Abstract
Objective
To identify clinicopathologic and genomic features associated with brain metastasis after resection of lung adenocarcinoma (LUAD) and to evaluate survival after brain metastasis.
Methods
Patients who underwent complete resection of stage I-IIIA LUAD between 2011 and 2020 were included. A subset of patients had broad-based panel next-generation sequencing performed on their tumors. Fine-Gray models for the development of brain metastasis were constructed, with death without brain metastasis as a competing risk.
Results
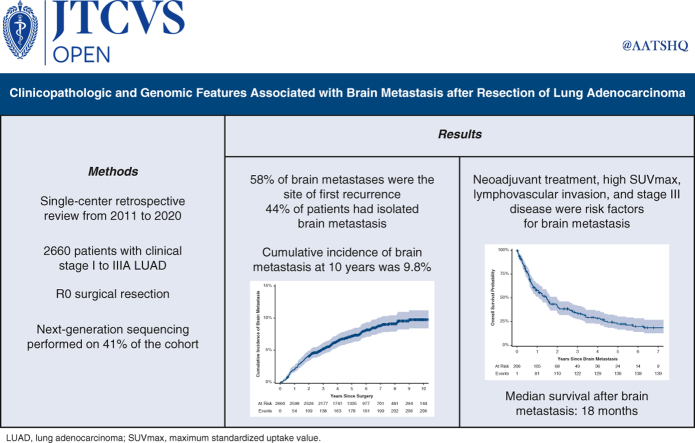
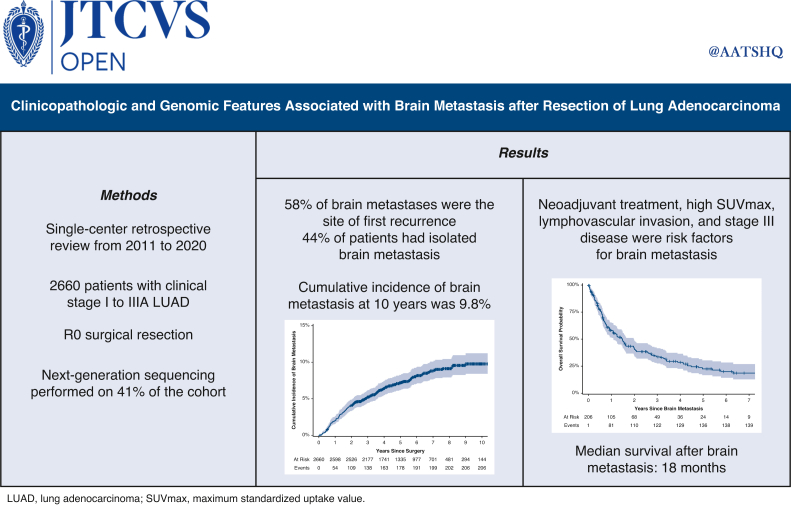
A total of 2660 patients were included. The median duration of follow-up was 71 months (95% confidence interval [CI], 69-73 months). The cumulative incidence of brain metastasis at 10 years was 9.8%. Among patients who developed a brain metastasis, the median time from surgery to brain metastasis was 21 months (interquartile range, 10-42 months). Higher maximum standardized uptake value of the primary tumor, neoadjuvant therapy, lymphovascular invasion, and stage III disease were associated with the development of brain metastasis. Among patients who underwent next-generation sequencing, a multivariable analysis identified neoadjuvant therapy, pathologic stage, and TP53 mutations as associated with development of brain metastasis. The median survival after brain metastasis was 18 months (95% CI, 13-24 months). Better performance status, lack of extracranial metastasis, stereotactic radiosurgery, and targeted therapy were associated with better survival after brain metastasis.
Conclusions
Brain metastasis is common after complete resection of LUAD and often occurs within 2 years. Markers of aggressive tumor biology, including higher maximum standardized uptake value, lymphovascular invasion, and TP53 mutations, and neoadjuvant therapy are associated with brain metastasis.
Key Words: lung adenocarcinoma, brain metastasis, lymphovascular invasion, neoadjuvant therapy
Graphical Abstract
Cumulative incidence of brain metastasis after surgical resection of lung adenocarcinoma.
Central Message.
Brain metastasis is common after complete resection of primary lung adenocarcinoma and occurs more frequently in patients with aggressive pathology and patients who have received neoadjuvant therapy.
Perspective.
Despite the high incidence of brain metastasis after surgical resection of lung adenocarcinoma, National Comprehensive Cancer Network guidelines do not recommend surveillance of the brain or prophylactic treatment. Identifying patients at high risk of brain metastasis may select those who could benefit from postoperative brain imaging or prophylactic treatment.
Despite a decreasing incidence of metastatic disease at the time of diagnosis, lung cancer remains the leading cause of cancer-related death in the United States.1 Even among patients with surgically resected non–small cell lung cancer (NSCLC), 30% to 76% will develop a recurrence and die of their disease.2 The brain is one of the most common sites of NSCLC metastasis; 20% to 40% of patients with NSCLC develop a brain metastasis during their disease course.3 Brain metastases impart a decreased life expectancy, with prior studies showing a median post–brain metastasis survival of only 3 to 12 months.3, 4, 5 Furthermore, patients with brain metastases have significantly worse quality of life than patients with metastases to other sites.6
Despite the high incidence of brain metastasis and the associated morbidity and mortality, Current National Comprehensive Cancer Network (NCCN) guidelines7 do not recommend central nervous system (CNS) surveillance after resection of NSCLC. If patients with a high risk of brain metastasis after surgical resection can be identified, surveillance or CNS-directed therapies may prove beneficial. Several studies have shown that lung adenocarcinoma (LUAD) histology is a significant risk factor for the development of brain metastasis in patients with NSCLC; however, to date no studies have investigated risk factors in patients with resectable LUAD alone.3, 4, 5 Consequently, we sought to determine the incidence of brain metastasis after complete resection of LUAD, identify clinicopathologic and genomic features associated with brain metastases, and elucidate factors that affect survival after detection of brain metastases in this population.
