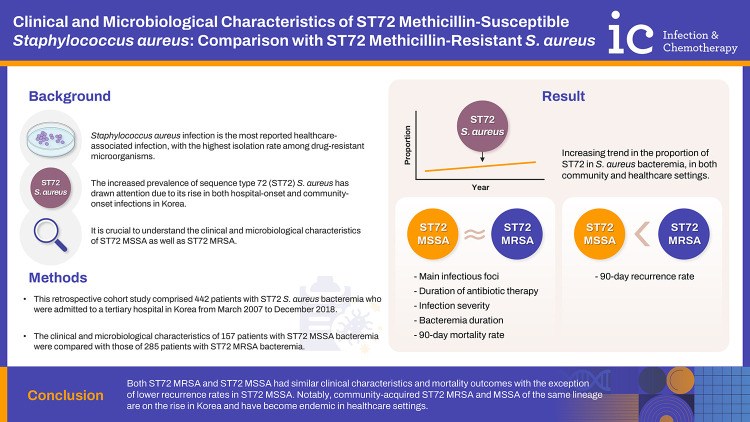
Abstract
Background
Sequence type 72 (ST72) is the predominant community-associated methicillin-resistant Staphylococcus aureus (MRSA) genotype in Korea. With an increasing prevalence of the ST72 S. aureus lineage, regardless of methicillin resistance, it is crucial to understand the clinical and microbiological characteristics of ST72 methicillin-susceptible S. aureus (MSSA) as well as ST72 MRSA.
Materials and Methods
In this retrospective cohort study, data from patients with S. aureus bacteremia (SAB) who were admitted to a tertiary hospital in Korea from March 2007 to December 2018 were collected. Multilocus sequence typing was used to identify ST72 isolates. The clinical and microbiological characteristics of ST72 MSSA were compared with those of ST72 MRSA among patients infected with SAB.
Results
Among the 442 SAB patients with ST72, 157 (35.5%) were infected with MSSA and 285 (64.5%) were infected with MRSA. There was a significant increase in the proportion of ST72 MSSA in both the community and hospital settings. Compared to ST72 MRSA, ST72 MSSA isolates were less likely to have multidrug resistance. The main infection foci, infection severity, and duration of bacteremia did not differ significantly between the two groups. The 90-day recurrence rate was significantly lower in the MSSA group (2.5% vs. 8.4%, P=0.03), while the 90-day mortality rate was comparable (28.0% vs. 23.9%, P=0.40).
Conclusion
ST72 MSSA had similar clinical features as ST72 MRSA in terms of infection site, severity, and 90-day mortality. Despite exhibiting lower levels of antibiotic resistance, ST72 MSSA has increased in the hospital environment concurrently with ST72 MRSA.
Keywords: ST72, Community-associated MRSA, Staphylococcus aureus bacteremia
Graphical Abstract
Introduction
Staphylococcus aureus infection is the most reported healthcare-associated infection, with the highest isolation rate among microorganisms with drug resistance [1]. With the recent rise of community-associated methicillin-resistant S. aureus (CA-MRSA), sequence type 72 (ST72) MRSA has become the predominant strain as a community-acquired lineage in Korea [2]. Compared to the USA300 clone, a representative CA-MRSA lineage in the United States (US) possessing the Panton-Valentine leukocidin (PVL) gene, the ST72 MRSA lineage has a unique feature: it lacks the PVL gene [3]. Despite the absence of potential virulence factors like PVL, ST72 MRSA is the most common genotype in hospital-onset infections as well as community-onset infections in Korea [4,5]. With the increase of the ST72 genotype in both MSSA and MRSA, it is important to understand the clinical features of ST72 MSSA as well as the changes in its proportion among clinical S. aureus isolates over time. In this study, clinical and microbiological characteristics of ST72 MSSA infections were compared with those of ST72 MRSA infections using a large S. aureus bacteremia (SAB) patient cohort.
Materials and Methods
1. Study population and design
This retrospective cohort study was conducted at Asan Medical Center, Seoul, Korea. We included hospital patients aged ≥18 years with a diagnosis of SAB from March 2007 until December 2018 at our tertiary teaching hospital. These patients had confirmed blood bacterial cultures for S. aureus. Patients infected with S. aureus that belonged to ST72 were included. The clinical characteristics and outcomes of patients with ST72 MSSA were compared with those with ST72 MRSA.
2. Ethics statement
This study was approved by the Institutional Review Board of Asan Medical Center (IRB number: 2013-0234). Informed consent was waived because of the nature of the study setting, and any collected data with identifiable factors were encrypted and stored anonymously before analysis.
