Abstract
Biotic stress significantly challenges the global citrus industry. Major post-harvest issues include diseases caused by Penicillium digitatum, Penicillium italicum, Geotrichum citri-aurantii, Alternaria alternata, and Phytophthora citrophthora. The negative impact of chemical fungicides on the environment and health necessitates eco-friendly alternatives. This study examines the effectiveness of sodium, potassium, and calcium silicates against common citrus diseases. In vitro tests evaluated mycelial growth inhibition using silicate concentrations from 0 to 10,000 ppm after 7 days at 25°C. Sodium silicate showed the highest efficacy, completely inhibiting P. digitatum and P. italicum at 2000 ppm. Potassium and calcium silicates achieved 100% inhibition against Penicillium spp. at a concentration of 1%. In vivo tests on Sidi Aissa clementines assessed the preventive and curative effects of 1, 2, and 6% silicate salt solutions. Sodium silicate prevented 41% of brown rot, 72% of sour rot, and 100% of green mold at 6%. Calcium silicate at 6% significantly reduced blue mold and black rot by 32% and 74%, respectively. Sodium silicate was most effective in curative treatments, suggesting its potential as a pre- or post-harvest spray to control P. digitatum, P. italicum, and G. citriaurantii.
Key words: P. digitatum, P. italicum, G. citri-aurantii, A. alternata, P. citrophthora, antifungal activity
Introduction
Citrus fruits consistently rank among the world’s top ten most valuable crops, with cultivation spanning over 10.2 million hectares globally, yielding approximately 161.8 million tons annually (Pereira Gonzatto and Scherer Santos, 2023). Leading countries in citrus cultivation include China, Brazil, the United States, South Africa, Peru, Turkey, and Morocco (Zhong and Nicolosi., 2020). Notably, Morocco has seen a steady rise in citrus production across various varieties during the 2022/2023 period. Tangerines/mandarins witnessed a significant increase, reaching 927,000 metric tons (MT), while oranges reached 783,000 MT and lemons/limes reached 35,000 MT. This uptick in production has made a substantial economic impact, driven by the export of 425,000 MT of tangerines/mandarins, 80,000 MT of oranges, and 7,000 MT of lemons/limes (USDA, 2023).
Citrus fruits face susceptibility to various pathogens during post-harvest stages, including handling, shipping, storage, and marketing, resulting in substantial losses and economic setbacks within the citrus industry (Wang et al., 2020). Biotic stress, notably infections stemming from pre- and post-harvest decay fungi, presents a formidable challenge in citrus cultivation (Ezzouggari et al., 2024). In recent times, brown rot, alternaria rot, sour rot, and green, and blue mold have emerged as the most pervasive diseases afflicting harvested citrus fruits (Saito and Xiao, 2017; Salvador López et al., 2022). Green mold, attributed to P. digitatum, stands out as the most prevalent (36.3%), followed by blue mold caused by P. italicum (23.3%), and sour rot initiated by G. citri-aurantii (18.7%) (Saito and Xiao, 2017). Alongside these ailments, alternaria rot poses a significant threat, particularly in hot and humid climates, affecting citrus fruits through various species of the Alternaria genus, with A. citri among the most common pathogens (Soylu and Kose, 2015; Saito and Xiao, 2017). Moreover, brown rot, attributable to Phytophthora spp., holds considerable economic importance, affecting all citrus species and exhibiting increased prevalence during periods of ample rainfall in the later stages of fruit development (Ramallo et al., 2019). In Morocco, fungal diseases are the most frequent sorting gaps in packinghouses. A study conducted in the Berkane region revealed that Geotrichum, Penicillium spp., and Phytophthora are major contributors, with average sorting gap rates of 40.3, 31.2, and 28.6%, respectively (Ben Yazid et al., 2020).
