Summary
Human intestinal organoids (HIOs) derived from human pluripotent stem cells (hPSCs) are promising resources for intestinal regenerative therapy as they recapitulate both endodermal and mesodermal components of the intestine. However, due to their hPSC-line-dependent mesenchymal development and spherical morphology, HIOs have limited applicability beyond basic research and development. Here, we demonstrate the incorporation of separately differentiated mesodermal and mid/hindgut cells into assembled spheroids to stabilize mesenchymal growth in HIOs. In parallel, we generate tubular intestinal constructs (assembled human intestinal tubules [a-HITs]) by leveraging the high aggregative property of assembled spheroids. Through rotational culture in a bioreactor, a-HITs self-organize to develop epithelium and supportive mesenchyme. Upon mesenteric transplantation, a-HITs mature into centimeter-scale tubular intestinal tissue with complex architectures. Our aggregation- and suspension-based approach offers basic technology for engineering tubular intestinal tissue from hPSCs, which could be ultimately applied to the generation of the human intestine for clinical application.
Keywords: human pluripotent stem cells, human intestinal organoids, regenerative medicine, transplantation, self-organization, suspension culture, cell aggregation
Graphical abstract

Highlights
-
•
PSC-derived hindgut and mesodermal cells are assembled into spheroids (as-Spheroids)
-
•
Integration of mesoderm stabilizes the cell-line-dependent HIO mesenchymal development
-
•
as-Spheroids are aggregated to form centimeter-scale human intestinal tubules (a-HITs)
-
•
a-HIT matures into tubular human intestine with continuous lumen upon transplantation
Motivation
Pluripotent stem cell-derived human intestinal organoids (HIOs) hold great promise as an in vitro human intestinal model with potential future translational medical applications. However, the unpredictable iPSC-line-dependent mesenchymal development and their morphological dissimilarity to the human intestine have stifled further advancements. To address these issues, we assembled separately differentiated mid/hindgut and mesodermal cells to stabilize the mesenchymal development in HIOs and, through controlled aggregation, generated a centimeter-scale human intestinal tubule (HIT), recapitulating the tubular macromorphology of the human intestine in vitro and producing mature human intestine in vivo.
Takahashi et al. integrate induced hindgut and mesodermal cells into intestinal spheroids (as-Spheroids) to stabilize mesenchymal development in human intestinal organoids. Further, as-Spheroids are linearly aggregated to form centimeter-scale human intestinal tubules (a-HITs) with a central continuous lumen, recapitulating the tubular intestinal macrostructure.
Introduction
The generation of functional, transplantable intestinal tissue has been the holy grail of intestinal regenerative medicine as an alternative for conventional organ transplantation. Recent advances using tissue-engineering approaches with adult intestinal stem cells have succeeded in autologous epithelialization of patient-derived decellularized intestinal scaffolds1 and the small intestinalization of colon epithelia.2 These intestinal epithelial organoid-based approaches are promising for restoring crucial small intestinal functions but require a pre-existing de-epithelialized intestine to biofabricate a sufficiently functional organ. On the other hand, human pluripotent stem cell (hPSC)-based approaches seek to generate both epithelial and stromal components of the intestine. hPSC-derived human intestinal organoids (HIOs) are characterized by the mesenchymal cells that co-develop with, surround, and support the intestinal epithelial layer, forming the basis of the mucosal and submucosal linings of the intestine.3,4,5,6 On the microscale, HIOs mimic certain functions, cellular compositions, and three-dimensional architectures of the intestine. However, on the macroscale, they are spherical, limiting their functional and morphological resemblance to the tubular intestinal structure. Further, with the current differentiation protocols, the degree of mesenchymal development seen in HIOs varies widely between hPSC strains and batches, likely due to variations in differentiation potentials,7,8,9,10,11 presenting a hurdle to consistent and reliable mucosal stratification. Recent studies in other hPSC fields have reported the integration of multiple progenitor cells into assembloids through the aggregation of single cells to generate more functionally complex models.12,13,14,15 The aggregation method has been applied by us and others to generate mid/hindgut spheroids for HIO culture.16,17,18,19 Our protocol, in particular, allows the generation of these aggregated spheroids, termed suspension spheroids (s-Spheroids), and subsequent differentiation to HIOs in suspension culture.17,18 Further, we reported the implementation of a rotational bioreactor to facilitate the long-term culture of millimeter-scale HIOs.17,18 Based on these findings, we postulated that the assembloid technique can be applied to HIO culture to stabilize mesenchymal development and the aggregative property of hPSC-derived progenitor cells can be leveraged to design the macromorphology of HIOs in a spatially unconstrained suspension culture system.
Here, we demonstrate the successful integration of separately differentiated induced pluripotent stem cell (iPSC)-derived mesodermal cells and mid/hindgut cells into assembled spheroids in suspension. Then, we control the aggregation axis of the assembled spheroids to generate centimeter-scale tubular intestinal constructs, which mature into intestinal tissue in vivo.
Results
Incorporation of splanchnic mesodermal cells to mid/hindgut spheroids for robust mesenchymal growth of HIOs
To ensure adequate and stable development of mesenchymal components in HIOs, we aimed to modify the s-Spheroid generation protocol17,18 by adding splanchnic mesodermal cells to mid/hindgut cells during the aggregative spheroid-forming process. iPSC-derived splanchnic mesodermal cells and mid/hindgut cells were induced as previously described and confirmed by the expressions of splanchnic mesodermal marker FOXF1 and mid/hindgut marker CDX2, respectively (Figure S1A).17,20,21 GFP-labelled mid/hindgut cells and tdTomato-labeled splanchnic mesodermal cells were seeded onto spheroid-forming plates, allowing their aggregation and integration into assembled suspension spheroids (as-Spheroids) (Figures 1A, 1B, and S1B; Video S1). At formation, as-Spheroids exhibited a higher expression of FOXF1 while maintaining a comparable expression of CDX2 to s-Spheroids, indicating successful integration of the mesodermal population and maintenance of the intestinal trajectory (Figures 1C, S1C, and S1D). When cultured in suspension, the initially random distribution of mid/hindgut and mesodermal cells in as-Spheroids self-organized into a well-defined distribution with CDX2+ epithelium surrounded by FOXF1+ mesenchyme (Figure 1D). After 2 weeks in suspension culture, as-Spheroids developed a visible mesenchymal component surrounding the epithelia, while s-Spheroids formed less mesenchyme (Figures 1E, S1E, and S1F). Further, the mesenchymal expressions of epithelia-supporting growth factor R-spondin322 were confirmed, indicating the benefit of mesenchymal support for epithelial growth23 as evidenced by the extensive budding structures seen in as-Spheroids (Figures S1F and S1G). Notably, the self-organization was significantly diminished when cultured in Matrigel-free suspension, implying the importance of extracellular matrix signaling for self-organization (Figure S1H). Upon further culture, as-Spheroids differentiated into assembled HIOs (a-HIOs), maintaining the organized layers of the assembled components with a central E-cadherin+ epithelium surrounded by vimentin+ mesenchyme regardless of the iPSC lines used, demonstrating the stability of this approach (Figures 1F, 1G, and S1I). The vast majority of mesenchymal cells were of integrated origin, even in the iPSC line, which normally develops endogenous mesenchyme (Figure S1J).
Figure 1.
Incorporation of splanchnic mesodermal cells to mid/hindgut spheroids for robust mesenchymal growth of HIOs
(A) Schematic overview of the generation and culture of as-Spheroids.
(B) Fluorescent image of GFP+ mid/hindgut cells and tdTomato+ splanchnic mesodermal cells forming morphologically homogeneous as-Spheroids. H: Line1, M: Line2. Scale bar, 200 μm.
(C) Whole-mount immunostaining of CDX2 and FOXF1 of s-Spheroids (left) and as-Spheroids (right). H: Line3, M: Line3. Scale bars, 100 μm.
(D) Whole-mount immunostaining of FOXF1 and CDX2 of day 11 as-Spheroid. H: Line3, M: Line3. Scale bar, 100 μm.
(E) Bright-field images of day 15 s-Spheroids (left) and as-Spheroids (right). Incorporation of mesodermal cells allows robust mesenchymal growth of HIOs. H: Line3, M: Line3. Scale bars, 200 μm.
(F) Fluorescent image of a day 28 a-HIO. The tdTomato+ mesenchyme surrounds the GFP+ epithelium. H: Line1, M: Line2. as-Spheroids were generated using EZ-BindShut. Scale bar, 500 μm.