3. Laboratory and molecular results
S. aureus isolates were identified using established laboratory methods, and their antimicrobial susceptibility was assessed following the standard criteria outlined by the MicroScan system (Dade Behring, West Sacramento, CA, USA) and the Clinical and Laboratory Standards Institute (CLSI) [6]. MRSA was confirmed by determining the minimum inhibitory concentration (MIC) of oxacillin and detecting the presence of the mecA gene. The minimal inhibitory concentration of vancomycin was determined by the broth microdilution (BMD) method according to the CLSI guidelines as well as by the E test (AB Biodisk, Solna, Sweden) according to the manufacturer’s instructions. Multilocus sequence typing (MLST), δ-hemolysin activity to determine accessory gene regulator (agr) functionality, and genotyping of staphylococcal protein A (spa) were performed as described previously [7,8].
4. Data collection and definitions
Electronic medical records were used to obtain the following information for all patients included in this study: age, gender, location of infection acquisition, past medical history, site of infection foci, severity presentation at admission, microbiological data, and clinical outcomes. Bloodstream infections caused by S. aureus were categorized into community-acquired, healthcare-associated, and hospital-acquired infections. Community-acquired bacteremia was defined as a bloodstream infection confirmed by a positive blood culture within 48 hours of admission. Healthcare-associated bacteremia was defined as a bloodstream infection confirmed by a positive blood culture obtained at the time of hospital admission or within 48 hours of admission if the patient had attended a hospital or hemodialysis clinic, or received intravenous chemotherapy in the 30 days before the bloodstream infection, or was hospitalized in an acute care hospital for 2 or more days in the 90 days before the bloodstream infection. Hospital-acquired bacteremia was defined by a positive blood culture obtained from patients who had been hospitalized for more than 48 hours [9]. The infection focus was established by an infectious diseases specialist based on microbiological data, radiological images, and the clinical course of the patient. The index day was the day on which blood for cultures was drawn from a patient later confirmed to have SAB. Persistent bacteremia was defined as culture-confirmed bacteremia persisting for more than 3 days. Catheter-related bloodstream infection was diagnosed in patients according to the Infectious Diseases Society of America guidelines, which provide comprehensive criteria for the diagnosis and management of intravascular catheter-related infections [10]. The Charlson comorbidity index was used to produce a composite score of comorbid conditions, allowing for the assessment of the cumulative burden of illness [11]. The Pitt bacteremia score was utilized to determine the severity of acute illness in patients with bloodstream infections. This score considers factors such as temperature, blood pressure, mechanical ventilation, cardiac arrest, and mental status to quantify the severity of bacteremia [12]. Factors associated with the clinical outcomes of the patients, such as mortality and the recurrence of bacteremia, were considered.
5. Statistical analysis
Statistical data analyses were performed using R Studio software (version 4.2.2, R Project for Statistical Computing, Vienna, Austria), using the ‘moonBook’ package (version 0.3.1, Hannarae, Seoul, Korea). Data visualization was conducted using the ‘ggplot2’ package (version 3.4.0, Springer-Verlag, New York, NY, USA) [13]. Clinical, microbiological, and genetic characteristics were compared between the ST72 MSSA and MRSA strains. Differences between the two groups for continuous and categorical variables were assessed using Mann-Whitney U tests or Fisher’s exact test as appropriate. Trend analysis was performed to determine a significant increase or decrease in the proportion of annual ST72 MSSA during the study period using the Cochran-Armitage test. Univariate and multivariate analyses using logistic regression models were performed to find independent risk factors for mortality and recurrence. All variables with P-values <0.10 in the univariate analyses were included in the multivariate analysis. Stepwise logistic regression using the backward elimination method was performed. A P-value less than 0.05 was set as the threshold for statistical significance.
