ABSTRACT
Studies have suggested that the administration of epidural analgesia (Epi) and oxytocin (OT) during labor affects offspring outcomes. However, the effects of their combined use remain unclear. This article aimed to review the outcomes of offspring exposed to Epi and OT, identify research gaps, and discuss future research directions. We searched the MEDLINE/PubMed, Web of Science, and Cochrane Library databases to identify studies describing offspring outcomes in the Epi, OT, Epi-OT, and control groups. We included one systematic review, six cohort studies, and one case-control study. The offspring outcomes at birth did not differ between the Epi-OT and Epi groups. In the first hour of life, the pre-feeding and sucking behaviors of the Epi-OT group showed an inverse correlation. At 2 days of age, the breastfeeding behavior and skin temperature patterns differed significantly between the Epi-OT and other groups. At 4 days of age, hyperbilirubinemia was more prevalent in the Epi-OT versus control group. Behavioral scores at 1 month differed little among the Epi-OT, Epi, and control groups. No eligible studies examined 1 month to 1 year of life. From 1 to >13 years of age, the risk of autism spectrum disorder was higher in the Epi and Epi-OT groups versus the control group. Most eligible studies were small and observational without randomization, and the results were inconsistent. Additional large cohort studies of various aspects of offspring development are required to assess the long-term effects of Epi-OT administration.
Key Words: epidural analgesia, opioids, oxytocin administration, offspring outcome, development
INTRODUCTION
Epidural analgesia (Epi) is widely administered and recommended for pain relief during labor in many countries1 and used in more than 70% of deliveries in the United States.2 Some studies have investigated the association between Epi use and offspring outcomes.3,4 Moreover, several studies have examined the association between Epi and neurological development and the risk of autism spectrum disorder (ASD) in offspring.5-9 A recent meta-analysis showed that the long-term neurobiological effects of Epi administered to laboring women may predispose the offspring to ASD.10
Oxytocin (OT), a peptide molecule produced in the hypothalamus and secreted by the pituitary gland, induces uterine contractions during childbirth and stimulates breast milk production.11 Synthetic OT, which has the same structure as endogenous OT, is widely used to induce or augment labor. Nearly one-third of all term deliveries in England involve the induction of labor.12 The reported neonatal outcomes immediately after birth in relation to OT administration remain controversial.13-17 Furthermore, similar to Epi, the long-term effects of exogenous OT administration on later social and neurological developments, such as bipolar disorder and ASD, have been studied.18-20
Epi use reportedly increases OT administration by more than two-fold,21 and their biological interactions were previously demonstrated.22-24 While many studies have examined their individual effects on offspring with promising results, little is known about their interactions and the effects of their combined use on offspring. Therefore, we conducted this narrative review to overview the outcomes of a group of offspring exposed to both Epi and OT during delivery, identify existing research gaps, and propose areas of future research.
MATERIALS AND METHODS
Research question
What are the offspring outcomes of exposure to combined Epi and OT during labor?
Search strategy and eligibility criteria
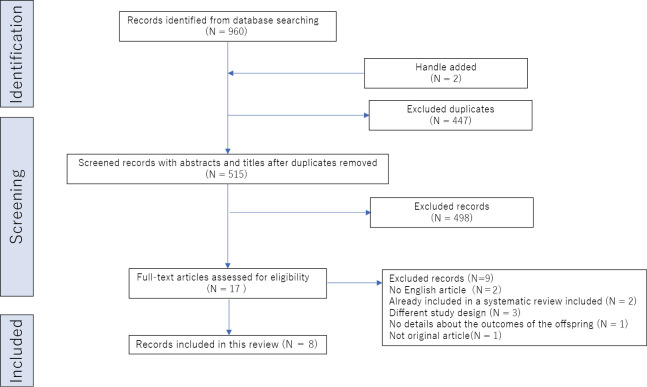
The search aimed to review the outcomes of offspring of mothers exposed to the combined use of Epi and OT and included all relevant evidence of short- and long-term offspring outcomes. It included the MEDLINE/PubMed, Web of Science, and Cochrane Library databases. The Medical Subject Headings (MeSH) search terms “oxytocin,” “administered,” “epidural,” “anesthesia,” “analgesia,” and “offspring” along with the listed indications were used to search the databases. A manual search of the reference lists of the retrieved articles yielded additional studies. Duplicate studies were removed. The titles and abstracts of all studies were screened by independent reviewers (including A.T.), and relevant full-text reviews were performed independently of the primary research question of each study (A.T. and Y.T.). The search was conducted on September 22, 2023. Only full-text human studies published in English were included. Exclusion criteria were non-English publication, case report design, previous inclusion in a systematic review or meta-analysis, and lack of relevance to the topic (eg, either Epi or OT was included as a covariate but not examined in combination use, Epi was compared with another type of anesthesia). Reasons for exclusion were non-English publication (n = 2), previous inclusion in a systematic review (n = 2), different study design (n = 3), lack of details about offspring outcomes (n = 1), and non-original article (n = 1; Figure 1).
Fig. 1.
Flowchart for selecting studies from the MEDLINE/PubMed, Web of Science, and Cochrane Library databases
RESULTS
The search yielded 960 studies. After the exclusion of 447 duplicate studies and the elimination of 515 studies based on the title and abstract screening, 17 studies were analyzed. The studies were reviewed in full; of them, eight investigated the outcomes in the offspring of mothers exposed to both Epi and OT during labor (Epi-OT group; Figure 1). These eight studies included one systematic review, six cohort studies, and one case-control study. This systematic review investigated the neonatal outcomes of the Epi-OT group. Two studies reported from birth to 1 hour after birth, two studies reported from birth to 2 days after birth, one study reported from birth to 4 days after birth, one study reported from birth to 1 month after birth, and one study reported from 1 year to 13 years (Table 1).
Table 1.