Frequently, fungicides serve as a remedy to mitigate postharvest damage inflicted on citrus by diverse fungi (Radouane et al., 2023). However, the extensive application of these chemical agents has fostered the emergence of highly resistant pathogen strains and the buildup of chemical residues in food, posing heightened risks to human health and the environment (Cheng et al., 2020). Consequently, the pressing need to explore alternative methods for disease management has led to the investigation of various approaches beyond synthetic fungicides. These methods include physical treatments such as hot water treatment, hot air treatment, and light irradiation (Kahramanoğlu et al., 2020; Kahramanoğlu et al., 2020); the use of antagonistic microorganisms like yeast, bacteria, and fungi (Wang et al., 2022; Ezzouggari et al., 2024); incorporation of nanomaterials (Ruffo et al., 2019); application of chitosan (El Guilli et al., 2016); plant extracts and essential oils (Bhatta, 2022); and the utilization of salts (Palou, 2018). Silicon, ranked as the second most abundant element on Earth after oxygen (Mvondo-She et al., 2021), holds the status of a Generally Recognized as Safe (GRAS) compound (Nikagolla et al., 2019). Currently, silicon has garnered attention for its beneficial effects on plants, enhancing their resilience against both biotic and abiotic stressors (Yavaş and Ünay, 2017). In the foreseeable future, silicon is poised to emerge as a sustainable solution for addressing challenges in horticulture. Among silicon compounds, potassium, calcium, and sodium silicates have been under scrutiny for their potential application in plant systems (ValdebenitoSanhueza et al., 2018). Silicate presence instigates resistance against fungal and bacterial pathogens through mechanisms such as the establishment of physical barriers (Datnoff et al., 2001) and the accumulation of phenolic and phytoalexin compounds (Seebold et al., 2001).
The presence of silicate can induce resistance to both fungal and bacterial pathogens through the formation of a physical barrier (Datnoff et al., 2001) and the accumulation of phenolic and phytoalexin compounds (Seebold et al., 2001). Additionally, this resistance is linked to the activation of genes responsible for producing glucanase, peroxidases, and chitinases (Rodrigues et al., 2005). The specific objective of this study was to evaluate and compare the efficacy of various silicate forms potassium, sodium, and calcium silicates in combating postharvest citrus fruit diseases caused by assorted fungi.
Materials and Methods
Fungal pathogen and preparation of inoculum
The pathogenic fungi employed in this study, namely Penicillium digitatum, Penicillium italicum, Geotrichum citriaurantii, Alternaria alternata, and Phytophthora citrophthora were sourced from the Plant Pathology and Postharvest Quality Laboratory at the Regional Center of Agricultural Research of Kénitra, Morocco. Fungal solutions were prepared by harvesting colonies grown on potato dextrose agar (PDA) medium and transferring them into glass test tubes containing 0.05% Tween 80 solution (Soto-Muñoz et al., 2020). The concentrations of the suspensions were standardized to 105 conidia/ml for P. digitatum and P. italicum, and 107 conidia/mL for G. citri-aurantii and A. alternata, utilizing a hemacytometer (Moscoso-Ramírez and Palou, 2014).
Plant material
The plant material utilized in this research comprised mature and healthy Citrus fruits of the Sidi Aissa clementine variety, harvested from the Sidi Allal Tazi experimental domain in Morocco. After harvesting, the samples were transported to the Plant Pathology and Postharvest Quality Laboratory at the Regional Center of Agricultural Research in Kenitra. Before commencing each experiment, the fruits underwent immersion in a 10% sodium hypochlorite solution for 2 minutes, followed by two rinses with sterile distilled water. Subsequently, two wounds were created on opposite sides along the equatorial axis of the fruits, with each wound measuring 2-3 mm in depth and 5 mm in width, utilizing a sterile cork-borer.
Soluble silicate compounds
In this study, three types of silicate: potassium silicate, sodium silicate, and calcium silicate, were evaluated through both in vitro and in vivo trials. Table 1 shows detailed information, such as the molecular formula, food additive E-number, and molecular weight of each silicate.
In vitro evaluation of the effectiveness of soluble silicate
The aim of this experiment was to evaluate the effects of soluble sodium silicate (Na2SiO3), potassium silicate (K2SiO3), and calcium silicate (CaSiO3) on the mycelial growth of the aforementioned fungi. Different volumes of the three silicates were individually mixed into a sterilized PDA medium before solidification, resulting in final concentrations of 0, 500, 2000, 4000, 6000, and 10000 ppm. These solutions were then poured into sterile 90 mm-diameter Petri dishes. Each dish was centrally inoculated with 17 μL of fungal suspension, while mycelial plug disks (5 mm) were used for P. citrophthora. Five plates were prepared for each silicate concentration and each fungus. Following a 7-day incubation period at 25°C, the linear growth of the fungi was measured using the methodology outlined by Abd-El-Kareem et al. (2019).