Results
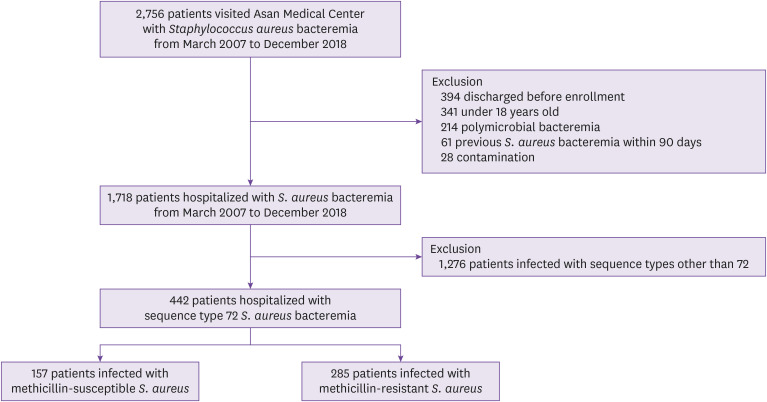
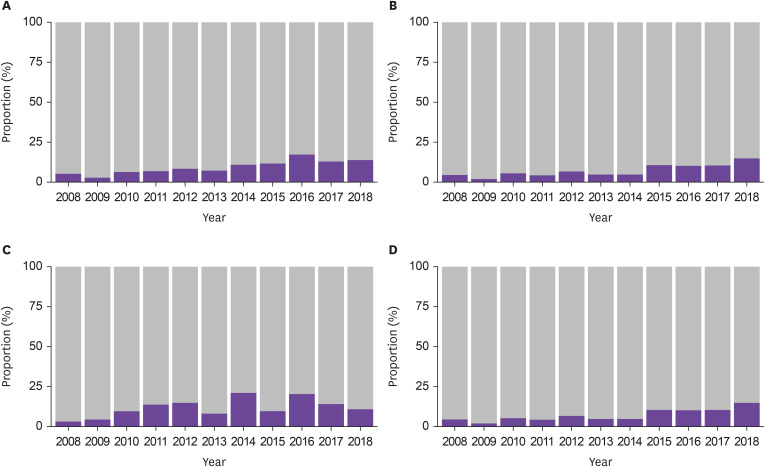
Among 1,718 patients hospitalized with SAB, 442 patients with ST72 S. aureus were identified, including 157 patients with MSSA (35.5%) and 285 patients with MRSA (64.5%) (Fig. 1). During the study period, the proportion of ST72 MSSA showed a significant increasing trend by year (P for trend <0.01, Fig. 2). Similar increasing trends were observed across community-acquired, healthcare-associated, and hospital-acquired infections (P<0.01, P=0.03, P<0.01, respectively, Fig. 2). The increasing trend of ST72 MSSA was observed within the MSSA subgroups (Supplementary Fig. 1). When stratified by mode of acquisition, ST72 MSSA had the third highest frequency in both the community-acquired and healthcare-associated bacteremia, and the highest frequency in the hospital-acquired bacteremia (Supplementary Table 1).
Figure 1. Flowchart of study population.
Figure 2. Changes in the proportion of ST72 in the MSSA cohort by year.
(A) Total number of Staphylococcus aureus bacteremia (SAB) (P for trend <0.01), (B) Community-acquired SAB (P for trend <0.01), (C) Healthcare-associated SAB (P for trend=0.03), (D) Hospital-acquired SAB (P for trend <0.01).
Stacked blue bars represent the proportion of ST72 MSSA.
ST, sequence type; MSSA, methicillin-susceptible Staphylococcus aureus.
1. Baseline and clinical characteristics
The baseline characteristics of 442 patients with ST72 MSSA and ST72 MRSA are summarized in Table 1. Patients with ST72 MSSA were younger than those with ST72 MRSA. Community-acquired infections were more common in the ST72 MSSA group than in the ST72 MRSA group (20.4% vs. 10.2%, P<0.01), but most of the infections in both groups occurred in healthcare-associated or hospital-acquired settings. There were no significant differences between the two groups in the prevalence of underlying diseases, Charlson comorbidity index, or predisposing conditions. The main focus of infection and the severity of infection were not significantly different between the two groups.
Table 1. Baseline and clinical characteristics of patients infected with ST72 Staphylococcus aureus .
| Characteristics | Total (n=442) | ST72 MSSA (n=157) | ST72 MRSA (n=285) | P | ||
|---|---|---|---|---|---|---|
| Age, years | 63 (53–71) | 59 (51–70) | 64 (54–72) | 0.05 | ||
| Age >60 years | 250 (56.6) | 76 (48.4) | 174 (61.1) | 0.01 | ||
| Male | 262 (59.3) | 95 (60.5) | 167 (58.6) | 0.77 | ||
| Mode of acquisition | <0.01 | |||||
| Community-acquired | 61 (13.8) | 32 (20.4) | 29 (10.2) | |||
| Healthcare-associated | 172 (38.9) | 63 (40.1) | 109 (38.2) | |||
| Hospital-acquired | 209 (47.3) | 62 (39.5) | 147 (51.6) | |||
| Comorbidity | ||||||
| Diabetes mellitus | 151 (34.2) | 49 (31.2) | 102 (35.8) | 0.39 | ||
| Hypertension | 189 (42.8) | 61 (38.9) | 128 (44.9) | 0.26 | ||
| Ischemic heart disease | 43 (9.7) | 13 (8.3) | 30 (10.5) | 0.55 | ||
| Heart failure | 21 (4.8) | 11 (7.0) | 10 (3.5) | 0.16 | ||
| End-stage kidney disease | 54 (12.2) | 17 (10.8) | 37 (13.0) | 0.61 | ||
| Liver cirrhosis | 70 (15.8) | 30 (19.1) | 40 (14.0) | 0.21 | ||
| Biliary disease | 15 (3.4) | 4 (2.6) | 11 (3.9) | 0.65 | ||