Overview of research studies
| Source | Aim | Participants | Groups of medical intervention | Age | Study design | Epidural analgesia | Oxytocin administration protocol | Offspring outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epi- OT | Epi | OT | OT im | Control | ||||||||
| Costley et al,27 2013 | To determine whether augmentation using Epi and OT will decrease the incidence of operative deliveries, reduce foetal and maternal morbidity. | A total of 319 infants born vaginally and their mothers who used Epi during labour were randomized into 2 groups as follows; OT group (n=154) and a placebo treatment group (n=165). | ○ | ○ | On birth | Systematic review and meta-analysis | Bupivacain | Initial infusion started from 2 mU/min and dose was doubled every 20 minutes up to rate of 16 mU/min (1989). Initial rate was 2 mu/min, increasing every 15 min to the following up to 2 mu/min (1995). | No statistically significant differences between the Epi and Epi-OT groups in Apgar scores less than seven at five minutes (RR, 3.06, 95% CI, 0.13–73.33; two studies, 319 women) and admission to a neonatal intensive care unit (RR, 1.07, 95% CI, 0.29–3.93; two studies, 319 women). | |||
| Brimdyr et al,28 2015 | To describe the effect of Epi and OT for breastfeeding during the first one hour of skin-to-skin contact on infants. | A total number of 63 low-risk full-term infants; the control group (n=10), the Epi group (n=16), the OT group (n=12), and the Epi-OT group (n=25). | ○ | ○ | ○ | ○ | During the first hour of life | Cohort study | Ropivacaine, fentanyl | Infusion increased by 1 mU/minute every 30 minutes, up to 24 mU/minute. | No differences in birthweight (P = 0.11), gestational age (P = 0.46) and one- and five-minute Apgar scores (P = 0.64, P = 0.65) in four groups. Epidural analgesia showed strong inverse correlations with the achieving suckling (P = 0.01) and the amount of oxytocin (P = 0.046). | |
| Brimdyr et al,29 2019 | To describe the effect of Epi and OT for breastfeeding behaviour during the first one hour of skin-to-skin contact on infants. | A total number of 63 low-risk full-term infants; the control group (n=10), the Epi group (n=16), the OT group (n=12), and the Epi-OT group (n=25) | ○ | ○ | ○ | ○ | During the first hour of life | Cohort study | Fentanyl | No data | Epi-OT group reached significantly lower stages of Widström’s 9 Stages by the end of the first hour than the control and the OT group (mean stage 5.47 ± SD 1.4 vs 7.10 ± 1.7; P = 0.03, and vs 7.17 ± 11; P = 0.01). | |
| Jonas et al,31 2007 | To assess the influence of Epi and OT during birth for the skin temperature pattern in neonate on two days after birth. | A total number of full-term low-risk 47 infants whose mothers were Swedish speaking in primipara; the OT group (n=9), the Epi-OT group (n=20), and the control group (n=18). | ○ | ○ | ○ | On day 2 after birth | Cohort study | Sufentanyl | No data | The Epi-OT group showed significantly higher body temperatures compared to the control group (mean ± SE, 35.07 ± 0.26 °C vs 34.19 ± 0.26 °C, P = 0.025) at the start. The Epi-OT group showed significantly negative temperature difference between the start and first ten minutes (mean ± SE, -0.62 ± 0.22 °C, P = 0.019) whereas the control group and the OT groups showed significantly increasing between the start and first ten minutes (mean ± SE 0.91 ± 0.2 °C; P = 0.001 and 1.47 ± 0.37 °C, P = 0.008). | ||
| Takahashi et al,32 2021 | To explore the association between the quality breastfeeding behaviors and Epi/OT use for mother during delivery. | A total number of 41 low-risk full-term Infants whose mothers were Swedish-speaking in primiparity; the control group (n=13), the OT group (n=5), the OT-im group (n=8), the Epi group (n=5) and the Epi-OT groups (n=10). | ○ | ○ | ○ | ○ | ○ | On day 2 after birth | Cohort study | Bupivacain, sufentanyl | The oxytocin infusion started from 10 IU in 500 mL, giving rise to a concentration of 20 mIU/mL (OT group). 10 IE oxytocin was administered by intramuscular injection (OT im group). | The control group showed a significantly longer duration of rooting than the other four groups. The median of total IBFAT scores were the lowest in the Epi group (8 vs the control group; 10 vs, the OT group; 10 vs the OT im group; 10.5 vs the Epi-OT group; 9). The Epi-OT group showed significantly lower scores than the control group in (medians, interquartile ranges Q25-Q75; 2, 1-2 vs 1, 0-2, P = 0.02). |
| Sima et al,34 1975 | To explore prenatal factors for the risk of neonatal jaundice. | A total number of 138 infants (46 in full-term and pre-term infants who diagnosed as hyperbilirubinaemia) and matched controls (n=92). | ○ | ○ | ○ | ○ | Around 4 days after birth | Matched control study | No data | No data | Hyperbilirubinemia case was independently associated with oxytocin administration for induction compared to the control group (52.2% vs 28.3%, P <0.01) and Epi use (39.1% vs 15.2%, P <0.01). More hyperbilirubinemia infants in the Epi-OT group than the control group (χ2 = 3.18, P < 0.05). | |
| Murray et al,35 1981 | To evaluate the effects of Epi-dosing on healthy neonates using the Neonatal Behaviour Assessment Scale (NBAS). | A total number of 60 full-term babies; the Epi group (n=20), the Epi with OT group (n=20), and the control group (n=15). | ○ | ○ | ○ | ○ | First -1 month day | Cohort study | Bupivacaine with epinephrine | No data | On the first day, there were significant differences in NBAS between the control and both the Epi and the Epi-OT groups in the motor process (P <0.01), state control (P <0.05), and physiological response (P <0.01), as well as their total score (P <0.01). The Epi-OT group showed significantly lower values in motor behaviour on the NBAS than the Epi group (mean score, 23 vs 32; P <0.01). However, these differences were attenuated as the day progressed. At the one month of life, there was no difference in the NBAS between the three groups (mean score, 64 vs 81 vs 72, in control, Epi, and Epi-OT groups; P >0.05). | |
| Qiu et al,37 2023 | To assess the association between the use of Epi and OT and the risk of ASD in children. | A total number of 205 994 singleton births with vaginal deliveries; the Epi group (n=49 679), the OT group (n=13 607), the Epi-OT group (n=104 201), and the control group (n=38 507). | ○ | ○ | ○ | ○ | 1 to over 4 to 13 years | Cohort study | No data | No data | Compared to the control group, the HRs for ASD were 0.90 (95% CI, 0.78–1.04), 1.20 (95% CI, 1.09–1.32), and 1.30 (95% CI, 1.20-1.42) in the OT, Epi, and Epi-OT groups respectively. The HR for ASD in the Epi-OT group was significantly higher than in the OT group (P = 0.02). | |