In vivo evaluation of the effectiveness of soluble silicate
The study utilized prepared fruits of the Sidi Aissa clementine variety to investigate the efficacy of Na, K, and Ca silicates in controlling postharvest citrus diseases. Two treatments were administered: preventive and curative, with a 24-hour interval between inoculation and treatment. The fruits underwent treatment by immersion for 2 minutes in silicate solutions prepared at various concentrations (1%, 2%, and 6%). Fruits immersed solely in sterile distilled water served as the negative control, while those treated with the fungicide Imazalil at 0.2% were designated as positive controls. Each fruit wound received a 40 µL conidial suspension, and ten repetitions were carried out for each type and concentration of silicate. Subsequently, all fruits were stored at 25±1°C and maintained at a relative humidity for 7 days.
Assessment methodology and determination of the inhibitory concentration 50
The inhibition rate of fungal development was determined in both in vitro and in vivo trials according to the flowing formula:
| % IC = ((dc - dt) / dc) × 100 |
where: % I represent the percentage of inhibition; dc is the control development rate; dt is the treatment development rate.
The concentration that induces 50% inhibition of fungal growth [inhibitory concentration 50 (IC50)] was determined through regression analysis of the log-probit transformed data.
Statistical analysis
The in vitro and in vivo results were analyzed using XLSTAT 2016 version. A one-way ANOVA was performed, and Duncan’s test was used to compare means between treatments. The statistical significance was judged at the 95% level of confidence (p=0.05). Data were presented as a mean ± standard deviation.
Results
In vitro antifungal activity of sodium, potassium, and calcium silicates against fungal diseases in citrus fruits
The in vitro results revealed pronounced inhibitory effects on fungal mycelium growth upon the application of sodium, potassium, and calcium silicates (p<0.0001) (Figures 1 and 2). Specifically, sodium silicate completely arrested colony growth of Penicillium spp. and G. citri-aurantii at concentrations of 2000 and 4000 ppm, respectively. Potassium silicate demonstrated significant inhibition, reducing mycelial growth by 72% for P. italicum and achieving complete inhibition (100%) for P. digitatum at 4000 ppm. Calcium silicate, when applied at a concentration of 10000 ppm, led to a 55% reduction in G. citri-aurantii mycelium growth. Regarding A. alternata, the most effective inhibition of radial mycelial growth was observed with sodium silicate (100%), followed by potassium silicate (30%), both at the highest concentration of 10000 ppm. Notably, complete inhibition (100%) of P. citrophthora was exclusively achieved with sodium silicate at 6000 ppm. Various concentrations of the three silicates were employed to establish the IC50 for each fungus, as outlined in Table 2. The results showed that sodium silicate displayed a slightly lower IC50 value compared to potassium and calcium silicate. Additionally, the IC50 values varied depending on the specific fungus.
Preventive activity bioassay
The effectiveness of silicate salts in preventing post-harvest citrus diseases was assessed following a 7-day treatment period, with the findings presented in Figures 3 and 4. Notably, sodium silicate exhibited superior protective effects compared to other silicates, particularly at a 1% concentration. Sodium silicate demonstrated efficacy rates of 53.12% and 81.61% against A. alternata and P. citrophthora, respectively, achieving complete inhibition (100%) against P. digitatum and G. citri-aurantii. Potassium silicate, applied at a 6% concentration, significantly suppressed the growth of blue and green molds by 32.86% and 68.91%, respectively. Additionally, at the same concentration, potassium silicate mitigated disease symptoms caused by A. alternata and P. citrophthora by up to 43.35%. In the case of calcium silicate, its efficacy against green and blue molds surpassed 37.26%, notably reaching 42.44% for sour rot at a concentration of 6%. These results underscore the potential of silicate salts as effective preventive measures against various post-harvest citrus diseases.
Curative activity bioassay
Regarding curative activity, a significant and noteworthy difference emerged when citrus fruits underwent treatment 24 hours after inoculation, distinguishing between treated and untreated fruits (Figures 5 and 6). The results revealed that P. digitatum was effectively suppressed by sodium silicate at a concentration of 2% (p<0.0001), a level of inhibition comparable to that achieved by the fungicide imazalil at 0.2% (2000 ppm). Across all other pathogens, a substantial inhibition rate exceeding 52.11% was observed at a concentration of 6%. Potassium silicate, at a 6% concentration, demonstrated a 40% inhibition rate against P. citrophthora. Conversely, calcium silicate, at a concentration of 6%, exhibited approximately 20% and 26% reductions in brown rot and green mold, respectively. However, no inhibitory effects were observed for potassium and calcium silicate in controlling black rot and sour rot at any of the tested concentrations.
Table 1.