Methods
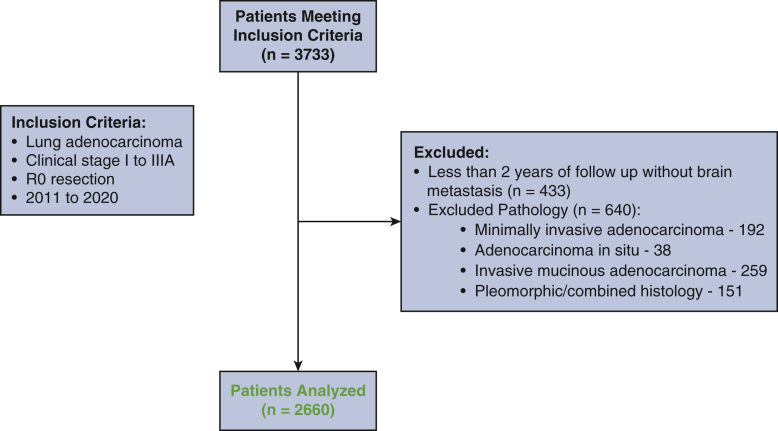
After approval from our Institutional Review Board (approval 18-931; approved September 7, 2018, with a waiver of individual consent), we performed a retrospective review of a prospectively maintained database. Patients who underwent complete resection of clinical stage I-IIIA LUAD (according to the eighth edition of the American Joint Committee on Cancer staging manual) between 2011 and 2020 were included. Exclusion criteria included incomplete resection; minimally invasive, in situ, or mucinous adenocarcinoma; and combined histologic types. Patients who did not have a recurrence in the brain with less than 2 years of follow-up also were excluded (Figure E1).
Figure E1.
Consort diagram.
Information on patient demographics, staging, surgery, pathology, recurrence, and survival was collected. All charts were reviewed manually to confirm the presence or absence of brain metastasis throughout the disease course. Brain metastasis was defined as a metastasis to the brain or leptomeningeal disease and was identified with imaging and/or molecular profiling.
As part of their clinical care, a subset of patients had next-generation sequencing (NGS) performed on their primary tumor using MSK-IMPACT, a broad-based NGS panel consisting of 341 to 505 cancer-related genes.8 The Wilcoxon rank-sum test was used to compare distributions of tumor mutational burden, fraction of genome altered, and mutations between groups. Only genes mutated in at least 2% of the population were included in the mutational analysis. P values were adjusted to correct for multiple comparisons using the false-discovery rate.
The primary endpoint was development of a brain metastasis, measured by cumulative incidence to account for the competing event of death, which would preclude the subsequent development of a brain metastasis. The secondary endpoint was survival after brain metastasis. Patient demographic and treatment details were summarized using median and interquartile range (IQR) for continuous variables and count and percentage for categorical variables. Fine-Gray models were used to estimate the association between clinicopathologic characteristics and development of a brain metastasis. Death without a brain metastasis was treated as a competing event. Characteristics that were significant on univariable analysis were included in the multivariable analysis. Survival after brain metastasis was calculated from the date of diagnosis of the brain metastasis to the date of death and was estimated using the Kaplan-Meier approach. Patients were censored on the date of the last available follow-up. Univariable and multivariable Cox models were constructed to estimate the association between patient factors and survival after brain metastasis. All statistical tests were 2-tailed, with a type I error rate (α) of 0.05. Statistical analyses were conducted using R version 4.3.2 (R Core Development Team).
Results
During the study period, a total of 2660 patients met the inclusion criteria. Most were white, ever-smokers, and had stage I disease (Table 1). Ten percent of the patients (n = 260 of 2660) received neoadjuvant therapy, and 26 of these patients had a pathologic complete response (ypT0N0) after therapy and were staged as pathologic stage 0. Adjuvant therapy was administered to 19% (n = 503 of 2660); the most common regimen for both was chemotherapy alone (Table E1). Most patients underwent lobectomy (61%; n = 1617 of 2660), and 37% of the tumors (n = 959 of 2612) had lymphovascular invasion (LVI) (Table 1).
Table 1.
Patient demographic characteristics (N = 2660)
| Characteristic | Value |
|---|---|
| Age at surgery, y, median (IQR) | 68 (62-74) |
| Sex, n (%) | |
| Female | 1728 (65) |
| Male | 932 (35) |
| Race, n (%) | |
| White | 2257 (85) |
| Asian | 192 (7) |
| Black | 109 (4) |
| Other | 102 (4) |
| Smoking history, n (%) | |
| Ever | 2075 (78) |
| Never | 585 (22) |
| Smoking pack-years, median (IQR) (N = 2656) | 23 (2-42) |
| Initial clinical stage, n (%) | |
| I | 2102 (79) |
| II | 329 (12) |
| IIIA | 229 (9) |
| SUVmax of the primary tumor, median (IQR) | 3.5 (1.8-7.7) |
| Neoadjuvant therapy, n (%) | 260 (10) |
| Procedure, n (%) | |
| Lobectomy | 1617 (61) |
| Pneumonectomy or bilobectomy | 59 (2) |
| Segmentectomy | 356 (13) |
| Wedge | 628 (24) |
| Lymphovascular invasion, n (%) (N = 2612) | 959 (37) |
| Visceral pleural invasion, n (%) (N = 2338) | 441 (19) |
| Pathologic stage, n (%) | |
| 0 | 26 (1) |
| I | 1994 (75) |
| II | 331 (12) |
| III | 309 (12) |
| Adjuvant therapy, n (%) | 503 (19) |
IQR, Interquartile range; SUVmax, maximum standardized uptake value.