| Chronic lung disease | 9 (2.0) | 2 (1.3) | 7 (2.5) | 0.62 | ||
| Solid tumor | 172 (38.9) | 63 (40.1) | 109 (38.2) | 0.78 | ||
| Hematologic malignancy | 42 (9.5) | 14 (8.9) | 28 (9.8) | 0.89 | ||
| Solid organ transplant | 16 (3.6) | 6 (3.8) | 10 (3.5) | >0.99 | ||
| Charlson comorbidity index | 3 (2–5) | 3 (2–5) | 2 (2–4) | 0.47 | ||
| Predisposing condition | ||||||
| Neutropenia | 29 (6.6) | 14 (8.9) | 15 (5.3) | 0.19 | ||
| Recent surgery within 30 days | 68 (15.4) | 19 (12.1) | 49 (17.2) | 0.20 | ||
| Anticancer chemotherapy | 83 (18.8) | 29 (18.5) | 54 (18.9) | >0.99 | ||
| Corticosteroid use | 105 (23.8) | 37 (23.6) | 68 (23.9) | >0.99 | ||
| Immunosuppressive drug use other than corticosteroid | 21 (4.8) | 6 (3.8) | 15 (5.3) | 0.65 | ||
| Central venous catheter | 126 (28.5) | 39 (24.8) | 87 (30.5) | 0.25 | ||
| Non-catheter indwelling devices | 79 (17.9) | 24 (15.3) | 55 (19.3) | 0.36 | ||
| Cardiac implantable electronic device | 4 (0.9) | 1 (0.6) | 3 (1.1) | >0.99 | ||
| Prosthetic heart valve | 16 (3.6) | 5 (3.2) | 11 (3.9) | 0.92 | ||
| Vascular graft | 37 (8.3) | 10 (6.4) | 27 (9.5) | 0.34 | ||
| Orthopedic implant | 18 (4.1) | 4 (2.6) | 14 (4.9) | 0.34 | ||
| Main focus of infection | ||||||
| Primary bacteremia | 66 (14.9) | 28 (17.8) | 38 (13.3) | 0.26 | ||
| Central catheter related | 83 (18.8) | 27 (17.2) | 56 (19.6) | 0.61 | ||
| Peripheral catheter related | 40 (9.0) | 18 (11.5) | 22 (7.7) | 0.25 | ||
| Pneumonia | 39 (8.8) | 12 (7.6) | 27 (9.5) | 0.64 | ||
| Skin or soft tissue | 47 (10.6) | 21 (13.4) | 26 (9.1) | 0.22 | ||
| Urinary tract | 9 (2.0) | 1 (0.6) | 8 (2.8) | 0.23 | ||
| Surgical wound | 22 (5.0) | 5 (3.2) | 17 (6.0) | 0.29 | ||
| Endocarditis | 19 (4.3) | 6 (3.8) | 13 (4.6) | 0.90 | ||
| Bone and joint infection | 50 (11.3) | 17 (10.8) | 33 (11.6) | 0.94 | ||
| Arteriovenous graft | 17 (3.9) | 3 (1.9) | 14 (4.9) | 0.19 | ||
| Metastatic infection | 79 (17.9) | 26 (16.6) | 53 (18.6) | 0.69 | ||
| Severity of infection | 0.84 | |||||
| No sepsis | 84 (19.0) | 31 (19.7) | 53 (18.6) | |||
| Sepsis | 308 (69.7) | 110 (70.1) | 198 (69.5) | |||
| Septic shock | 50 (11.3) | 16 (10.2) | 34 (11.9) | |||
| Pitt bacteremia score | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.19 | ||
Data are the number of patients (%) or median (interquartile range).
ST, sequence type; MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus.
2. Microbiological characteristics
ST72 MRSA isolates were more likely to have resistance against antibiotics, such as clindamycin (20.4% vs. 3.8%, P<0.01), ciprofloxacin (7.7% vs. 1.3%, P=0.01), and erythromycin (26.0% vs. 5.1%, P<0.01). In contrast, MSSA isolates were more likely to be resistant to fusidic acid (83.4% vs. 0.7%, P<0.01). S. aureus isolates with a higher vancomycin MIC (>1 mg/L) were significantly more frequent in the ST72 MRSA group, using the E test (77.9% vs. 45.3%, P<0.01) or the BMD method (14.4% vs. 1.3% for MIC=2, P<0.01). agr dysfunction was more prominent in the MSSA group than the MRSA group (19.7% vs. 10.9%, P=0.02). In ST72 MRSA, the spa types were diverse, with t324, t664, and t148 being the main types, whereas in ST72 MSSA, t126 was observed with high frequency and was the predominant spa type (Table 2).