(G) Immunostaining of vimentin and E-cadherin of a day 28 a-HIO with high-magnification inset. The vimentin+ mesenchymal layer surrounds the E-cadherin+ epithelial layer. H: Line1, M: Line2. as-Spheroids were generated using EZ-BindShut. Scale bars, 200 μm and 100 μm (inset).
H, hindgut cells; M, mesodermal cells.
Mid/hindgut and splanchnic mesodermal cells were differentiated from GFP-labeled and tdTomato labeled iPSCs, respectively. Scale bar, 200 μm.
a-HIOs engraft and mature into human intestinal tissue
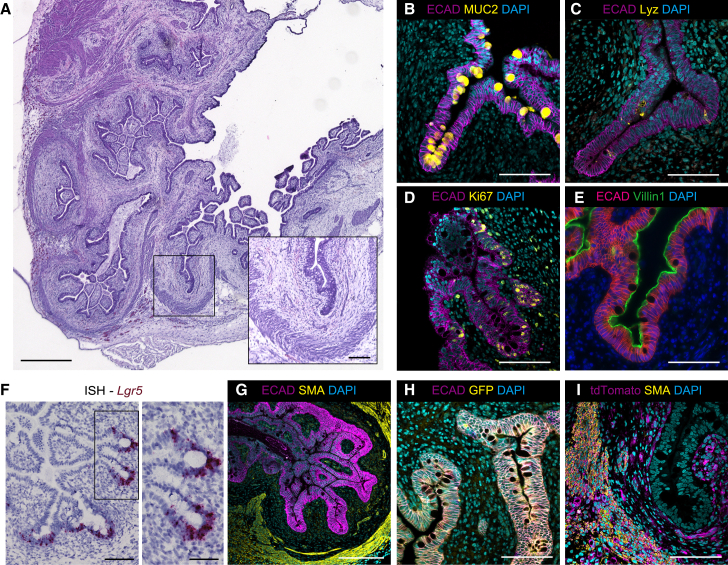
We then examined whether a-HIOs recapitulate mature intestinal structures in vivo. Following 4 weeks of in vitro maturation, a-HIOs were transplanted onto the mesenteries of immunocompromised mice based on established protocols.24,25,26 The mice were euthanized after 8 weeks to examine the engrafted tissue. Histological analysis revealed complex epithelium consisting of differentiated cells of the intestine including goblet cells, Paneth cells, proliferative cells, and enterocytes (Figures 2A–2E). Further, the epithelium formed many crypt-like invaginations containing localized LGR5+ intestinal stem cells, indicating the emergence of an organized mature crypt-villus axis (Figure 2F). The mesenchyme surrounding the well-developed epithelium formed a submucosal layer and an organized smooth muscle actin (SMA)+ smooth muscle layer, recapitulating both the mucosal and submucosal linings of the human intestine (Figure 2G). While the maturation observed is comparable to previously reported transplanted HIOs,5,25,26 the integration of mesodermal cells ensures the presence of mesenchymal components required for successful engraftment15 and supporting epithelial development. The epithelial components developed from GFP+ mid/hindgut cells, while the mesenchymal components developed from tdTomato+ mesodermal cells, demonstrating a remarkable lack of deviation or spontaneous differentiation during in vivo maturation (Figures 2H and 2I).
Figure 2.
In vivo maturation of a-HIOs upon transplantation
(A) H&E staining of engrafted a-HIOs. Invaginated crypt-like structure is surrounded by mesenchymal layers (inset). Scale bars, 500 μm and 100 μm (inset).
(B–E) Immunostaining of MUC2 (B), lysozyme (C), Ki67 (D), and villin1 (E) in E-cadherin+ epithelial cell layer of engrafted a-HIOs. Scale bars, 100 μm.
(F) In situ hybridization of Lgr5 in engrafted a-HIOs. LGR5+ cells are localized at the bottom of the crypt-like invaginations (inset). Scale bars, 100 μm (left) and 50 μm (inset).
(G) Immunostaining of E-cadherin and SMA of engrafted a-HIOs. Scale bar, 200 μm.
(H and I) Immunostaining of E-cadherin/GFP (H) and tdTomato/SMA (I) of engrafted a-HIOs. Scale bars, 100 μm. as-Spheroids were generated using EZ-BindShut. H: Line1, M: Line2.
H, hindgut cells; M, mesodermal cells.
Generation of tubular intestinal construct from as-Spheroids
Through the formation of as-Spheroids, mid/hindgut cells and mesodermal cells have demonstrated a considerable capacity to aggregate and integrate into a coherent single spheroid, which undergoes self-organization and maturation in vitro and in vivo. We postulated that this aggregative and self-organizing capacity can be gently guided to provide cues to form organ-like macroscopic architecture, in this case a tubular intestinal structure. To that end, we engineered a custom-made ultra-low-attachment tissue culture plate containing parallel 1,000-μm-wide furrows (Org-SP plate, AGC Techno Glass, Japan) to segregate sedimenting spheroids into linear concentrations and control their aggregation and fusion in a single linear axis (Figures 3A and S2A). As expected, seeded as-Spheroids fused within the furrows to form elongated centimeter-scale tubules, termed assembled human intestinal tubules (a-HITs) (Figures 3B, 3C, and S2B; Video S2). s-Spheroids can similarly be formed into intestinal tubules (HITs) with less FOXF1+ mesodermal cells (Figures S2C and S2D). Of note, conventional spontaneous mid/hindgut spheroids only formed fragmented HITs with uneven diameters (Figure S2E), indicating that the superior aggregative property of s-Spheroids is critical for the generation of HITs (Videos S2 and S3). To culture a-HITs, we applied the rotational bioreactor, which has been shown to enhance the growth of large HIOs.17,18 Upon rotational culture, a-HITs self-organized to form a GFP+ tubular core surrounded by a tdTomato+ layer, similar to as-Spheroids (Figure S2F). Immunostaining confirmed that the tubular core is E-cadherin+ epithelia, while the surrounding cells are vimentin+ mesenchyme, demonstrating hallmark characteristics of established HIOs (Figure S2G).3,5 However, a-HITs contained multiple small pockets of luminal space separated by mesenchymal cells, defeating the purpose of a tubular intestinal construct (Figures S2G and S2H). This multi-luminal problem, which is characterized by lackluster epithelia and mesenchymal overgrowth, was observed when cultured in the widely adopted HIO medium consisting of epidermal growth factor (EGF), BMP inhibitor Noggin, and Wnt agonist R-Spondin1 (ENR). To facilitate the development of a continuous lumen in a-HITs, we aimed to boost the epithelial proliferation by modifying and optimizing the culture condition of a-HITs. We added Wnt3a, IGF1, FGF2, A83-01, and nicotinamide to the medium to enhance epithelial growth and tubulogenesis and validated their effects on HIOs and a-HIOs.17,27,28,29,30,31 The modified assembloid medium resulted in enhanced epithelial growth in non-a-HIOs as evidenced by the formation of budding structures, as well as a significant increase in Ki67+ proliferative cells (Figures S3A–S3C). HIOs cultured in the assembloid medium lacked a well-developed mesenchymal population due to exogenous Wnt signal activation, consistent with a previous report (Figure S3A).32 However, a-HIOs formed a stable mesenchymal layer around the epithelium despite Wnt activation, indicating that cellular assembly is sufficient to overcome suboptimal conditions for endogenous mesenchymal development (Figure S3D). Whole-mount immunostaining confirmed that the epithelium is positive for CDX2 and E-cadherin, while the mesenchyme is positive for FOXF1 and vimentin, indicating the maintenance of the intestinal differentiation trajectory after 2 weeks of suspension culture (Figures S3E and S3F). To further investigate the effects on the two populations, the epithelial and mesenchymal proliferations of a-HIOs at days 14 and 28 cultured with ENR or the assembloid medium were compared. At day 14, the epithelial proliferation was significantly higher than the mesenchymal proliferation in the assembloid medium, while at day 28, assembloid epithelia were significantly more proliferative than ENR epithelia (Figures S3G and S3H). Taken together, these data indicate that the modified assembloid medium, in conjunction with the assembloid technique, enhances the proliferation of the epithelium without sacrificing the mesenchymal components while maintaining the desired differentiation trajectory. This condition, validated with a-HIOs, was applied to a-HITs. Similar to a-HIOs, a-HITs cultured in the assembloid medium exhibited extensive budding epithelia, suggesting increased proliferation (Figure S4A). The self-organization of the epithelium and mesenchyme was not affected by the medium modification (Figures 3D and S4B). Despite exogenous Wnt signaling, integrated mesoderm-derived mesenchymal cells have developed surrounding the epithelia, some differentiating into subepithelial myofibroblasts (Figures S4C and S4D). Importantly, with the boosted epithelia, a-HITs developed a central continuous lumen along the intestinal tubule in vitro (Figures 3E, S4E, and S4F).