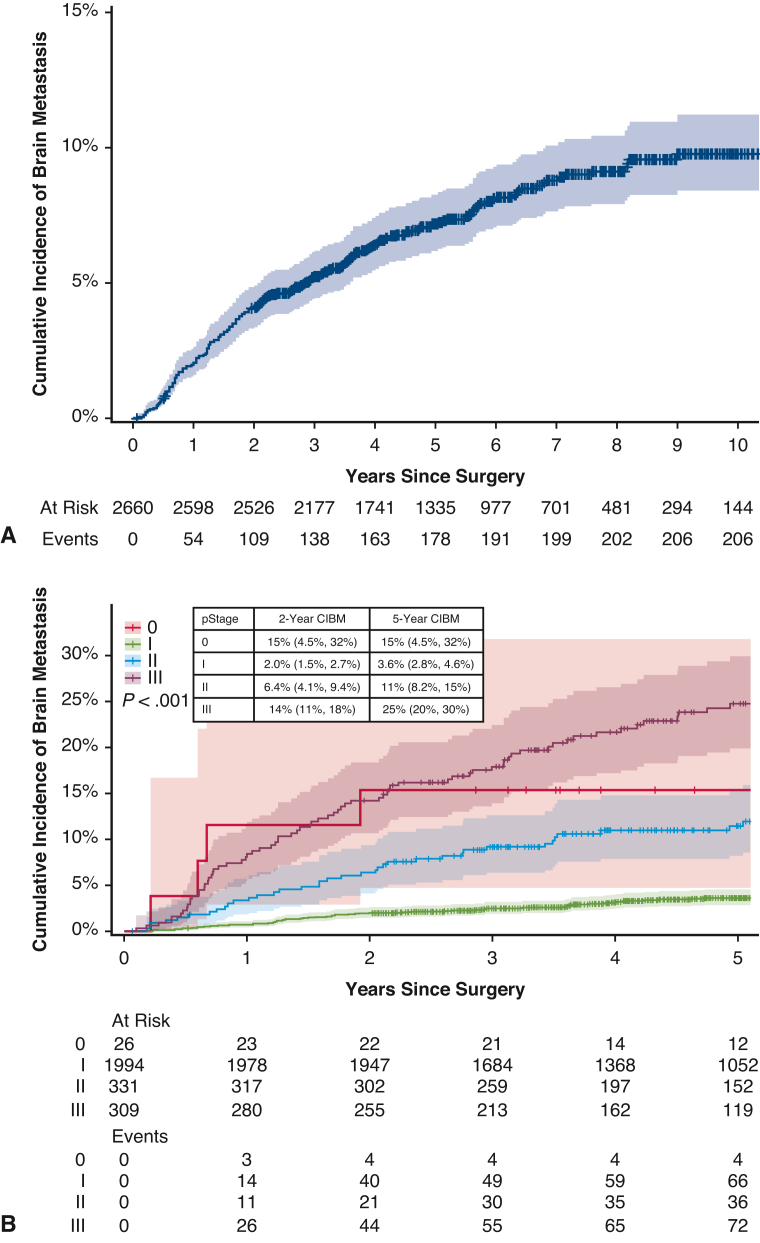
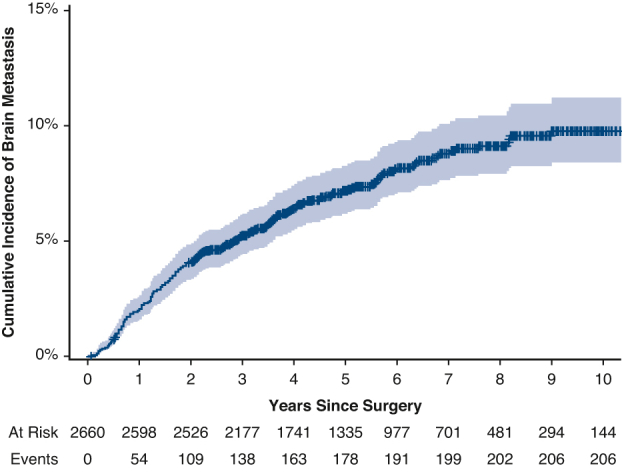
The median follow-up was 71 months (95% confidence interval [CI], 69-73 months). The cumulative incidence of brain metastasis was 4.1% (95% CI, 3.4%-4.9%) at 2 years and 9.8% (95% CI, 8.4%-11%) at 10 years (Figure 1). This differed significantly by stage, as the 2-year cumulative incidence was 2.0% (95% CI, 1.5%-2.7%) for patients with stage I disease but 25% (95% CI, 20%-30%) for patients with stage III disease. Among the 206 patients who developed a brain metastasis, the median time from surgery to detection of the brain metastasis was 21 months (IQR, 10-42 months).
Figure 1.
Cumulative incidence (with 95% confidence interval) of brain metastasis (CIBM) after surgical resection of lung adenocarcinoma in the overall cohort (A) and by pathologic stage (B).
Preoperative brain imaging was performed in 37% of the patients (n = 992 of 2660) in the overall cohort and in 65% (n = 134 of 206) of those who developed a brain metastasis; rates of imaging increased with disease stage (Table E2). Furthermore, one-half (n = 74 of 158) of the patients who did not undergo preoperative brain imaging and whose disease was upstaged at the time of surgery underwent postoperative brain imaging; of these patients, 4% (n = 3 of 74) had a brain metastasis diagnosed on postoperative imaging.
Of the patients who had disease recurrence after surgery, 36% (n = 206 of 573) had a recurrence in the brain during their disease course. The brain was the site of first recurrence in 58% of patients (n = 119 of 206) who had a recurrence in the brain. Most of the patients with recurrence (n = 132 of 206) were symptomatic at the time of diagnosis, and nearly one-half of these patients (n = 89 of 206) had isolated brain metastases and did not have recurrence in any extracranial location during subsequent follow-up (Tables E3 and E4). The median number of brain metastases at diagnosis was 2 (IQR, 1-3), and the median size of these metastases was 1.6 cm (IQR, 0.9-2.7 cm) (Table E5).
On multivariable analysis, a higher maximum standardized uptake value (SUVmax) of the primary tumor on preoperative positron emission tomography scan, receipt of neoadjuvant therapy, LVI, and pathologic stage III disease were associated with the development of a brain metastasis (Table 2). A subset analysis of patients who developed brain metastases within 2 years of surgery showed similar results, except that visceral pleural invasion, and not LVI, was a significant pathologic risk factor (Table E6). Additional subset analyses of patients who developed isolated brain metastases had similar results (Table E7).
Table 2.
