Abstract
In this issue of Molecular Cell, Vaisvila et al.1 report a tour de force functional characterization of a large and highly diverse set of polynucleotide cytosine deaminase (PCD) enzymes, which is already propelling new biotechnology applications.
The hydrolytic removal of the amino (NH2) group from cytosine and modified cytosine nucleobases can occur spontaneously or enzymatically (inset, Figure 1). This simple chemical reaction, however, can cause profound changes in polynucleotide substrates – in RNA by changing codons, regulatory elements, structural motifs, and/or functionality, and in DNA by creating pre-mutagenic lesions with both immediate and downstream consequences. The first enzyme demonstrated to catalyze C-to-U in RNA is APOBEC1, which introduces a premature stop codon in the APOB mRNA (hence, APOB mRNA editing catalytic subunit-1).2 This important discovery in 1993 helped usher-in a new field centered on “RNA editing”; that is, until the discovery of DNA cytosine deamination in 2002 and revelations that DNA editors outnumber RNA editors, DNA editing likely preceded RNA editing evolutionarily, and even APOBEC1 can edit DNA.3–5
Figure 1. Biological functions and technological applications of PCDs.
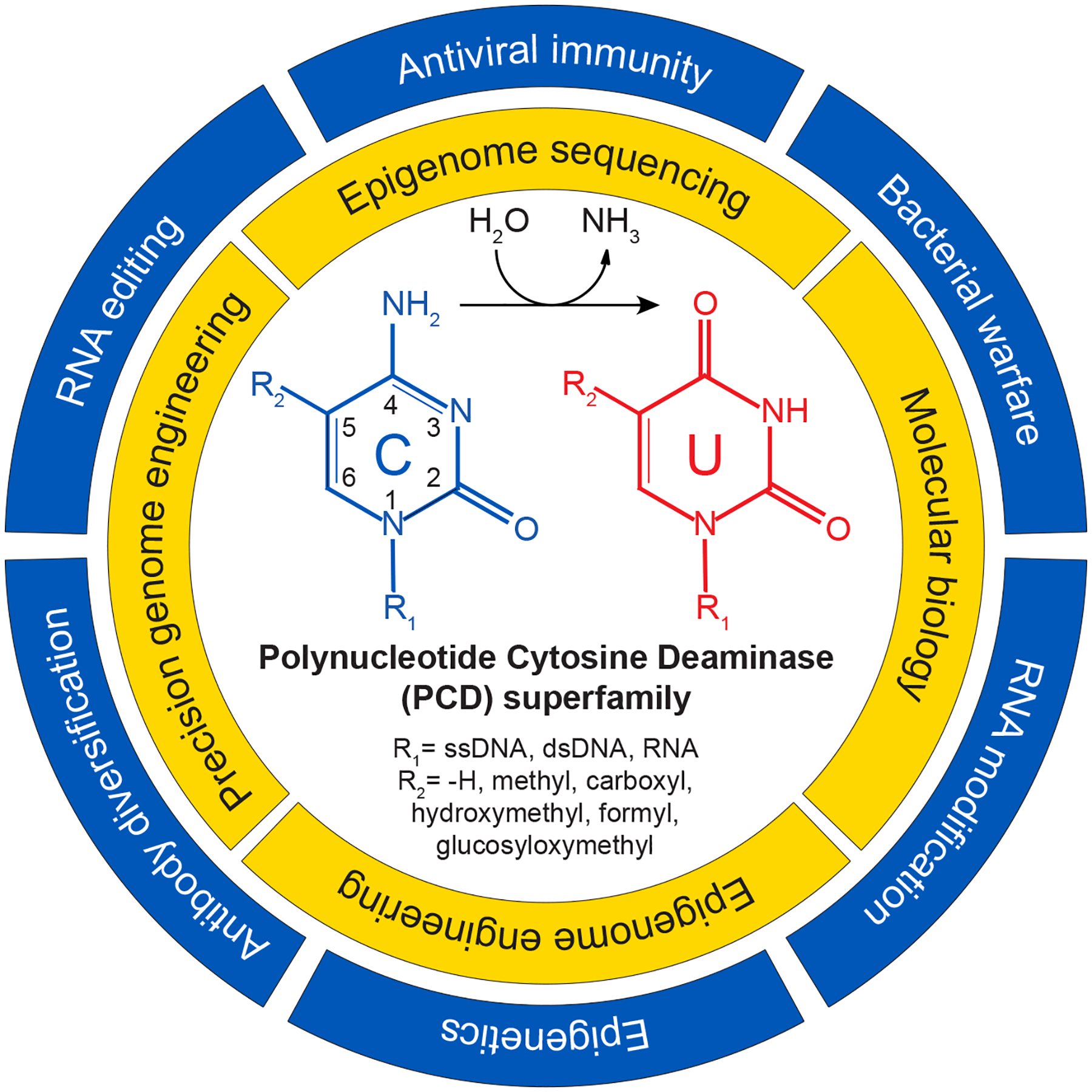
Outer ring: Biological functions of PCDs (pathological functions are not listed such as tumor evolution by APOBEC3s in humans). Inner ring: Technological applications of PCDs. Center: Schematic of hydrolytic deamination at the 4-position of a model cytosine nucleobase. R1 = a polynucleotide. R2 = indicated chemical group at the 5-position of the cytosine ring.