Figure 3.
Generation of tubular intestinal tissue through controlled assembly of as-Spheroids
(A) Schematic overview of the generation and culture of a-HITs.
(B) Fluorescent image of a-HITs at formation generated from as-Spheroids with high-magnification inset. Mid/hindgut and splanchnic mesodermal cells are derived from GFP-labeled and tdTomato-labeled iPSCs, respectively. H: Line1, M: Line2. Scale bars, 2 mm (left) and 500 μm (right).
(C) Immunostaining of CDX2 and FOXF1 of a-HIT at formation. H: Line3, M: Line3. Scale bar, 100 μm.
(D) Fluorescent image of a day 18 a-HIT cultured in the assembloid medium. tdTomato+ mesenchymal layer surrounds the GFP+ epithelium. H: Line1, M: Line2. Scale bar, 1 mm.
(E) Bright-field image of a day 14 a-HIT cultured in the assembloid medium with high-magnification inset. Dotted white line indicates the boundary of a continuous lumen (Lu). H: Line3, M: Line3. Scale bars, 1 mm and 500 μm (inset).
H, hindgut cells; M, mesodermal cells.
Mid/hindgut and splanchnic mesodermal cells were differentiated from GFP-labeled and tdTomato labeled iPSCs, respectively. Scale bar, 500 μm.
Centimeter-scale a-HITs cultured in vitro matured into intestinal tubular tissue in vivo
When transplanted onto the mouse mesentery along the distal ileum, a-HITs cultured in the assembloid medium engrafted and matured into centimeter-scale intestinal tubular tissue with highly organized intestinal architecture (Figures 4A and 4B). Notably, the integration of mesodermal cells into HITs markedly improved the engraftment rate of HITs, demonstrating the significance of mesenchymal support for successful in vivo engraftment (Figure S5A). Qualitatively, the central lumen was largely maintained through the in vivo maturation process, in contrast to a-HITs cultured in ENR medium forming multiple small lumens (Figures 4B and S5B). Quantitatively, the assembloid medium led to significantly larger and fewer lumens compared to the ENR medium, indicating a higher degree of lumen continuity (Figures S5C and S5D).
Figure 4.
a-HITs recapitulate mature tubular intestinal tissue in vivo
(A) Bright-field images of a transplanted (right) and engrafted a-HITs (middle and right) on the mesentery. a-HITs grew into multi-centimeter-scale intestinal tissue through in vivo maturation for 8 weeks. H: Line1, M: Line2. Scale bars, 5 mm.
(B) H&E staining of an engrafted a-HIT with high-magnification inset at top left. Crypt-like invaginations are surrounded by organized mesenchymal layers (inset). H: Line3, M: Line3. Scale bars, 1 mm and 100 μm (inset).
(C) Immunostaining of αSMA and E-cadherin of an engrafted a-HIT with high magnification on the top left. H: Line1, M: Line2. Scale bars, 2 mm and 100 μm (inset).
(D–H) Immunostaining of MUC2 (D), chromogranin A (E), villin1 (F), sucrase isomaltase (G), and lysozyme (H) of E-cadherin+ epithelial layer of the engrafted a-HIT. H: Line3, M: Line3. Scale bars, 100 μm and 10 μm (inset).
(I) In situ hybridization of Lgr5 of the engrafted a-HIT. H: Line1, M: Line2. Scale bar, 200 μm.
(J) Immunostaining of vimentin and αSMA of engrafted a-HIT. H: Line3, M: Line3. Scale bars, 100 μm.
(K) Immunostaining of E-cadherin and Wnt2b of engrafted a-HIT. H: Line3, M: Line3. Scale bars, 100 μm.
H, hindgut cells; M, mesodermal cells.
Immunostaining confirmed the development of organized, layered structures consisting of the intestinal epithelium, subepithelial myofibroblasts, and smooth muscle (Figures 4B and 4C). Further histological analyses revealed various differentiated epithelial cells and LGR5+ intestinal stem cells, indicative of mature epithelia, supported by αSMA+/vimentin+ subepithelial myofibroblasts expressing Wnt2b and R-spondin3 (Figures 4D–4K and S5E–S5G). The developed epithelium and surrounding mesenchymal tissue recapitulate the mucosa and submucosa of the intestine, demonstrating the successful generation of multi-centimeter human intestine from iPSCs. These results demonstrate that the a-HIT formed from linear aggregation of as-Spheroids is of comparable quality to previously reported HIOs5,25,26 as an iPSC-derived intestinal tissue while possessing a centimeter-scale intestinal tubular macrostructure.
Discussion
In this study, we showed that the incorporation of separately differentiated mesodermal cells allow robust mesenchymal growth in HIOs. By leveraging the exceptional aggregation property of mid/hindgut spheroids and the self-organization ability of hPSC-derived progenitor cells, we also demonstrated successful generation of centimeter-scale tubular intestinal constructs, which recapitulate mature intestinal tissue in vivo.
Thus far, mesenchymal development in HIOs depended on the coincidental generation of a mesodermal population during mid/hindgut differentiation, which is line dependent and unpredictable.3,4,7,8,9 Following previous reports on the integration of several iPSC-derived progenitors into assembloids,12,13,14,15 we aimed to integrate splanchnic mesodermal cells into s-Spheroids to form as-Spheroids. The two cell types derived from iPSCs self-organized in a similar fashion to conventional HIOs, indicating that assembled mesodermal cells self-organize similarly to endogenously developed mesenchymal components. The splanchnic mesoderm used in this study follows an established protocol that generates early posterior foregut-like mesoderm and therefore may have subtle differences from the endogenous mesenchyme.33 Intestine-specific mesoderm derivation has not been reported yet and represents a limitation to this approach. However, as early iPSC-derived cells tend to possess high cellular plasticity, and the observed expression of epithelia-supporting factors in vitro and the formation of comparable mucosal and submucosal intestinal linings in vivo, the differences between the endogenous and integrated mesenchymal populations seem to be insignificant. The results demonstrate the validity of this strategy to stabilize mesenchymal development as well as the potential to incorporate diverse cell populations, including neural cells, immune cells, and endothelial cells, and the need to further advance region-specific differentiation protocols to biofabricate a more complete and physiologically relevant and specific intestinal model.
In current literature, this remarkable aggregative property has mainly been used to increase model complexity and probe cell-cell interactions.12,13,14,15 Previously reported methods on gastrointestinal assembloids generally involve inexact proximity-based assembly through mass centrifugation or co-culture within Matrigel micro-droplets.15,21 On the other hand, our single-cell-based approach allows tight control of cell type ratios and generates highly aggregative structurally homogeneous assembloids. The tubular intestinal construct generated through the controlled fusion of these assembloids can be seen as a proof of concept for the use of as-Spheroids as building blocks to generate complex macrostructures.
Several approaches to biofabricating large tissue constructs have been recently reported, but most require dedicated bioprinters and predesigned cell positioning.34,35,36 The method described herein only requires spheroid-forming plates and construct-forming plates, providing a more accessible entry point to tissue engineering. Further, the method only shapes the tissue on the macroscale but relies on the cells’ intrinsic self-organizing ability to give rise to organ-like cellular composition and distribution from the initial random mosaic of cell types, providing a platform to probe the processes during organogenesis. However, the cells’ finite capacity to self-organize may become the limiting factor to the scalability of this approach beyond the centimeter scale and may require further refinement to introduce more interventional guidance during the tissue-engineering process. To optimize the self-organization, growth, and maturation of a-HITs, we employed a rotational bioreactor, given the benefits to growth and differentiation reported in millimeter-scale iPSC-derived organoids.17,37 Upon mesenteric transplantation, a-HITs matured into centimeter-scale tubular intestinal tissue with a diameter comparable to mouse intestine, presenting a tangible step in terms of size and morphology toward the clinically significant goal of generating a human-sized intestine. The aggregation- and suspension-based approach we described here has a potentially wide applicability to the field of tissue engineering to generate other endoderm-derived tubular organs.