Clinicopathologic features associated with brain metastasis
| Clinicopathologic feature | Univariable analysis |
Multivariable analysis |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age at surgery | 0.97 (0.96-0.98) | <.001 | 0.99 (0.97-1.00) | .087 |
| Smoking pack-y | 1.00 (0.99-1.00) | .7 | — | — |
| SUVmax of the primary tumor | 1.08 (1.06-1.09) | <.001 | 1.04 (1.01-1.06) | <.001 |
| Neoadjuvant therapy | 6.12 (4.59-8.16) | <.001 | 2.39 (1.62-3.53) | <.001 |
| Procedure (vs lobectomy) | ||||
| Pneumonectomy or bilobectomy | 1.93 (1.02-3.64) | .042 | 0.92 (0.43-1.96) | .8 |
| Segmentectomy | 0.29 (0.15-0.54) | <.001 | 0.63 (0.32-1.25) | .2 |
| Wedge resection | 0.34 (0.22-0.53) | <.001 | 0.81 (0.49-1.33) | .4 |
| Lymphovascular invasion | 3.05 (2.29-4.06) | <.001 | 1.46 (1.02-2.10) | .039 |
| Visceral pleural invasion | 2.37 (1.76-3.19) | <.001 | 1.20 (0.86-1.67) | .3 |
| Pathologic stage (vs I) | ||||
| 0 | 4.59 (1.60-13.1) | .005 | 1.09 (0.30-4.02) | .9 |
| II | 3.66 (2.54-5.28) | <.001 | 1.54 (0.94-2.71) | .085 |
| III | 7.47 (5.47-10.2) | <.001 | 2.21 (1.18-4.11) | .013 |
| EGFR mutation present | 1.30 (0.95-1.78) | .10 | — | — |
| Adjuvant therapy | 4.37 (3.32-5.76) | <.001 | 1.42 (0.89-2.28) | .14 |
Bold type indicates statistical significance. HR, Hazard ratio; CI, confidence interval; SUVmax, maximum standardized uptake value.
Forty-one percent of patients in the overall cohort (n = 1085 of 2660) had targeted NGS performed on their primary tumor. Tumors that metastasized to the brain had a higher tumor mutational burden, higher fraction of genome altered, and more commonly had TP53 and NKX2-1 mutations compared with tumors that did not metastasize to the brain (Tables E8 and E9). When genomic features were combined with statistically significant clinicopathologic features, neoadjuvant therapy, pathologic stage, and TP53 mutations were independently associated with development of a brain metastasis (Table 3).
Table 3.
Clinicopathologic and genomic features associated with brain metastasis
| Clinicopathologic or genomic feature | Multivariable analysis |
|
|---|---|---|
| HR (95% CI) | P value | |
| SUVmax of the primary tumor | 1.01 (0.98-1.05) | .4 |
| Neoadjuvant therapy | 2.10 (1.19-3.70) | .010 |
| Lymphovascular invasion | 1.73 (0.92-3.25) | .088 |
| Pathologic stage (vs I) | ||
| 0 | 0 (0.00-0.00) | <.001 |
| II | 2.00 (0.99-4.04) | .054 |
| III | 2.67 (1.32-5.42) | .006 |
| TP53 mutation | 1.67 (1.01-2.77) | .046 |
| NKX2-1 mutation | 1.80 (0.85-3.78) | .12 |
| Fraction of genome altered | 3.32 (0.81-13.6) | .10 |
| Tumor mutational burden | 1.00 (0.98-1.02) | >.9 |
Bold type indicates statistical significance. HR, Hazard ratio; CI, confidence interval; SUVmax, maximum standardized uptake value.
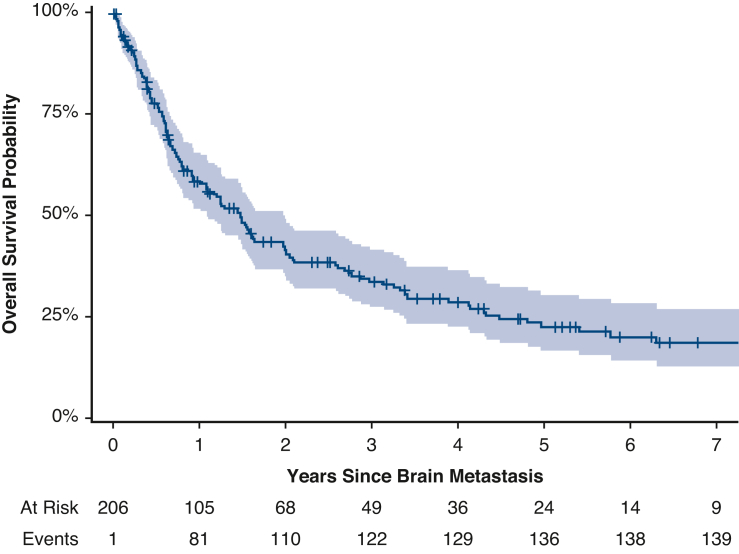
The median overall survival after diagnosis of a brain metastasis was 18 months (95% CI, 13-24 months) (Figure 2), and 5-year overall survival after diagnosis of a brain metastasis was 22% (95% CI, 17%-30%). Patients underwent a variety of treatments for their brain metastases; most patients (57%; n = 118 of 206) underwent stereotactic radiosurgery (SRS) (Table E5). On multivariable analysis, higher performance status, lack of extracranial metastasis, SRS, and targeted therapy were associated with better survival after diagnosis of a brain metastasis (Table 4).
Figure 2.
Overall survival (95% confidence interval) after diagnosis of brain metastasis.
Table 4.