Table 2. Microbiological characteristics of ST72 Staphylococcus aureus .
| Characteristics | Total (n=442) | ST72 MSSA (n=157) | ST72 MRSA (n=285) | P | |
|---|---|---|---|---|---|
| Antibiotics resistance | |||||
| Clindamycin | 64 (14.5) | 6 (3.8) | 58 (20.4) | <0.01 | |
| Ciprofloxacin | 24 (5.4) | 2 (1.3) | 22 (7.7) | 0.01 | |
| Erythromycin | 82 (18.6) | 8 (5.1) | 74 (26.0) | <0.01 | |
| Fusidic acid | 133 (30.1) | 131 (83.4) | 2 (0.7) | <0.01 | |
| Gentamicin | 50 (11.3) | 14 (8.9) | 36 (12.6) | 0.31 | |
| Rifampin | 7 (1.6) | 2 (1.3) | 5 (1.8) | >0.99 | |
| TMP/SMX | 2 (0.5) | 0 | 2 (0.7) | 0.54 | |
| Tetracycline | 9 (2.0) | 1 (0.6) | 8 (2.8) | 0.17 | |
| Vancomycin MIC by BMD method | <0.01 | ||||
| 0.5 | 8 (1.8) | 3 (1.9) | 5 (1.8) | ||
| 1 | 391 (88.5) | 152 (96.8) | 239 (83.9) | ||
| 2 | 43 (9.7) | 2 (1.3) | 41 (14.4) | ||
| Vancomycin MIC by E test method | <0.01 | ||||
| 0.5 | 9 (2.0) | 9 (5.7) | 0 | ||
| 0.75 | 30 (6.8) | 23 (14.6) | 7 (2.5) | ||
| 1 | 110 (24.9) | 54 (34.4) | 56 (19.6) | ||
| 1.5 | 221 (50.0) | 61 (38.9) | 160 (56.1) | ||
| 2 | 68 (15.4) | 10 (6.4) | 58 (20.4) | ||
| 3 | 4 (0.9) | 0 | 4 (1.4) | ||
| agr group | 0.69 | ||||
| I | 436 (98.6) | 155 (98.7) | 281 (98.6) | ||
| II | 1 (0.2) | 0 | 1 (0.4) | ||
| III | 1 (0.2) | 0 | 1 (0.4) | ||
| Not determined | 4 (0.9) | 2 (1.3) | 2 (0.7) | ||
| agr dysfunction | 62 (14.0) | 31 (19.7) | 31 (10.9) | 0.02 | |
| spa typesa | <0.01 | ||||
| t324 | 145 (32.8) | 11 (7.0) | 134 (47.0) | ||
| t126 | 106 (24.0) | 104 (66.2) | 2 (0.7) | ||
| t664 | 45 (10.2) | 2 (1.3) | 43 (15.1) | ||
| t148 | 34 (7.7) | 3 (1.9) | 31 (10.9) | ||
| t2461 | 9 (2.0) | 1 (0.6) | 8 (2.8) | ||
| t2431 | 7 (1.6) | 0 | 7 (2.5) | ||
| others | 87 (19.7) | 30 (19.1) | 57 (20.0) | ||
Data are the number of patients (%).
aOnly spa types with more than 5 cases are shown.
ST, sequence type; MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus; TMP/SMX, trimethoprim/sulfamethoxazole; MIC, minimal inhibitory concentration; BMD, broth microdilution.
3. Treatment outcomes
Table 3 outlines the outcomes of ST72 MSSA and ST72 MRSA patients. The frequencies of eradicable foci, as well as the removal of these foci, did not significantly differ between the groups. The total duration of antibiotics therapy, the duration of the bacteremia period, and persistent bacteremia for more than 3 days were not significantly different between the groups. The median length of hospital stay was shorter in patients with ST72 MSSA (20 days vs. 25 days, P=0.03). Clinical outcomes, including 30-day and 90-day mortality, were similar in both groups. The 90-day recurrence rate was lower in the ST72 MSSA group than in the ST72 MRSA group (2.5% vs. 8.4%, P=0.03). In the multivariable logistic regression analysis, ST72 MSSA infections were significantly correlated with a lower 90-day recurrence rate compared with ST72 MRSA infections (odds ratio, 0.28; 95% confidence interval, 0.05–1.69; P=0.01), while there was no significant association between ST72 MSSA and 90-day mortality (Table 4).
Table 3. Treatment and clinical outcomes of patients infected with ST72 Staphylococcus aureus .
| Variables | Total (n=442) | ST72 MSSA (n=157) | ST72 MRSA (n=285) | P | |
|---|---|---|---|---|---|
| Removable infection focus | 233 (52.7) | 81 (51.6) | 152 (53.3) | 0.26 | |
| Focus removal | 208 (89.3) | 77 (95.1) | 131 (86.2) | 0.11 | |
| Duration of antibiotics | 21 (14–36) | 20 (15–35) | 23 (15–39) | 0.25 | |
| Length of hospital stay | 35 (14–43) | 20 (14–36) | 25 (14–46) | 0.03 | |
| Duration of bacteremia | 1 (1–4) | 1 (1–3) | 1 (1–4) | 0.30 | |
| Persistent bacteremia (>3d) | 167 (37.8) | 55 (35.0) | 112 (39.3) | 0.29 | |
| 30-day mortality | 59 (13.3) | 21 (13.4) | 38 (13.3) | >0.99 | |
| 30-day recurrence | 5 (1.1) | 0 | 5 (1.8) | 0.17 | |
| 90-day mortality | 112 (25.3) | 44 (28.0) | 68 (23.9) | 0.40 | |
| 90-day recurrence | 28 (6.3) | 4 (2.5) | 24 (8.4) | 0.03 | |
Data are the number of patients (%) or median days (interquartile range).