Limitations of the study
As we briefly mentioned, there are several cell populations missing from the current iteration of a-HITs, including endothelial, neural, and immune cells. The successful assembly of mid/hindgut and mesodermal cells into HIOs and HITs points to the potential of assembling additional cellular components for a more complete intestinal model. In the current form, we identified a variety of differentiated epithelial and stromal cells in transplanted a-HITs; however, the degree to which they confer intestinal functions has not been established. Functional analyses, including anastomosis between HIT and the host intestine, would shed further light on the potential of this model. Despite a-HITs representing a step up in the scale of iPSC-derived tissue, they are still far from the human scale. New strategies that lean more toward bioengineering approaches and less on self-organization may need to be devised to make the next step up in scalability.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to the lead contact, Tomohiro Mizutani (tmizutani.gast@tmd.ac.jp).
Materials availability
Org-SP plates were developed in collaboration with AGC Techno Glass (Shizuoka, Japan). The purchase of the custom-made Org-SP plates can be ordered directly from the manufacturer, AGC Techno Glass.
Data and code availability
-
•
All data analyzed and reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this work is available from the lead contact upon request.
Acknowledgments
We thank Dr. Yukio Nakamura for the gift of the human iPSC line HiPS-RIKEN-2F and Dr. Hideki Masaki for the gift of the human iPSC line PB001. We also thank our laboratory members for materials, discussions, and technical support. This work has been supported by funding from JSPS KAKENHI JP20K22920, JP21H02895, and JP22K07957 (to T.M.); JP20K21597 and JP22H00465 (to R.O.); and JP23K19612 (to J.T.), as well as AMED JP22bm1123007 (to T.M.) and JP21bm0404055 (to R.O.); FOREST Program JPMJFR2113 (to T.M.); TMDU priority research areas grant (to J.T. and T.M.); JST SPRING JPMJSP2120 (to H.Y.S., S. Kato, and S. Kawasaki); Naoki Tsuchida Research Grant (to T.M. and R.O.); and the Japan Foundation for Applied Enzymology (to J.T.).
Author contributions
J.T. and T.M. conceived the idea and designed the experiments; J.T., H.Y.S., and T.M. conducted the experiments with assistance from S. Kato, S. Kawasaki, and S.N. J.T., H.Y.S., S. Kawasaki, and T.M. analyzed the results. J.T., H.Y.S., and T.M. wrote the manuscript. T.M. and R.O. supervised the project. All authors contributed to the discussion and interpretation of the results.
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| CDX2 (1:200) | BioGenex | Cat#MU392A-5U; RRID: AB_2650531 |
| FOXF1 (1:200) | R&D | Cat#AF4798; RRID: AB_2105588 |
| E-cadherin (1:100) | R&D | Cat#AF648; RRID: AB_355504 |
| MUC2 (1:100) | Santa Cruz | Cat#Sc15334; RRID: AB_2146667 |
| Chromogranin A (1:1000) | DiaSorin | Cat#SP-1 |
| Villin (1:100) | Novus Biologicals | Cat#NBP1-85335; RRID: AB_11020888 |
| Ki67 (1:50) | Novus Biologicals | Cat#NB600-1252; RRID: AB_2142376 |
| alpha-Smooth Muscle Actin (1:150) | Novus Biologicals | Cat#NB600-531; RRID: AB_10000930 |
| Vimentin (1:200) | Novus Biologicals | Cat#NB300-223; RRID: AB_10003206 |
| Lysozyme (1:1000) | Sigma | Cat#HPA048284; RRID: AB_2680339 |
| Sucrase Isomaltase (1:100) | Santa Cruz | Cat#Sc393424; RRID:AB_2891093 |
| Wnt2b (1:500) | Invitrogen | Cat#710888 |
| tdTomato (1:300) | SICGEN | Cat# AB8181 RRID: AB_2722750 |
| GFP (1:100) | Santa Cruz | Cat# sc-9996 RRID: AB_627695 |
| Alexa 488 donkey anti-rabbit IgG (1:200) | Invitrogen | Cat#A21206; RRID: AB_2535792 |
| Alexa 488 goat anti-rabbit IgG (1:200) | Invitrogen | Cat#A11034; RRID: AB_2576217 |
| Alexa 488 donkey anti-mouse IgG (1:200) | Invitrogen | Cat#A21202; RRID: AB_141607 |
| Alexa 488 rabbit anti-goat IgG (1:200) | Invitrogen | Cat#A11078; RRID: AB_141838 |
| Alexa 488 chicken anti-goat IgG (1:200) | Invitrogen | Cat#A21467; RRID: AB_2535870 |
| Alexa 594 donkey anti-rabbit IgG (1:200) | Invitrogen | Cat#A21207; RRID: AB_141637 |
| Alexa 594 chicken anti-goat IgG (1:200) | Invitrogen | Cat#A21468; RRID: AB_141859 |
| Alexa 594 donkey anti-goat IgG (1:200) | Invitrogen | Cat#A11058; RRID: AB_2534105 |
| Alexa 594 donkey anti-chicken IgG (1:200) | Jackson Immuno | Cat#703-585-155; RRID: AB_2340377 |
| Alexa 594 donkey anti-chicken IgG (1:200) | Invitrogen | Cat#A78951; RRID: AB_2921073 |
| Alexa 594 donkey anti-mouse IgG (1:200) | Invitrogen | Cat#A21203; RRID: AB_141633 |
| Chemicals, peptides, and recombinant proteins | ||
| Recombinant murine EGF | Peprotech | Cat#31509 |
| Recombinant mouse Noggin | R&D | Cat#1967 |
| Recombinant mouse R-spondin1 | R&D | Cat#3474-RS |
| A83-01 | Tocris | Cat#2939 |
| Recombinant Human IGF-1 | BioLegend | Cat#590906 |
| Nicotinamide | Sigma | Cat#N0636 |
| Afamin/Wnt3a conditioned medium | MBL lifescience | Cat#J2-001 |
| Activin A | Nacalai Tesque | Cat#18585-81 |
| CHIR99021 | Cayman Chemicals | Cat#13122 |
| Recombinant human FGF4 | Peprotech | Cat#100-31 |
| Recombinant human BMP-4 | R&D | Cat# 314-BP |
| Wnt-C59 | Chemscene LLC | Cat#CS-1420 |
| PIK90 | Cayman Chemicals | Cat#10010749 |
| Retinoic acid | Sigma Aldrich | Cat#R2625 |
| Purmorphamine | Tocris Bioscience | Cat#4551 |
| Y-27632 | Fujifilm Wako Pure Chemical | Cat#253-00513 |
| FGF-basic(154a.a.), Human, Recombinant | Peprotech | Cat#100-18B |
| Recombinant Human IGF-I | BioLegend | Cat#590906 |
| Cellartis DEF-CS™ 500 culture system | Takara Bio | Cat#Y50101 |
| Advanced DMEM/F12 | Thermo Fisher | Cat#12634010 |
| DMEM, high glucose | Thermo Fisher | Cat#11965-092 |
| RPMI 1640 with L-Gln, liquid | Nacalai Tesque | Cat#30264-85 |
| Embryonic stem cell Fetal Bovine Serum | Thermo Fisher | Cat#16141079 |
| GlutaMAX™ Supplement | Thermo Fisher | Cat#35050-061 |
| HEPES buffer | Nacalai Tesque | Cat#17557-94 |
| Penicillin-Streptomycin Mixed Solution | Nacalai Tesque | Cat#26253-84 |
| Matrigel Growth Factor Reduced | Corning | Cat#356231 |
| N-2 supplement (100X) | Thermo Fisher | Cat#17502-048 |
| B-27 Supplement (50X), serum free | Thermo Fisher | Cat#17504-044 |
| B-27 Supplement (50X), minus vitamin A | Thermo Fisher | Cat#12587010 |
| TrypLE Express Enzyme | Thermo Fisher | Cat#12604013 |
| Accumax | Nacalai Tesque | Cat#17087-54 |
| Hoechst 33342 | Thermo Fisher | Cat#H3570 |
| VECTASHIELD mounting medium with DAPI | VECTOR Laboratories | Cat#H1200 |
| DAPI solution (1 mg/mL) | Nacalai Tesque | Cat#19178-91 |
| The P3 Primary Cell 4D-Nucleofector X Kit S | Lonza | Cat#V4XP-3032 |
| Critical commercial assays | ||
| RNAscope 2.5HD Assay-Red | Advanced Cell Diagnostics | Cat#322350 |
| RNeasy Mini kit | QIAGEN | Cat#74106 |
| QuantiTect Reverse Transcription Kit | QIAGEN | Cat#205313 |
| Experimental models: Cell lines | ||