Clinicopathologic features associated with survival after brain metastasis
| Clinicopathologic feature | Univariable analysis |
Multivariable analysis |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age at diagnosis of brain metastasis | 1.02 (1.00-1.04) | .036 | 1.00 (0.98-1.02) | >.9 |
| Karnofsky performance status | 0.97 (0.95-0.98) | <.001 | 0.98 (0.96-0.99) | .008 |
| Number of brain metastases | 1.04 (1.02-1.07) | .002 | 1.02 (0.99-1.05) | .2 |
| Location of brain metastasis (vs both supratentorial and infratentorial) | ||||
| Supratentorial | 0.71 (0.48-1.05) | .086 | — | — |
| Infratentorial | 0.69 (0.35-1.35) | .3 | — | — |
| Leptomeningeal disease only | 1.74 (0.88-3.41) | .11 | — | — |
| Size of largest metastasis | 0.93 (0.81-1.08) | .3 | — | — |
| Extracranial metastasis present | 1.73 (1.24-2.41) | .001 | 1.52 (1.00-2.32) | .049 |
| Craniotomy | 0.48 (0.33-0.70) | <.001 | 0.75 (0.45-1.27) | .3 |
| Stereotactic radiosurgery | 0.41 (0.29-0.58) | <.001 | 0.35 (0.21-0.57) | <.001 |
| Whole-brain radiation | 1.58 (1.11-2.26) | .012 | 1.12 (0.67-1.85) | .7 |
| Targeted therapy | 0.63 (0.43-0.93) | .019 | 0.42 (0.26-0.70) | <.001 |
| Immunotherapy | 1.11 (0.76-1.61) | .6 | — | — |
| Chemotherapy | 1.03 (0.73-1.45) | .9 | — | — |
Bold type indicates statistical significance. HR, Hazard ratio; CI, confidence interval.
Discussion
To our knowledge, this is the largest study to examine risk factors for brain metastasis after surgical resection of NSCLC and the only study to analyze this topic after resection for LUAD specifically (Figure 3). Brain metastasis was common after complete resection of LUAD, with a cumulative incidence of 9.8% at 10 years, 30% for patients with stage III disease. Previous studies have reported rates of brain metastasis of 5% to 31% after resection of NSCLC, with rates varying by differences in the initial stage and duration of follow-up.5,9, 10, 11, 12
Figure 3.
Design, results, and implications of our study.
In line with previous studies, in the present study, LVI and pathologic stage were associated with development of a brain metastasis.5,9,13 Although SUVmax has not been previously identified as a risk factor for brain metastasis in patients with LUAD, it is a known factor in poor prognosis for patients with NSCLC in several other settings.14
Our study also shows that neoadjuvant therapy was associated with the development of a brain metastasis after surgical resection of LUAD. Previous studies that investigated risk factors for brain metastasis in similar cohorts excluded patients who underwent neoadjuvant therapy,9,13 and thus this association has not been examined until now. Furthermore, among patients in our cohort with the same disease stage, those who underwent neoadjuvant therapy had significantly higher rates of brain metastases but lower rates of extracranial metastases compared to those who underwent surgery alone (Table 5). One possible explanation for this association is the known poor penetrance and efficacy of chemotherapy in the brain.3,15, 16, 17 Cox and colleagues16 reported that the addition of chemotherapy to definitive radiation in patients with unresectable or medically inoperable stage II-III NSCLC did not reduce the rate of brain metastases despite significantly reducing metastases to other sites. Moreover, in a randomized controlled trial of neoadjuvant chemotherapy followed by surgery versus surgery alone, the rate of brain metastasis was twice as high in the neoadjuvant chemotherapy group compared to the surgery alone group.15 Thus, patients who undergo neoadjuvant therapy with poor CNS penetrance may have a higher risk of brain metastasis, owing to delayed surgery.
Table 5.
Site of metastasis by neoadjuvant therapy and pathologic stage
| Pathologic stage, site of metastasis | Overall, n/N (%) | Neoadjuvant therapy, n/N (%) | No neoadjuvant therapy, n/N (%) | P value |
|---|---|---|---|---|
| Stage I | <.001 | |||
| Brain | 77/277 (28) | 12/18 (67) | 65/259 (25) | |
| Extracranial only | 200/277 (72) | 6/18 (33) | 194/259 (75) | |
| Stage II | .018 | |||
| Brain | 45/128 (35) | 13/23 (57) | 32/105 (30) | |
| Extracranial only | 83/128 (65) | 10/23 (43) | 73/105 (70) | |
| Stage III | .004 | |||
| Brain | 80/171 (47) | 45/76 (59) | 35/95 (37) | |
| Extracranial only | 91/171 (53) | 31/76 (41) | 60/95 (63) |
Bold type indicates statistical significance.
Of note, CNS penetrance has been understudied in the expanding use of neoadjuvant immunotherapy. Although recent randomized trials18,19 have reported local versus distant recurrence, site-specific recurrence is an important outcome for evaluating the CNS penetrance of these modern agents, particularly as they are being used in patients with earlier-stage disease. The PACIFIC trial may provide some insight into the CNS efficacy of immunotherapy, as the addition of durvalumab to definitive chemoradiation decreased brain metastases from 12% to 7%.20 Additionally, targeted therapies appear to have efficacy in the CNS; the ADAURA trial showed an 85% decrease in CNS disease or death with the use of adjuvant osimertinib.21
From a tumor genomic perspective, only TP53 mutations were associated with a higher risk of brain metastasis, which are known to be more common in primary LUAD tumors that metastasize.22 Contrary to findings from studies performed primarily in Asian populations, EGFR mutations were not associated with development of brain metastases in our Western cohort of patients with early-stage disease.3,4,13 Differences in EGFR mutation rates between patients of Western and Asian populations may explain this discrepancy, as the one other study analyzing brain metastases in white patients also found no association with EGFR mutations.23,24
Survival after brain metastasis (median, 18 months) was longer in our cohort than previous estimates of 3 to 12 months for patients with NSCLC of any stage.3, 4, 5 Because the NCCN guidelines do not recommend CNS surveillance,7 the vast majority of brain metastases were diagnosed when patients became symptomatic. Yokoi and colleagues25 surveilled 128 patients with NSCLC with brain computed tomography for 2 years after resection and found better survival in patients with brain metastases diagnosed on surveillance. In our cohort, patients who had a high SUVmax on positron emission tomography, underwent neoadjuvant therapy, had visceral pleural invasion, and had stage III disease were at an increased risk of brain metastases in the 2 years after their operation. Given the survival benefit of craniotomy and/or SRS in patients with small, isolated brain metastases, CNS surveillance for 2 years postoperatively in these high-risk patients may be beneficial.7,17
Limitations of this study include its retrospective nature, which restricted the variables available to include in our analyses. NGS was performed on less than one-half of the patients in our cohort, potentially obscuring the identification of other genomic factors associated with brain metastases. Finally, we could not perform a subgroup analysis of the impact of the type of neoadjuvant therapy on brain metastasis development owing to an insufficient sample size.