The properties of the soluble silicate employed in this study.
| Compounds | Chemical formula | Molecular weight (g/mol) | E-number1 | Purity (%) |
|---|---|---|---|---|
| Potassium silicate | K2SiO3 | 154,280 | E-560 | 99 |
| Sodium meta silicate | Na2SiO3 | 122,063 | E-211 | 99 |
| Calcium silicate | Ca2SiO4 | 172,239 | E-552 | 99 |
1E-number refers to a code assigned by the European Union.
Table 2.
Inhibitory concentration 50 values of sodium, potassium, and calcium silicates of each fungus.
| IC 50 (ppm) Pathogen | Na silicate | K silicate | Ca silicate |
|---|---|---|---|
| P. italicum | 826.65 | 2897.58 | 4488.82 |
| P. digitatum | 651.17 | 1082.47 | 2178.19 |
| G. citri-aurantii | 1809.08 | 148284 | 10378.01 |
| A. alternata | 4694.02 | 24405.71 | 36321.92 |
| P. citrophthora | 2207.05 | 34857.91 | 75515.66 |
| Mean | 10187.97 | 206527.67 | 128882.60 |
Figure 1.
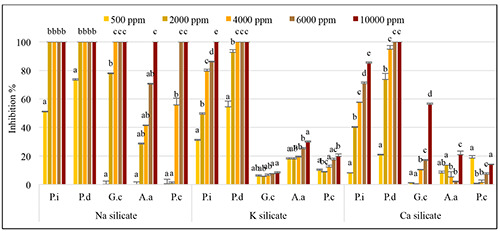
Percentage of growth inhibition of P. italicum, P. digitatum, G. citri-aurantii, A. alternata and P. citrophthora induced by Sodium, Potassium, and Calcium Silicates. Data represent average inhibition rate (%) ± standard deviation. Values having the same letter, above histogram bars, are not significantly different according to the Duncan test (p<0.05).
Figure 2.
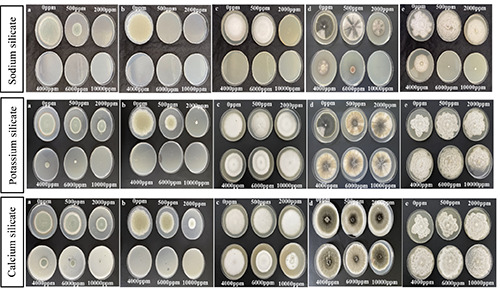
Effectiveness of silicate salts at various concentrations on the radial mycelial growth of P. italicum (a), P. digitatum (b), G. citriaurantii (c), A. alternata (d) and P. citrophthora (e) on PDA plates after 7 days at 25°C.
Discussion
The primary post-harvest diseases affecting citrus fruits include green mold, blue mold, and sour rot, caused by P. digitatum, P. italicum, and Geotrichum citri-aurantii, respectively. Less prevalent but still significant are A. alternata and P. citrophthora. These pathogens pose a considerable threat to citrus exports due to the substantial production losses they induce and their detrimental impact on citrus quality. Addressing citrus rot presents a significant challenge, leading to the exploration of various solutions such as beneficial yeasts, bacteria, fungi, biofungicides, plant extracts, essential oils, chitosan, and GRAS substances, along with synthetic elicitors. Physical approaches like heat exposure or irradiation are also under consideration (Palou et al., 2016; Papoutsis et al., 2019). Despite the promising inhibitory capabilities of certain methods, their use is limited due to their high cost and strict environmental requirements, including specific pH, particular temperature, and precise water activity coverage (Li et al., 2019). The use of organic and inorganic salts classified as GRAS compounds or as food additives by national or international legislation constitutes a promising and non-polluting alternative to conventional fungicides for controlling post-harvest diseases of fresh horticultural produce, including fresh citrus fruits (D’Aquino and Palma, 2019). These salts are highly soluble, easy to handle and apply, and widely available at relatively low costs, making them suitable for commercial implementation in existing fresh produce packing warehouses (Palou, 2018). This makes them well-suited for addressing issues related to fruit residues, improving water quality, and meeting the requirements of organic agriculture.
Figure 3.
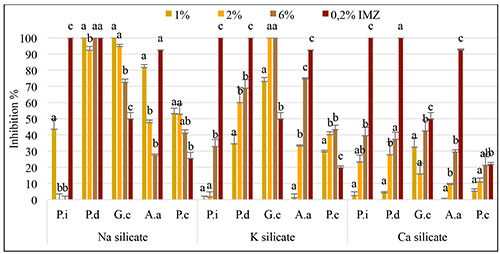
Preventive activity of sodium, potassium, and calcium silicates at different concentrations for controlling blue and green molds, sour rot, black rot and brown rot on artificially inoculated clementine ‘Sidi Aissa’ fruits. Data represent average inhibition rate (%) ± standard deviation. Values having the same letter, above histogram bars, are not significantly different according to the Duncan test (p<0.05).