| Human induced pluripotent stem cell line: HiPS-RIKEN-2F | RIKEN BRC Cell Bank (Japan) | HPS0014 |
| Human induced pluripotent stem cell line: PB001 | Kindly gifted by Dr Hideki Masaki (Institute of Medical Science, the University of Tokyo) | N/A |
| Human induced pluripotent stem cell line: TkdA3-4 | Institute of Medical Science, the University of Tokyo | N/A |
| Experimental models: Organisms/strains | ||
| NOD.Cg-Prkdcscid/Il2rgtm1wji/SzJ (NSG) mice | The Jackson Laboratory Japan | N/A |
| Oligonucleotides | ||
| RNAscope Probe-Hs-LGR5 | Advanced Cell Diagnostics | Cat#311021 |
| RNAscope Probe-Hs-RSPO3-01 | Advanced Cell Diagnostics | Cat#429851 |
| Primer: CDX2 Fwd: CTCGGCAGCCAAGTGAAAAC | This paper | N/A |
| Primer: CDX2 Rev: CTCCTTTGCTCTGCGGTTCT | This paper | N/A |
| Primer: FOXF1 Fwd: AGCAGCCGTATCTGCACCAGAA | This paper | N/A |
| Primer: FOXF1 Rev: CTCCTTTCGGTCACACATGCTG | This paper | N/A |
| Primer: β-actin Fwd: GGATGCAGAAGGAGATCACTG | This paper | N/A |
| Primer: β-actin Rev: CGATCCACACGGAGTACTTG | This paper | N/A |
| Recombinant DNA | ||
| PB-CMV-MCS-EF1-GreenPuro cDNA Cloning and Expression Vector | System Biosciences | Cat# PB513B-1 |
| Super piggyBac Transposase expression vector | System Biosciences | Cat# PB200A-1 |
| Software and algorithms | ||
| Prism 9 | GraphPad Software; Dotmatics | N/A |
| CellSens | Olympus | N/A |
| R v4.3.3 | R Foundation | N/A |
| ImageJ v1.54k | National Institute of Health | N/A |
| Other | ||
| EZSPHERE® 6-well plate | AGC Techno Glass Co. Ltd. | Cat#4810-900SP |
| EZ-BindShut® 96-well plate | AGC Techno Glass Co. Ltd. | Cat#4870-800SP |
| Org-SP plate | AGC Techno Glass Co. Ltd. | N/A |
| Ultra-low attachment 6-well plate | Corning | Cat#3471 |
| CellPet 3D-iPS rotary suspension culture system | JTEC Corp. | CELLPET iPS/3S/MA-2.1 |
| Culture vessel syringe (10 mL) | JTEC Corp. | CELLPET VES/S10/6 |
| Culture vessel syringe (30 mL) | JTEC Corp. | CELLPET VES/S30/6 |
| Culture vessel syringe (50 mL) | JTEC Corp. | CELLPET VES/S50/6 |
| SUMILON STEMFULL 15mL Centrifuge tube | Sumitomo Bakelite Co., Ltd | Cat#MS-90150 |
| SUMILON ProteoSave SS 50mL Centrifuge tube | Sumitomo Bakelite Co., Ltd | Cat#MS-52550 |
| Beriplast P Combi-Set Tissue adhesion 0.5 mL | CSL Behring | N/A |
| VICRYL | ETHICON | J107G |
| All-in-one Fluorescence Microscope | KEYENCE | BZ-X810 |
| Laser-scanning confocal microscope | Olympus | FLUOVIEW FV3000 |
| Stereomicroscope system | OLYMPUS | SZX7 |
| 4D-NucleofectorR System | Lonza | N/A |
| StepOnePlus Real-Time PCR | Applied Biosystems | N/A |
Experimental model and study participant details
Animals
All experiments were carried out with 8–10 weeks old non-obese diabetic severe combined immunodeficiency (NOD/SCID) interleukin-2 receptor gamma chain (IL2Rγ)null (NSG) mice (purchased from The Jackson Laboratory Japan, Inc.). All mice were housed in the animal facility at Tokyo Medical and Dental University. All experiments were performed with approval of the Animal Care and Use Committee of Tokyo Medical and Dental University (A2023-202A).
Human iPSC lines and culture
The iPSC line HiPS-RIKEN-2F (Line1) was generated from umbilical cord fibroblasts of a Japanese male and reprogrammed by retroviral expression of Oct3/4, Klf4 Sox2, and c-Myc (OKSM).38 This line was obtained from RIKEN BRC Cell Bank (Tsukuba, Japan). The iPSC line PB001 (Line2) derived from peripheral blood cells and reprogrammed by a Sendai virus vector expressing OKSM.39 PB001 was labeled by a lentiviral vector expressing tdTomato. PB001 was gifted by Dr. Hideki Masaki (Institute of Medical Science, the University of Tokyo). The iPSC line TkdA3-4 (Line3) was established from peripheral blood cells and reprogrammed by retroviral expression of OKSM.40 This line was obtained from Institute of Medical Science, the University of Tokyo. The iPSC lines were maintained using Cellartis DEF-CS 500 culture system (Takara Bio) based on manufacturer’s protocol and passaged by TrypLE Express Enzyme (Thermo Fisher).
Method details
GFP induction to iPSC
HiPS-RIKEN-2F was labeled with GFP using electroporation (4D-NucleofectorR System, Lonza) based on previously described method with some modifications.41 We used piggyBac transposon vector expressing GFP (#PB513B-1, System Bioscinences). Briefly, 1.0 x 106 dissociated cells were collected to Nucleocuvette containing 82 μL Nucleofector Solution, 18 μL Supplement, 7.2 μL piggyBac vector expressing GFP and 2.8 μL transposase, and electroporated with 4D-NucleofectorR System using program CB-150. Electroporated cells were plated at 5.0 x 105 cells/well on 6-well plate and cultured with Cellartis DEF-CS 500 culture system. Seven days after electroporation, cells were exposed to 1 μg/mL puromycin for seven days. After puromycin selection, cells were maintained using Cellartis DEF-CS 500 culture system and used for further experiments.
Differentiation of iPSCs into mid/hindgut cells
Mid/hindgut differentiation was performed as previously described with slight modifications.3,4,5,6,42,43 Briefly, iPSCs were initially treated with Activin A (100 ng/mL, Nacalai Tesque) and CHIR99021 (3 μM, Cayman Chemicals) in RPMI1640, followed by Activin A (100 ng/mL) for two days in RPMI1640 (Nacalai Tesque) with increased concentration (0.2% and 2%) of fetal bovine serum (FBS). Then definitive endoderm (DE) was exposed to FGF4 (100 ng/mL, Peprotech) and CHIR99021(3 μM) in RPMI1640 containing 2% FBS for four days. Mid/hindgut cells were dissociated into single cells using TrypLE Express Enzyme, resuspended with 12 mL DMEM, counted, and used for generation of s-Spheroids or as-Spheroids.
Differentiation of iPSCs into splanchnic mesodermal cells
iPSCs were differentiated into splanchnic mesodermal cells based on a previously reported method.15,20,21 Briefly, iPSCs were treated with Activin A (30 ng/mL), CHIR99021 (6 μM), basic FGF (20 ng/mL, Peprotech), BMP4 (40 ng/mL, R&D) and PIK90 (100 nM, Cayman Chemicals) on day 0, Wnt-C59 (1 μM, Chemscene LLC), BMP4 (30 ng/mL) and A83-01 (1 μM, Tocris Bioscience) on day 1, basic FGF (20 ng/mL), Wnt-C59 (1 μM), BMP4 (30 ng/mL), A83-01(1 μM) and retinoic acid (2 μM, Sigma Aldrich) on day 2–3, retinoic acid (2 μM) and purmorphamine (PMA) (2 μM, Tocris Bioscience) on day 4–5. The basal medium composed of AdDMEM/F12, GlutaMAX (1x, Thermo Fisher), HEPES (15 mM, Nacalai Tesque), N-2 supplement (1x, Thermo Fisher), B-27 supplement without vitamin A (1x, Thermo Fisher) and Penicillin-Streptomycin Mixed Solution (1x, Nacalai Tesque). On day 6, confluent splanchnic mesodermal cells were dissociated into single cells using Accumax (Nacalai Tesque) supplemented with Y-27632 (10 μM, Fujifilm Wako Pure Chemical). Dissociated cells were strained using a 70 μm cell filter, resuspended with 12mL DMEM (Thermo Fisher), counted and used for generation of as-Spheroids.