Conclusions
Brain metastasis was common after surgical resection of LUAD, even among a cohort of patients with predominantly stage I disease. Brain metastases occurred more commonly within 2 years of the operation; however, cases were diagnosed up to 10 years after the operation. The brain was most commonly the site of first recurrence, and almost one-half of patients developed isolated brain metastases. Higher SUVmax, neoadjuvant therapy, LVI, and stage were associated with the development of a brain metastasis after surgical resection of LUAD. Further investigation of the benefit of postoperative CNS surveillance in high-risk populations is warranted.
Conflict of Interest Statement
G.R. has financial relationships with Scanlan International, Medtronic, and Merck. J.E.C serves as a consultant for AstraZeneca, Bristol-Myers Squibb, Flame Biosciences, Regeneron-Sanofi, Guardant Health, and Arcus Biosciences and receives institutional research funding from AztraZeneca, Bristol-Myers Squibb, Merck, and Novartis. P.I. serves on the advisory board of AstraZeneca and receives institutional grant support from Incyte. D.G. reports research funding from Merck, AstraZeneca, Varian, and Bristol Myers Squibb and serves on advisory boards for MedLearning Group, Medtronic, GRAIL, Johnson & Johnson, AstraZeneca, and Varian. P.S.A. reports research funding from ATARA Biotherapeutics; is a scientific advisory board member and consultant for ATARA Biotherapeutics, Bayer, Bio4T2, Carisma Therapeutics, Imugene, ImmPactBio, Johnson & Johnson, Orion, and Outpace Bio; has patents, royalties, and intellectual property for T cell therapies, which have been licensed to ATARA Biotherapeutics; and has an issued patent method for detection of cancer cells using virus and pending patent applications on PD-1 dominant negative receptor, a wireless pulse-oximetry device, and an ex vivo malignant pleural effusion culture system. Memorial Sloan Kettering Cancer Center has licensed intellectual property related to mesothelin-targeted chimeric antigen receptors and T cell therapies to ATARA Biotherapeutics. B.J.P. has received honoraria from Intuitive Surgical, AstraZeneca, and Medtronic; serves as a consultant to CEEVRA; and has received institutional research support from Intuitive Surgical. J.M.I. has served on advisory boards for AstraZeneca and Merck and as an uncompensated steering board member for Genentech; has received institutional research support from ArcherDx/Invitae, Guardant Health, GRAIL, and Intuitive Surgical and travel support from Intuitive Surgical; and has equity/ownership interest in LumaCyte. M.J.B. is a consultant for AstraZeneca, Iovance Biotherapeutics, and Intuitive Surgical and receives research support from Obsidian Therapeutics. S.S. serves on the AstraZeneca Advisory Board. D.M. serves on a steering committee for AstraZeneca; serves as a consultant for Johnson & Johnson, Bristol-Myers Squibb, AstraZeneca, and Boston Scientific; and has been an invited speaker for Merck and Genentech. D.R.J. serves on an advisory council for AstraZeneca and receives research grant support from Merck.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
Funding was provided by National Institutes of Health/National Cancer Institute Grants R01 CA217169 and R01 CA240472 (to D.R.J.) and Cancer Center Support Grant P30 CA008748 to Memorial Sloan Kettering Cancer Center.
Approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board (18-391; September 7, 2018), with a waiver of individual consent.
Appendix E1
Table E1.
Neoadjuvant and adjuvant therapy
| Therapy | Neoadjuvant therapy∗ | Adjuvant therapy† |
|---|---|---|
| Overall cohort (N = 2660) | ||
| Chemotherapy alone | 191 (73) | 270 (54) |
| Chemoradiation | 21 (8) | 95 (19) |
| Radiation alone | 0 | 88 (17) |
| Targeted therapy alone | 14 (5) | 36 (7) |
| Chemotherapy and targeted therapy | 5 (2) | 2 (0.4) |
| Targeted therapy and radiation | 0 | 5 (1) |
| Immunotherapy alone | 24 (9) | 3 (0.6) |
| Chemotherapy and immunotherapy | 5 (2) | 3 (0.6) |
| Immunotherapy and radiation | 0 | 1 (0.2) |
| Patients who developed brain metastasis (N = 206) | ||
| Chemotherapy alone | 56 (76) | 44 (44) |
| Chemoradiation | 8 (11) | 16 (16) |
| Radiation alone | 0 | 31 (31) |
| Targeted therapy alone | 4 (5) | 5 (5) |
| Chemotherapy and targeted therapy | 4 (5) | 0 |
| Targeted therapy and radiation | 0 | 2 (2) |
| Immunotherapy alone | 2 (3) | 1 (1) |
N = 260 for the overall cohort; N = 74 for patients who developed brain metastasis.
N = 503 for the overall cohort; N = 99 for patients who developed brain metastasis.
Table E2.
Preoperative brain imaging by stage and cohort
| Clinical stage | No. (%) |
|---|---|
| Overall cohort (N = 2660) | |
| IA (N = 1859) | 459 (25) |
| IB (N = 242) | 112 (46) |
| IIA (N = 93) | 56 (61) |
| IIB (N = 237) | 159 (67) |
| IIIA (N = 229) | 206 (90) |
| Patients who developed brain metastasis (N = 206) | |
| IA (N = 80) | 28 (35) |
| IB (N = 18) | 9 (50) |
| IIA (N = 9) | 5 (56) |
| IIB (N = 35) | 29 (83) |
| IIIA (N = 64) | 63 (98) |
Table E3.
Indications for brain imaging that resulted in a diagnosis of brain metastasis (N = 206)
| Indication | No. (%) |
|---|---|
| Symptomatic | 132 (64) |
| Workup after first recurrence | 42 (20) |
| Incidental | 9 (4) |
| Workup after progression | 10 (5) |
| Postoperative imaging | 3 (1) |
| Unknown | 10 (5) |
Table E4.