Figure 4.

In vivo silicates effect of sodium, potassium and calcium silicate on the severity of green mold on artificially inoculated clementine ‘Sidi Aissa’ fruits (preventive treatment).
Hence, our aim was to evaluate the efficacy and consistency of various post-harvest treatments utilizing GRAS substances such as potassium silicate, sodium silicate, and calcium silicate as a practical approach to combat citrus rot. This study employs a sequential methodology to optimize the application parameters of these silicate salts (potassium, sodium, and calcium silicates). Initially, their impact on linear growth was assessed, followed by two in vivo tests focusing on both preventive and curative actions against citrus diseases. Preliminary in vitro trials were conducted to demonstrate the antifungal effectiveness of the treatment compounds. All types of silicates tested significantly reduced mycelial growth during the in vitro experiments. Notably, sodium silicate exhibited complete suppression of Penicillium spp., G. citriaurantii, P. citrophthora, and A. alternata at concentrations of 2000, 4000, 6000, and 10000 ppm, respectively. Moreover, potassium silicate demonstrated complete inhibition of mycelial growth in P. digitatum and P. italicum at concentrations of 4000 and 10,000 ppm, respectively. Furthermore, only calcium silicate-enriched Petri dishes at 10,000 ppm exhibited the ability to completely suppress P. digitatum and P. italicum. However, potassium silicate and sodium silicate were identified as the least effective soluble silicate types in inhibiting the growth of G. citri-aurantii, A. alternata, and P. citrophthora. These findings contrast with those of Biggs et al. (1997), who reported the effectiveness of calcium silicate incorporated into PDA in inhibiting the growth of Monilinia fructicola (G. Wint.) Honey, the causal agent of brown rot in peach fruit when compared to the control group. The in vivo experiments revealed variable outcomes, indicating disparities depending on the types of silicates, application methods (preventive or curative), and specific pathogens. The three silicate salts have the capacity to prevent the development of green and blue molds, sour rot, alternaria rot, and brown rot.
Figure 5.
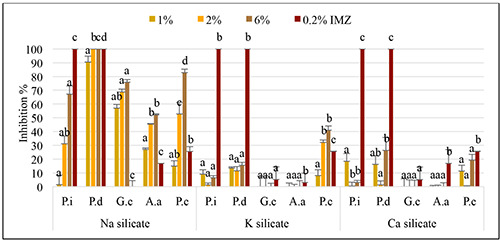
The curative activity of sodium, potassium, and calcium silicates at different concentrations for controlling blue and green molds, sour rot, black rot, and brown rot on artificially inoculated clementine ‘Sidi Aissa’ fruits. Data represent average inhibition rate (%) ± standard deviation. Values having the same letter, above histogram bars, are not significantly different according to the Duncan test (p<0.05).
Figure 6.

In vivo effect of sodium silicate on the severity of different pathogens evaluated on artificially inoculated clementine ‘Sidi Aissa’ fruits (curative treatment).
However, potassium and calcium silicate treatments are not effective in controlling the development of sour rot and alternaria rot in the curative activity; the results underscore the crucial importance of timing in the application. Indeed, applying the salts before harvest allows for extended interaction with pathogens present on the fruit, potentially altering their inoculum density and the environmental conditions within wounds, thus potentially inducing tissue resistance (Youssef et al., 2012). Abd-El-Kareem et al. (2019), evaluating the same silicate salts in controlling Fusarium solani and Rhizoctonia solani, the causal agents of black root disease of strawberry plants, found that the complete inhibition of mycelium growth of R. solani and F. solani was achieved with concentrations of 4 g/L and 6 g/L, respectively. Additionally, a similar effect of sodium silicate showed in this study at the concentration of 10000 ppm has been reported by Zhao et al. (2023), which resulted in complete suppression of 100% on P. italicum, G. citri-aurantii, C. gloeosporioides and P. digitatum. Furthermore, in a previous study, 90 mM of potassium silicate reduced the severity of green and blue molds by 74% and 67%, respectively. Additionally, its curative effects on diminishing gray mold and blue mold on ‘Valencia’ oranges by 23% and 40%, respectively, were also documented (Moscoso-Ramírez and Palou, 2014). Effective inhibition of fungal growth was observed with the incorporation of 100 and 200 mM of sodium silicate into PDA, achieving rates of 52% and 90%, respectively, after a 7-day incubation at 25°C, as demonstrated by Li et al. (2009). According to Mbili (2012), potassium silicate decreased significantly grey mold incidence on “Golden Delicious” apples, showing a reduction of 75% at a concentration of 10 mg/L to 72.5% at a concentration of 100 mg/L. Other studies report that potassium silicate exhibits the lowest efficacy among GRAS salts, resulting in a 50% inhibition of growth in C. gloeosporioides at the highest concentration of 2% (Martínez-Blay et al., 2020). However, calcium silicate did not show a significant effect on inhibiting Rigidoporus microporus (Hadi et al., 2021).