ST, sequence type; MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus.
Table 4. OR for recurrence and mortality at 90 days in patients with ST72 MSSA compared to those with ST72 MRSA.
| Analyses | Recurrence | Mortality | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Univariate | 0.97 | 0.93–1.00 | 0.07 | 1.04 | 0.96–1.14 | 0.34 |
| Multivariatea | 0.28 | 0.05–1.69 | 0.01 | 0.81 | 0.15–4.17 | 0.80 |
aMultivariate analyses were adjusted for age, sex, mode of acquisition, persistent bacteremia, underlying comorbidities, Charlson comorbidity index, predisposing conditions, focus of infection, metastatic infection, sepsis grade, Pitt bacteremia score, antibiotic resistance, duration of antibiotics, minimal inhibitory concentration by broth microdilution, agr dysfunction, and removal of infection focus.
OR, odds ratios; ST, sequence type; MRSA, methicillin-resistant Staphylococcus aureus; CI, confidence interval.
Discussion
In this retrospective cohort study, the clinical characteristics of patients with MSSA infections were compared with patients with MRSA among patients infected with sequence type 72 S. aureus, which is the predominant genotype in both the community and healthcare settings in Korea. We found that the prevalence of ST72 MSSA increased annually in both community and hospital settings, and the majority of ST72 MSSA infections were healthcare-associated or hospital-acquired. No significant differences in clinical characteristics and outcomes were observed between ST72 MSSA and ST72 MRSA, except for a higher recurrence rate in the ST72 MRSA group. These results indicate that ST72 MSSA shares clinical and microbiological traits with ST72 MRSA, which has achieved epidemiological success in both community and hospital settings in Korea.
Over the past two decades, the emergence and spread of community-associated MRSA have been reported globally, including USA300 (ST8) in the US, USA300-LV in South America, ST72 in Korea [14], and ST59 in China [15] and Taiwan [16]. The emergence of CA-MRSA, with the replacement of traditional hospital MRSA strains, has become a public health threat in Korea as well as the US [17,18]. We observed a significant annual increase in ST72 MSSA within the S. aureus bacteremia cohort, mirroring the rise of ST72 MRSA reported in our previous study [19]. Given the rise of ST72 MSSA in both community and hospital settings, along with its predominance in healthcare-related cases, this implies a successful adaptation and establishment of ST72 MSSA in healthcare environments. Unlike CA-MRSA clones from various countries, such as USA300, ST72 has a unique feature. It lacks the PVL gene, which has been recognized as a key virulence factor. Therefore, the successful establishment of this PVL-negative ST72 lineage regardless of methicillin resistance in Korea suggests that factors unique to ST72, other than PVL, may contribute to its prevalence. Therefore, further research on the pathogenesis of ST72 will provide crucial insights into the recent global epidemic of CA-MRSA.
In our study, we observed that ST72 MSSA displayed lower resistance rates to various non-beta lactam antibiotics compared to its MRSA counterpart. This observation is in line with a recent genomic analysis conducted in China, which demonstrated that ST72 MSSA harbored fewer antimicrobial resistance genes compared to ST72 MRSA [20]. It is well-documented that CA-MRSA, including the ST72 strain, typically carries smaller staphylococcal cassette chromosome (SCC)mec elements (IV or V), resulting in a lower multidrug resistance profile compared with HA-MRSA lineages that carry larger SCCmec elements (II or III) [14,21]. Given these findings, we infer that the role of antibiotic resistance in the spread of ST72 within both community and hospital settings may be relatively minor. On the other hand, the uniquely higher resistance to fusidic acid observed in MSSA compared to MRSA is thought to be a consequence of the long-standing, unrestricted use of fusidic acid in Korea for the prevention and treatment of wound infections [22].
We found a lower recurrence rate in the ST72 MSSA group compared to the ST72 MRSA group, and this finding was robust after adjustment for various confounders. Since methicillin resistance is a well-known risk factor for the recurrence of S. aureus infections, this is likely to explain the higher recurrence rate for ST72 MRSA than ST72 MSSA [23]. However, there were no significant differences between the two groups in terms of clinical features, distribution of infection foci, or treatment outcomes other than recurrence. In the genome analysis of ST72 isolates, there was no significant difference in the number of virulence genes between MRSA and MSSA, suggesting that the virulence potential of MRSA and MSSA may be comparable [20]. Taken together, these findings suggest a close relationship of both genotype and phenotype between ST72 MRSA and ST72 MSSA, considering their clinical characteristics, which are similar apart from the recurrence rate, which could be attributed to differences in methicillin resistance.