ASD: autism spectrum disorder
HR: hazard ratio
RR: risk ratio
IBFAT: Infant Breastfeeding Assessment Tool
Epi: epidural analgesia
Epi with OT group: infants from mothers exposed to both intravenous oxytocin and Epi
Epi group: infants from mothers exposed Epi without intravenous oxytocin
OT group: infants from mothers exposed intravenous oxytocin without Epi
OT-im group: infants from mothers exposed intramuscular oxytocin without Epi
Control group: infants from mothers exposed to neither intravenous
At birth
A Cochrane review of two randomized controlled trials25,26 examined the offspring outcomes of the Epi-OT group.27 This study found no statistically significant differences between offspring in the Epi-OT group and those who received Epi without oxytocin during labor (Epi group) in terms of 5-minute Apgar score of <7 (risk ratio, 3.06; 95% confidence interval [CI], 0.13–73.33) or admission to the neonatal intensive care unit (NICU; risk ratio, 1.07; 95% CI, 0.29–3.93).27
First hour of life
Two cohort studies examined the same offspring in different analyses: one focused on the achievement of suckling behavior28 and the other focused on the offsprings’ behaviors in terms of Widström’s nine stages,29 which describe the progression of newborn behavior during skin-to-skin contact with the mother during the first hour of life.30 The offspring were divided into four groups: exposure to neither OT nor Epi (control), exposure to intravenous OT without Epi (OT), Epi, and Epi-OT.28,29 There were no intergroup differences in birth weight (P = 0.11), gestational age (P = 0.46), and 1- and 5-minute Apgar scores (P = 0.64 and P = 0.65, respectively).28,29 However, for the Epi group, strong inverse correlations were found between the amount of fentanyl administered and achievement of suckling (P = 0.01) and the amount of OT (P = 0.046).28 The Epi-OT group reached significantly lower stages of Widström’s nine stages by the end of the first hour than the control and OT groups (mean ± standard deviation, 5.47 ± 1.4 vs 7.10 ± 1.7, P = 0.03 vs 7.17 ± 11, P = 0.01, respectively).29
Second day of life
Two cohort studies assessed the outcomes of the Epi-OT group on the second day of life.31,32 The skin temperature of the infants was assessed for 30 minutes when in skin-to-skin contact with their mothers.31 Another study showed offspring behavior with duration of rooting and assessed their feeding behavior and suckling quality using the Infant Breast Feeding Assessment Tool,32 which assesses infant state, readiness, rooting, latching, suckling behaviors, and maternal satisfaction with the breastfeeding experience.33 In the first experiment, the offspring were divided into the OT, Epi-OT, and control groups. The Epi-OT group had a significantly higher body temperature than the control group (mean ± standard error [SE], 35.07 ± 0.26 °C vs 34.19 ± 0.26 °C, P = 0.025) when the offspring were placed in skin-to-skin contact with their mothers.31 In addition, the Epi-OT group showed a significantly lower temperature difference between the start of breastfeeding and 10 minutes into the session (mean ± SE, -0.62 ± 0.22 °C, P = 0.019) whereas the control and OT groups showed significantly increasing temperatures between the start of breastfeeding and 10 minutes into the session (mean ± SE, 0.91 ± 0.2 °C, P = 0.001; and 1.47 ± 0.37 °C, P = 0.008).31
A later study assessed the feeding behavior quality of the infants in the control, Epi, Epi-OT, OT, and intramuscular OT without Epi groups.31 In that study, offspring in the control group had a significantly longer rooting duration than those in the other four groups.32 The median total Infant Breast Feeding Assessment Tool scores were lowest in the Epi group (Epi, 8; control, 10; OT, 10; intramuscular OT without Epi, 10.5; Epi-OT, 9). The Epi-OT group showed significantly lower Infant Breast Feeding Assessment Tool scores than the control group, especially for rooting (median [interquartile range], 2 [1–2] vs 1 [0–2], respectively; P = 0.02).32
Fourth day of life
One case-control study was conducted in the hospital at 4 days after birth to evaluate prenatal factors related to the risk of neonatal jaundice and hyperbilirubinemia in the offspring.34 Compared to control, hyperbilirubinemia was independently associated with OT administration (52.2% vs 28.3%, P < 0.01) and Epi use (39.1% vs 15.2%, P < 0.01).34 More infants in the Epi-OT versus the control group had hyperbilirubinemia (χ2 = 3.18, P < 0.05).34
Birth to 1 month of age
One observational study assessed neonatal outcomes from birth to 1 month of age by assessing neonatal behavior using the Neonatal Behavioral Assessment Scale (NBAS),35 a neurobehavioral assessment tool that was designed to describe newborns’ responses to their new environment and document their behavioral repertoire.36 On the first day of life, the Epi and Epi-OT groups showed significantly lower NBAS scores than the control group in terms of motor process (P < 0.01), state control (P < 0.05), and physiological response (P < 0.01) as well as total score (P < 0.01).35 In addition, the Epi-OT group showed significantly lower motor behavior scores on the NBAS than the Epi group (mean, 23 vs 32; P < 0.01).35 By the fifth day of life, the differences in NBAS scores among the three groups were smaller, but the Epi and Epi-OT groups showed significantly poorer state organization than the control group (P < 0.05).35 Moreover, the Epi-OT group showed significantly fewer feedings than those in the Epi group (mean, 7.5 vs 6.6, P < 0.05) and fewer minutes of crying per day than those in the Epi group (mean, 105.0 vs 71.4, P < 0.05).35 Although the Epi and Epi-OT groups received fewer feedings per day than the control group (mean, 66 vs 67 vs 93 times; P < 0.05) at 3 weeks of age, no differences were noted between the Epi and Epi-OT groups.35 Finally, at 1 month of life, there was no difference in NBAS scores among the three groups (mean, 64 vs 81 vs 72 in the control, Epi, and Epi-OT groups, respectively; P > 0.05).35
One month to 1 year
There were no studies in this area of infants from 1 month to 1 year of age.