Further investigation is warranted into the potential of GRAS salts for controlling citrus rots, particularly in terms of their capacity to halt or slow down fruit-to-fruit transmission. Additionally, there is a need for research to evaluate the effectiveness of aqueous solutions containing these salts in eradicating or deactivating food-borne microorganisms on citrus surfaces, especially concerning regulatory frameworks like the Food Safety Modernization Act in the USA (Soto-Muñoz et al., 2020). It is also crucial to conduct compatibility studies with common fruit sanitizers such as chlorine derivatives and peracetic acid. Beyond serving as alternatives to banned fungicides like propiconazole in the EU, these salts exhibit promise in reducing other significant postharvest citrus ailments such as green and blue molds (Radulović et al., 2023). Importantly, their commercial application in citrus packinghouses as aqueous solutions would necessitate minimal facility modifications, leveraging their designation as GRAS compounds or food additives. This classification facilitates their registration as postharvest anti-fungal treatments, potentially expediting industry adoption and compliance with fruit safety standards.
Conclusions
This study highlights the effectiveness of sodium, potassium, and calcium silicates as viable, eco-friendly alternatives to traditional chemical fungicides in combating the primary post-harvest ailments of citrus fruits. Sodium silicate emerged as the most effective treatment, significantly inhibiting key pathogens such as Penicillium digitatum, Penicillium italicum, and Geotrichum citriaurantii. It demonstrated strong preventive and curative effects, particularly against brown rot, sour rot, and green mold. Calcium silicate also showed promising results in reducing blue mold. This proposition carries significant weight in tackling citrus rots, particularly P. digitatum, solely responsible for approximately 90% of total post-harvest citrus fruit losses, and sour rot induced by G. citri-aurantii. Additionally, their straightforward application and widespread availability render them accessible choices for citrus growers seeking sustainable disease control strategies. Nonetheless, further research is imperative to fine-tune application protocols and comprehend the long-term ramifications before widespread implementation. The potential antifungal prowess of this compound could be augmented through synergistic combinations with other treatments such as heat, minimal doses of fungicides, and biological antagonists. Nevertheless, the findings presented underscore the promising role of GRAS compounds in enhancing the sustainability and resilience of citrus fruit production systems.
Acknowledgments
This research was supported by the Ministry of Agriculture and Fisheries as part of the MCRDV project in a collaboration between INRA (Plant Pathology and Postharvest Quality Laboratory) and ENAM (the Phytopathology Unit of the National School of Agriculture) and PROVIDENCE VERTE.
Funding Statement
Funding: this research was funded by the MCRDV project.
Availability of data and materials
The data are contained within the manuscript.