Additionally, our study underscores the importance of continuous surveillance and detailed molecular epidemiological studies to monitor the evolving patterns of S. aureus infections. The rise of ST72 MSSA, alongside ST72 MRSA, highlights the dynamic nature of S. aureus epidemiology and the potential for specific clones to dominate in different clinical settings. Understanding the factors contributing to the success of ST72, beyond antibiotic resistance, is crucial for developing targeted strategies to control its spread and mitigate its impact on public health.
This study has a few limitations. First, this study may have potential biases due to being an observational study conducted at a single institution, and its generalizability may be limited in areas dominated by genotypes other than ST72. However, this epidemic of PVL-negative ST72 in Korea could provide key evidence of other major virulence factors in addition to PVL [24]. Second, we conducted genotyping, including MLST and spa typing. However, a Chinese study that performed whole genome sequencing showed that there could be various clades within ST72, and it found that the number of antibiotic resistance genes and virulent genes can vary depending on clades [20]. Therefore, clinical features, modes of acquisition, and clinical outcomes can vary according to the clades.
In this study, we found that the prevalence of ST72 MSSA had increased in both the community and hospital settings. Both ST72 MRSA and ST72 MSSA had similar clinical characteristics and mortality outcomes; however, recurrence rates were lower in the ST72 MSSA group. It is important to note that community-acquired ST72 MRSA and MSSA of the same lineage are on the rise in Korea and have become endemic in healthcare settings. These findings suggest that continued surveillance and research on the pathogenesis of ST72 in healthcare settings is necessary to identify its mechanisms, associated risk factors, and appropriate treatment strategies.
Footnotes
Funding: None.
Conflict of Interest: SOL is editorial board of Infect Chemother; however, he did not involve in the peer reviewer selection, evaluation, and decision process of this article. Otherwise, no potential conflicts of interest relevant to this article was reported.
- Conceptualization: SB, JH.
- Data curation: SB.
- Formal analysis: JH.
- Funding acquisition: YSK.
- Investigation: JH.
- Methodology: SB, JH.
- Resources: SB, YSK, SOL.
- Software: JH, SB.
- Supervision: SB.
- Validation: YSK, EC, JJ, MJK, YPC, SHK, SOL, SHC.
- Visualization: SB.
- Writing - original draft: JH.
- Writing - review & editing: SB.
SUPPLEMENTARY MATERIALS
Changes in the proportion of ST72 in the MSSA cohort by year within MSSA subgroups.
Multilocus sequence typing of Staphylococcus aureus bacteremia stratified by mode of acquisition
References
- 1.Kim D, Yoon EJ, Hong JS, Choi MH, Kim HS, Kim YR, Kim YA, Uh Y, Shin KS, Shin JH, Park JS, Park KU, Won EJ, Kim SH, Shin JH, Kim JW, Lee S, Jeong SH. Major bloodstream infection-causing bacterial pathogens and their antimicrobial resistance in South Korea, 2017-2019: phase I report from Kor-GLASS. Front Microbiol. 2022;12:799084. doi: 10.3389/fmicb.2021.799084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park SY, Chung DR, Yoo JR, Baek JY, Kim SH, Ha YE, Kang CI, Peck KR, Lee NY, Song JH. Sequence type 72 community-associated meticillin-resistant Staphylococcus aureus emerged as a predominant clone of nasal colonization in newly admitted patients. J Hosp Infect. 2016;93:386–389. doi: 10.1016/j.jhin.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Otto M. Community-associated MRSA: what makes them special? Int J Med Microbiol. 2013;303:324–330. doi: 10.1016/j.ijmm.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi SH, Lee J, Jung J, Kim ES, Kim MJ, Chong YP, Kim SH, Lee SO, Choi SH, Woo JH, Kim YS. A longitudinal study of adult patients with Staphylococcus aureus bacteremia over 11 years in Korea. J Korean Med Sci. 2021;36:e104. doi: 10.3346/jkms.2021.36.e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee YW, Bae S, Yang E, Chung H, Kim E, Jung J, Kim MJ, Chong YP, Kim SH, Choi SH, Lee SO, Kim YS. Clinical and microbiological characteristics of hospital-acquired methicillin-resistant Staphylococcus aureus bacteremia caused by a community-associated PVL-negative strain. Open Forum Infect Dis. 2021;8:ofab424. doi: 10.1093/ofid/ofab424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standard Institute (CLSI) Performance standards for antimicrobial susceptibility testing. 28th ed. CLSI supplement M100. Wayne, PA: CLSI; 2018. [Google Scholar]
- 7.Traber KE, Lee E, Benson S, Corrigan R, Cantera M, Shopsin B, Novick RP. agr function in clinical Staphylococcus aureus isolates. Microbiology (Reading) 2008;154:2265–2274. doi: 10.1099/mic.0.2007/011874-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakoulas G, Eliopoulos GM, Moellering RC, Jr, Wennersten C, Venkataraman L, Novick RP, Gold HS. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob Agents Chemother. 2002;46:1492–1502. doi: 10.1128/AAC.46.5.1492-1502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, Reller LB, Sexton DJ. Health care--associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 10.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Chow JW, Yu VL. Combination antibiotic therapy versus monotherapy for Gram-negative bacteraemia: a commentary. Int J Antimicrob Agents. 1999;11:7–12. doi: 10.1016/s0924-8579(98)00060-0. [DOI] [PubMed] [Google Scholar]
- 13.The R Foundation. The R project for statistical computing. [Accessed 30 November 2023]. Available at: https://www.r-project.org.