Over 1 year
A large cohort study of 205,994 singleton vaginal births investigated the risk of ASD from 1 year to 13 years. The study included the control (n = 38,507), Epi (n = 49,679), OT (n = 13,607), and Epi-OT (n = 104,201) groups.37 Compared to the control group, the hazard ratio for ASD was 1.20 (95% CI, 1.09–1.32) in the Epi group and 1.30 (95% CI, 1.20–1.42) in the Epi-OT group, respectively, and 0.90 (95% CI, 0.78–1.04) in the OT group after the adjustment for potential confounders.37 A significant interaction was observed between Epi and OT in terms of ASD risk (P = 0.02), with the hazard ratio for ASD in the Epi-OT group being significantly higher than that in the OT group (P = 0.02).37
DISCUSSION
This narrative review identified eight studies of neurological and biobehavioral outcomes in the Epi-OT group. Regarding neonatal outcomes at birth, such as Apgar score or NICU admission, no differences were noted between the Epi-OT and Epi groups,27 or among the control, OT, and Epi-OT groups.28,29 Regarding infants’ pre-feeding and suckling behaviors during the first hour of life, a strong inverse correlation was found in the Epi-OT group.28,29 At 2 days after birth, the breastfeeding behavior and skin temperature patterns in the Epi-OT group were significantly different from those in the other three groups.31,32 At 4 days of age, more patients in the Epi-OT group versus the control group had hyperbilirubinemia.34 From the first day to 1 month of age, the effects of Epi and synthetic OT on neonatal behavior, particularly motor behavior, were strongest on the first day, but this trend was attenuated by the fifth day.35 At 1 month of age, the Epi-OT group showed no differences in NBAS scores from the Epi or the control groups.35 Between 1 month and 1 year of age, no eligible studies were conducted in this area. From 1 to 13 years of age, the risk of ASD was higher in the Epi and Epi-OT than the control group, while it remained unchanged in the OT group.37
All outcomes described above are suggested to be associated with neurodevelopmental impairments.38-53 The development of ASD, a neurobiological condition characterized by deficits in social communication, restricted interests, and repetitive behaviors,54 is influenced by both genetic and environmental factors that affect the developing brain.55,56 Although more than 1,000 genes are suggested to be associated with ASD, most variations have only a small effect on its risk, and there is insufficient evidence to prove their causal relationship with ASD.57 Several environmental factors are also associated with ASD, including maternal smoking, air pollution exposure, and vitamin intake; however, there is insufficient evidence of their causal association with ASD.58 Low Apgar scores are reportedly associated with neurological delay or cerebral palsy, and NICU admission is a prognostic factor for long-term cognitive status in offspring.38,39 Regarding ASD, limited evidence has shown an association between low Apgar scores and NICU admission40-42; however, these factors often result from preterm birth or low birth weight, which are associated with a risk of neurological problems including ASD.42,43,59 Feeding and suckling behaviors affect differentiation of the oropharyngeal and cranial nerves, which in turn affect neurological development.44,52,53
Suckling behavior reportedly reflects neonatal development, and neonatal behavior during the first hour after birth is influenced by fetal development.60 Feeding difficulties as well as oral, motor, and sensory problems are common in individuals with ASD.45,46 Body and skin temperatures are regulated by the hypothalamus. Studies have shown that temperature instability in newborns is associated with neurodevelopmental disorders.47,61 Persistent hyperbilirubinemia can cause jaundice in newborns, leading to brain damage and neurological abnormalities such as hearing loss and motor delay.62 Additionally, neonatal hyperbilirubinemia is reportedly associated with an increased risk of ASD.48,49 Moreover, NBAS scores are correlated with neurological development, and low scores are an early indicator of later developmental difficulties.63,64 Lower NBAS scores are also associated with an increased risk of ASD.50,51
Regarding the potential physiological mechanisms of Epi exposure in these neonatal outcomes, the local anesthesia and opioids used for Epi was reported to diffuse from the epidural space into the fetus.65-68 Epi administration for vaginal delivery reportedly affects an offspring’s development,69,70 while its use for cesarean delivery does not.71 Bupivacaine and ropivacaine, commonly used as Epi drugs during delivery, reportedly inhibit the gamma-aminobutyric acid response in rat pyramidal neurons,72 and a reduction in synaptic inputs has been implicated in an ASD model mice.73,74 Opioids induce leukoencephalopathy and edema in infants67 and promote autism-like behavior through hypermethylation of the glutamate receptor gene in the mouse brain.75,76 Moreover, recent studies reported the DNA methylation of Epi in immune-related signaling pathways in the umbilical cord blood of offspring.77 However, the mechanisms underlying the long-term effects of maternal Epi use on the fetal or neonatal brain or the later development of ASD remain unclear.
The same associations as Epi for the neurological and physiological effects of maternal OT administration on the brains of infants have been reported, such as low Apgar scores; NICU admission; pre-feeding, feeding, and neonatal behaviors; hyperbilirubinemia; and neurodevelopmental disorders such as bipolar disorder or ASD.14,78-80 Although the transport of OT from maternal plasma to the placenta has been reported,81 a recent review concluded that it is not expected to occur at high levels when synthetic OT is used at the recommended dose.82 Regarding the potential physiological mechanisms of synthetic OT administration in terms of neonatal outcomes, studies of rodent pup models showed that the induction of high doses of synthetic OT in dams resulted in pup brain damage in pups with oligodendrocyte cell death and microglial phagocytosis in the prefrontal cortex,83-85 which is known to be associated with psychiatric disorders.86 In addition, synthetic OT administration increased the methylation of OT receptors in the vole brain.87 Most importantly, as with Epi, the mechanisms underlying the long-term effects of OT on the fetal or neonatal brain or subsequent ASD development remain unclear. Epi inhibits exogenous OT secretion in animals and increases the need for synthetic OT in humans.22-24 However, the drug interaction effect of the combined use of Epi and OT on offspring and their neurodevelopment remains unclear.
More importantly, the medical necessity and benefits of epidural analgesia and oxytocin administration require consideration. Epidural anesthesia is beneficial for pain relief during childbirth.1 Oxytocin administration according to proper protocols to induce or accelerate labor shortens delivery time and reduces the incidence of clinical chorioamnionitis.88
Some studies examined the outcomes of offspring from birth to 13 years of age exposed to both Epi and OT during labor and reported inconsistent results. However, the research in this area is insufficient. Conducting randomized controlled trials for this purpose presents ethical challenges. Most of the studies included in this review were small and observational; only one large cohort study specifically focused on ASD. Further population-based long-term cohort studies of several aspects of offspring development are needed in this area to provide additional information for both women and clinicians considering Epi and synthetic OT administration during labor.
LIMITATIONS
This study has several limitations. First, with the exception of one systematic review that included two randomized controlled trials, most of the included studies were small and observational, which may make it difficult to exclude the possibility of bias. Second, we did not evaluate all Epi protocols (eg, anesthesia drug, dose, or duration) or OT protocols (eg, dose or duration) because they differed completely among studies, some of which lacked detailed data. Finally, we did not describe offspring developmental outcomes in terms of genetic and environmental background factors since the included studies provided limited offspring and family information.
ARTICLE INFORMATION
Credit authorship contribution statement
Tachi Asuka: Conceptualization: Writing – original draft, Writing – review and editing. Takahashi Yuki: Conceptualization, Funding acquisition, Writing – review and editing. Kotani Tomomi: Writing – review and editing.
Declaration of competing interests
The authors declare no competing interests.
Funding
This study was funded by the Japan Society for the Promotion of Science, KAKENHI (grant no. 19K11030).
Data availability
No data were used for the research described herein.
Acknowledgment
We thank Dr Hasegawa Kiyoshi (Dokkyo Medical University, Mibu, Tochigi, Japan) for his constructive feedback on the manuscript drafts. We also thank Editage (https://www.editage.jp/) for the English language editing.