Now, two-plus decades onward, this editing dogma is shattered once again with the functional characterization of nearly 400 new polynucleotide cytosine deaminase (PCD) members by Vaisvila and colleagues in this issue of Molecular Cell1 and by Huang et al. in a recent issue of Cell.6 The former study used sequence-based bioinformatics to identify candidate genes and a cell-free system to express proteins for functional characterization in vitro (via mass spectrometry and next-generation sequencing of substrate nucleic acids). The latter identified candidate PCDs using structural inferences (AlphaFold2), cloned candidates using conventional molecular approaches, and tested functionality in cellular systems. Both groups reported a remarkably broad and sporadic distribution of PCDs across biology and significant diversity in PCD enzymatic activities. For instance, PCDs can be found in branches of all kingdoms of life with the vast majority thus far in eubacteria, and polynucleotide substrates range from single-stranded (ss)DNA to double-stranded (ds)DNA to ssRNA and nucleobase targets from normal cytosine to 5-methylcytosine (5mC) to 5-carboxycytosine and 5-glucosyloxymethylcytosine (inset, Figure 1). These studies are limited in part by assays available and substrates tested and, therefore, some enzymes may have different biological substrates (e.g., polynucleotides such as dsRNA, RNA/DNA hybrid, or Z-DNA/RNA and non-polynucleotide substrates such as modified or unmodified (deoxy)nucleosides/tides).
The precise biological functions of this greatly expanded polynucleotide cytosine deaminase (PCD) superfamily will take years to sort out, but many are likely to function as toxins in microbial warfare (outer circle, Figure 1). In support, elegant studies had already characterized a double-stranded DNA deaminase, DddA, in Burkholderia cenocepacia that functions as a secreted antimicrobial toxin.7 Earlier bioinformatic studies had also predicted both ancient origins and incredible diversity in PCDs.8 A sporadic distribution in different phylogenetic branches and extreme divergence even within branches strongly suggested roles in antimicrobial defense (bacteria and bacteriophage). As for present-day mammalian APOBEC3s, many of these bacterial PCDs may be under pressure to diversify in order to defend host species from infections or compete with other species in challenging environments. Both offensive and defensive roles are supported by evidence for recurrent gene loss as well as horizontal transfer for gene gain across species.
The explosion in bona fide PCD members also enables exciting technological advancements (inner circle, Figure 1). The first is in epigenome mapping where the gold standard for decades has been bisulfite sequencing. This technique is robust for interrogating short target sequences such as promoters but challenging to implement genome-wide. This issue was overcome with an appreciation that the biochemical activities of TET dioxygenases and human APOBEC3A could be combined to purposefully distinguish normal and 5-modified genomic cytosines prior to sequencing.9 Enzymatic methyl-sequencing (EM-Seq) is a recent example: TET2 and T4-phage beta-glucosyltransferase (T4-BGT) convert all 5mC (and rarer, larger 5-modified C) into bulkier products non-recognizable by human APOBEC3A, and then all remaining normal C nucleobases (after denaturation) are deaminated by APOBEC3A into uracils prior to sequencing.10 Here, Vaisvila and colleagues report modification-sensitive DNA deaminases, such as MsddA, that exhibit strong preferences for normal C (over all modified C’s) in both ssDNA and dsDNA. Thus, a single enzyme method can now be used for epigenome sequencing (SEM-seq). Future structural studies will be required to explain these unique properties including the remarkable capacity to efficiently accommodate both ssDNA and dsDNA cytosines.
Second, the large number of PCDs reported by the Vaisvila and Huang papers also dramatically expands the cytosine base editor (CBE) toolbox for genome engineering (inner circle, Figure 1). Since breakthrough work first fused APOBEC1 to Cas9/gRNA complexes (the original CBE),11 deaminases have been on center-stage in the field of precision genome medicine but constrained in utility due to strong intrinsic preferences for TC dinucleotides. In comparison, the new repertoire of enzymes exhibits a wide range of local preferences, from completely unbiased enzymes recognizing virtually any DNA cytosine (NCN) to some with strong di- and tri-nucleotide preferences and others with curiously large (canonical restriction enzyme-like) preferences. Huang et al. also report the smallest single-stranded DNA deaminases to-date, such as mini-Sdd7, that they exploit as a CBE to engineer soybeans. Prior to this, several plant species such as soybeans had resisted CBE engineering (for unknown reasons). The small size of these enzymes also makes them more amenable to virus-mediated transduction (e.g., recombinant adenovirus-associated virus, rAAV), which could enhance utility in multiple experimental and therapeutic settings such as genomic medicine.