Generation of s-Spheroids and as-Spheroids
For generation of as-Spheroids, single cell suspensions were transferred to an ultra-low-attachment 15 mL conical tube (SUMILON STEMFUL, Sumitomo Bakelite) at a ratio of 75% mid/hindgut and 25% splanchnic mesodermal cells. For s-Spheroids, only mid/hindgut cells were used.17,18 The tube was centrifuged at 300 x g for 3 min and supernatant was discarded. The basal medium (HIO basal medium) composed of AdDMEM/F12, GlutaMAX (1x), HEPES (15 mM), N-2 supplement (1x), B-27 supplement (1x, Thermo Fisher), Penicillin-Streptomycin Mixed Solution (1x) and used for subsequent culture. Approximately 1.6 x 106 total cells were seeded on each well of EZSPHERE 6-well plate (AGC Techno Glass Co. Ltd.), exposed to CHIR99021 (3 μM), FGF4 (100 ng/mL), PMA (2 μM) and Y-27632 (10 μM), and cultured for 24 h in 37°C, 5% CO2 humidified incubator. To generate large-sized as-Spheroids, 1 x 105 total cells were seeded on each well of EZ-BindShut 96-well plate (AGC Techno Glass Co. Ltd.).
Culture of s-Spheroids and as-Spheroids
s-Spheroids and as-Spheroids were cultured in suspension based on a previously described method.17,18 Briefly, s-Spheroids and as-Spheroids were collected from EZSPHERE 6-well plate and transferred to ultra-low-attachment plate. The culture medium composition was as follows; HIO-basal medium containing Matrigel (10% (v/v), Corning), EGF (100 ng/mL, Peprotech), Noggin (100 ng/mL, R&D) for the initial three days, R-Spondin1 (500 ng/mL, R&D), and PMA (2 μM). Large s-Spheroids generated by EZ-BindShut 96-well plates were cultured in a culture vessel syringe (JTEC Corp.) on a CellPet 3D-iPS rotary suspension culture system (JTEC Corp.) from day 3 as previously described.17,18 Medium was changed twice a week. Bright-field, fluorescent and time-lapse images were captured using BZ-X810 (KEYENCE).
Engineering custom-made tissue culture plates (Org-SP plates)
We developed the custom-made tissue culture plates containing parallel furrows (Org-SP plate) in collaboration with AGC Techno Glass Co. Ltd. (Shizuoka, Japan).
Generation of assembled human intestinal tubules (a-HITs)
The medium composition for generating a-HITs is as follows; HIO basal medium supplemented with CHIR99021 (3 μM), FGF4 (100 ng/mL), PMA (2 μM) and Y-27632 (10 μM). Spheroids were collected from EZSPHERE 6-well plates and transferred to a low-attachment conical 50 mL tube (SUMILON ProteoSave; Sumitomo Bakelite). Typically, spheroids from 12 wells of EZSPHERE 6-well plate were applied to 1 well of Org-SP plate (2700 x 12 = 32,400 spheroids per well). The tube was centrifuged at 100 x g for 3 min and the supernatant was discarded. One mL of medium was added to the tube, and the spheroid-suspension was seeded into one well of the Org-SP plate using a wide-bore 1000 μL pipette. The medium was added up to 12 mL/well. The plate was placed in 37°C, 5% CO2 humidified incubator for 24 h to allow for the aggregation of spheroids.
Collection and culture of a-human intestinal tubules
a-HITs were cultured in suspension in HIO-basal medium supplemented with Matrigel (20% for the initial three days and 10% for the rest) and growth factors. The combination of growth factors was as follows; (1) ENR: EGF (100 ng/mL), Noggin (100 ng/mL) for the initial three days, R-Spondin1 (500 ng/mL) and 2 μM PMA (2) the assembloid medium: Afamin/Wnt3a conditioned medium (10% (v/v), MBL lifescience), EGF (100 ng/mL), R-Spondin1 (500 ng/mL), nicotinamide (10 mM, Sigma), IGF-1 (50 ng/mL, BioLegend), basic FGF (25 ng/mL), A8301 (500 nM) and PMA (2 μM). a-HITs collected from the Org-SP plate were gently transferred to 6-well ultra-low attachment plates using wide-bore 1000 μL pipette and cultured in 37°C, 5% CO2 humidified incubator. After three days of static culture on ultra-low-attachment plates, a-HITs were transferred to a culture vessel syringe and cultured in rotary suspension on a CellPet 3D-iPS rotary suspension culture system. The rotation speed started from 10 rpm, and gradually increased as a-HITs grew. Medium was changed twice a week.
Mesenteric transplantation of a-HIOs and a-HITs
Experiments were performed on female 8–10 weeks old NSG mice (20–24 g in weight) were used for experiments. Prior to transplantation, a-HIOs and a-HITs were cultured in vitro for four weeks and two to four weeks respectively. Mesenteric transplantation was performed based on a previously described method with slight modifications.17,24,25,26 In brief, mice were anesthetized with 2% inhaled isoflurane, and a 2 cm incision of the abdominal wall and peritoneum was created. The mesentery was gently pulled over along with the distal ileum from the incision using cotton swabs. A single a-HIO or a-HIT was placed on mice mesentery along the distal ileum and fixed with fibrin sealant (Beriplast, CSL Behring). After transplantation, the abdominal wall and peritoneum was sutured using bioabsorbable thread (4-0 VICRYL, ETHICHON). Eight weeks after transplantation, mice were euthanized and the engrafted tissues were processed for histological analyses.
RNA isolation and qRT-PCR
RNeasy Mini Kit (Qiagen) was used for RNA extraction, and QuantiTect Reverse Transcription Kit (Qiagen) for reverse transcription, based on the manufacture’s protocol. Primer sequences are detailed in key resource table. We performed qRT-PCR on a StepOnePlus Real-Time PCR (Applied Biosystems) using QuantiTect SybrGreen Master mix (Qiagen). The relative expression of each gene was calculated by the ΔΔ Ct method and normalized to beta-actin. GraphPad Prism 9 (GraphPad Software; Dotmatics) was used for statistical analysis.
Immunofluorescent staining
Immunofluorescent staining of spheroids and a-HITs was performed based on a previously described method.17 Cultured spheroids and a-HITs were fixed for 2 h in 4% paraformaldehyde (PFA) at 4°C, rinsed with phosphate-buffered saline (PBS) and incubated in 30% sucrose-PBS buffer overnight. Transplanted spheroids and a-HITs were fixed for two days in PFA using horizontal rotator at 4°C, rinsed with PBS and incubated with 30% sucrose-PBS buffer for two days. Samples were frozen in the OCT compound and cryosectioned. Slides were washed in PBS with 0.02% Tween 20 (PBS-T), processed with antigen retrieval, washed with PBS-T three times, blocked with blocking buffer (Blocking One, Nacalai Tesque) for an hour at room temperature, incubated with primary antibody in blocking buffer overnight. To detect CDX2 and FOXF1, slides were permeabilized by PBS containing 0.2% Triton X-100 for 10 min at room temperature prior to antigen retrieval. Slides were then washed in PBS-T three times, incubated with secondary antibody for an hour at room temperature, washed three times in PBS-T and mounted using VECTASHIELD mounting medium with DAPI (VECTOR Laboratories). Fluorescent images were captured using either laser-scanning confocal microscope (FLUOVIEW FV3000, Olympus) or BZ-X810 (KEYENCE). Detailed information of primary and secondary antibodies is described in the key resources table.
Whole-mount immunostaining of a-HIOs and a-HITs
Whole-mount immunostaining of a-HIOs and a-HITs were performed based on a previously reported protocol with slight modifications.44 a-HIOs and a-HITs were fixed overnight at 4°C in 4% PFA, washed with PBS and permeabilized in PBS supplemented with 1% Triton X-100 at room temperature for an hour. They were incubated with primary antibodies diluted in PBS containing 0.1% Triton X-100 and 0.2% BSA (organoid washing buffer; OWB44) for two days at 4°C. They were then washed with OWB three times (each time 1 h) and incubated with secondary antibodies for two days. Subsequently, they were washed with OWB two times, incubated with OWB containing 0.1% DAPI or Hoechst 33242 for 1h, and washed with OWB two times. Samples were incubated in fructose-glycerol clearing solution (60% (v/v) glycerol and 2.5 M fructose) for 20 min at room temperature. Images were captured using laser-scanning confocal microscope (FLUOVIEW FV3000, OLYMPUS) and Z stacks were analyzed and assembled by FV3000 software (Olympus). Ki67+ cells and DAPI+ cells were counted using CellSens software (Olympus), percentage was calculated and GraphPad Prism 9 (GraphPad Software; Dotmatics) was used for statistical analysis.