Abbreviations
- ASD
autism spectrum disorder
- CI
confidence interval
- Epi
epidural analgesia
- Epi-OT
exposure to both epidural analgesia and oxytocin
- NBAS
Neonatal Behavioral Assessment Scale
- NICU
neonatal intensive care unit
- OT
oxytocin
REFERENCES
- 1.World Health Organization. WHO recommendations: intrapartum care for a positive childbirth experience. https://iris.who.int/bitstream/handle/10665/260178/9789241550215-eng.pdf?sequence=1. Published February 2018. Accessed November 1, 2021. [PubMed]
- 2.Butwick AJ, Bentley J, Wong CA, Snowden JM, Sun E, Guo N. United States state-level variation in the use of neuraxial analgesia during labor for pregnant women. JAMA Netw Open. 2018;1(8):e186567. doi: 10.1001/jamanetworkopen.2018.6567. [DOI] [PMC free article] [PubMed]
- 3.Kearns RJ, Shaw M, Gromski PS, Iliodromiti S, Lawlor DA, Nelson SM. Association of epidural analgesia in women in labor with neonatal and childhood outcomes in a population cohort. JAMA Netw Open. 2021;4(10):e2131683. doi: 10.1001/jamanetworkopen.2021.31683. [DOI] [PMC free article] [PubMed]
- 4.Wang K, Cao L, Deng Q, et al. The effects of epidural/spinal opioids in labour analgesia on neonatal outcomes: a meta-analysis of randomized controlled trials. Can J Anaesth. 2014;61(8):695–709. doi: 10.1007/s12630-014-0185-y. [DOI] [PubMed]
- 5.Qiu C, Lin JC, Shi JM, et al. Association between epidural analgesia during labor and risk of autism spectrum disorders in offspring. JAMA Pediatr. 2020;174(12):1168–1175. doi: 10.1001/jamapediatrics.2020.3231. [DOI] [PMC free article] [PubMed]
- 6.Wall-Wieler E, Bateman BT, Hanlon-Dearman A, Roos LL, Butwick AJ. Association of epidural labor analgesia with offspring risk of autism spectrum disorders. JAMA Pediatr. 2021;175(7):698–705. doi: 10.1001/jamapediatrics.2021.0376. [DOI] [PMC free article] [PubMed]
- 7.Hanley GE, Bickford C, Ip A, et al. Association of epidural analgesia during labor and delivery with autism spectrum disorder in offspring. JAMA. 2021;326(12):1178–1185. doi: 10.1001/jama.2021.14986. [DOI] [PMC free article] [PubMed]
- 8.Mikkelsen AP, Greiber IK, Scheller NM, Lidegaard Ø. Association of labor epidural analgesia with autism spectrum disorder in children. JAMA. 2021;326(12):1170–1177. doi: 10.1001/jama.2021.12655. [DOI] [PMC free article] [PubMed]
- 9.Murphy MSQ, Ducharme R, Hawken S, et al. Exposure to intrapartum epidural analgesia and risk of autism spectrum disorder in offspring. JAMA Netw Open. 2022;5(5):e2214273. doi: 10.1001/jamanetworkopen.2022.14273. [DOI] [PMC free article] [PubMed]
- 10.Wang X, Li J, Liu D. Effects of epidural analgesia exposure during parturition on autism spectrum disorder in newborns: a systematic review and meta-analysis based on cohort study. Front Psychiatry. 2022;13:974596. doi: 10.3389/fpsyt.2022.974596. [DOI] [PMC free article] [PubMed]
- 11.Vigneaud V, Ressler C, Swan JM, Roberts CW, Katsoyannis PG, Gordon S. The synthesis of an octapeptide amide with the hormonal activity of oxytocin. J Am Chem Soc. 1953;75(19):4879–4880. doi: 10.1021/ja01115a553. [DOI]
- 12.NHS Digital. NHS Maternity Statistics, England – 2020-21. https://digital.nhs.uk/data-and-information/publications/statistical/nhs-maternity-statistics/2020-21. Published November 2021. Accessed February 7, 2024.
- 13.Oscarsson ME, Amer-Wåhlin I, Rydhstroem H, Källén K. Outcome in obstetric care related to oxytocin use. A population-based study. Acta Obstet Gynecol Scand. 2006;85(9):1094–1098. doi: 10.1080/00016340600804530. [DOI] [PubMed]
- 14.Espada-Trespalacios X, Ojeda F, Perez-Botella M, et al. Oxytocin administration in low-risk women, a retrospective analysis of birth and neonatal outcomes. Int J Environ Res Public Health. 2021;18(8):4375. doi: 10.3390/ijerph18084375. [DOI] [PMC free article] [PubMed]
- 15.Bugg GJ, Siddiqui F, Thornton JG. Oxytocin versus no treatment or delayed treatment for slow progress in the first stage of spontaneous labour. Cochrane Database Syst Rev. 2013;6:CD007123. doi: 10.1002/14651858.CD007123.pub3. [DOI] [PMC free article] [PubMed]
- 16.Baranowska B, Kajdy A, Kiersnowska I, et al. Oxytocin administration for induction and augmentation of labour in polish maternity units – an observational study. BMC Pregnancy Childbirth. 2021;21(1):764. doi: 10.1186/s12884-021-04190-w. [DOI] [PMC free article] [PubMed]
- 17.Hidalgo-Lopezosa P, Hidalgo-Maestre M, Rodríguez-Borrego MA. Labor stimulation with oxytocin: effects on obstetrical and neonatal outcomes [in English, in Portuguese, in Spanish]. Rev Lat Am Enfermagem. 2016;24:e2744. doi: 10.1590/1518-8345.0765.2744. [DOI] [PMC free article] [PubMed]
- 18.Freedman D, Brown AS, Shen L, Schaefer CA. Perinatal oxytocin increases the risk of offspring bipolar disorder and childhood cognitive impairment. J Affect Disord. 2015;173:65–72. doi: 10.1016/j.jad.2014.10.052. [DOI] [PMC free article] [PubMed]
- 19.Weisman O, Agerbo E, Carter CS, et al. Oxytocin-augmented labor and risk for autism in males. Behav Brain Res. 2015;284:207–212. doi: 10.1016/j.bbr.2015.02.028. [DOI] [PubMed]
- 20.Soltys SM, Scherbel JR, Kurian JR, et al. An association of intrapartum synthetic oxytocin dosing and the odds of developing autism. Autism. 2020;24(6):1400–1410. doi: 10.1177/1362361320902903. [DOI] [PMC free article] [PubMed]