- 14.Chuang YY, Huang YC. Molecular epidemiology of community-associated meticillin-resistant Staphylococcus aureus in Asia. Lancet Infect Dis. 2013;13:698–708. doi: 10.1016/S1473-3099(13)70136-1. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Li X, Liu W, Huang W, Fu Q, Li M. Molecular characteristic and virulence gene profiles of community-associated methicillin-resistant Staphylococcus aureus isolates from pediatric patients in Shanghai, China. Front Microbiol. 2016;7:1818. doi: 10.3389/fmicb.2016.01818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang YC, Chen CJ. Community-associated meticillin-resistant Staphylococcus aureus in children in Taiwan, 2000s. Int J Antimicrob Agents. 2011;38:2–8. doi: 10.1016/j.ijantimicag.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Seybold U, Kourbatova EV, Johnson JG, Halvosa SJ, Wang YF, King MD, Ray SM, Blumberg HM. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin Infect Dis. 2006;42:647–656. doi: 10.1086/499815. [DOI] [PubMed] [Google Scholar]
- 18.Park SH, Park C, Yoo JH, Choi SM, Choi JH, Shin HH, Lee DG, Lee S, Kim J, Choi SE, Kwon YM, Shin WS. Emergence of community-associated methicillin-resistant Staphylococcus aureus strains as a cause of healthcare-associated bloodstream infections in Korea. Infect Control Hosp Epidemiol. 2009;30:146–155. doi: 10.1086/593953. [DOI] [PubMed] [Google Scholar]
- 19.Yang E, Kim E, Chung H, Lee YW, Bae S, Jung J, Kim MJ, Chong YP, Kim SH, Choi SH, Lee SO, Kim YS. Changing characteristics of S. aureus bacteremia caused by PVL-negative, MRSA strain over 11 years. Sci Rep. 2021;11:15677. doi: 10.1038/s41598-021-95115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou W, Jin Y, Zhou Y, Wang Y, Xiong L, Luo Q, Xiao Y. Comparative genomic analysis provides insights into the evolution and genetic diversity of community-genotype sequence type 72 Staphylococcus aureus isolates. mSystems. 2021;6:e0098621. doi: 10.1128/mSystems.00986-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim ES, Song JS, Lee HJ, Choe PG, Park KH, Cho JH, Park WB, Kim SH, Bang JH, Kim DM, Park KU, Shin S, Lee MS, Choi HJ, Kim NJ, Kim EC, Oh MD, Kim HB, Choe KW. A survey of community-associated methicillin-resistant Staphylococcus aureus in Korea. J Antimicrob Chemother. 2007;60:1108–1114. doi: 10.1093/jac/dkm309. [DOI] [PubMed] [Google Scholar]
- 22.Baek YS, Jeon J, Ahn JW, Song HJ. Antimicrobial resistance of Staphylococcus aureus isolated from skin infections and its implications in various clinical conditions in Korea. Int J Dermatol. 2016;55:e191–e197. doi: 10.1111/ijd.13046. [DOI] [PubMed] [Google Scholar]
- 23.Kreisel K, Boyd K, Langenberg P, Roghmann MC. Risk factors for recurrence in patients with Staphylococcus aureus infections complicated by bacteremia. Diagn Microbiol Infect Dis. 2006;55:179–184. doi: 10.1016/j.diagmicrobio.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Voyich JM, Otto M, Mathema B, Braughton KR, Whitney AR, Welty D, Long RD, Dorward DW, Gardner DJ, Lina G, Kreiswirth BN, DeLeo FR. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis. 2006;194:1761–1770. doi: 10.1086/509506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Changes in the proportion of ST72 in the MSSA cohort by year within MSSA subgroups.
Multilocus sequence typing of Staphylococcus aureus bacteremia stratified by mode of acquisition