References
- Abd-El-Kareem F, Elshahawy IE, Abd-Elgawad MMM, 2019. Effectiveness of silicon and silicate salts for controlling black root rot and induced pathogenesis-related protein of strawberry plants. Bull Natl Res Cent 43:91. [Google Scholar]
- Ben Yazid J, Chafik Z, Chedadi T, Bibi I, Kharmach E, 2020. Assessment of the main causes of sorting gaps in the citrus packinghouses of Berkane, Morocco. Crop Production and Environment. Available from: https://techagro.org/index.php/ MJAS/article/view/870/940. [Google Scholar]
- Bhatta UK, 2022. Alternative management approaches of citrus diseases caused by Penicillium digitatum (green mold) and Penicillium italicum (blue mold). Front Plant Sci 12:833328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs AR, El-Kholi MM, El-Neshawy S, Nickerson R, 1997. Effects of calcium salts on growth, polygalacturonase activity, and infection of peach fruit by monilinia fructicola. Plant Dis 81:399-403. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Lin Y, Cao H, Li Z, 2020. Citrus postharvest green mold: recent advances in fungal pathogenicity and fruit resistance. Microorganisms 8:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aquino S, Palma A, 2019. Reducing or replacing conventional postharvest fungicides with low toxicity acids and salts. In: Palou L, Smilanick JL, eds. Postharvest pathology of fresh horticultural produce. CRC Press, Boca Raton, FL, pp 595-632. [Google Scholar]
- Datnoff LE, Seebold KW, Correa-V FJ, 2001. The use of silicon for integrated disease management: reducing fungicide applications and enhancing host plant resistance. Studies in Plant Science 8:171-84. [Google Scholar]
- El Guilli M, Hamza A, Clément C, Ibriz M, Ait Barka E, 2016. Effectiveness of postharvest treatment with chitosan to control citrus green mold. Agriculture 6:12. [Google Scholar]
- Ezzouggari R, Bahhou J, Taoussi M, Kallali NS, Aberkani K, 2024. Yeast Warriors: exploring the potential of yeasts for sustainable citrus post-harvest disease management. Agronomy 14:288. [Google Scholar]
- Hadi SMHSA, Zakaria L, Sidique SNM, Mahyudin MM, Nor NMIM, 2021. The potential of soluble silicon for managing white root disease in rubber (Hevea brasiliensis). Aust J Crop Sci 15:1346-54. [Google Scholar]
- Kahramanoğlu İ, Chen C, Chen Y, Chen J, Gan Z, Wan C, 2020. Improving storability of “nanfeng” mandarins by treating with postharvest hot water dipping. J Food Qual 2020. doi: 10.1155/2020/8524952. [Google Scholar]
- Kahramanoğlu İ, Nisar MF, Chen C, Usanmaz S, Chen J, Wan C, 2020. Light: an alternative method for physical control of postharvest rotting caused by fungi of citrus fruit. J Food Qual 2020. doi: 10.1155/2020/8821346. [Google Scholar]
- Li L, Tang X, Ouyang Q, Tao N, 2019. Combination of sodium dehydroacetate and sodium silicate reduces sour rot of citrus fruit. Postharvest Biol Technol 151:19-25. [Google Scholar]
- Li YC, Bi Y, Ge YH, Sun XJ, Wang Y, 2009. Antifungal activity of sodium silicate on fusarium sulphureum and its effect on dry rot of potato tubers. J Food Sci 74:M213-8. [DOI] [PubMed] [Google Scholar]
- Martínez-Blay V, Pérez-Gago MB, de la Fuente B, Carbó R, Palou L, 2020. Edible coatings formulated with antifungal GRAS salts to control citrus anthracnose caused by Colletotrichum gloeosporioides and preserve postharvest fruit quality. Coatings 10:730. [Google Scholar]
- Mbili NC, 2012. Evaluation of integrated control of postharvest grey mould and blue mould of pome fruit using yeast, potassium silicate and hot water treatments. Available from: https://researchspace.ukzn.ac.za/server/api/core/bitstreams/11 8ce4c6-2eb0-47fe-980d-f1d8f491ef9f/content. [Google Scholar]
- Moscoso-Ramírez PA, Palou L, 2014. Preventive and curative activity of postharvest potassium silicate treatments to control green and blue molds on orange fruit. Eur J Plant Pathol 138:721-32. [Google Scholar]
- Mvondo-She MA, Gatabazi A, Laing MD, Ndhlala AR, 2021. A review on the role of silicon treatment in biotic stress mitigation and citrus production. Agronomy 11:2198. [Google Scholar]
- Nikagolla NGDN, Udugala-Ganehenege MY, Daundasekera WAM, 2019. Postharvest application of potassium silicate improves keeping quality of banana. J Horticult Sci Biotechnol 94:735-43. [Google Scholar]
- Palou L, 2018. Postharvest treatments with GRAS salts to control fresh fruit decay. Horticulturae 4:46. [Google Scholar]