- 21.Zhang J, Klebanoff MA, DerSimonian R. Epidural analgesia in association with duration of labor and mode of delivery: a quantitative review. Am J Obstet Gynecol. 1999;180(4):970–977. doi: 10.1016/s0002-9378(99)70669-1. [DOI] [PubMed]
- 22.Brunton PJ, Bales J, Russell JA. Allopregnanolone and induction of endogenous opioid inhibition of oxytocin responses to immune stress in pregnant rats. J Neuroendocrinol. 2012;24(4):690–700. doi: 10.1111/j.1365-2826.2012.02295.x. [DOI] [PubMed]
- 23.Morris MS, Domino EF, Domino SE. Opioid modulation of oxytocin release. J Clin Pharmacol. 2010;50(10):1112–1117. doi: 10.1177/0091270010361256. [DOI] [PubMed]
- 24.Jonas W, Johansson LM, Nissen E, Ejdebäck M, Ransjö-Arvidson AB, Uvnäs-Moberg K. Effects of intrapartum oxytocin administration and epidural analgesia on the concentration of plasma oxytocin and prolactin in response to suckling during the second day postpartum. Breastfeed Med. 2009;4(2):71–82. doi: 10.1089/bfm.2008.0002. [DOI] [PubMed]
- 25.Saunders NJ, Spiby H, Gilbert L, et al. Oxytocin infusion during second stage of labour in primiparous women using epidural analgesia: a randomised double-blind placebo-controlled trial. BMJ. 1989;299(6713):1423–1426. doi: 10.1136/bmj.299.6713.1423. [DOI] [PMC free article] [PubMed]
- 26.Shennan AH, Smith R, Browne D, Edmonds DK, Morgan B. The elective use of oxytocin infusion during labour in nulliparous women using epidural analgesia. Int J Obstet Anesth. 1995;4(2):78–81. doi: 10.1016/0959-289x(95)82996-N. [DOI] [PubMed]
- 27.Costley PL, East CE. Oxytocin augmentation of labour in women with epidural analgesia for reducing operative deliveries. Cochrane Database Syst Rev. 2013;7:CD009241. doi: 10.1002/14651858.CD009241.pub3. [DOI] [PMC free article] [PubMed]
- 28.Brimdyr K, Cadwell K, Widström AM, et al. The association between common labor drugs and suckling when skin-to-skin during the first hour after birth. Birth. 2015;42(4):319–328. doi: 10.1111/birt.12186. [DOI] [PMC free article] [PubMed]
- 29.Brimdyr K, Cadwell K, Widström AM, Svensson K, Phillips R. The effect of labor medications on normal newborn behavior in the first hour after birth: a prospective cohort study. Early Hum Dev. 2019;132:30–36. doi: 10.1016/j.earlhumdev.2019.03.019. [DOI] [PubMed]
- 30.Brimdyr K, Cadwell K, Svensson K, Takahashi Y, Nissen E, Widström AM. The nine stages of skin-to-skin: practical guidelines and insights from four countries. Matern Child Nutr. 2020;16(4):e13042. doi: 10.1111/mcn.13042. [DOI] [PMC free article] [PubMed]
- 31.Jonas W, Wiklund I, Nissen E, Ransjö-Arvidson AB, Uvnäs-Moberg K. Newborn skin temperature two days postpartum during breastfeeding related to different labour ward practices. Early Hum Dev. 2007;83(1):55–62. doi: 10.1016/j.earlhumdev.2006.05.001. [DOI] [PubMed]
- 32.Takahashi Y, Uvnäs-Moberg K, Nissen E, Lidfors L, Ransjö-Arvidson AB, Jonas W. Epidural analgesia with or without oxytocin, but not oxytocin alone, administered during birth disturbs infant pre-feeding and sucking behaviors and maternal oxytocin levels in connection with a breastfeed two days later. Front Neurosci. 2021;15:673184. doi: 10.3389/fnins.2021.673184. [DOI] [PMC free article] [PubMed]
- 33.Matthews MK. Developing an instrument to assess infant breastfeeding behaviour in the early neonatal period. Midwifery. 1988;4(4):154–165. doi: 10.1016/s0266-6138(88)80071-8. [DOI] [PubMed]
- 34.Sima DG, Neligan GA. Factors affecting the increasing incidence of severe non-haemolytic neonatal jaundice. Br J Obstet Gynaecol. 1975;82(11):863–867. doi: 10.1111/j.1471-0528.1975.tb00590.x. [DOI] [PubMed]
- 35.Murray AD, Dolby RM, Nation RL, Thomas DB. Effects of epidural anesthesia on newborns and their mothers. Child Dev. 1981;52(1):71–82. [PubMed]
- 36.Brazelton TB. The Brazelton Neonatal Behavior Assessment Scale: introduction. Monogr Soc Res Child Dev. 1978;43(5–6):1–13. [PubMed]
- 37.Qiu C, Carter SA, Lin JC, et al. Association of labor epidural analgesia, oxytocin exposure, and risk of autism spectrum disorders in children. JAMA Netw Open. 2023;6(7):e2324630. doi: 10.1001/jamanetworkopen.2023.24630. [DOI] [PMC free article] [PubMed]
- 38.Persson M, Razaz N, Tedroff K, Joseph KS, Cnattingius S. Five and 10 minute Apgar scores and risks of cerebral palsy and epilepsy: population-based cohort study in Sweden. BMJ. 2018;360:k207. doi: 10.1136/bmj.k207. [DOI] [PMC free article] [PubMed]
- 39.Royer AS, Busari JO. A systematic review of the impact of intensive care admissions on post discharge cognition in children. Eur J Pediatr. 2021;180(12):3443–3454. doi: 10.1007/s00431-021-04145-5. [DOI] [PMC free article] [PubMed]
- 40.Modabbernia A, Sandin S, Gross R, et al. Apgar score and risk of autism. Eur J Epidemiol. 2019;34(2):105–114. doi: 10.1007/s10654-018-0445-1. [DOI] [PMC free article] [PubMed]
- 41.Polo-Kantola P, Lampi KM, Hinkka-Yli-Salomäki S, Gissler M, Brown AS, Sourander A. Obstetric risk factors and autism spectrum disorders in Finland. J Pediatr. 2014;164(2):358–365. doi: 10.1016/j.jpeds.2013.09.044. [DOI] [PubMed]
- 42.Arafa A, Mahmoud O, Salah H, Abdelmonem AA, Senosy S. Maternal and neonatal risk factors for autism spectrum disorder: a case-control study from Egypt. PLoS One. 2022;17(6):e0269803. doi: 10.1371/journal.pone.0269803. [DOI] [PMC free article] [PubMed]
