SACP vs DHCA ∗OR [95% CI] |
|---|---|
| Primary end point | |
| Mortality (in hospital) | 4.56 [0.54-38.3] |
| Secondary end points | |
| Acute renal failure | 0.55 [0.18-1.69] |
| Unplanned catheter reintervention | 2.17 [0.25-18.94] |
| Postoperative LOS (d) | 1.00 [0.99-1.004] |
Odds for neurologic events were not computed because there are no events in the DHCA group. SACP, Selective antegrade cerebral perfusion; DHCA, deep hypothermic circulatory arrest; OR, odds ratio; LOS, length of stay.
Models adjusted for age and repair type.
Discussion
The optimal way to protect the brain during neonatal arch reconstruction continues to be a matter of debate despite decades of research on this topic. Cerebral perfusion strategies and our ability to monitor cerebral protection have continued to evolve over the last 30 years but without any convincing evidence that one remains superior to the other. This has led centers to reevaluate this topic in the last decade, but without any large-scale randomized trials, we continue to fall short of any conclusive data to suggest an optimal way to protect the brain and visceral organs. The current study examines our institutional data over the past 10 years. We have transitioned over the last decade from DHCA to SACP. Part of this change was based on a theoretical benefit of SACP and that providing some amount of oxygenated blood to the brain should be better than its pure absence to limit ischemic insult, although there are no current data to support its superiority compared with DHCA. The other reason for the transition was institutional preference, which has now led our group to almost exclusively use SACP during aortic arch reconstruction of any type. With this in mind, we wanted to review our data and examine our results comparing the 2 cerebral perfusion techniques.
We examined 165 patients who were a mixture of single ventricle and biventricular circulations. We compared 2 groups, one being “simple” and the other “complex” to best compare patient groups among each other. Our results suggest that there is no difference in stroke, seizures, renal failure, or mortality irrespective of the cerebral perfusion strategy used during aortic arch reconstruction. This held true even when stratified by type of repair, simple or complex. This is not surprising given that similar results have been demonstrated by other centers. Most recently, Hsia and colleagues12 showed that postoperative seizures, and thus a surrogate for neurologic injury, were similar between those undergoing DHCA or SACP. Their cohort was large and heavily weighted toward DHCA, which they corrected for by propensity matching and comparing to a smaller SACP group. Although not significant, our rates of neurologic events were higher among SACP than DHCA. However, the neurologic event rate seen in our SACP group was similar to a previous report by another center who primarily used DHCA.12 The move toward SACP began in the early to mid-2000s after the Boston Circulatory Arrest Trial when DHCA was shown to have more neurologic perturbation compared to low-flow bypass.10 Several institutions started examining whether SACP would provide a benefit in comparison with DHCA, but we still have no data to support one technique over the other. There are many advances that have been made since the original Boston Circulatory Arrest Trial in the early 1990s, which most people still use as the gold standard for comparing the effectiveness of DHCA to other techniques. Alpha stat blood gas management was used as the principal method during that trial, which was later found to be inferior compared with pH stat in a subsequent trial performed by the same institution.13 It is now common practice for pH stat blood gas management to be used during cooling to moderate or deep hypothermia for pediatric cardiac cases. The equipment, safety, and efficiency of the cardiopulmonary bypass machine has drastically improved since the 1990s and early 2000s, which has no doubt improved our ability to safely perform complex neonatal cardiac operations that require aortic arch reconstruction. These factors coupled with continued surgical improvement are reasons why DHCA has likely continued to be noninferior to SACP when studied and compared in the neonatal population over the last 15 years.
A study using the single ventricle reconstruction trial follow-up data showed that SACP had no positive impact on short-term outcomes compared with DHCA.14 The duration of safe DHCA is time sensitive. The inflexion point of when neurologic damage starts to exponentially rise has traditionally been thought of as 40 minutes, which originated from the Boston data approximately 30 years ago. With modern techniques such as pH stat blood gas management and a goal hematocrit more than 30 while on cardiopulmonary bypass, is this time limit still accurate? Most studies, including our own, have similar SACP times compared with DHCA times.5, 6, 7,9 For our complex cases overall, our median DHCA time was 40 minutes compared with 51 minutes for SACP (Table 3). For those who underwent a Norwood procedure within our complex cases, the median DHCA time was 50 minutes compared with 52 minutes for the SACP group (data not shown). A theoretical benefit of using SACP is it gives the surgeon a sense of safety during the operation and a feeling of not needing to rush through the reconstruction, which can provide a calming effect during a stressful environment. Some argue that this can be dangerous, giving the surgeon a false sense of safety. We would argue that because DHCA and SACP times tend to be similar, this is unlikely to be of concern. Although most agree that SACP can safely be performed upward of 60 minutes without increasing neurologic insult, the absolute safe duration is unknown.5, 6, 7,9 Most aortic arch reconstructions of any kind (advancements, patch augmentation, or Norwood reconstruction) can be performed in less than 60 minutes, with the majority being completed between 35 and 50 minutes.5, 6, 7,9 Thus, SACP becomes an attractive option for many surgeons who do not want to feel threatened when approaching and exceeding 40 minutes of neurologic ischemic time with DHCA.