Sites of metastasis in patients with brain metastases (N = 206)
| Site | No. (%) |
|---|---|
| Brain only | 89 (43) |
| Lung | 59 (29) |
| Lymph node | 61 (30) |
| Bone | 58 (28) |
| Pleura | 18 (9) |
| Liver | 32 (16) |
| Adrenal | 24 (12) |
| Other | 14 (7) |
Table E5.
Characteristics and treatments for brain metastasis (N = 206)
| Characteristic or treatment | Value |
|---|---|
| Age at diagnosis of brain metastasis, y, median (IQR) | 68 (61-76) |
| Karnofsky performance status, median (IQR) | 80 (73-90) |
| Number of brain metastases, median (IQR) | 2 (1-3) |
| Location of brain metastasis, n (%) | |
| Supratentorial | 121 (59) |
| Infratentorial | 16 (8) |
| Both supratentorial and infratentorial | 50 (24) |
| Leptomeningeal disease only | 12 (6) |
| Unknown | 7 (3) |
| Size of largest metastasis, cm, median (IQR) | 1.6 (0.9-2.7) |
| Extracranial metastasis present, n (%) | 87 (42) |
| Craniotomy, n (%) | 66 (32) |
| Stereotactic radiosurgery, n (%) | 118 (57) |
| Whole-brain radiation, n (%) | 49 (24) |
| Targeted therapy, n (%) | 53 (26) |
| Immunotherapy, n (%) | 52 (25) |
| Chemotherapy, n (%) | 71 (34) |
IQR, Interquartile range.
Table E6.
Clinicopathologic features associated with brain metastasis within 2 years of surgery
| Clinicopathologic feature | Univariable analysis |
Multivariable analysis |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age at surgery | 0.99 (0.97-1.01) | .2 | — | — |
| Smoking pack-years | 1.01 (1.00-1.01) | .019 | 1.01 (1.00-1.01) | .050 |
| SUVmax of the primary tumor | 1.08 (1.06-1.10) | <.001 | 1.05 (1.03-1.08) | <.001 |
| Neoadjuvant therapy | 4.76 (2.92-7.78) | <.001 | 3.36 (2.05-5.52) | <.001 |
| Procedure (vs lobectomy) | ||||
| Pneumonectomy or bilobectomy | 1.93 (1.02-3.64) | .042 | — | — |
| Segmentectomy | 0.29 (0.15-0.54) | <.001 | — | — |
| Wedge resection | 0.34 (0.22-0.53) | <.001 | — | — |
| Lymphovascular invasion | 1.63 (1.00-2.67) | .051 | — | — |
| Visceral pleural invasion | 1.82 (1.17-2.81) | .007 | 1.58 (1.02-2.44) | .040 |
| Pathologic stage (vs I) | ||||
| 0 | 8.37 (2.96-23.7) | <.001 | 1.11 (0.30-4.11) | .9 |
| II | 3.26 (1.92-5.52) | <.001 | 1.53 (0.83-2.85) | .2 |
| III | 7.61 (4.96-11.7) | <.001 | 2.85 (1.70-4.80) | <.001 |
| EGFR mutation present | 0.88 (0.54-1.41) | .6 | — | — |
| Adjuvant therapy | 1.11 (0.55-2.24) | .8 | — | — |
Bold type indicates statistical significance. HR, Hazard ratio; CI, confidence interval; SUVmax, maximum standardized uptake value.
Table E7.
Clinicopathologic features associated with isolated brain metastasis
| Clinicopathologic feature | Multivariable analysis |
|
|---|---|---|
| HR (95% CI) | P value | |
| Age at surgery | 1.00 (0.98-1.03) | .9 |
| SUVmax of the primary tumor | 1.04 (1.00-1.07) | .044 |
| Neoadjuvant therapy | 1.76 (0.93-3.37) | .085 |
| Procedure (vs lobectomy) | ||
| Pneumonectomy or bilobectomy | 1.08 (0.37-3.16) | .9 |
| Segmentectomy | 0.95 (0.37-2.41) | >.9 |
| Wedge resection | 0.47 (0.16-1.36) | .2 |
| Lymphovascular invasion | 1.41 (0.81-2.46) | .2 |
| Visceral pleural invasion | 1.22 (0.75-1.99) | .4 |
| Pathologic stage (vs stage I) | ||
| 0 | 4.76 (1.08-21.0) | .040 |
| II | 3.85 (1.71-8.67) | .001 |
| III | 4.95 (1.74-14.1) | .003 |
| Adjuvant therapy | 0.84 (0.44-1.60) | .6 |
Bold type indicates statistical significance. HR, Hazard ratio; CI, confidence interval; SUVmax, maximum standardized uptake value.
Table E8.
Genomic differences between tumors that did and did not metastasize to the brain
| Gene | Mutational analysis, number altered |
||
|---|---|---|---|
| Brain metastasis cohort (N = 77) | No brain metastasis cohort (N = 1008) | Q value | |
| TP53 | 45 (58) | 358 (36) | .002 |
| NKX2-1 | 8 (10) | 34 (3.3) | .041 |
| RB1 | 5 (6.5) | 22 (2.2) | .19 |
| ERBB2 | 5 (6.5) | 32 (3.2) | .53 |
| MYC | 5 (6.5) | 32 (3.2) | .53 |
| SETD2 | 5 (6.5) | 45 (4.5) | .88 |
| CDK4 | 5 (6.5) | 47 (4.7) | .88 |
| STK11 | 11 (14) | 123 (12) | .88 |
| EGFR | 21 (27) | 56 (31) | .88 |
| RBM10 | 9 (12) | 145 (14) | .88 |
| TERT | 6 (7.8) | 68 (6.7) | .88 |
| KRAS | 29 (38) | 359 (36) | .89 |
| MDM2 | 5 (6.5) | 63 (6.3) | .93 |
| CDKN2A | 5 (6.5) | 70 (6.9) | 1 |
Bold type indicates statistical significance.
Table E9.