- 43.Crump C, Sundquist J, Sundquist K. Preterm or early term birth and risk of autism. Pediatrics. 2021;148(3):e2020032300. doi: 10.1542/peds.2020-032300. [DOI] [PMC free article] [PubMed]
- 44.McBride MC, Danner SC. Sucking disorders in neurologically impaired infants: assessment and facilitation of breastfeeding. Clin Perinatol. 1987;14(1):109–130. [PubMed]
- 45.Parish-Morris J, Sariyanidi E, Zampella C, et al. Oral-motor and lexical diversity during naturalistic conversations in adults with autism spectrum disorder. Proc Conf. 2018;2018:147–157. doi: 10.18653/v1/w18-0616. [DOI] [PMC free article] [PubMed]
- 46.Nadon G, Feldman DE, Dunn W, Gisel E. Association of sensory processing and eating problems in children with autism spectrum disorders. Autism Res Treat. 2011;2011:541926. doi: 10.1155/2011/541926. [DOI] [PMC free article] [PubMed]
- 47.Laptook AR, McDonald SA, Shankaran S, et al. Elevated temperature and 6- to 7-year outcome of neonatal encephalopathy. Ann Neurol. 2013;73(4):520–528. doi: 10.1002/ana.23843. [DOI] [PMC free article] [PubMed]
- 48.Hung TW, Tsai JD, Pan HH, Chen HJ, Liao PF, Sheu JN. Is neonatal hyperbilirubinemia exposure associated with a risk of autism spectrum disorder? A nationwide cohort study. Am J Perinatol. 2021;38(12):1244–1253. doi: 10.1055/s-0040-1708033. [DOI] [PubMed]
- 49.Lozada LE, Nylund CM, Gorman GH, Hisle-Gorman E, Erdie-Lalena CR, Kuehn D. Association of autism spectrum disorders with neonatal hyperbilirubinemia. Glob Pediatr Health. 2015;2:2333794X15596518. doi: 10.1177/2333794X15596518. [DOI] [PMC free article] [PubMed]
- 50.Lv MN, Zhang H, Shu Y, Chen S, Hu YY, Zhou M. The neonatal levels of TSB, NSE and CK-BB in autism spectrum disorder from Southern China. Transl Neurosci. 2016;7(1):6–11. doi: 10.1515/tnsci-2016-0002. [DOI] [PMC free article] [PubMed]
- 51.Shoaff JR, Nugent K, Brazelton TB, Korrick SA. Early infant behavioural correlates of social skills in adolescents. Paediatr Perinat Epidemiol. 2021;35(2):247–256. doi: 10.1111/ppe.12723. [DOI] [PMC free article] [PubMed]
- 52.Maynard TM, Zohn IE, Moody SA, LaMantia AS. Suckling, Feeding, and swallowing: behaviors, circuits, and targets for neurodevelopmental pathology. Annu Rev Neurosci. 2020;43:315–336. doi: 10.1146/annurev-neuro-100419-100636. [DOI] [PMC free article] [PubMed]
- 53.Delaney AL, Arvedson JC. Development of swallowing and feeding: prenatal through first year of life. Dev Disabil Res Rev. 2008;14(2):105–117. doi: 10.1002/ddrr.16. [DOI] [PubMed]
- 54.Barbaresi WJ, Katusic SK, Voigt RG. Autism: a review of the state of the science for pediatric primary health care clinicians. Arch Pediatr Adolesc Med. 2006;160(11):1167–1175. doi: 10.1001/archpedi.160.11.1167. [DOI] [PubMed]
- 55.Folstein S, Rutter M. Infantile autism: a genetic study of 21 twin pairs. J Child Psychol Psychiatr. 1977;18(4):297–321. doi: 10.1111/j.1469-7610.1977.tb00443.x. [DOI] [PubMed]
- 56.Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatr. 2011;68(11):1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed]
- 57.Myers SM, Challman TD, Bernier R, et al. Insufficient evidence for “autism-specific” genes. Am J Hum Genet. 2020;106(5):587–595. doi: 10.1016/j.ajhg.2020.04.004. [DOI] [PMC free article] [PubMed]
- 58.Modabbernia A, Velthorst E, Reichenberg A. Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol Autism. 2017;8:13. doi: 10.1186/s13229-017-0121-4. [DOI] [PMC free article] [PubMed]
- 59.Harel-Gadassi A, Friedlander E, Yaari M, et al. Risk for ASD in preterm infants: a three-year follow-up study. Autism Res Treat. 2018;2018:8316212. doi: 10.1155/2018/8316212. [DOI] [PMC free article] [PubMed]
- 60.Pineda R, Harris R, Foci F, Roussin J, Wallendorf M. Neonatal Eating Outcome Assessment: tool development and inter-rater reliability. Acta Paediatr. 2018;107(3):414–424. doi: 10.1111/apa.14128. [DOI] [PMC free article] [PubMed]
- 61.Tan CL, Knight ZA. Regulation of body temperature by the nervous system. Neuron. 2018;98(1):31–48. doi: 10.1016/j.neuron.2018.02.022. [DOI] [PMC free article] [PubMed]
- 62.Le Pichon JB, Riordan SM, Watchko J, Shapiro SM. The neurological sequelae of neonatal hyperbilirubinemia: definitions, diagnosis and treatment of the kernicterus spectrum disorders (KSDs). Curr Pediatr Rev. 2017;13(3):199–209. doi: 10.2174/1573396313666170815100214. [DOI] [PubMed]
- 63.Ohgi S, Arisawa K, Takahashi T, et al. Neonatal Behavioral Assessment Scale as a predictor of later developmental disabilities of low birth-weight and/or premature infants. Brain Dev. 2003;25(5):313–321. doi: 10.1016/s0387-7604(02)00233-4. [DOI] [PubMed]
- 64.Canals J, Esparó G, Fernández-Ballart JD. Neonatal behaviour characteristics and psychological problems at 6 years. Acta Paediatr. 2006;95(11):1412–1417. doi: 10.1080/08035250600760790. [DOI] [PubMed]
- 65.Kuhnert BR, Zuspan KJ, Kuhnert PM, Syracuse CD, Brown DE. Bupivacaine disposition in mother, fetus, and neonate after spinal anesthesia for cesarean section. Anesth Analg. 1987;66(5):407–412. doi: 10.1213/00000539-198705000-00006. [DOI] [PubMed]
- 66.Johnson RF, Cahana A, Olenick M, et al. A comparison of the placental transfer of ropivacaine versus bupivacaine. Anesth Analg. 1999;89(3):703–708. doi: 10.1213/00000539-199909000-00032. [DOI] [PubMed]