The real question remains: Is there a technique that will provide a better long-term neurocognitive outlook for these patients? This question, although studied, has not had the rigorous attention that it needs. There are multiple studies that have looked at short-term outcomes and some that have used the original Boston data for longer term outcomes.1,5,7, 8, 9, 10,12,14 One could argue that the Boston patients who underwent operation in the original Circulatory Arrest Trial, which has the longest follow-up data available, are not a great comparison considering the advances that have been made with the cardiopulmonary bypass machine and surgical technique. This should be an active area of national research now that we have a large number of patients around the country receiving SACP (43% national STS data6) and many centers still using DHCA routinely (32% national STS data6). A multi-institutional trial with long-term developmental and neurocognitive follow-up data would be extremely valuable to the field of congenital cardiac surgery.
Limitations
Our study has several limitations. First, this was a retrospective study at a single center with limited power and small numbers to generalize to other centers or detect differences in outcomes. Our outcomes may be due to unmeasured variables and not due to cerebral perfusion strategies.
Conclusions
There were no significant short-term differences and a low rate of neurologic events in both groups during aortic arch reconstruction among neonates and infants. Longer follow-up is necessary to evaluate the impact of cerebral perfusion strategy on neurocognitive development later in life.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
This protocol was approved and consent for this study was waived by the Institutional Review Board under exempt category 4 (IRB-300012193, 12/1/2023).
References
- 1.Bellinger D.C., Wypij D., Rivkin M.J., et al. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation. 2011;124:1361–1369. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dent C.L., Spaeth J.P., Jones B.V., et al. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg. 2006;131:190–197. doi: 10.1016/j.jtcvs.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Mahle W.T., Tavani F., Zimmerman R.A., et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106(12 suppl 1):I109–I111. [PubMed] [Google Scholar]
- 4.Andropoulos D.B., Hunter J.V., Nelson D.P., et al. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J Thorac Cardiovasc Surg. 2010;139:543–556. doi: 10.1016/j.jtcvs.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Algra S.O., Jansen N.J., van der Tweel I., et al. Neurological injury after neonatal cardiac surgery: a randomized, controlled trial of 2 perfusion techniques. Circulation. 2014;129:224–233. doi: 10.1161/CIRCULATIONAHA.113.003312. [DOI] [PubMed] [Google Scholar]
- 6.Meyer D.B., Jacobs J.P., Hill K., Wallace A.S., Bateson B., Jacobs M.L. Variation in perfusion strategies for neonatal and infant aortic arch repair: contemporary practice in the STS congenital heart surgery database. World J Pediatr Congenit Heart Surg. 2016;7(5):638–644. doi: 10.1177/2150135116658458. [DOI] [PubMed] [Google Scholar]
- 7.Kornilov I.A., Sinelnikov Y.S., Soinov I.A., et al. Outcomes after aortic arch reconstruction for infants: deep hypothermic circulatory arrest versus moderate hypothermia with selective antegrade cerebral perfusion. Eur J Cardiothorac Surg. 2015;48:e45–e50. doi: 10.1093/ejcts/ezv235. [DOI] [PubMed] [Google Scholar]
- 8.Kulyabin Y.Y., Bogachev-Prokophiev A.V., Soynov I.A., et al. Clinical assessment of perfusion techniques during surgical repair of coarctation of aorta with aortic arch hypoplasia in neonates: a pilot prospective randomized study. Semin Thoracic Surg. 2020;32:860–871. doi: 10.1053/j.semtcvs.2020.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg C.S., Bove E.L., Devaney E.J., et al. A randomized clinical trial of regional cerebral perfusion versus deep hypothermic circulatory arrest: outcomes for infants with functional single ventricle. J Thorac Cardiovasc Surg. 2007;133:880–887. doi: 10.1016/j.jtcvs.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 10.Wypij D., Newburger J.W., Rappaport L.A., et al. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1397–1403. doi: 10.1016/s0022-5223(03)00940-1. [DOI] [PubMed] [Google Scholar]
- 11.Englum B.R., He X., Gulack B.C., et al. Hypothermia and cerebral protection strategies in aortic arch surgery: a comparative effectiveness analysis from the STS Adult Cardiac Surgery Database. Eur J Cardiothorac Surg. 2017;52:492–498. doi: 10.1093/ejcts/ezx133. [DOI] [PubMed] [Google Scholar]
- 12.Hsia J., Abend N.S., Gaynor J.W., et al. Incidence of postoperative seizures in neonates following cardiac surgery with regional cerebral perfusion and deep hypothermic circulatory arrest. JTCVS Open. 2023;16:771–783. doi: 10.1016/j.xjon.2023.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.du Plessis A.J., Jonas R.A., Wypij D., et al. Perioperative effects of alpha-stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. 1997;114:991–1001. doi: 10.1016/S0022-5223(97)70013-8. [DOI] [PubMed] [Google Scholar]
- 14.Migally K., Rettiganti M., Gossett J.M., Reemtsen B., Gupta P. Impact of regional cerebral perfusion on outcomes among neonates undergoing Norwood operation. World J Pediatr Congenit Heart Surg. 2019;10:261–267. doi: 10.1177/2150135118825274. [DOI] [PubMed] [Google Scholar]