In situ hybridization
Hybridization steps were performed on sections of engrafted tissue. We performed in situ hybridization with an RNAscope 2.5HD Assay-Red (Advanced Cell Diagnostics) as per the manufacturer’s instructions. LGR5 RNAscope target probe and RSPO3-01 RNAscope target probe were used to detect LGR5 and R-spondin3, respectively. Hs-PPIB and DapB probes were used as positive and negative controls, respectively. Images were captured using BZ-X810.
Ki67+ cell counting of a-HIOs
Cell counting was performed with ImageJ software and Fiji plugins. In brief, immunostained slides were imaged using laser-scanning confocal microscopy and analyzed with Fiji automatic particle counting. Ki67+ cells were counted as proliferative cells, and DAPI+ cells were counted as total cell count to calculate the Ki67+ ratios. The counting was performed separately for epithelial and mesenchymal cells and GraphPad Prism 9 was used for statistical analysis and visualization.
Lumen continuity analysis
Luminal area relative to the tissue area was used as a proxy for lumen continuity. Lumen and tissue areas were measured from H&E-stained sections of transplanted a-HITs using ImageJ. The relative luminal area was calculated for each lumen in the sections by dividing the lumen area by the tissue area of the transplanted a-HIT. Relative areal data from 12 a-HITs were collated for statistical analysis and visualization, which were performed with R (v4.3.3).
Quantification and statistical analysis
All error bars indicate the SD. The quantified data represent the findings of three or more independent experiments. Statistical analyses of qRT-PCR were performed using the Prism 9 software program (GraphPad). Two-group comparison was performed using unpaired Student’s t test, except for Figure S4D which was performed using Mann-Whitney U test. p < 0.05 was considered statistically significant.
Published: December 10, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.crmeth.2024.100930.
Supplemental information
References
- 1.Meran L., Massie I., Campinoti S., Weston A.E., Gaifulina R., Tullie L., Faull P., Orford M., Kucharska A. Engineering transplantable jejunal mucosal grafts using patient-derived organoids from children with intestinal failure. Nat. Med. 2020;26:1593–1601. doi: 10.1038/s41591-020-1024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugimoto S., Ohta Y., Fujii M., Matano M., Shimokawa M., Nanki K., Date S., Nishikori S., Nakazato Y., Nakamura T., et al. Reconstruction of the Human Colon Epithelium In Vivo. Cell Stem Cell. 2018;22:171–176.e5. doi: 10.1016/j.stem.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Spence J.R., Mayhew C.N., Rankin S.A., Kuhar M.F., Vallance J.E., Tolle K., Hoskins E.E., Kalinichenko V.V., Wells S.I., Zorn A.M., et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCracken K.W., Howell J.C., Wells J.M., Spence J.R. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat. Protoc. 2011;6:1920–1928. doi: 10.1038/nprot.2011.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watson C.L., Mahe M.M., Múnera J., Howell J.C., Sundaram N., Poling H.M., Schweitzer J.I., Vallance J.E., Mayhew C.N., Sun Y., et al. An in vivo model of human small intestine using pluripotent stem cells. Nat. Med. 2014;20:1310–1314. doi: 10.1038/nm.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Workman M.J., Mahe M.M., Trisno S., Poling H.M., Watson C.L., Sundaram N., Chang C.-F., Schiesser J., Aubert P., Stanley E.G., et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat. Med. 2017;23:49–59. doi: 10.1038/nm.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arora N., Imran Alsous J., Guggenheim J.W., Mak M., Munera J., Wells J.M., Kamm R.D., Asada H.H., Shvartsman S.Y., Griffith L.G. A process engineering approach to increase organoid yield. Development. 2017;144:1128–1136. doi: 10.1242/dev.142919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carcamo-Orive I., Hoffman G.E., Cundiff P., Beckmann N.D., D'Souza S.L., Knowles J.W., Patel A., Papatsenko D., Abbasi F., Reaven G.M., et al. Analysis of Transcriptional Variability in a Large Human iPSC Library Reveals Genetic and Non-genetic Determinants of Heterogeneity. Cell Stem Cell. 2017;20:518–532.e9. doi: 10.1016/j.stem.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cahan P., Daley G.Q. Origins and implications of pluripotent stem cell variability and heterogeneity. Nat. Rev. Mol. Cell Biol. 2013;14:357–368. doi: 10.1038/nrm3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kajiwara M., Aoi T., Okita K., Takahashi R., Inoue H., Takayama N., Endo H., Eto K., Toguchida J., Uemoto S., Yamanaka S. Donor-dependent variations in hepatic differentiation from human-induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA. 2012;109:12538–12543. doi: 10.1073/pnas.1209979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estrada H.Q., Patel S., Rabizadeh S., Casero D., Targan S.R., Barrett R.J. Development of a Personalized Intestinal Fibrosis Model Using Human Intestinal Organoids Derived From Induced Pluripotent Stem Cells. Inflamm. Bowel Dis. 2022;28:667–679. doi: 10.1093/ibd/izab292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen J., Revah O., Miura Y., Thom N., Amin N.D., Kelley K.W., Singh M., Chen X., Thete M.V., Walczak E.M., et al. Generation of Functional Human 3D Cortico-Motor Assembloids. Cell. 2020;183:1913–1929.e26. doi: 10.1016/j.cell.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miura Y., Li M.-Y., Birey F., Ikeda K., Revah O., Thete M.V., Park J.-Y., Puno A., Lee S.H., Porteus M.H., Pașca S.P. Generation of human striatal organoids and cortico-striatal assembloids from human pluripotent stem cells. Nat. Biotechnol. 2020;38:1421–1430. doi: 10.1038/s41587-020-00763-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koike H., Iwasawa K., Ouchi R., Maezawa M., Giesbrecht K., Saiki N., Ferguson A., Kimura M., Thompson W.L., Wells J.M., et al. Modelling human hepato-biliary-pancreatic organogenesis from the foregut–midgut boundary. Nature. 2019;574:112–116. doi: 10.1038/s41586-019-1598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eicher A.K., Kechele D.O., Sundaram N., Berns H.M., Poling H.M., Haines L.E., Sanchez J.G., Kishimoto K., Krishnamurthy M., Han L., et al. Functional human gastrointestinal organoids can be engineered from three primary germ layers derived separately from pluripotent stem cells. Cell Stem Cell. 2022;29:36–51.e6. doi: 10.1016/j.stem.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onozato D., Yamashita M., Nakanishi A., Akagawa T., Kida Y., Ogawa I., Hashita T., Iwao T., Matsunaga T. Generation of Intestinal Organoids Suitable for Pharmacokinetic Studies from Human Induced Pluripotent Stem Cells. Drug Metab. Dispos. 2018;46:1572–1580. doi: 10.1124/dmd.118.080374. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi J., Mizutani T., Sugihara H.Y., Nagata S., Kato S., Hiraguri Y., Takeoka S., Tsuchiya M., Kuno R., Kakinuma S., et al. Suspension culture in a rotating bioreactor for efficient generation of human intestinal organoids. Cell Rep. Methods. 2022;2 doi: 10.1016/j.crmeth.2022.100337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi J., Sugihara H.Y., Kato S., Nagata S., Okamoto R., Mizutani T. Protocol to generate large human intestinal organoids using a rotating bioreactor. STAR Protoc. 2023;4 doi: 10.1016/j.xpro.2023.102374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitstick A.L., Poling H.M., Sundaram N., Lewis P.L., Kechele D.O., Sanchez J.G., Scott M.A., Broda T.R., Helmrath M.A., Wells J.M., Mayhew C.N. Aggregation of cryopreserved mid-hindgut endoderm for more reliable and reproducible hPSC-derived small intestinal organoid generation. Stem Cell Rep. 2022;17:1889–1902. doi: 10.1016/j.stemcr.2022.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han L., Chaturvedi P., Kishimoto K., Koike H., Nasr T., Iwasawa K., Giesbrecht K., Witcher P.C., Eicher A., Haines L., et al. Single cell transcriptomics identifies a signaling network coordinating endoderm and mesoderm diversification during foregut organogenesis. Nat. Commun. 2020;11:4158. doi: 10.1038/s41467-020-17968-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song A.T., Sindeaux R.H.M., Li Y., Affia H., Agnihotri T., Leclerc S., van Vliet P.P., Colas M., Guimond J.-V., Patey N., et al. Developmental role of macrophages modeled in human pluripotent stem cell-derived intestinal tissue. Cell Rep. 2024;43 doi: 10.1016/j.celrep.2023.113616. [DOI] [PubMed] [Google Scholar]