- 67.McPherson C, Haslam M, Pineda R, Rogers C, Neil JJ, Inder TE. Brain injury and development in preterm infants exposed to fentanyl. Ann Pharmacother. 2015;49(12):1291–1297. doi: 10.1177/1060028015606732. [DOI] [PMC free article] [PubMed]
- 68.de Barros Duarte L, Moisés EC, Carvalho Cavalli R, Lanchote VL, Duarte G, da Cunha SP. Distribution of fentanyl in the placental intervillous space and in the different maternal and fetal compartments in term pregnant women. Eur J Clin Pharmacol. 2009;65(8):803–808. doi: 10.1007/s00228-009-0645-4. [DOI] [PubMed]
- 69.Golub MS, Germann SL. Perinatal bupivacaine and infant behavior in rhesus monkeys. Neurotoxicol Teratol. 1998;20(1):29–41. doi: 10.1016/s0892-0362(97)00068-8. [DOI] [PubMed]
- 70.Standley K, Soule AB 3rd, Copans SA, Duchowny MS. Local-regional anesthesia during childbirth: effect on newborn behaviors. Science. 1974;186(4164):634–635. doi: 10.1126/science.186.4164.634. [DOI] [PubMed]
- 71.Sprung J, Flick RP, Wilder RT, et al. Anesthesia for cesarean delivery and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;111(2):302–310. doi: 10.1097/ALN.0b013e3181adf481. [DOI] [PMC free article] [PubMed]
- 72.Breton JD, Stuart GJ. GABA(B) receptors in neocortical and hippocampal pyramidal neurons are coupled to different potassium channels. Eur J Neurosci. 2017;46(12):2859–2866. doi: 10.1111/ejn.13777. [DOI] [PubMed]
- 73.Lazaro MT, Taxidis J, Shuman T, et al. Reduced prefrontal synaptic connectivity and disturbed oscillatory population dynamics in the CNTNAP2 model of autism. Cell Rep. 2019;27(9):2567–2578. doi: 10.1016/j.celrep.2019.05.006. [DOI] [PMC free article] [PubMed]
- 74.Barón-Mendoza I, Maqueda-Martínez E, Martínez-Marcial M, De la Fuente-Granada M, Gómez-Chavarin M, González-Arenas A. Changes in the number and morphology of dendritic spines in the hippocampus and prefrontal cortex of the C58/J mouse model of autism. Front Cell Neurosci. 2021;15:726501. doi: 10.3389/fncel.2021.726501. [DOI] [PMC free article] [PubMed]
- 75.Blackwood CA, Cadet JL. The molecular neurobiology and neuropathology of opioid use disorder. Curr Res Neurobiol. 2021;2:100023. doi: 10.1016/j.crneur.2021.100023. [DOI] [PMC free article] [PubMed]
- 76.Sheng Z, Liu Q, Cheng C, et al. Fentanyl induces autism-like behaviours in mice by hypermethylation of the glutamate receptor gene Grin2b. Br J Anaesth. 2022;129(4):544–554. doi: 10.1016/j.bja.2022.04.027. [DOI] [PubMed]
- 77.Wang Y, Tzeng JY, Huang Y, Maguire R, Hoyo C, Allen TK. Duration of exposure to epidural anesthesia at delivery, DNA methylation in umbilical cord blood and their association with offspring asthma in Non-Hispanic Black women. Environ Epigenet. 2022;9(1):dvac026. doi: 10.1093/eep/dvac026. [DOI] [PMC free article] [PubMed]
- 78.Litorp H, Sunny AK, Kc A. Augmentation of labor with oxytocin and its association with delivery outcomes: a large-scale cohort study in 12 public hospitals in Nepal. Acta Obstet Gynecol Scand. 2021;100(4):684–693. doi: 10.1111/aogs.13919. [DOI] [PubMed]
- 79.Marín Gabriel MA, Olza Fernández I, Malalana Martínez AM, et al. Intrapartum synthetic oxytocin reduce the expression of primitive reflexes associated with breastfeeding. Breastfeed Med. 2015;10(4):209–213. doi: 10.1089/bfm.2014.0156. [DOI] [PMC free article] [PubMed]
- 80.Buchan PC. Pathogenesis of neonatal hyperbilirubinaemia after induction of labour with oxytocin. Br Med J. 1979;2(6200):1255–1257. doi: 10.1136/bmj.2.6200.1255. [DOI] [PMC free article] [PubMed]
- 81.Malek A, Blann E, Mattison DR. Human placental transport of oxytocin. J Matern Fetal Med. 1996;5(5):245–255. doi:. [DOI] [PubMed]
- 82.Uvnäs-Moberg K. The physiology and pharmacology of oxytocin in labor and in the peripartum period. Am J Obstet Gynecol. 2024;230(3S):S740–S758. doi: 10.1016/j.ajog.2023.04.011. [DOI] [PubMed]
- 83.Boksa P, Zhang Y, Nouel D. Maternal oxytocin administration before birth influences the effects of birth anoxia on the neonatal rat brain. Neurochem Res. 2015;40(8):1631–1643. doi: 10.1007/s11064-015-1645-7. [DOI] [PubMed]
- 84.Hirayama T, Hiraoka Y, Kitamura E, et al. Oxytocin induced labor causes region and sex-specific transient oligodendrocyte cell death in neonatal mouse brain. J Obstet Gynaecol Res. 2020;46(1):66–78. doi: 10.1111/jog.14149. [DOI] [PubMed]
- 85.Kitamura E, Koike M, Hirayama T, et al. Susceptibility of subregions of prefrontal cortex and corpus callosum to damage by high-dose oxytocin-induced labor in male neonatal mice. PLoS One. 2021;16(8):e0256693. doi: 10.1371/journal.pone.0256693. [DOI] [PMC free article] [PubMed]
- 86.Gamo NJ, Arnsten AF. Molecular modulation of prefrontal cortex: rational development of treatments for psychiatric disorders. Behav Neurosci. 2011;125(3):282–296. doi: 10.1037/a0023165. [DOI] [PMC free article] [PubMed]
- 87.Kenkel WM, Perkeybile AM, Yee JR, et al. Behavioral and epigenetic consequences of oxytocin treatment at birth. Sci Adv. 2019;5(5):eaav2244. doi: 10.1126/sciadv.aav2244. [DOI] [PMC free article] [PubMed]
- 88.Hermesch AC, Kernberg AS, Layoun VR Caughey AB, Caughey AB. Oxytocin: physiology, pharmacology, and clinical application for labor management. Am J Obstet Gynecol. 2024;230(3S):S729–S739. doi: 10.1016/j.ajog.2023.06.041. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used for the research described herein.