- Palou L, Ali A, Fallik E, Romanazzi G, 2016. GRAS, plant- and animal-derived compounds as alternatives to conventional fungicides for the control of postharvest diseases of fresh horticultural produce. Postharvest Biol Technol 122:41-52. [Google Scholar]
- Papoutsis K, Mathioudakis MM, Hasperué JH, Ziogas V, 2019. Non-chemical treatments for preventing the postharvest fungal rotting of citrus caused by Penicillium digitatum (green mold) and Penicillium italicum (blue mold). Trends Food Sci Technol 86:479-91. [Google Scholar]
- Pereira Gonzatto M, Scherer Santos J, 2023. Introductory Chapter: world citrus production and research. In: Pereira Gonzatto M, Scherer Santos J, eds. Citrus research - horticultural and human health aspects. IntechOpen, London, UK. [Google Scholar]
- Radouane N, Adadi H, Ezrari S, Kenfaoui J, Belabess Z, Mokrini F, Barka EA, Lahlali R, 2023. Exploring the bioprotective potential of halophilic bacteria against major postharvest fungal pathogens of citrus fruit Penicillium digitatum and Penicillium italicum. Horticulturae 9:922. [Google Scholar]
- Radulović J, Lučić M, Nešić A, Onjia A, 2023. Multivariate assessment and risk ranking of pesticide residues in citrus fruits. Foods 12:2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramallo AC, Cerioni L, Olmedo GM, Volentini SI, Ramallo J, Rapisarda VA, 2019. Control of Phytophthora brown rot of lemons by pre-and postharvest applications of potassium phosphite. Eur J Plant Pathol 154:975-82. [Google Scholar]
- Rodrigues FÁ, Jurick WM, Datnoff LE, Jones JB, Rollins JA, 2005. Silicon influences cytological and molecular events in compatible and incompatible rice-Magnaporthe grisea interactions. Physiol Mol Plant Pathol 66:144-59. [Google Scholar]
- Ruffo SR, Youssef K, Hashim AF, Ippolito A, 2019. Nanomaterials as alternative control means against postharvest diseases in fruit crops. Nanomaterials 9:1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Xiao CL, 2017. Prevalence of postharvest diseases of mandarin fruit in California. Plant Health Progress 18:204-10. [Google Scholar]
- Salvador López JM, Vandeputte M, Van Bogaert INA, 2022. Oleaginous yeasts: Time to rethink the definition? Yeast 39:553-606. [DOI] [PubMed] [Google Scholar]
- Seebold KW, Kucharek TA, Datnoff LE, Correa-Victoria FJ, Marchetti MA, 2001. The influence of silicon on components of resistance to blast in susceptible, partially resistant, and resistant cultivars of rice. Phytopathology 91:63-9. [DOI] [PubMed] [Google Scholar]
- Soto-Muñoz L, Taberner V, de la Fuente B, Jerbi N, Palou L, 2020. Curative activity of postharvest GRAS salt treatments to control citrus sour rot caused by Geotrichum citri-aurantii. Int J Food Microbiol 335:108860. [DOI] [PubMed] [Google Scholar]
- Soylu EM, Kose F, 2015. Antifungal activities of essential oils against citrus black rot disease agent alternaria alternata. J Essential Oil Bearing Plants 18:894-903. [Google Scholar]
- USDA, 2023. Citrus: world markets and trade. Available from: https://apps.fas.usda.gov/psdonline/circulars/citrus.pdf. [Google Scholar]
- Valdebenito-Sanhueza RM, Vargas VR, Pereira JC, Tochetto N, Longhi GH, 2018. Silicon effects in postharvest apple rots in Brazil. Acta Horticulturae 1194:215-20. [Google Scholar]
- Wang S, Ruan C, Yi L, Deng L, Yao S, Zeng K, 2020. Biocontrol ability and action mechanism of Metschnikowia citriensis against Geotrichum citri-aurantii causing sour rot of postharvest citrus fruit. Food Microbiol 87:103375. [DOI] [PubMed] [Google Scholar]
- Wang Z, Sui Y, Li J, Tian X, Wang Q, 2022. Biological control of postharvest fungal decays in citrus: a review. Crit Rev Food Sci Nutr 62:861-70. [DOI] [PubMed] [Google Scholar]
- Yavaş İ, Ünay A, 2017. The role of silicon under biotic and abiotic stress conditions. Türkiye Tarımsal Araştırmalar Dergisi 4. doi: 10.19159/tutad.300023. [Google Scholar]
- Youssef K, Ligorio A, Sanzani SM, Nigro F, Ippolito A, 2012. Control of storage diseases of citrus by pre- and postharvest application of salts. Postharvest Biol Technol 72:57-63. [Google Scholar]
- Zhao J, Wang Y, Liu Q, Liu S, Pan H, Cheng Y, Long C, 2023. The GRAS Salts of Na2SiO3 and EDTA-Na2 control citrus postharvest pathogens by disrupting the cell membrane. Foods 12:2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, Nicolosi E, 2020. Citrus origin, diffusion, and economic importance. In: Gentile A, La Malfa S, Deng Z, eds. The citrus genome. Springer Cham, pp 5-21. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are contained within the manuscript.