There are many other nuggets of information in these publications that will undoubtedly require follow-up and expansion. For instance, Vaisvila and colleagues describe several enzymes, such as HcDa01, that exhibit strong RNA cytosine editing activity, which may be amenable to specific epigenomic applications. They also report an expansive range of enzymes capable of cytosine deamination in dsDNA. This property has already been exploited for DddA to edit mitochondrial dsDNA in a Cas9/gRNA-independent (CRISPR-free) manner.12 The availability of many new enzymes in this dsDNA editing class, including several with different local preferences, will undoubtedly expand prospects for organelle genome engineering in humans, animals, and plants. For every PCD toxin, there is also likely to be an equally effective, self-preserving antitoxin. Perhaps this explosion of PCDs will also enable the Holy Grail of genome engineering to be achieved – the highly efficient editing of specific sites without local or distal collateral damage to genomes.
ACKNOWLEDGEMENTS
Research in the Harris laboratory is supported by NIAID R37-AI064046, NCI P01-CA234228, NCI P50-CA247749, and a Recruitment of Established Investigators Award from the Cancer Prevention and Research Institute of Texas (CPRIT RR220053). RSH is an Investigator of the Howard Hughes Medical Institute and the Ewing Halsell President’s Council Distinguished Chair at University of Texas Health San Antonio.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Vaisvila R, Johnson SR, Yan B, Dai N, Bourkia BM, Corrêa IR Jr., Yigit E, and Sun Z (2024). Discovery of novel cytosine deaminase activities enables powerful tools for methylome analysis Molecular Cell xxx. [DOI] [PubMed]
- 2.Teng B, Burant CF, and Davidson NO (1993). Molecular cloning of an apolipoprotein B messenger RNA editing protein. Science 260, 1816–1819. [DOI] [PubMed] [Google Scholar]
- 3.Harris RS, Petersen-Mahrt SK, and Neuberger MS (2002). RNA editing enzyme APOBEC1 and some of its homologs can act as DNA mutators. Mol Cell 10, 1247–1253. [DOI] [PubMed] [Google Scholar]
- 4.Conticello SG (2008). The AID/APOBEC family of nucleic acid mutators. Genome Biol 9, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen-Mahrt SK, Harris RS, and Neuberger MS (2002). AID mutates E. coli suggesting a DNA deamination mechanism for antibody diversification. Nature 418, 99–103. [DOI] [PubMed] [Google Scholar]
- 6.Huang J, Lin Q, Fei H, He Z, Xu H, Li Y, Qu K, Han P, Gao Q, Li B, et al. (2023). Discovery of deaminase functions by structure-based protein clustering. Cell 186, 3182–3195 e3114. 10.1016/j.cell.2023.05.041. [DOI] [PubMed] [Google Scholar]
- 7.de Moraes MH, Hsu F, Huang D, Bosch DE, Zeng J, Radey MC, Simon N, Ledvina HE, Frick JP, Wiggins PA, et al. (2021). An interbacterial DNA deaminase toxin directly mutagenizes surviving target populations. Elife 10. 10.7554/eLife.62967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iyer LM, Zhang D, Rogozin IB, and Aravind L (2011). Evolution of the deaminase fold and multiple origins of eukaryotic editing and mutagenic nucleic acid deaminases from bacterial toxin systems. Nucleic Acids Res 39, 9473–9497. 10.1093/nar/gkr691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schutsky EK, Nabel CS, Davis AKF, DeNizio JE, and Kohli RM (2017). APOBEC3A efficiently deaminates methylated, but not TET-oxidized, cytosine bases in DNA. Nucleic Acids Res 45, 7655–7665. 10.1093/nar/gkx345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaisvila R, Ponnaluri VKC, Sun Z, Langhorst BW, Saleh L, Guan S, Dai N, Campbell MA, Sexton BS, Marks K, et al. (2021). Enzymatic methyl sequencing detects DNA methylation at single-base resolution from picograms of DNA. Genome Res 31, 1280–1289. 10.1101/gr.266551.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komor AC, Kim YB, Packer MS, Zuris JA, and Liu DR (2016). Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424. 10.1038/nature17946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mok BY, de Moraes MH, Zeng J, Bosch DE, Kotrys AV, Raguram A, Hsu F, Radey MC, Peterson SB, Mootha VK, et al. (2020). A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing. Nature 583, 631–637. 10.1038/s41586-020-2477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