- 22.Goto N., Goto S., Imada S., Hosseini S., Deshpande V., Yilmaz Ö.H. Lymphatics and fibroblasts support intestinal stem cells in homeostasis and injury. Cell Stem Cell. 2022;29:1246–1261.e6. doi: 10.1016/j.stem.2022.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J., Horiuchi S., Kuramochi S., Kawasaki T., Kawasumi H., Akiyama S., Arai T., Morinaga K., Kimura T., Kiyono T., et al. Human intestinal organoid-derived PDGFRα + mesenchymal stroma enables proliferation and maintenance of LGR4 + epithelial stem cells. Stem Cell Res. Ther. 2024;15:16. doi: 10.1186/s13287-023-03629-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poling H.M., Wu D., Brown N., Baker M., Hausfeld T.A., Huynh N., Chaffron S., Dunn J.C.Y., Hogan S.P., Wells J.M., et al. Mechanically induced development and maturation of human intestinal organoids in vivo. Nat. Biomed. Eng. 2018;2:429–442. doi: 10.1038/s41551-018-0243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortez A.R., Poling H.M., Brown N.E., Singh A., Mahe M.M., Helmrath M.A. Transplantation of human intestinal organoids into the mouse mesentery: A more physiologic and anatomic engraftment site. Surgery. 2018;164:643–650. doi: 10.1016/j.surg.2018.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh A., Poling H.M., Sundaram N., Brown N., Wells J.M., Helmrath M.A. Evaluation of transplantation sites for human intestinal organoids. PLoS One. 2020;15 doi: 10.1371/journal.pone.0237885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato T., Stange D.E., Ferrante M., Vries R.G.J., Van Es J.H., Van den Brink S., Van Houdt W.J., Pronk A., Van Gorp J., Siersema P.D., Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto S., Fujii S., Sato A., Ibuka S., Kagawa Y., Ishii M., Kikuchi A. A combination of Wnt and growth factor signaling induces Arl4c expression to form epithelial tubular structures. EMBO J. 2014;33:702–718. doi: 10.1002/embj.201386942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumoto S., Fujii S., Kikuchi A. Arl4c is a key regulator of tubulogenesis and tumourigenesis as a target gene of Wnt–β-catenin and growth factor–Ras signalling. J. Biochem. 2017;161:27–35. doi: 10.1093/jb/mvw069. [DOI] [PubMed] [Google Scholar]
- 30.Mihara E., Hirai H., Yamamoto H., Tamura-Kawakami K., Matano M., Kikuchi A., Sato T., Takagi J. Active and water-soluble form of lipidated Wnt protein is maintained by a serum glycoprotein afamin/α-albumin. Elife. 2016;5 doi: 10.7554/eLife.11621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujii M., Matano M., Toshimitsu K., Takano A., Mikami Y., Nishikori S., Sugimoto S., Sato T. Human Intestinal Organoids Maintain Self-Renewal Capacity and Cellular Diversity in Niche-Inspired Culture Condition. Cell Stem Cell. 2018;23:787–793.e6. doi: 10.1016/j.stem.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 32.Tamminen K., Balboa D., Toivonen S., Pakarinen M.P., Wiener Z., Alitalo K., Otonkoski T. Intestinal Commitment and Maturation of Human Pluripotent Stem Cells Is Independent of Exogenous FGF4 and R-spondin1. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kishimoto K., Iwasawa K., Sorel A., Ferran-Heredia C., Han L., Morimoto M., Wells J.M., Takebe T., Zorn A.M. Directed differentiation of human pluripotent stem cells into diverse organ-specific mesenchyme of the digestive and respiratory systems. Nat. Protoc. 2022;17:2699–2719. doi: 10.1038/s41596-022-00733-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Machino R., Matsumoto K., Taniguchi D., Tsuchiya T., Takeoka Y., Taura Y., Moriyama M., Tetsuo T., Oyama S., Takagi K., et al. Replacement of Rat Tracheas by Layered, Trachea-Like, Scaffold-Free Structures of Human Cells Using a Bio-3D Printing System. Adv. Healthcare Mater. 2019;8 doi: 10.1002/adhm.201800983. [DOI] [PubMed] [Google Scholar]
- 35.Nikolaev M., Mitrofanova O., Broguiere N., Geraldo S., Dutta D., Tabata Y., Elci B., Brandenberg N., Kolotuev I., Gjorevski N., et al. Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature. 2020;585:574–578. doi: 10.1038/s41586-020-2724-8. [DOI] [PubMed] [Google Scholar]
- 36.Brassard J.A., Nikolaev M., Hübscher T., Hofer M., Lutolf M.P. Recapitulating macro-scale tissue self-organization through organoid bioprinting. Nat. Mater. 2021;20:22–29. doi: 10.1038/s41563-020-00803-5. [DOI] [PubMed] [Google Scholar]
- 37.DiStefano T., Chen H.Y., Panebianco C., Kaya K.D., Brooks M.J., Gieser L., Morgan N.Y., Pohida T., Swaroop A. Accelerated and Improved Differentiation of Retinal Organoids from Pluripotent Stem Cells in Rotating-Wall Vessel Bioreactors. Stem Cell Rep. 2018;10:300–313. doi: 10.1016/j.stemcr.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujioka T., Shimizu N., Yoshino K., Miyoshi H., Nakamura Y. Establishment of induced pluripotent stem cells from human neonatal tissues. Hum. Cell. 2010;23:113–118. doi: 10.1111/j.1749-0774.2010.00091.x. [DOI] [PubMed] [Google Scholar]
- 39.Masaki H., Kato-Itoh M., Umino A., Sato H., Hamanaka S., Kobayashi T., Yamaguchi T., Nishimura K., Ohtaka M., Nakanishi M., Nakauchi H. Interspecific in vitro assay for the chimera-forming ability of human pluripotent stem cells. Development. 2015;142:3222–3230. doi: 10.1242/dev.124016. [DOI] [PubMed] [Google Scholar]
- 40.Takayama N., Nishimura S., Nakamura S., Shimizu T., Ohnishi R., Endo H., Yamaguchi T., Otsu M., Nishimura K., Nakanishi M., et al. Transient activation of c-MYC expression is critical for efficient platelet generation from human induced pluripotent stem cells. J. Exp. Med. 2010;207:2817–2830. doi: 10.1084/jem.20100844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steyer B., Bu Q., Cory E., Jiang K., Duong S., Sinha D., Steltzer S., Gamm D., Chang Q., Saha K. Scarless Genome Editing of Human Pluripotent Stem Cells via Transient Puromycin Selection. Stem Cell Rep. 2018;10:642–654. doi: 10.1016/j.stemcr.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munera J.O., Sundaram N., Rankin S.A., Hill D., Watson C., Mahe M., Vallance J.E., Shroyer N.F., Sinagoga K.L., Zarzoso-Lacoste A., et al. Differentiation of Human Pluripotent Stem Cells into Colonic Organoids via Transient Activation of BMP Signaling. Cell Stem Cell. 2017;21:51–64. doi: 10.1016/j.stem.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crespo M., Vilar E., Tsai S.-Y., Chang K., Amin S., Srinivasan T., Zhang T., Pipalia N.H., Chen H.J., Witherspoon M., et al. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat. Med. 2017;23:878–884. doi: 10.1038/nm.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dekkers J.F., Alieva M., Wellens L.M., Ariese H.C.R., Jamieson P.R., Vonk A.M., Amatngalim G.D., Hu H., Oost K.C., Snippert H.J.G., et al. High-resolution 3D imaging of fixed and cleared organoids. Nat. Protoc. 2019;14:1756–1771. doi: 10.1038/s41596-019-0160-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mid/hindgut and splanchnic mesodermal cells were differentiated from GFP-labeled and tdTomato labeled iPSCs, respectively. Scale bar, 200 μm.
Mid/hindgut and splanchnic mesodermal cells were differentiated from GFP-labeled and tdTomato labeled iPSCs, respectively. Scale bar, 500 μm.
Data Availability Statement
-
•
All data analyzed and reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this work is available from the lead contact upon request.