Global genomic features
| Feature | Brain metastasis cohort, median (IQR) | No brain metastasis cohort, median (IQR) | P value |
|---|---|---|---|
| Tumor mutational burden | 6.1 (3.5-11.8) | 5.3 (2.6-8.8) | .005 |
| Fraction of genome altered | 0.10 (0.02-0.25) | 0.04 (0.01-0.13) | <.001 |
Bold type indicates statistical significance. IQR, Interquartile range.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Pignon J.P., Tribodet H., Scagliotti G.V., et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 3.Chen S., Hua X., Jia J., et al. Risk factors for brain metastases in patients with non–small cell lung cancer: a meta-analysis of 43 studies. Ann Palliat Med. 2021;10(4):3657–3672. doi: 10.21037/apm-20-1722. [DOI] [PubMed] [Google Scholar]
- 4.An N., Jing W., Wang H., et al. Risk factors for brain metastases in patients with non–small-cell lung cancer. Cancer Med. 2018;7(12):6357–6364. doi: 10.1002/cam4.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Won Y.W., Joo J., Yun T., et al. A nomogram to predict brain metastasis as the first relapse in curatively resected non–small cell lung cancer patients. Lung Cancer. 2015;88(2):201–207. doi: 10.1016/j.lungcan.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Peters S., Bexelius C., Munk V., Leighl N. The impact of brain metastasis on quality of life, resource utilization and survival in patients with non–small-cell lung cancer. Cancer Treat Rev. 2016;45:139–162. doi: 10.1016/j.ctrv.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology: non–small cell lung cancer, version 3.2023. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- 8.Cheng D.T., Mitchell T.N., Zehir A., et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17(3):251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hubbs J.L., Boyd J.A., Hollis D., Chino J.P., Saynak M., Kelsey C.R. Factors associated with the development of brain metastases: analysis of 975 patients with early-stage non–small cell lung cancer. Cancer. 2010;116(21):5038–5046. doi: 10.1002/cncr.25254. [DOI] [PubMed] [Google Scholar]
- 10.Karacz C.M., Yan J., Zhu H., Gerber D.E. Timing, sites, and correlates of lung cancer recurrence. Clin Lung Cancer. 2020;21(2):127–135.e3. doi: 10.1016/j.cllc.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yun P.J., Wang G.C., Chen Y.Y., et al. Brain metastases in resected non–small cell lung cancer: the impact of different tyrosine kinase inhibitors. PLoS One. 2019;14(5) doi: 10.1371/journal.pone.0215923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carolan H., Sun A.Y., Bezjak A., et al. Does the incidence and outcome of brain metastases in locally advanced non–small cell lung cancer justify prophylactic cranial irradiation or early detection? Lung Cancer. 2005;49(1):109–115. doi: 10.1016/j.lungcan.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Deng C., Zhang Y., Fu F., et al. Genetic-pathological prediction for timing and site-specific recurrence pattern in resected lung adenocarcinoma. Eur J Cardiothorac Surg. 2021;60(5):1223–1231. doi: 10.1093/ejcts/ezab288. [DOI] [PubMed] [Google Scholar]
- 14.Berghmans T., Dusart M., Paesmans M., et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non–small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol. 2008;3(1):6–12. doi: 10.1097/JTO.0b013e31815e6d6b. [DOI] [PubMed] [Google Scholar]
- 15.Gilligan D., Nicolson M., Smith I., et al. Preoperative chemotherapy in patients with resectable non–small cell lung cancer: results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet. 2007;369(9577):1929–1937. doi: 10.1016/S0140-6736(07)60714-4. [DOI] [PubMed] [Google Scholar]
- 16.Cox J.D., Scott C.B., Byhardt R.W., et al. Addition of chemotherapy to radiation therapy alters failure patterns by cell type within non–small cell carcinoma of lung (NSCCL): analysis of radiation therapy oncology group (RTOG) trials. Int J Radiat Oncol Biol Phys. 1999;43(3):505–509. doi: 10.1016/s0360-3016(98)00429-5. [DOI] [PubMed] [Google Scholar]
- 17.Suh J.H., Kotecha R., Chao S.T., Ahluwalia M.S., Sahgal A., Chang E.L. Current approaches to the management of brain metastases. Nat Rev Clin Oncol. 2020;17(5):279–299. doi: 10.1038/s41571-019-0320-3. [DOI] [PubMed] [Google Scholar]
- 18.Forde P.M., Spicer J., Lu S., et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973–1985. doi: 10.1056/NEJMoa2202170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wakelee H., Liberman M., Kato T., et al. Perioperative pembrolizumab for early-stage non–small-cell lung cancer. N Engl J Med. 2023;389(6):491–503. doi: 10.1056/NEJMoa2302983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spigel D.R., Faivre-Finn C., Gray J.E., et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. J Clin Oncol. 2022;40(12):1301–1311. doi: 10.1200/JCO.21.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y.L., Tsuboi M., He J., et al. Osimertinib in resected EGFR-mutated non–small-cell lung cancer. N Engl J Med. 2020;383(18):1711–1723. doi: 10.1056/NEJMoa2027071. [DOI] [PubMed] [Google Scholar]
- 22.Lengel H.B., Mastrogiacomo B., Connolly J.G., et al. Genomic mapping of metastatic organotropism in lung adenocarcinoma. Cancer Cell. 2023;41(5):970–985.e3. doi: 10.1016/j.ccell.2023.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou W., Christiani D.C. East meets West: ethnic differences in epidemiology and clinical behaviors of lung cancer between East Asians and Caucasians. Chin J Cancer. 2011;30(5):287–292. doi: 10.5732/cjc.011.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanic K., Zwitter M., Hitij N.T., Kern I., Sadikov A., Cufer T. Brain metastases in lung adenocarcinoma: impact of EGFR mutation status on incidence and survival. Radiol Oncol. 2014;48(2):173–183. doi: 10.2478/raon-2014-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokoi K., Miyazawa N., Arai T. Brain metastasis in resected lung cancer: value of intensive follow-up with computed tomography. Ann Thorac Surg. 1996;61(2):546–550. doi: 10.1016/0003-4975(95)01096-3. discussion 551. [DOI] [PubMed] [Google Scholar]